Abstract
The mechanism of skin allograft rejection has been thought to require presentation of graft antigen by resident epidermal Langerhans cells (LCs). We have previously engineered mice that have a selective and constitutive absence of epidermal LCs. By using donor skin from these LC-deficient mice, we show that LCs are not required for rejection of major (FVB→B6) or minor (H-Y, male→female on B6 background) antigen-mismatched skin grafts. On the FVB background, where H-Y mismatched grafts are normally maintained indefinitely, grafts lacking LCs are efficiently rejected. Thus, LCs in the donor graft are required for long-term skin engraftment, which supports a regulatory role for LCs in skin graft acceptance.
INTRODUCTION
The current understanding of solid-tissue graft rejection holds that graft antigens are presented to T cells by antigen-presenting cells (APCs) in secondary lymphoid tissues (Rosenberg and Singer, 1992; Lakkis et al., 2000). Dendritic cells (DCs) are professional APCs that are central for many adaptive immune responses (Banchereau et al., 2000). DCs of donor origin present in the graft at the time of transplantation migrate to regional lymph nodes and are thought to be important for antigraft T-cell priming (Lechler et al., 2001). However, host DCs that acquire graft antigens also participate in rejection, and the relative contribution of donor and host-derived DCs is unclear (Benichou et al., 1999; Chen et al., 2003; Reed et al., 2003). In addition, there are many subtypes of DCs that differ in location, marker expression, and function (Banchereau et al., 2000). Certain subtypes, such as plasmacytoid DCs and DCs, that have been exposed to IL-10 or tissue growth factor-β can promote regulatory responses (Ochando et al., 2006; Svensson and Kaye, 2006). The identity and characterization of the DC subtype(s) that participate in rejection of graft tissue are critical to understanding the mechanism and may provide targets for novel therapeutic intervention.
In skin, the major DC subpopulation are Langerhans cells (LCs), which reside in a dense network throughout the epidermis (Romani et al., 2003). It has been long presumed that donor LCs present in skin grafts migrate to regional lymph nodes where they stimulate antigraft T-cell responses. This is based on the observations that LCs migrate out of skin grafts and that intact lymphatic connections to grafted skin are required for rejection (Barker and Billingham, 1968; Larsen et al., 1990). In addition, the observation that skin grafts are more immunogenic than other solid organs, such as heart, has been attributed to the large number of LCs serving as professional APCs in the skin (He et al., 2004). Moreover, the degree of immunogenicity of skin from different anatomic locations correlated with the density of LCs (Mathieson et al., 1975; Sena et al., 1976; Chen and Silvers, 1983). Although LCs are the most numerous DC subtype in the skin, the data supporting their role in graft rejection are correlative and have not been formally demonstrated. There are other APCs in the skin, including dermal DCs, macrophages, and host DC, that are recruited during inflammation, all of which could participate in graft rejection (Romani et al., 2003; Larregina and Falo, 2005; Le Borgne et al., 2006).
We have recently generated bacterial artificial chromosome (BAC) transgenic mice that use the genomic locus containing the LC-specific gene, Langerin, to selectively express diphtheria toxin subunit A (DTA) in LCs (Kaplan et al., 2005). These Langerin-DTA mice have a constitutive and complete absence of LCs in the epidermis, but other DCs in the skin and secondary lymphoid tissues are unaffected. Contact hypersensitivity (CHS) to cutaneously applied haptens, the classic assay for adaptive skin immune responses, was enhanced by approximately twofold in the absence of LCs. This showed that LCs are not required and that they in fact downregulate CHS responses.
To examine the requirement of LCs in skin graft rejection directly, Langerin-DTA mice were used as skin donors in a series of skin graft experiments.
