Abstract
We evaluated the effect of the levonorgestrel intrauterine device (LNG-IUD) on genital HIV-1 RNA shedding and inflammation among 25 HIV-infected women. Blood, endocervical, and cervicovaginal lavage samples were collected from HIV-infected women not taking antiretrovirals before LNG-IUD insertion and 1-, 3-, and 6-months thereafter. HIV-1 RNA was quantitated by real-time RT-PCR. Inflammatory markers were measured by EIA. Genital HIV-1 RNA shedding and inflammatory markers did not differ between LNG-IUD placement and month 6, with the exception of interleukin-1B, which increased (0.42 log10; 95% CI: 0.10, 0.75). The LNG-IUD did not increase genital HIV-1 RNA shedding after 6-months of use.
Keywords: HIV infection, IUD, genital HIV shedding, levonorgestrel
INTRODUCTION
The World Health Organization (WHO) recommends the provision of reliable contraception to HIV-infected women as a primary strategy for the prevention of pediatric HIV-1 infection resulting from unintended pregnancies (1). The levonorgestrel releasing intrauterine device (LNG-IUD) is one of the most effective, long-term, reversible contraceptive methods available (2). In addition to its contraceptive benefits, the LNG-IUD has been shown to decrease menstrual blood loss and reduce the incidence of pelvic inflammatory disease (PID) in healthy IUD users (3, 4).
In HIV-infected women (including women with AIDS who are clinically well on antiretroviral therapy [ARVs]), WHO’s medical eligibility criteria for contraceptive use ranks initiation of LNG-IUD as a Category 2 device, meaning the advantages generally outweigh the theoretical or proven risks (5). A major theoretical risk of LNG-IUD use by HIV-1–infected women is increased infectiousness secondary to irregular menstrual bleeding, hormonal effects, or recruitment of inflammatory cells to the genital tract (6–8). The primary objective of this study was to determine if LNG-IUD increased genital HIV-1 RNA after 6 months of use. We hypothesized that LNG-IUD use would not increase genital HIV-1 RNA and might improve hemoglobin concentration (Hb) among HIV-1–infected women.
METHODS
HIV-1–infected women attending City Council Family Planning Centers and the Family AIDS Care and Education Services clinic at the Centre for Respiratory Disease Research in Nairobi, Kenya were approached to participate in the study. Eligibility criteria included ages 18 to 50 years; HIV-1 infection; no ARVs within 90 days of study entry; CD4 count >250 cells/mm3; history of a prior pregnancy > 20 weeks gestation; desired LNG-IUD contraception for at least 6 months; willing and able to comply with the protocol; and able to provide informed consent. Exclusion criteria included hysterectomy; untreated gonorrhea, chlamydia, syphilis, or PID (defined as pelvic pain plus either adnexal or cervical motion tenderness); menopause (defined as lack of menses for ≥1 year); endometritis or infected abortion within 90 days; use of injectable contraception within 180 days or any other hormonal contraception within 30 days of study entry; or pregnancy. Institutional Review Boards at University of Washington and Kenya Medical Research Institution granted study approval. Written informed consent was obtained from all participants.
At screening and all subsequent study visits, participants completed a structured questionnaire and underwent pelvic examination to collect samples. Visible blood precluded sample collection and the visit was rescheduled. Vaginal fluid was collected from the posterior vaginal fornix using three Dacron swabs (Puritan Hardwood Products, Guilford, Maine). The first swab was used for Gram stain to diagnose bacterial vaginosis (BV) by Nugent’s criteria (9). The second swab was used for KOH microscopy to detect yeast. The third swab was used to culture Trichomonas vaginalis (TV) (InPouch™; Biomed Diagnostics, White City, Oregon). Cervical swabs were used to collect secretions for Neisseria gonorrheoea and Chlamydia trachomatis detection using PCR (Amplicor, Roche Diagnostic Systems, Indianapolis, IN). After collection of endocervical secretions, three saturated filter-paper wicks (Sno-Strip™ Chavin Pharmaceuticals, Romford, United Kingdom) were transferred to cryotubes containing 500μL of 4M guanidinium isothiocyanate and 2-mercaptoethanol and frozen at −70°C. Cervical vaginal lavage (CVL) was performed last and consisted of flushing the cervix and vagina five times with 7mL of lithium chloride (10). CVL fluid was stored at −70°C until analyzed at the University of Washington, Retrovirology Laboratory.
