Abstract
Oxidative damage is a well-established driver of aging. Evidence of oxidative stress exists in aged and degenerated discs, but it is unclear how it affects disc metabolism. In this study, we first determined whether oxidative stress negatively impacts disc matrix metabolism using disc organotypic and cell cultures. Mouse disc organotypic culture grown at atmospheric oxygen (20% O2) exhibited perturbed disc matrix homeostasis, including reduced proteoglycan synthesis and enhanced expression of matrix metalloproteinases, compared to discs grown at low oxygen levels (5% O2). Human disc cells grown at 20% O2 showed increased levels of mitochondrial-derived superoxide anions and perturbed matrix homeostasis. Treatment of disc cells with the mitochondria-targeted reactive oxygen species (ROS) scavenger XJB-5-131 blunted the adverse effects caused by 20% O2. Importantly, we demonstrated that treatment of accelerated aging Ercc1−/Δmice, previously established to be a useful in vivo model to study age-related intervertebral disc degeneration (IDD), also resulted in improved disc total glycosaminoglycan content and proteoglycan synthesis. This demonstrates that mitochondrial-derived ROS contributes to age-associated IDD in Ercc1−/Δmice. Collectively, these data provide strong experimental evidence that mitochondrial-derived ROS play a causal role in driving changes linked to aging-related IDD and a potentially important role for radical scavengers in preventing IDD.
Keywords: Aging, oxidative stress, reactive oxygen species (ROS), intervertebral discs, radical scavenger, nitroxide, matrix proteoglycan
INTRODUCTION
Intervertebral disc degeneration (IDD) is the underlying cause of many spine-related disorders, which result in tremendous socioeconomic burden 1. Aging is a major etiological factor of IDD, as evidenced by the dramatic increase of IDD with age 2, 3. Intervertebral discs exhibit many age-related degenerative changes, including an increase in the number and size of disc fissures and accumulation of degraded matrix molecules 4. With aging, there is progressive loss of disc water and matrix proteoglycan 5, which results in altered biomechanics and pathologic outcomes such as spine stiffness, spinal stenosis, and disabling chronic back pain 5, 6. Physiologic disc vascularity disappears in the second decade of life in humans, and this likely contributes to a reduction in nutrient supply to the disc, accumulation of cellular waste products, and an increasingly acidic environment (pH 6.3–6.6) that can severely compromise cell function or cause cell death 7.
The free radical theory of aging posits that aging is the result of time-dependent accumulation of cellular and molecular damage caused by reactive oxygen species (ROS), in particular mitochondrial-derived ROS 8, 9, 10. Numerous studies have shown that oxidative damage increases with age in many tissues and various organisms 11. Endogenous ROS originate as byproducts of normal oxygen-utilizing metabolic processes and the cellular response to pro-inflammatory cytokines 12, 13. The major site of ROS production in non-immune cells is in the mitochondria. Although residing in an environment of low oxygen tension, cells of normal intact disc tissue are not entirely anaerobic and still carry out some level of oxidative metabolism 14. With aging, however, there is an accumulation of fissures, resulting in neovascularization and exposure of the otherwise hypoxic resident cells to higher oxygen tension 15. Injury to the intervertebral discs can also cause inflammation-driven ROS production 16, 17.
There is growing evidence indicating that oxidative damage accumulates with aging in discs. Immunohistochemical analysis revealed a higher level of carboxymethyl-lysine (CML), a biomarker of oxidized protein, in degenerated intervertebral discs from aged patients compared to young normal discs 18. Activation of NF-κB, a transcription factor centrally involved in cellular response to oxidative and inflammatory stress, also increases in old discs 18. In addition, elevated levels of advanced glycation end products (AGEs), caused by nonenzymatic glycosylation and oxidation of proteins and lipids, are found in disc tissues from aged individuals compared to young 19, 20. Disc tissue is rich in collagen, and the long half-life of collagen (>100 years for collagen type II) 21 makes it particularly susceptible to progressive accumulation of AGEs. The best characterized AGEs in intervertebral discs are pentosidine and carboxymethyl-lysine. The former is found in collagen and directly increases with donor age in human cartilage. Pentosidine, which cross-links collagen molecules, might play an important role in increased collagen stiffness and the weakening of cartilage biomechanics with old age 19.
What remains to be determined is whether oxidative damage drives disc aging or is a product of it. Previously we reported spontaneous age-dependent disc degeneration in a murine model (Ercc1−/Δ mice) of a human progeroid syndrome caused by deficiency of the DNA repair endonuclease ERCC1-XPF 22. These mice showed loss of disc height, premature loss of disc proteoglycan (PG) and reduced matrix PG synthesis. We used these mice as a model highly sensitive to oxidative damage to ask if reducing mitochondrial-derived ROS production was sufficient to attenuate changes associated with aging-related IDD. To test this hypothesis, the mice were chronically and systemically treated with XJB-5-131, a mitochondria-targeted ROS scavenger previously demonstrated to be therapeutic in rodent models of hemorrhagic shock and sepsis 23. GAG content and PG synthesis of the intervertebral discs of treated and untreated mice were assessed. In addition, we studied the effects of exogenous oxidative stress on matrix homeostasis of normal mouse discs and human disc cells in vitro.