RESULTS AND DISCUSSION
LCs are not required for major or minor antigen-mismatched graft rejection
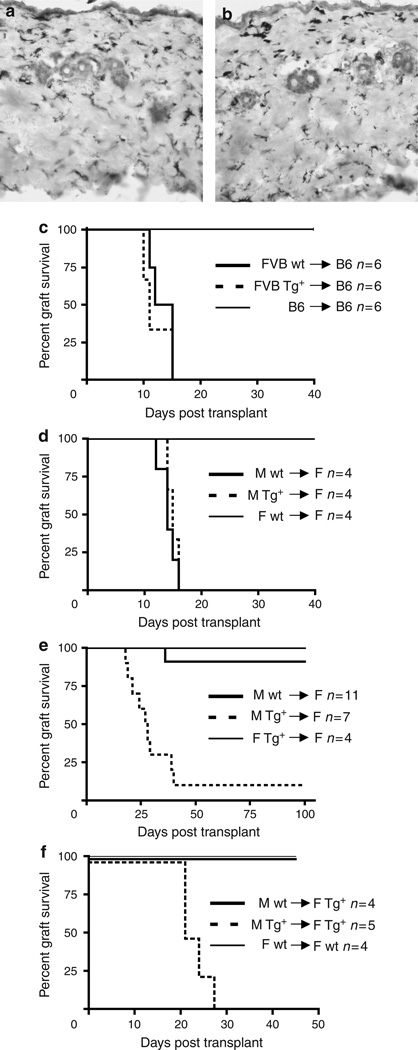
To investigate the requirement for LCs in skin graft rejection, we focused first on a fully allogeneic skin transplant model. Partial thickness skin was harvested from the flanks of Langerin-DTA (Tg+) and littermate controls (wt), which were generated and maintained on a pure FVB (H-2q) genetic background. Except for the absence of LCs, wt and Tg+ partial thickness grafts are histologically virtually indistinguishable and both contain similar numbers of dermal major histocompatibility complex (MHC) II APCs (Figure 1a and b). Donor skin was transplanted onto the flanks of C57BL/6 (B6, H-2b) recipients and monitored for signs of rejection (Figure 1c). Unlike syngeneic B6 grafts that were maintained indefinitely, both Tg+ and wt FVB grafts were rapidly rejected with similar kinetics.
Figure 1. LCs are required for skin graft acceptance, but not rejection.
Partial thickness grafts from FVB Tg+ (a) or control (b) mice were stained with for MHC II and counterstained with hematoxylin. Original magnification × 200. (c) Grafts harvested from wt (solid line) and Tg+ (broken line) FVB (H-2q) mice and grafted onto fully mismatched B6 (H-2b) mice. Both Tg+ and wt grafts were rejected with similar kinetics. Control syngeneic B6 grafts (thin line) were accepted indefinitely (> 100 days). (d) Skin grafts harvested from wt or Tg+ B6 males and transplanted onto B6 littermate females were rejected with similar kinetics. Control B6 female skin was accepted indefinitely. (e) Wt or Tg+ FVB male skin was placed on FVB littermate females. Tg+ grafts lacking LCs were rejected much more frequently than wt skin (P<0.001). Female Tg+ skin placed on littermate wt females was accepted indefinitely. (f) Wt or Tg+ FVB male skin was placed on Tg+ FVB littermate females. Tg+ grafts lacking LCs were rejected much more frequently than wt skin (P<0.001). Control female wt skin placed on littermate wt females was accepted indefinitely.
We next examined male→female (H-Y) minor mismatch grafts. Male donor skin from Tg+ and wt mice, which had been backcrossed four generations onto the B6 background, was transplanted onto littermate wt female recipients. As with the fully allogeneic system, we observed brisk rejection of both wt and Tg+ grafts with similar kinetics (Figure 1d). These data clearly establish that LCs are not required for efficient rejection of either MHC-mismatched or minor histocompatibility antigen-mismatched skin allografts.