Participants returned to the clinic two weeks after the screening visit to obtain test results. Women with chlamydia, gonorrhea or PID were treated according to local guidelines but were not enrolled in the study; however, one woman was inadvertently enrolled after treatment for gonorrhea. Subjects with TV, BV, or yeast were treated prior to enrollment.
Eligible participants were scheduled for enrollment and IUD placement within 7 days of the onset of menses. At enrollment, 1-, 3-, and 6-month follow-up visits, a urine pregnancy test was performed and a blood sample was collected to measure plasma HIV-1 RNA, CD4 count, and complete blood count. At enrollment, endocervical secretions and CVL fluid were collected for HIV-1 RNA quantitation followed by insertion of the LNG-IUD according to manufacturer’s instructions (11). HIV-1 RNA was measured as it is a good surrogate for infectivity, whereas the relationship between DNA and infectivity is uncertain (12). At each follow-up visit, participants also underwent testing for sexually transmitted infections (STIs). Participants were counseled on STI prevention and consistent use of condoms. CD4 count was enumerated using flow cytometry (FACS-Calibur, Becton Dickinson, Cowley, Oxfordshire, UK). Hemoglobin concentration was performed by an automated cell counter and measured by a colorimeter. An independently validated real-time HIV-1 RNA PCR assay was used to quantitate plasma HIV-1 RNA with a 60 copies/mL lower limit of detection (LLOD); endocervical wick HIV-1 RNA with a 1575 HIV-1 RNA copies/mL LLOD; and CVL HIV-1 RNA with a 30 copies/mL LLOD (13–15). To improve the sensitivity and precision of HIV-1 RNA detection, both endocervical wicks and CVL were collected; as genital HIV-1 RNA varies least in endocervical wicks and most in CVL (14). Interleukin (IL)-1β, IL-8, and secretory leukocyte protease inhibitor (SLPI) were measured in CVL fluid using enzyme immunoassays.
Data for plasma, endocervical, and CVL RNA as well as IL-β, IL-8 and SLPI were log10-transformed, as these data were not normally distributed. Mean HIV-1 RNA, cytokine, SLPI and Hb concentrations and standard deviations (SD) were calculated at each visit. The primary outcome was the change in endocervical and CVL HIV-1 RNA between enrollment and 6-month visits. Secondary outcomes included changes in concentration of pro-inflammatory cytokines, SLPI, and Hb; LNG-IUD removal/expulsion; incident STI; and PID. In this proof of principle study, paired analyses were performed to allow each subject to serve as her own control. Wilcoxon sign rank test was used to compare continuous outcomes and McNemar’s test was used to compare categorical outcomes. Linear regression with generalized estimating equations, an independent correlation structure, and robust standard errors were used to assess genital HIV-1 RNA over time (days since IUD placement) adjusted for plasma HIV-1 RNA. Sample size estimate was based upon mean and within-individual SD of 3.903 and 0.55 log10 HIV-1 RNA copies/ml, respectively (14). Fifteen participants were necessary to provide 90% power to detect a 1 log10 copies/ml difference significant at the 5% level. All statistical tests were assessed using a 2-sided alpha of 0.05 using Stata version 10.1 (StataCorp, Inc., College Station, TX).