METHODS
Disc cell culture
Human nucleus pulposus (hNP) cells were isolated from surgical specimens and cultured as previously described 24. hNP cells were isolated from 8 patients (mean age 43 ± 4 years, and average Thompson 25 degeneration grade 2.8 ± 0.7) who underwent elective surgical procedures for degenerative spine disease. Disc cells were grown in monolayer cultures (37°C, 5% CO2, 5% O2 and bicarbonate buffering to maintain pH at 7.2) at passage zero. At passage one, cells were reseeded and grown at either 5% O2 or 20% O2. Cell cultures at passage 1 were used for the assays described below.
Ex vivo disc organotypic culture
Wild-type (C57BL/6) mouse disc organotypic cultures of isolated functional spine units (FSU), each consisting of vertebra, disc, vertebra, were performed as previously described 26. Thoracic FSUs were cultured in complete growth medium [F-12/D-MEM containing 10% fetal calf serum (FCS), 1% PS (10000 units/mL penicillin, 10 mg/mL streptomycin), and 25 μg/mL L-ascorbic acid] at either 5% O2 or 20% O2.
XJB-5-131 treatment
XJB-5-131 was synthesized and purified as described previously 27. hNP cells cultured at 20% O2 tension were treated with or without 0.5 μM XJB-5-131 in complete growth media (F12, 10% FBS, 1% PS, and 25 μg/mL L-ascorbic acid) for seven days, with culture media and XJB-5-131 refreshed every other day. For PG synthesis and gene expression experiments, a total of five individual assays +/− XJB-5-131 were done. For in vivo treatment, Ercc1−/Δ mice, previously established to have age-associated disc degeneration 22, were injected with XJB-5-131 intraperitoneally (2 mg/kg) three times per week beginning at 5 weeks of age before the animals had significant disc PG loss and continued until 18–20 weeks of age. A total of ten Ercc1−/Δ mice were treated with XJB-5-131 and another set of ten Ercc1−/Δ mice were treated with vehicle only (sunflower seed oil). Disc tissues were isolated for analyses postmortem. Experiments involving mice were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and in accordance with the NIH guidelines for humane care of animals.
1,9-Dimethylmethylene blue (DMMB) colorimetric assay for sulfated glycosaminoglycans
NP tissue isolated from six lumbar IVDs of each mouse was pooled and digested with papain at 60°C for two hours. Glycosaminoglycans (GAG) content was measured in duplicate by the DMMB procedure 28 using chondroitin-6-sulfate (Sigma C-8529) as a standard. The DNA concentration of each sample was measured using the PicoGreen assay (Molecular Probes) and used to normalize the GAG values. Average values from six independent trials were calculated and reported ± standard error.
Quantitation of matrix synthesis
Proteoglycan (PG) synthesis by hNP cell culture was measured by 35S-sulfate incorporation as described previously 29. PG synthesis was also measured in mouse disc organotypic cultures of isolated functional spine units (FSU). Briefly, four thoracic FSUs were cultured in complete growth medium for two days to equilibrate after the trauma of surgical dissection, followed by three days of labeling incubation with 35S-sulfate (20 μCi/mL). PG synthesis was then measured by quantifying the amount of incorporated 35S-sulfate per μg of DNA as described 29. Relative PG syntheses of cultures grown at 5% vs. 20% oxygen were calculated by setting the PG synthesis level from hNP cells or FSU grown at 5% O2 as one hundred percent.
Mitochondrial superoxide anion measurement
hNP cells were seeded on 35mm glass bottom dishes (MatTek Corporation, Ashland, MA) and incubated with 5 μM MitoSOX™ Red (Invitrogen, Eugene, OR) for 15 minutes at 37 °C. Cells were washed with PBS, the media replaced and the dish inserted in a closed, thermo-controlled (37 °C) stage top incubator (Tokai Hit Co., Shizuoka-ken, Japan) atop the motorized stage of an inverted Nikon TiE fluorescent microscope (Nikon Inc., Melville, NY) equipped with a 40X oil immersion optic (Nikon, CFI PlanFluor, NA 1.3) and NIS Elements Software. MitoSOX™ Red was excited using a Lumencor diode-pumped light engine (SpectraX, Lumencor Inc., Beaverton OR) and detected using a DsRed longpass filter set (Chroma Technology Corp) and Photometrics CoolSNAP HQ2 camera (Photometrics, Tucson, AZ). Data was collected in 2–12 cells per stage position, with 15 stage positions in each condition.