LCs are required for graft acceptance across an H-Y barrier
Because we had previously found that CHS is augmented in Langerin-DTA mice, indicating that LCs participate in the regulation of cutaneous immune responses, we were somewhat surprised that LC-deficient skin was not rejected more rapidly than LC-replete skin. The rapid tempo of rejection in both the FVB→B6 and B6 H-Y models raised the possibility that detection of any regulatory effect of LCs could have been obscured. Significant variability in the rejection response to H-Y antigen between different strains of mice is well documented and is thought to result from different T-cell responses to the MHC–peptide complexes generated in varying MHC haplotypes (Simpson et al., 1997). B6 mice reject H-Y disparate grafts, whereas other non-H-2b strains, such as C3H (H-2k) mice, do not.
We therefore next examined male→female (H-Y) minor mismatch grafts on the FVB (H-2q) background. Male donor skin from FVB Tg+ and wt mice was transplanted onto FVB wt females. On the FVB background, we found indefinite (> 100 days) graft survival of wt male skin when it was transplanted onto wt female mice in 10/11 instances (Figure 1e). The absence of LCs, however, led to the rejection of Tg+ grafts over the course of 18–40 days in all but a single animal (Figure 1e). As Tg+ grafts express DTA, it is conceivable that the grafts could be rejected on the basis of recognition of the toxin and not H-Y. In addition, the gene CLEC4F, a poorly studied lectin expressed by Kupffer cells, is located adjacent to the gene for Langerin on the BAC (Hoyle and Hill, 1988). Although it is not thought to be expressed in the skin, CLEC4F could also serve as a rejection antigen. To control for this possibility, we transplanted female Tg+ grafts onto wt female recipients (Figure 1e, thin line). The skin from female Tg+ mice expresses DTA and other putative antigens encoded by the BAC but not male-specific antigens. These grafts were maintained indefinitely, demonstrating that expression of antigens other than H-Y are not sufficient to mediate rejection. We also transplanted male grafts onto Tg+ female recipients. Tg+ recipients contain the BAC DNA from birth and are tolerant to any potential rejection antigens that might be encoded by the BAC. Just as with wt recipients, Tg+ male grafts were rapidly rejected, whereas wt male grafts were maintained (> 50 days) (Figure 1f). Thus, rejection of male grafts that lack LC is based solely on recognition of male-specific antigens.
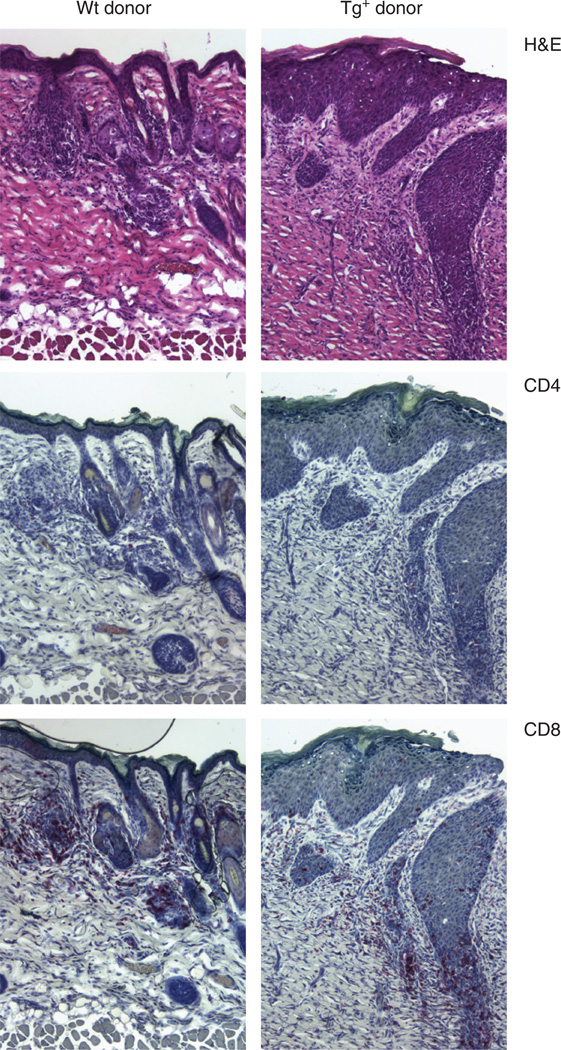
Grafts from wt male donors maintained graft size and dense hair growth. In contrast, Tg+ grafts that were rejected developed hair loss and a greater than 90% reduction in graft size. This is reflected in histology obtained on day 40 after transplantation. Microscopic remnants of the rejection response are evident in Tg+ grafts histologically as severely acanthotic tissue that lacks hair follicles and has a CD8-dominant mononuclear cell infiltrate (Figure 2). In contrast, grafted Tg− skin shows intact graft tissue with a mildly acanthotic epidermis and the presence of hair follicles. As in Tg+ donor grafts, there is also a CD8-dominant mononuclear cell infiltrate.