RESULTS
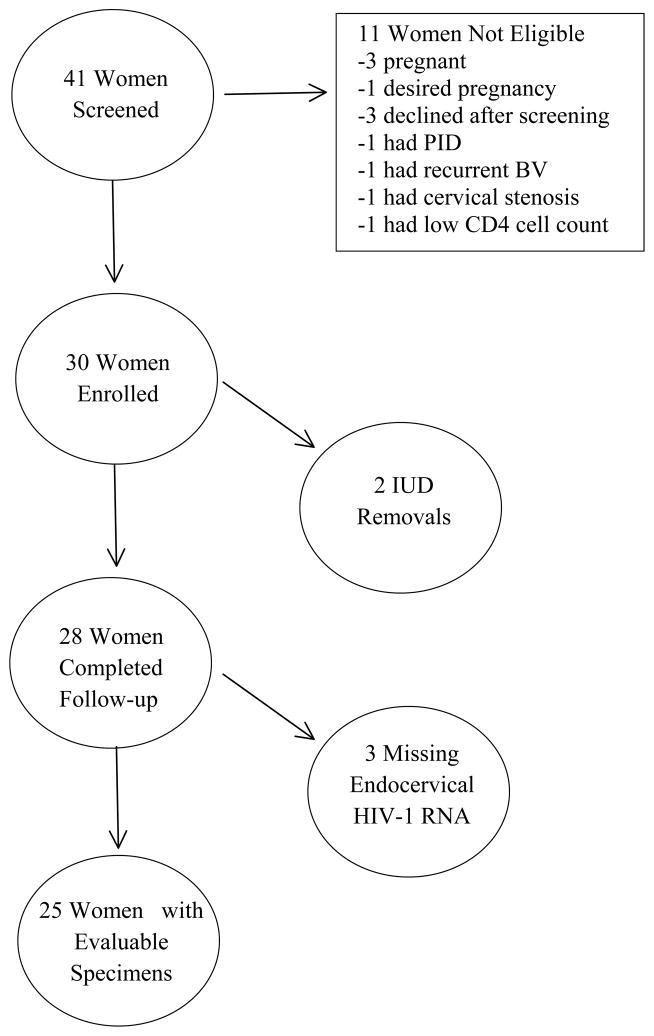
Between July 2008 and July 2009, 41 women were screened, 11 were ineligible and 30 were enrolled (Figure 1). The median age of participants was 31 years (range: 21–42) and the median number of live children was 2 (range: 1–4). Close to half (48%) were married or had a steady partner and the majority (72%) were employed. Enrollment CD4 count, HIV-1 RNA, and Hb are presented in Table 1.
Figure 1.
Study Population
Table 1.
HIV-1 RNA, proinflammatory cytokine concentration, hemoglobin and CD4+ cell count at enrollment and by month of follow-up (N=25)
| Enrollment | 1 month | 3 months | 6 months | p-value*** | |
|---|---|---|---|---|---|
| Log10 plasma HIV-1 RNA** | 3.79 ± 1.04 | 4.12 ± 1.10 | 4.40 ± 0.84 | 3.87 ± 1.03 | 0.34 |
| Log10 endocervical HIV-1 RNA | 4.26 ± 0.91 | 3.99 ± 0.85 | 4.06 ± 0.78 | 4.01 ± 0.94 | 0.55 |
| Detectable endocervical HIV-1 RNA (>1575) | 17 (68) | 16 (64) | 18 (72) | 14 (56) | 0.26 |
| Log10 CVL HIV-1 RNA | 2.46 ± 0.77 | 2.39 ± 0.65 | 2.40 ± 0.65 | 2.47 ± 1.13 | 0.60 |
| Detectable HIV-1 RNA by CVL (>60) | 15 (60) | 15 (60) | 17 (68) | 14 (56) | 0.71 |
| CD4+ cell count (cells/mm3) | 617 ± 234 | 548 ± 214 | 496 ± 209 | 493 ± 196 | <0.001 |
| Hemoglobin (g/dL) | 12.81 ± 1.80 | 12.82* ± 1.65 | 13.00 ± 1.92 | 13.20 ± 1.45 | 0.16 |
| Anemia† | 7 (25) | 7 (29)* | 6 (24) | 4 (17)* | 0.50 |
| Log10 IL-1β | 1.53 ± 0.73 | 1.88 ± 0.85 | 1.84 ± 0.68 | 2.05 ± 0.93 | 0.02 |
| Log10 IL-8 | 3.07 ± 0.63 | 3.12 ± 0.60 | 3.09 ± 0.53 | 3.29 ± 0.57 | 0.08 |
| Log10 SLPI | 5.55 ± 0.54 | 5.08 ± 0.45 | 5.30 ± 0.45 | 5.53 ± 0.45* | 0.85 |
Result are displayed as mean ± standard deviation or N (%)
N=24
Excludes samples that were inhibited or partially inhibited (N at enrollment = 19; N at 1 month = 15; N at 3 months = 18; N at 6 months =18).