Gene expression by RT-PCR
Total RNA was isolated using the reagents and protocol per the Qiagen RNeasy Mini Kit and QIAshredder (Valencia, CA). Gene expression was analyzed by real-time RT-PCR using the Bio-Rad iScript One-Step RT-PCR Kit (Hercules, CA) and the Bio-Rad iCycler IQ5 detection system. The cycle threshold (Ct) values were obtained, and data normalized to GAPDH expression using the ΔΔCt method to calculate relative mRNA levels 30. Validated PCR primers used in these experiments are shown in Table 1.
Table 1.
Validated primer sequences used for RT-PCR
| Gene | Mouse primer sequences (5′ to 3′) | Human primer sequences (5′ to 3′) |
|---|---|---|
| GAPDH | Rev: GAGGCCGGTGCTGAGTAT | Rev: ACCCACTCCTCCACCTTTGAC |
| For: GCGGAGATGATGACCCTTTTGG | For: TCCACCACCCTGTTGCTGTAG | |
| ADAMTS-4 | Rev: GCAACGCAGGGCAGAGATAC | Rev: TCACTGACTTCCTGGACAATGG |
| For: CTACTGTGTTTTAGCAGGAG | For: ACTGGCGGTCAGCATCATAGT | |
| ADAMTS-5 | Rev: CAAGCGTTTAATGTCTTCAATCCTTA | Rev: CTGACCTACCACGAAAGCAGATC |
| For: ACTGCTGGGTGGCATCGT | For: ATGCCGGACACACGGAGTA | |
| MMP1 | Rev: TCTTTATGGTCCAGGCGATGAA | Rev: GAGCTCAACTTCCGGGTAGA |
| For: CCTCTTCTATGAGGCGGGGAT | For: CCCAAAAGCGTGTGACAGTA | |
| MMP3 | Rev: GGTACAGAGCTGTGGGAAGTC | Rev: CAAGGAGGCAGGCAAGACAGC |
| For: GATGAGCACACAACCACACAC | For: GCCACGCACAGCAACAGTAGG | |
| Aggrecan | Rev: ATACCCCATCCACACGCCCCG | Rev: AAGAATCAAGTGGAGCCGTGTGTC |
| For: GCGAAGCAGTACACATCATAGG | For: GCGGAGACCAGTGTGAACTTGATT | |
| Collagen II | Rev: ATGACAATCTGGCTCCCAAC | |
| For: GAACCTGCTATTGCCCTCCTG |
Statistical analysis
Values represent the average of six measurements (duplicates x three independent experiments) ± standard error (SE), with 95% confidence intervals calculated to determine statistical significance. The confidence intervals were calculated based on the t-distribution because of the small sample size.
RESULTS
Culturing human disc cells at 20% O2 increases the production of mitochondrial-derived O2−
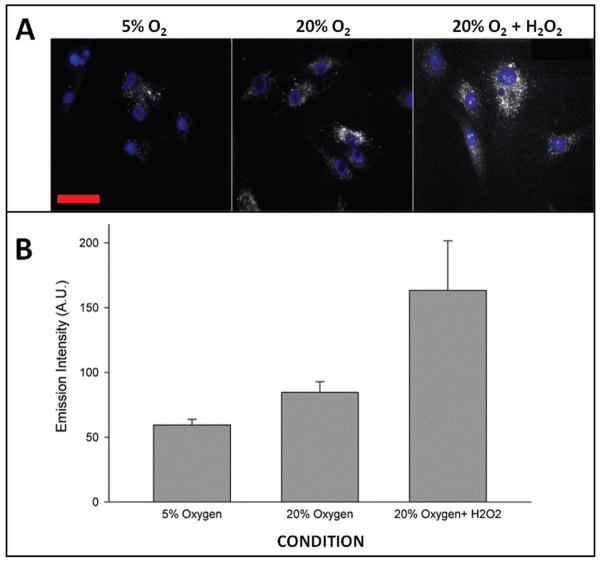
High oxygen tension generally boosts cellular oxidative metabolism, which can lead to increased production of ROS. To establish that culturing hNP cells at high oxygen (20% O2) increases endogenous production of ROS, we measured the superoxide anion (O2−) levels in the mitochondria of hNP cells grown at different oxygen tensions (5% O2 vs 20% O2). hNP cells grown at 20% O2 have more superoxide anion production in the mitochondria as measured by live cell imaging using MitoSox compared to cells cultured at 5% O2 (p<0.05, Fig. 1A and B). As a positive control, treatment of hNP cells with H2O2 led to a large increase in the intracellular superoxide radical anions. These data demonstrate that increased atmospheric oxygen can be used to induce endogenous oxidative stress in human NP cells.
Figure 1. Increased intracellular superoxide anion (O2−) level in human NP cells exposed to high oxygen tension.
A. Mitosox detection of superoxide anions in hNP cells grown at 5% vs. 20% O2. Treatment with 0.5 mM H2O2 was included as a positive control. The red bar represents 50 μm. B. Quantitation of mitochondrial superoxide in hNP cells by measurement of fluorescence produced by oxidation of MitoSOX reagent by superoxide anions.