Figure 2. CD8+ cells infiltrate both wt and Tg+ FVB H-Y grafts.
Graft tissue was harvested from FVB females 40 days after grafting with male wt (left panels) or Tg+ (right panels) skin. Sequential transverse sections were stained with H&E (hematoxylin and eosin) (top panels), anti-CD4 (middle panels), or anti-CD8 (bottom panels). Grafts from three mice in each group were examined and representative sections are shown. Original magnification × 100.
Investigation of LC function in graft acceptance
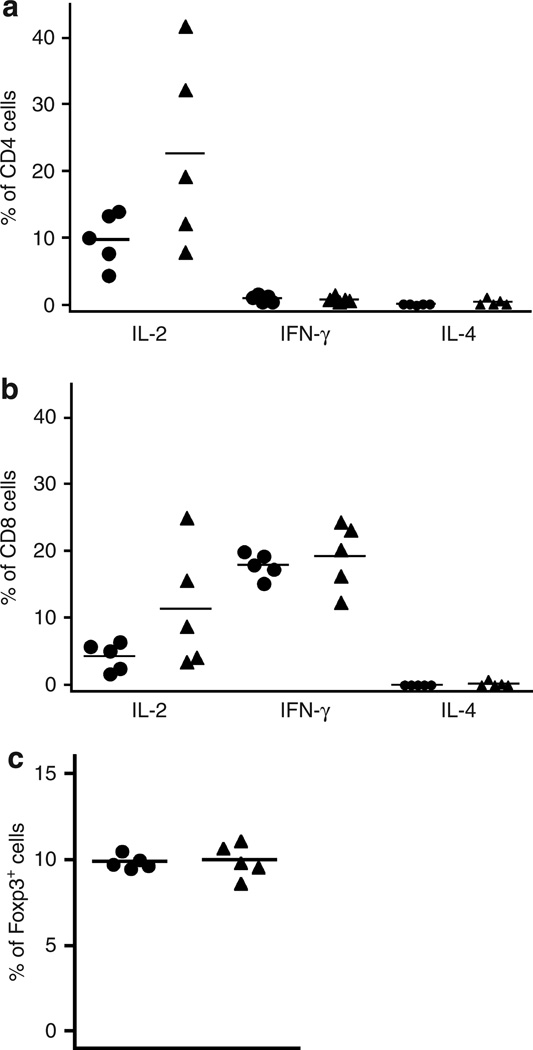
We have previously demonstrated that the absence of LCs leads to increased priming when mice are sensitized with a cutaneously applied hapten (Kaplan et al., 2005). Thus, we next examined whether alterations in T-cell responses could explain the enhanced rejection of Tg+ grafts. Responses were examined 14 days after transplantation when evidence of graft rejection first becomes evident. When we examined the percentage of cytokine-producing CD4+ T cells in skin-draining lymph nodes from mice transplanted with Tg+ or wt grafts, we observed little production of IFN-γ or IL-4 but did observe a nonsignificant increase in IL-2-producing cells in mice transplanted with Tg+ skin (Figure 3a). There was no difference in IFN-γ-producing CD8+ T cells between Tg− and Tg+, but, as with CD4+ T cells, there was a trend toward an increase in IL-2-producing cells in Tg+ recipients (Figure 3b). Similar results were obtained when comparing the total number of cytokine-producing cells (data not shown). We did not also observe a significant difference in the total numbers or percentages of activated CD4+ or CD8+ T cells in lymph node or spleen based on the levels of CD44 and CD62L expression (data not shown).