Compares enrollment value to the value at the 6 month follow-up visit; Wilcoxon sign rank test used for continuous outcomes and McNemar’s test used for categorical outcomes.
Hemoglobin <12.0 (g/dL)
Over 90% of the women continued the IUD for six-months with no incident pregnancies and few reports of intermenstrual bleeding. Vaginal symptoms (odor, discharge, or irritation/itching) were reported in 21% (16 out of 75) of all visits. One participant reported abdominal pain. No participant reported dyspareunia.
At screening, one participant tested positive for gonorrhea and was treated. This participant also tested positive for both gonorrhea and chlamydia at enrollment. She was treated again. There were no cases of chlamydia or TV at screening. During follow-up, there were 3 additional cases of gonorrhea, 1 case of TV, and no cases of PID.
Plasma HIV-1 RNA did not change over time. Endocervical HIV-1 RNA decreased at each follow-up visit compared to enrollment; however, these changes were not statistically significant (linear coefficient adjusted for plasma RNA = −0.0005; 95% CI; −0.002, 0.001). HIV-1 RNA concentration in CVL was similar at enrollment and follow-up (linear coefficient adjusted for plasma RNA = 0.0005; 95% CI −0.002, 0.003) (Table 1).
Compared to enrollment, mean Hb increased over time with the highest Hb (13.2 g/dL) observed at month 6. In addition, we observed a decrease in the proportion of women who were anemic (Hb <12.0 g/dL), though neither of these differences were statistically significant (Table 1).
IL-1β levels increased significantly between enrollment and 6 months after IUD placement (mean difference = 0.42; 95% CI: 0.10, 0.75). IL-8 (mean difference = 0.22; 95% CI: −0.007, 0.45) and SLPI (mean difference = −0.02; 95% CI: −0.22, 9.25) levels were similar at enrollment and 6 months after IUD placement (Table 1).
DISCUSSION
This is one of the first investigations to evaluate the effects of LNG-IUD on genital HIV-1 RNA among HIV-infected women not taking ARVs in a resource-limited setting. We did not find significant changes in genital HIV-1 RNA (endocervical or cervicovaginal) six months after placement of the LNG-IUD. This finding is consistent with a smaller prospective study among 12 HIV-infected women using LNG-IUD conducted in Finland (7). Among the 10 participants who were taking ARVs in this study, the proportion with detectable genital HIV-1 RNA did not change after 1 year. Furthermore, genital HIV-1 RNA was not detected in either of the 2 participants who were not taking ARVs. A study of genital proviral DNA and copper IUD use also reported that genital HIV-1 proviral DNA did not differ significantly from baseline to 4-months of use (16). Despite the observational designs, these prospective studies support the safety of levonorgestrel-releasing or copper IUD use in HIV-infected women.
We observed a slight, albeit not statistically significant, improvement in Hb and a decrease in the proportion of women with anemia six months after LNG-IUD insertion. This finding was not surprising since suppression of the endometrial epithelium is the main contraceptive mechanism of the LNG-IUD (2). The reduced blood loss is beneficial to women with anemia and may also reduce male partners’ exposure to potentially infectious virus.