High oxygen tension perturbed PG homeostasis in human intervertebral disc cells
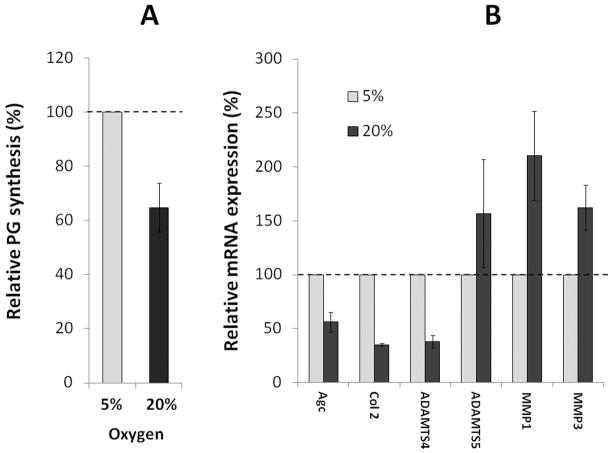
To test if high oxygen tension exerts oxidative stress on human disc cells, hNP cells cultured at 20% O2 were compared to those grown at 5% O2. hNP cells exposed to 20% O2 showed decreased PG synthesis and aggrecan gene expression after seven days (p<0.05, Fig. 2A and B). Since the NP is rich in collagen type 2, expression of this matrix gene was also measured. The mRNA level of collagen type 2 was 2.5 fold lower in hNP cells cultured at 20% O2 compared to hNP cells grown at 5% O2. Moreover, increased expression (1.5–2 fold) of the major matrix metalloproteinases, MMP-1, MMP-3, and ADAMTS5 was observed in hNP cells grown at 20% O2 relative to cells grown at 5% (Fig. 2B). Thus exposure of normal human disc cells to conditions that increase endogenous oxidative stress resulted in perturbations in matrix homeostasis associated with aging-related IDD. One notable exception is ADAMTS4, whose gene expression was decreased in hNP cells grown at 20% O2 compared to those grown at 5% O2.
Figure 2. Decreased PG synthesis and altered gene expression in human NP cells following exposure to high oxygen tension.
hNP cells were cultured at 5% O2 and 20% O2 for seven days. The effect of oxygen level on proteoglycan synthesis was measured by 35S-sulfate incorporation (A) and expression of genes encoding aggrecan and matrix metalloproteinases by real-time qRT-PCR (B). Relative percentage changes in PG synthesis and gene expression are shown with those of cells grown at 5% O2 normalized to 100%.
Treatment of hNP cells with XJB-5-131 mitochondrial-targeted radical scavenger rescued PG synthesis
If high oxygen tension perturbs matrix homeostasis in disc cells via the action of ROS, then depleting ROS is expected to blunt the negative effects of high oxygen on disc matrix metabolism. To test this idea, hNP cells grown at 20% oxygen for seven days were treated with the mitochondria-targeted ROS scavenger XJB-5-131 (0.5 μM, media and treatment refreshed every other day). Cells treated with XJB-5-131 showed a 30% increase in new PG synthesis (p<0.05, Fig. 3A) and a 50% increase in aggrecan gene expression (Fig. 3B) compared to hNP cells that were not treated with XJB-5-131. Thus, depleting endogenously produced, mitochondrial-derived superoxide anions mitigates the perturbation of matrix synthesis and aggrecan gene expression in intervertebral disc cells.
Figure 3. XJB-5-131 treatment increased PG synthesis in hNP cell cultures.
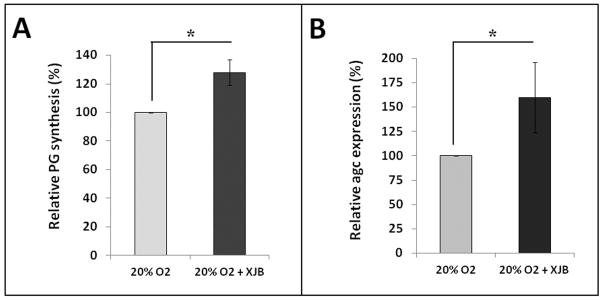
hNP cells were cultured at 20% O2 with or without 0.5 μM XJB-5-131 for seven days. The relative percentage change on proteoglycan synthesis was measured by 35S-sulfate incorporation (A) and aggrecan gene expression by RT-PCR (B). * p<0.05.