Figure 3. No differences in T-cell phenotype in Wt and Tg+ FVB H-Y graft recipients.
Cells were harvested from skin-draining lymph nodes of FVB female 14 days after grafting with male wt (circle) and Tg+ (triangle) skin. Cells were stimulated ex vivo for 3 hours with PMA, and ionomycin in the presence of IL-2. Cytokine expression was measured by intracellular flow cytometry. There was no significant difference in the percentages of CD4+ (a) or CD8+ (b) T cells expressing IFN-γ, IL-2, or IL-4 in recipients grafted with Tg+ or wt skin. (c) Day 14 after transplantation of skin grafts from FVB wt or Tg+ males, the percentage of CD4+CD25+Foxp3+ cells was quantified by flow cytometry. There was no significant difference between the groups.
The development of regulatory T cells (Treg) has also been shown to potentiate the long-term acceptance of allografts in mouse models (Qin et al., 1993; Graca et al., 2000). We therefore evaluated whether the absence of LCs affected Treg generation in graft recipients. We quantified the percentage of CD4+CD25+Foxp3+ in mice grafted with Tg+ and wt skin (Figure 3c) and found equal percentage and numbers of Tregs in both groups. Thus, we found no evidence that the absence of LCs induced obvious differences in T-cell cytokine production, activation state, or induction of Tregs that would account for the dramatic differences in rejection responses. However, these conclusions are tempered by the lack of an antigen-specific marker in this H-2q system, and it remains possible that there are effects on antigen-specific T cells that were undetectable in analyses of bulk T cells.
Skin graft rejection, like CHS, is a classic assay of the cutaneous immune response. The fact that grafts lacking epidermal LCs are efficiently rejected demonstrates now in two distinct in vivo systems that LCs are not required for skin immune responses. Moreover, we observed that minor mismatched skin grafts (FVB, H-Y) are not normally rejected on the FVB background but are efficiently rejected if the donor skin lacks LCs.
The vigorous rejection response elicited by allogeneic skin has been attributed to the high density of APCs in this tissue (He et al., 2004). The ability of LCs to migrate from transplanted skin and their ability to stimulate host T cells in vitro were part of the seminal studies that suggested the importance of donor APC-host T-cell interaction in transplant organ rejection (Larsen et al., 1990). We have clearly demonstrated that donor-derived LCs present in the skin graft tissue are not required for rejection. Skin contains numerous resident APCs other than LCs in the dermis, such as dermal DCs and macrophages (Romani et al., 2003; Larregina and Falo, 2005). In addition, monocytes and monocytic DCs are known to migrate into sites of inflammation (Le Borgne et al., 2006; Leon et al., 2007). Although little is known regarding the specific expression of male-associated antigens by particular DC subsets, male-specific genes encoded on the Y chromosome are expressed in all cell types. Thus, graft resident DCs, other than LCs that present endogenous antigens and/or host-derived DCs that present acquired antigens, are clearly sufficient for graft rejection.
The requirement of LCs for graft acceptance in at least certain instances further extends the concept that an important function of LCs is regulating cutaneous immune responses, consistent with our prior observation that LCs regulate CHS responses. There is considerable evidence that DCs participate in regulation of immune responses through both the presentation of antigen by DCs in an immature state and by DCs with regulatory properties (Steinman et al., 2003). It is interesting to note that LCs retain their regulatory capacity despite the surgically induced trauma of transplantation and suggest that they may have an intrinsic regulatory capacity. Indeed, another DC subset, plasmacytoid DCs, has been recently shown to generate regulatory effects in allografted mice treated with CD40L blockade (Ochando et al., 2006). Bone marrow-derived and epithelial DCs conditioned with factors, such as human thymic stromal lymphopoietin, IL-10, and tissue growth factor-β, have been shown to promote tolerance to presented antigens (Rimoldi et al., 2005; Svensson and Kaye, 2006). Keratinocytes are a major source of both human thymic stromal lymphopoietin and tissue growth factor-β, which raises the possibility that LCs may be acting similarly (Soumelis et al., 2002; Li et al., 2006).