Although there were no cases of PID, our sample size and length of follow-up was limited. The risk of PID is highest within the first 20 days of IUD insertion, which suggests bacteria are transferred from an infected cervix into the uterus at the time of insertion (17). Therefore, screening for gonorrhea and chlamydia is recommended when possible. The type of test should be chosen in accordance with local guidance. We screened for STIs four times over a 6-month time frame, which is not feasible in clinical practice. HIV-infected women should maintain timely access to medical services for rigorous STI screening, testing, and prompt treatment. In addition, STI prevention through condom use should be advised. There is evidence to support STI and PID treatment with the IUD in-situ, as long as there is adequate clinical follow-up (18, 19). Furthermore, the LNG-IUD itself has not been shown to increase the risk of PID in users who acquire a cervical infection (20).
Although there was a slight increase in IL-1β levels, there was no corresponding increase in endocervical or cervicovaginal HIV-1 RNA suggesting the LNG-IUD may be associated with slight inflammation in the genital tract but not sufficient to increase HIV-1 RNA levels.
Our study has its limitations. The small sample size may have hindered our ability to detect differences; however, the paired analysis was designed to increase power. In addition, we followed participants for 6 months and it is possible that longer follow-up may have resulted in differences in HIV-1 shedding or PID cases. Irregular vaginal bleeding caused by LNG-IUD typically occurs within the first 3 months after insertion (2); therefore, increased shedding as a result of LNG-IUD should have been detected. Furthermore, IUD associated PID is six times higher within the first 20 days of insertion versus later times, so it is unlikely that additional time would have detected any cases attributed to IUD use (17). Next, we did not evaluate participants for semen exposure, which may influence SLPI concentrations but not proinflammatory cytokines (21). Last, because we excluded women with CD4 counts ≤250 cells/mm3, our findings may not be applicable to women with AIDS or unknown CD4 counts.
In summary, the LNG-IUD was well tolerated in our population of HIV-1 infected women living in a resource-limited setting. No significant change in genital HIV-1 RNA shedding was observed. Potential additional health benefits, namely reduced menstrual blood loss and reduced anemia, make it a highly attractive contraceptive option for HIV-infected women, including those not taking ARVs.
Acknowledgments
Source of Funding:
This research was supported by a grant from the Puget Sound Partners for Global Health (Grant #2007-32); the American College of Obstetrics & Gynecology; UW CFAR, P30 AI-27757; AI-38858; and HD-40540.
We would like to thank the Director of KEMRI. We are especially grateful to the study participants who volunteered for the study and the study staff at City Council Family Planning Centers and Family AIDS Care and Education Services (FACES) clinic at the Centre for Respiratory Disease Research. We thank Joan Dragavon and Socorro Harb from the UW-ACTG Virology Specialty Laboratory for technical support.
Footnotes
This research was conducted in Nairobi, Kenya.
Please address reprint requests to above author.
Conflict of Interest: No conflicts declared.
This abstract was presented at the 17th Conference on Retroviruses and Opportunistic Infections in San Francisco, CA; February 16–19, 2010.