Oxidative stress perturbs disc matrix homeostasis in disc organotypic cultures from normal mice
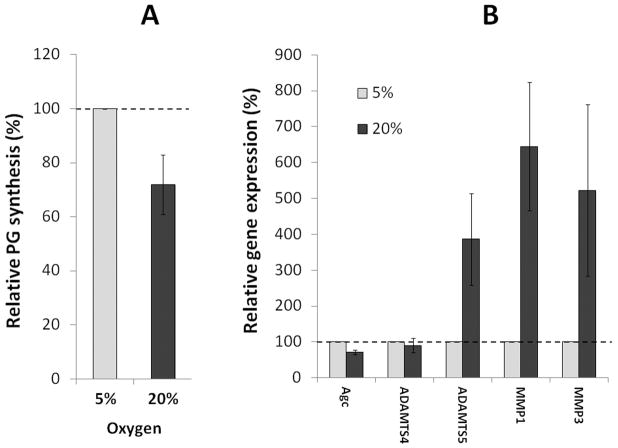
Our in vitro study suggested that ROS plays a role in driving matrix homeostatic imbalance in disc cell cultures. The next important question is whether oxidative stress drives similar changes in vivo or ex vivo where disc cells reside within their native matrix environment. To interrogate the latter, we increased oxidative stress in intervertebral discs isolated from wild-type mice (C57BL/6) by culturing them ex vivo at 20% O2 26. Isolated functional spine units (FSUs) were cultured in either at 5% O2 to mimic the physiologic hypoxic disc environment or at 20% O2 to induce oxidative stress. New PG synthesis, measured by 35S incorporation, by the disc organ cultured at 20% O2 was decreased by 30% compared to congenic FSUs cultured at 5% O2 (p<0.05, Fig. 4A). Isolated FSUs were cultured for seven days before 35S labeling to equilibrate after the surgical dissection and also to adapt to either the high oxygen (20% O2) or low oxygen (5% O2) environment. High oxygen tension decreased expression of the extra cellular matrix protein aggrecan, as measured by qRT-PCR, by 30%, but increased expression of catabolic factors, including the key matrix metalloproteinases ADAMTS5 (4x), MMP1 (6.5x) and MMP3 (5x) (Fig. 4B). Interestingly, ADAMTS4 gene expression was unaffected (Fig. 4B). Thus high oxygen tension perturbs disc PG homeostasis in vitro by decreasing matrix synthesis and increasing gene expression of catabolic factors.
Figure 4. Effects of high oxygen tension on disc matrix metabolism in an ex vivo disc organotypic culture system.
Wild-type mouse FSU cultured at 5% O2 (low oxygen) or 20% O2 (condition of increased oxidative stress) were assayed for (A) PG synthesis (35S-sufate incorporation) and (B) gene expression (qRT-PCR) of aggrecan and matrix metalloproteinases that degrade extracellular matrix proteins (ADAMTS4, ADAMTS5, MMP1 and MMP3). Relative percentage changes in PG synthesis and gene expression are shown with those of cells grown at 5% O2 normalized to 100%.
XJB-5-131 mitochondrial-targeted radical scavenger improved disc matrix proteoglycan content in a mouse model of accelerated aging
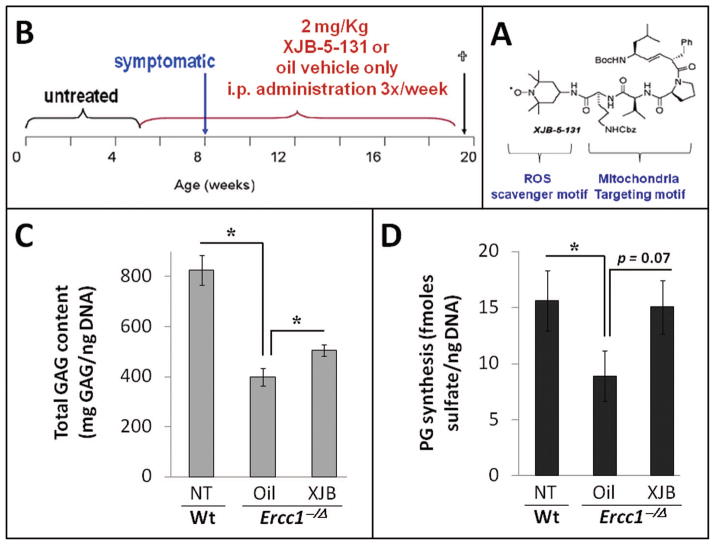
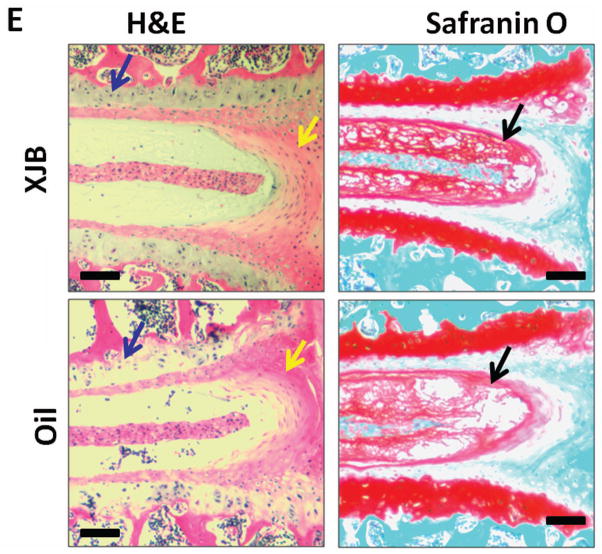
Our in vitro and ex vivo experiments both indicated that ROS-mediated oxidative stress perturbs disc PG homeostasis. We next determined whether endogenous ROS also negatively impacts disc health in vivo. To accomplish this, we employed the accelerated aging Ercc1−/Δ mice previously established as a highly sensitive model to oxidative damage due in part to their genetic deficiency in DNA repair 31. Because the mitochondria are the primary sites of endogenous ROS production, we designed the study to ask if reducing mitochondrial-derived ROS production can attenuate changes associated with aging-related IDD. Ercc1−/Δ mice were treated chronically and systemically with the mitochondria-targeted ROS scavenger XJB-5-131 for 13 weeks (2 mg/kg i.p. 3x per week) beginning at 5 weeks of age, which is prior to onset of symptoms associated with IDD (Fig. 5A and Fig. 5B). If ROS is detrimental to intervertebral disc, we expect that prolong systemic administration of XJB-5-131 will counter the negative effects of ROS and hence improve the overall disc matrix content in treated Ercc1−/Δ mice. Indeed, this XJB-5-131 treatment regimen resulted in a significant although modest improvement