As H-Y graft rejection is known to involve the generation of CD4 helper and CD8 effector cells, we anticipated finding T-cell differences in Tg+ and Tg− graft recipients. Our inability to detect any significant differences in T cells in mice that reject or accept their graft could arise from a number of factors. Changes in T-cell phenotype or activation state could have been too subtle to be detected in the polyclonal populations we examined or may be occurring within the graft and not in the skin-draining lymph node or spleen. Assays for anti-H-Y responses are relatively insensitive without the use of tetramers or in vitro restimulation with immunodominant peptides, neither of which have been developed for the H-2q haplotype. Although there was no difference in the number of Foxp3+ cells, LCs may promote the development of Treg populations that do not express Foxp3 (Shevach, 2006). Alternatively, there may be Foxp3+ Tregs induced in an antigen-specific manner, but which are too few to affect the total number of Foxp3+ cells. LCs may also primarily affect other cell types, such as plasmacytoid DCs or NK (natural killer) T cells, that have recently been reported to exert tolerogenic effects in solid organ transplantation (Oh et al., 2005; Ochando et al., 2006). Finally, LCs present in the graft tissue could exert their regulatory effect directly on graft-infiltrating effector cells. Although we demonstrated that LCs exert their regulatory effect during the priming and not elicitation phase during CHS to haptens, their role in preventing skin graft rejection may be different.
Two groups have developed LC ablation models using inducible expression of the diphtheria toxin receptor followed by injection of toxin and observed that LCs are either not required or are partially redundant for CHS responses (Bennett et al., 2005; Kissenpfennig et al., 2005). Enhanced CHS responses were not observed in either system. It is unclear whether the absence of enhanced CHS responses in these mice is due to the unanticipated elimination of Langerin + dermal DC and Langerin + CD8+ DCs when LCs are ablated (Douillard et al., 2005; Kissenpfennig et al., 2005; Bursch et al., 2007; Ginhoux et al., 2007; Poulin et al., 2007) or reflects the differences between the absence of LCs throughout ontogeny (Langerin-DTA) and inducible LC ablation in adult mice.
The fact that FVB H-Y grafts lacking LCs are rejected when transplanted onto normal animals indicates that the regulatory effect of LC is transferable with the skin. Any potential systemic effects of the constitutive absence of LCs in Langerin-DTA mice cannot be the cause for enhanced responses. Thus, in addition to ruling out a role for LCs as a requirement for skin rejection, our studies establish that the regulatory role of LCs in Langerin-DTA mice is unlikely to result from the absence of LCs during ontogeny.
MATERIALS AND METHODS
Mice
C57BL/6 (H-2b, hereafter B6) and FVB (H-2q) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and Harlan (Indianapolis, IN). The Langerin-DTA mice were described previously (Kaplan et al., 2005). They were derived and have been maintained on a pure FVB background or backcrossed onto B6 (generation 4). Mice were housed in microisolator cages and fed autoclaved food and acidified water. The Yale Institutional Animal Care and Use Committee approved all mouse protocols.
Antibodies
Fluorochrome- or biotin-labeled antibodies to CD4 (RM4-5), CD8α (53-6.7), IFN-γ (XMG1.2), and IL-2 (JES6-5H4) were purchased from BD Pharmingen (San Diego, CA). Anti-Foxp3 (FJK-16s) was purchased from eBiosciences (San Diego, CA). Anti I-A/I-E (M5/ 114.15.2) was purchased from BioLegend (San Diego, CA).
Skin grafting
Partial thickness skin transplantation was performed using abdominal skin or tail skin (Figure 1f) from donor mice as described previously (Obhrai et al., 2006). Grafts were monitored daily for the first 4 weeks after transplantation and then every other day. Rejection was defined as >90% graft loss.