References
- 1.World Health Organization. PMTCT strategic vision 2010–2015: prevention mother-to-child transmission of HIV to reach the UNGASS and Milennium Development Goals. Vol. 2010 Switzerland: 2010. Feb 2, [Google Scholar]
- 2.Luukkainen T, Toivonen J. Levonorgestrel-releasing IUD as a method of contraception with therapeutic properties. Contraception. 1995;52:269–76. doi: 10.1016/0010-7824(95)00210-2. [DOI] [PubMed] [Google Scholar]
- 3.Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception. 1994;49:56–72. doi: 10.1016/0010-7824(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 4.Kaunitz AM, Meredith S, Inki P, Kubba A, Sanchez-Ramos L. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104–16. doi: 10.1097/AOG.0b013e3181a1d3ce. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Medical eligibility criteria for contraceptive use. Switzerland: 2009. [PubMed] [Google Scholar]
- 6.Ghanem KG, Shah N, Klein RS, et al. Influence of sex hormones, HIV status, and concomitant sexually transmitted infection on cervicovaginal inflammation. J Infect Dis. 2005;191:358–66. doi: 10.1086/427190. [DOI] [PubMed] [Google Scholar]
- 7.Heikinheimo O, Lehtovirta P, Suni J, Paavonen J. The levonorgestrel-releasing intrauterine system (LNG-IUS) in HIV-infected women--effects on bleeding patterns, ovarian function and genital shedding of HIV. Hum Reprod. 2006;21:2857–61. doi: 10.1093/humrep/del264. [DOI] [PubMed] [Google Scholar]
- 8.Phillips SJ, Curtis KM, Polis CB. Effect of hormonal contraceptive methods on HIV disease progression: a Systematic Review. AIDS. 2012 doi: 10.1097/QAD.0b013e32835bb672. [DOI] [PubMed] [Google Scholar]
- 9.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell C, Paul K, Agnew K, Gaussman R, Coombs RW, Hitti J. Estimating volume of cervicovaginal secretions in cervicovaginal lavage fluid collected for measurement of genital HIV-1 RNA levels in women. J Clin Microbiol. 2011;49:735–6. doi: 10.1128/JCM.00991-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. [Accessed August 24, 2012];Mirena Placement-and-removal. at http://www.mirena-us.com/hcp/placement-and-removal/precise-placement.jsp.
- 12.Baeten JM, Kahle E, Lingappa JR, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011;3:77ra29. doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuckerman RA, Lucchetti A, Whittington WL, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007;196:1500–8. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]
- 14.Coombs RW, Wright DJ, Reichelderfer PS, et al. Variation of human immunodeficiency virus type 1 viral RNA levels in the female genital tract: implications for applying measurements to individual women. J Infect Dis. 2001;184:1187–91. doi: 10.1086/323660. [DOI] [PubMed] [Google Scholar]
- 15.Baeten JM, Strick LB, Lucchetti A, et al. Herpes simplex virus (HSV)-suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial. J Infect Dis. 2008;198:1804–8. doi: 10.1086/593214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson BA, Morrison CS, Sekadde-Kigondu C, et al. Effect of intrauterine device use on cervical shedding of HIV-1 DNA. AIDS. 1999;13:2091–7. doi: 10.1097/00002030-199910220-00012. [DOI] [PubMed] [Google Scholar]
- 17.Farley TM, Rosenberg MJ, Rowe PJ, Chen JH, Meirik O. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339:785–8. doi: 10.1016/0140-6736(92)91904-m. [DOI] [PubMed] [Google Scholar]
- 18.Larsson B, Wennergren M. Investigation of a copper-intrauterine device (Cu-IUD) for possible effect on frequency and healing of pelvic inflammatory disease. Contraception. 1977;15:143–9. doi: 10.1016/0010-7824(77)90012-9. [DOI] [PubMed] [Google Scholar]
- 19.Teisala K. Removal of an intrauterine device and the treatment of acute pelvic inflammatory disease. Ann Med. 1989;21:63–5. doi: 10.3109/07853898909149184. [DOI] [PubMed] [Google Scholar]
- 20.Soderberg G, Lindgren S. Influence of an intrauterine device on the course of an acute salpingitis. Contraception. 1981;24:137–43. doi: 10.1016/0010-7824(81)90086-x. [DOI] [PubMed] [Google Scholar]
- 21.Agnew KJ, Aura J, Nunez N, et al. Effect of semen on vaginal fluid cytokines and secretory leukocyte protease inhibitor. Infect Dis Obstet Gynecol. 2008;2008:820845. doi: 10.1155/2008/820845. [DOI] [PMC free article] [PubMed] [Google Scholar]