in disc total GAG content (p<0.05, Fig. 5C) and PG synthesis (p≤0.07, Fig. 5D). These positive effects were also evident histologically, including greater safranin O staining for PG in the NP region and higher cellularity in the endplate and AF region in XJB-treated mice compared to vehicle only (oil) control (Fig. 5E). The results suggest that endogenously produced ROS, in part, drives age-related IDD in accelerated aging Ercc1−/Δ mice and that systemic depletion of oxygen free radicals reduced disc degenerative changes.
Figure 5. Effects of XJB-5-131 treatment on disc matrix metabolism in Ercc1−/Δ mice.
A. Schematic diagram of the treatment regimen of Ercc1−/Δ mice. B. XJB-5-131 chemical structure. C. DMMB assay for total GAG of NP tissue (average values ± standard error). D. Proteoglycan synthesis as measured by 35S-sulfate incorporation by disc organotypic culture (average values ± standard error). * p<0.05. E. Safranin O and H&E histological staining of disc sections of XJB-treated Ercc1−/Δ mice and oil control. Endplate (blue arrows), nucleus pulposus (black arrows), and annulus fibrosus (yellow arrows) are indicated. The bars represent 200 μm.
DISCUSSION
The present study demonstrates that exposure of disc cells to high oxygen tension increases the levels of mitochondrial ROS and matrix homeostatic imbalance. High oxygen tension decreased proteoglycan synthesis and increased gene expression of matrix metalloproteinases in disc cell culture model systems. Treatment with the mitochondria-targeted ROS scavenger, XJB-5-131, moderately ameliorated these adverse effects, both in vitro and in vivo. These findings suggest that oxidative stress associated with ROS produced by exposure to high oxygen tension plays an important role in driving metabolic changes in disc cells. Our study extends previous disc research studies citing evidence of oxidative stress in aged intervertebral disc tissue by investigating how oxidative stress affects disc cell matrix metabolism.
The source of ROS-driven oxidative damage in intervertebral discs could originate from oxidative metabolism, especially in aged or degenerated discs with neovascularization as a result of accumulated annular and endplate fissures 15, 32, 33. This would result in exposure of the otherwise hypoxic resident disc cells to higher oxygen tension. Mitochondrial oxygen consumption, a hallmark of oxidative metabolism, was reported in freshly isolated outer annulus cells and in cultures of nucleus pulposus cells 14. Another major source of ROS-driven oxidative damage in intervertebral discs could originate from cellular response to pro-inflammatory cytokines, evidenced from studies showing elevated levels of pro-inflammatory cytokines in aged and degenerated disc tissues 12, 13, 18–20. For example, strong nitrosylation in degenerated discs, particularly NP tissue, indicates the presence of the inflammatory mediator nitric oxide (NO). NO has been shown to suppress PG synthesis in disc 17, 34. Treatment of disc cells with peroxynitrite, a product of the reaction between NO and superoxide anion, induces inflammatory gene expression in intervertebral disc cells 34. A similar phenomenon has been documented in articular cartilage where old rats have increased levels of intracellular ROS in their cartilage compared to young rats 35. Recently, dysfunction of chondrocyte mitochondria, the primary site of ROS production from cellular aerobic metabolism, was implicated in the establishment and progression of osteoarthritis 36.