Flow cytometry
To measure intracellular cytokine production, lymphocytes were stimulated ex vivo with PMA and ionomycin for 3 hours in the presence of IL-2 (10U per well) and monensin (Obhrai et al., 2006). Cells were then washed, stained for surface markers, fixed, permeabilized with 0.25% saponin, and incubated with anti-cytokine antibody for 1 hour at room temperature. All samples were analyzed on an LSR-II flow cytometer (BD Biosciences, Mountain View, CA). The data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
Immunohistochemistry
Transverse tissue sections were obtained and processed as described (Hannum et al., 2000). The sections were stained with biotinylated anti-CD4, MHC II, or CD8 followed by streptavidin alkaline-phosphatase (Invitrogen, Carlsbad, CA), and developed with Fastred as described (Hannum et al., 2000).
Statistical methods
The significance in skin graft rejection incidence was calculated by Breslow–Gehan–Wilcoxon rank sum. Differences in the number of cytokine-expressing cells were evaluated using Student’s t-test.
ACKNOWLEDGMENTS
This work was supported by NIH Grants K08 AR651092 (DHK). JSO was supported by a grant from the National Kidney Foundation and the American Heart Association 775031N. MO was supported by a grant from the American Society of Transplantation. MJS was supported by NIH grant ROI-AR4407.
Abbreviations
- APC
antigen-presenting cell
- BAC
bacterial artificial chromosome
- CHS
contact hypersensitivity
- DC
dendritic cell
- DTA
diphtheria toxin subunit A
- LC
Langerhans cell
- MHC
major histocompatibility complex
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Barker CF, Billingham RE. The role of afferent lymphatics in the rejection of skin homografts. J Exp Med. 1968;128:197–221. doi: 10.1084/jem.128.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162:352–358. [PubMed] [Google Scholar]
- Bennett CL, van Rijn E, Jung S, Inaba K, Steinman RM, Kapsenberg ML, et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, et al. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HD, Silvers WK. Influence of Langerhans cells on the survival of H-Y incompatible skin grafts in rats. J Invest Dermatol. 1983;81:20–23. doi: 10.1111/1523-1747.ep12537487. [DOI] [PubMed] [Google Scholar]
- Chen Y, Demir Y, Valujskikh A, Heeger PS. The male minor transplantation antigen preferentially activates recipient CD4+ T cells through the indirect presentation pathway in vivo. J Immunol. 2003;171:6510–6518. doi: 10.4049/jimmunol.171.12.6510. [DOI] [PubMed] [Google Scholar]
- Douillard P, Stoitzner P, Tripp CH, Clair-Moninot V, Ait-Yahia S, McLellan AD, et al. Mouse lymphoid tissue contains distinct subsets of langerin/CD207 dendritic cells, only one of which represents epidermal-derived Langerhans cells. J Invest Dermatol. 2005;125:983–994. doi: 10.1111/j.0022-202X.2005.23951.x. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Collin MP, Bogunovic M, Abel M, Leboeuf M, Helft J, et al. Blood-derived dermal Langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204:3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graca L, Honey K, Adams E, Cobbold SP, Waldmann H. Cutting edge: anti-CD154 therapeutic antibodies induce infectious transplantation tolerance. J Immunol. 2000;165:4783–4786. doi: 10.4049/jimmunol.165.9.4783. [DOI] [PubMed] [Google Scholar]
- Hannum LG, Haberman AM, Anderson SM, Shlomchik MJ. Germinal center initiation, variable gene region hypermutation, and mutant B cell selection without detectable immune complexes on follicular dendritic cells. J Exp Med. 2000;192:931–942. doi: 10.1084/jem.192.7.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Schenk S, Zhang Q, Valujskikh A, Bayer J, Fairchild RL, et al. Effects of T cell frequency and graft size on transplant outcome in mice. J Immunol. 2004;172:240–247. doi: 10.4049/jimmunol.172.1.240. [DOI] [PubMed] [Google Scholar]
- Hoyle GW, Hill RL. Molecular cloning and sequencing of a cDNA for a carbohydrate binding receptor unique to rat Kupffer cells. J Biol Chem. 1988;263:7487–7492. [PubMed] [Google Scholar]
- Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal Langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, et al. Dynamics and function of Langerhans cells in vivo dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Lakkis FG, Arakelov A, Konieczny BT, Inoue Y. Immunologic “ignorance” of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med. 2000;6:686–688. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
- Larregina AT, Falo LD., Jr Changing paradigms in cutaneous immunology: adapting with dendritic cells. J Invest Dermatol. 2005;124:1–12. doi: 10.1111/j.1523-1747.2004.23554.x. [DOI] [PubMed] [Google Scholar]
- Larsen CP, Steinman RM, Witmer-Pack M, Hankins DF, Morris PJ, Austyn JM. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990;172:1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne M, Et, Goubier A, Lira SA, Sirard JC, van Rooijen N, et al. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity. 2006;24:191–201. doi: 10.1016/j.immuni.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Lechler R, Ng WF, Steinman RM. Dendritic cells in transplantation—friend or foe? Immunity. 2001;14:357–368. doi: 10.1016/s1074-7613(01)00116-9. [DOI] [PubMed] [Google Scholar]
- Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- Mathieson BJ, Flaherty L, Bennett D, Boyse EA. Differences in the rejection of trunk skin and tail skin allografts involving weak histocompatibility loci. Transplantation. 1975;19:525–527. [PubMed] [Google Scholar]
- Obhrai JS, Oberbarnscheidt MH, Hand TW, Diggs L, Chalasani G, Lakkis FG. Effector T cell differentiation and memory T cell maintenance outside secondary lymphoid organs. J Immunol. 2006;176:4051–4058. doi: 10.4049/jimmunol.176.7.4051. [DOI] [PubMed] [Google Scholar]
- Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- Oh K, Kim S, Park SH, Gu H, Roopenian D, Chung DH, et al. Direct regulatory role of NKT cells in allogeneic graft survival is dependent on the quantitative strength of antigenicity. J Immunol. 2005;174:2030–2036. doi: 10.4049/jimmunol.174.4.2030. [DOI] [PubMed] [Google Scholar]
- Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains Langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204:3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, et al. “Infectious” transplantation tolerance. Science. 1993;259:974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- Reed AJ, Noorchashm H, Rostami SY, Zarrabi Y, Perate AR, Jeganathan AN, et al. Alloreactive CD4 T cell activation in vivo: an autonomous function of the indirect pathway of alloantigen presentation. J Immunol. 2003;171:6502–6509. doi: 10.4049/jimmunol.171.12.6502. [DOI] [PubMed] [Google Scholar]
- Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- Romani N, Holzmann S, Tripp CH, Koch F, Stoitzner P. Langerhans cells—dendritic cells of the epidermis. APMIS. 2003;111:725–740. doi: 10.1034/j.1600-0463.2003.11107805.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg AS, Singer A. Cellular basis of skin allograft rejection: an in vivo model of immune-mediated tissue destruction. Annu Rev Immunol. 1992;10:333–358. doi: 10.1146/annurev.iy.10.040192.002001. [DOI] [PubMed] [Google Scholar]
- Sena J, Wachtel SS, Murphy G. A comparison of the survival of H-Y incompatible ear, tail, and body skin grafts. Transplantation. 1976;21:412–416. doi: 10.1097/00007890-197605000-00010. [DOI] [PubMed] [Google Scholar]
- Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Simpson E, Scott D, Chandler P. The male-specific histo-compatibility antigen, H-Y: a history of transplantation, immune response genes, sex determination and expression cloning. Annu Rev Immunol. 1997;15:39–61. doi: 10.1146/annurev.immunol.15.1.39. [DOI] [PubMed] [Google Scholar]
- Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Svensson M, Kaye PM. Stromal-cell regulation of dendritic-cell differentiation and function. Trends Immunol. 2006;27:580–587. doi: 10.1016/j.it.2006.10.006. [DOI] [PubMed] [Google Scholar]