The mitochondria-targeted ROS scavenger XJB-5-131 is efficacious in rodent models of hemorrhagic shock, sepsis, Huntington’s disease, brain trauma and hyperoxic acute lung injury 23, 37, 38. Treatment of the DNA repair-deficient Ercc1−/Δ mice with XJB-5-131 delayed the onset of age-associated disorders, including kyphosis, lethargy, and cachexia 38, 39. Here, we demonstrate that XJB-5-131 also ameliorates age-dependent IDD in this aging animal model. Interestingly, treatment with the red wine polyphenol resveratrol, a well-studied antioxidant, also is reported to have pro-anabolic and anti-catabolic effects on the disc in vitro and in vivo 40, 41. Furthermore, treatment with the antioxidant glutathione also protects hNP cells from hydrogen peroxide-induced impaired matrix metabolism 42. These, together with our findings, provide strong evidence supporting the harmful effects of oxidative stress in disc tissue and the potential therapeutic effects of blocking oxidative damage using antioxidants.
It should be noted that there are differences in cell composition and phenotypes between mouse and human discs. Large vacuolated notochordal cells (NCs) exist in high number in disc nucleus pulposus of mice throughout their lives. In contrast, these clusters of vacuolated NCs disappear in humans before maturity, which coincides with the appearance of chondrocyte-like matured NP cells (MNPCs). Recent research suggests that the notochordal cells differentiate into these MNPCs rather than dying-off and being replaced by a new population of MNPCs. However, NCs and MNPCs are phenotypically different in several respects. First, cell viability and proliferation of NCs are highly dependent on hypoxic conditions, much more so than those of MNPCs. Second, NCs are more metabolically active than MNPCs and more sensitive to nutritional deprivation. Extrapolation from mouse to human therefore has some limitations. Nevertheless, in the above studies as well as our own, oxidative stress has detrimental effects on both murine and human disc cells.
In summary, oxidative damage has been well established as a key driver of aging. Previous studies reported evidence of oxidative stress in intervertebral disc tissue via detection of biomarkers of oxidative stress. Here, we show that ROS, a molecular species driving oxidative damage, were produced in disc cells at high oxygen tension. Production of ROS at high oxygen tension also correlates with reduced PG synthesis and enhanced expression of catabolic factors. Depleting superoxide radical anion in disc cells using XJB-5-131 appeared to rescue matrix homeostatic imbalance. Therefore, this study provides, for the first time, experimental evidence of the link between oxidative damage and disc matrix homeostasis and a proof of concept that mitochondria-targeted free radical scavengers could be therapeutic in delaying the onset of age-related IDD.
Acknowledgments
This work was supported by NIH grant AG033046 (Nam Vo), NIH ES016114 (Laura Niedernhofer), NIH GM067082 (Peter Wipf), NIH AR051456 (Paul Robbins), and the Albert B. Ferguson, Jr. M.D. Orthopaedic Fund of the Pittsburgh Foundation. The authors would like to thank Dr. Jennifer Davoren for the preparation of XJB-5-131. Author contributions: The contributions from the following individuals are gratefully acknowledged. Salony Maniar and Claudette C for MitoSox/live cell imaging. Qing Dong for histology. Sowa G, Robbins PD, Niedernhofer LJ, Kang J (experimental design, data interpretation, intellectual inputs, and manuscript preparation), Nasto LA, Ngo K, Dong Q (matrix synthesis, histology and immunohistochemistry), Robinson, AR, Clauson CL, (XJB-5-131 treatment). All authors were involved in drafting the article or revising it critically for important scientific content.
References
- 1.Hart LG, Deyo RA, Cherkin DC. Physician office visits for low back pain. Frequency, clinical evaluation, and treatment patterns from a U.S. national survey. Spine (Phila Pa 1976) 1995;20:11–9. doi: 10.1097/00007632-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Miller JA, Schmatz C, Schultz AB. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine. 1988;13:173–8. [PubMed] [Google Scholar]
- 3.Zhao CQ, Wang LM, Jiang LS, Dai LY. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev. 2007;6:247–61. doi: 10.1016/j.arr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Boos N, et al. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine (Phila Pa 1976) 2002;27:2631–44. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Roughley PJ, Alini M, Antoniou J. The role of proteoglycans in aging, degeneration and repair of the intervertebral disc. Biochem Soc Trans. 2002;30:869–74. doi: 10.1042/bst0300869. [DOI] [PubMed] [Google Scholar]
- 6.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 2006;31:2151–61. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 7.Bibby SR, Jones DA, Ripley RM, Urban JP. Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine (Phila Pa 1976) 2005;30:487–96. doi: 10.1097/01.brs.0000154619.38122.47. [DOI] [PubMed] [Google Scholar]
- 8.Salmon AB, Richardson A, Perez VI. Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med. 48:642–55. doi: 10.1016/j.freeradbiomed.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niedernhofer LJ, Robbins PD. Signaling mechanisms involved in the response to genotoxic stress and regulating lifespan. Int J Biochem Cell Biol. 2008;40:176–80. doi: 10.1016/j.biocel.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkwood TB. Biological theories of aging: an overview. Aging (Milano) 1998;10:144–6. [PubMed] [Google Scholar]
- 11.Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mech Ageing Dev. 2004;125:811–26. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Tiku ML, Liesch JB, Robertson FM. Production of hydrogen peroxide by rabbit articular chondrocytes. Enhancement by cytokines. J Immunol. 1990;145:690–6. [PubMed] [Google Scholar]
- 13.McMillan TJ, et al. Cellular effects of long wavelength UV light (UVA) in mammalian cells. J Pharm Pharmacol. 2008;60:969–76. doi: 10.1211/jpp.60.8.0004. [DOI] [PubMed] [Google Scholar]
- 14.Boubriak O, Zhou S, Urban J. Oxygen metabolism of intervertebral disc cells: comparison between cells from the bovine outer annulus and nucleus pulposus. International Society for the Study of the Lumbar Spine Conference Special poster presentation (SP36); 2010. [Google Scholar]
- 15.Ali R, Le Maitre CL, Richardson SM, Hoyland JA, Freemont AJ. Connective tissue growth factor expression in human intervertebral disc: implications for angiogenesis in intervertebral disc degeneration. Biotech Histochem. 2008;83:239–45. doi: 10.1080/10520290802539186. [DOI] [PubMed] [Google Scholar]
- 16.Ulrich JA, Liebenberg EC, Thuillier DU, Lotz JC. ISSLS prize winner: repeated disc injury causes persistent inflammation. Spine (Phila Pa 1976) 2007;32:2812–9. doi: 10.1097/BRS.0b013e31815b9850. [DOI] [PubMed] [Google Scholar]
- 17.Kang JD, Stefanovic-Racic M, McIntyre LA, Georgescu HI, Evans CH. Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine (Phila Pa 1976) 1997;22:1065–73. doi: 10.1097/00007632-199705150-00003. [DOI] [PubMed] [Google Scholar]
- 18.Nerlich AG, et al. Immunomorphological analysis of RAGE receptor expression and NF-kappaB activation in tissue samples from normal and degenerated intervertebral discs of various ages. Ann N Y Acad Sci. 2007;1096:239–48. doi: 10.1196/annals.1397.090. [DOI] [PubMed] [Google Scholar]
- 19.Bank RA, Bayliss MT, Lafeber FP, Maroudas A, Tekoppele JM. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem J. 1998;330 ( Pt 1):345–51. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sivan SS, et al. Age-related accumulation of pentosidine in aggrecan and collagen from normal and degenerate human intervertebral discs. Biochem J. 2006;399:29–35. doi: 10.1042/BJ20060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verzijl N, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027–31. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- 22.Vo N, et al. Accelerated aging of intervertebral discs in a mouse model of progeria. J Orthop Res. 2010;28:1600–7. doi: 10.1002/jor.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fink MP, et al. Hemigramicidin-TEMPO conjugates: novel mitochondria-targeted anti-oxidants. Biochem Pharmacol. 2007;74:801–9. doi: 10.1016/j.bcp.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Studer RK, et al. p38 MAPK inhibition in nucleus pulposus cells: a potential target for treating intervertebral disc degeneration. Spine. 2007;32:2827–33. doi: 10.1097/BRS.0b013e31815b757a. [DOI] [PubMed] [Google Scholar]
- 25.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–8. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 26.Wang D, et al. Bupivacaine decreases cell viability and matrix protein synthesis in an intervertebral disc organ model system. Spine J. 2011;11:139–46. doi: 10.1016/j.spinee.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wipf P, et al. Mitochondrial targeting of selective electron scavengers: synthesis and biological analysis of hemigramicidin-TEMPO conjugates. J Am Chem Soc. 2005;127:12460–1. doi: 10.1021/ja053679l. [DOI] [PubMed] [Google Scholar]
- 28.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 29.Gilbertson L, et al. The effects of recombinant human bone morphogenetic protein-2, recombinant human bone morphogenetic protein-12, and adenoviral bone morphogenetic protein-12 on matrix synthesis in human annulus fibrosis and nucleus pulposus cells. Spine J. 2008;8:449–56. doi: 10.1016/j.spinee.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Niedernhofer LJ, et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–43. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- 32.Johnson WE, et al. Immunohistochemical detection of Schwann cells in innervated and vascularized human intervertebral discs. Spine (Phila Pa 1976) 2001;26:2550–7. doi: 10.1097/00007632-200112010-00007. [DOI] [PubMed] [Google Scholar]
- 33.Freemont AJ, et al. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197:286–92. doi: 10.1002/path.1108. [DOI] [PubMed] [Google Scholar]
- 34.Poveda L, Hottiger M, Boos N, Wuertz K. Peroxynitrite induces gene expression in intervertebral disc cells. Spine (Phila Pa 1976) 2009;34:1127–33. doi: 10.1097/BRS.0b013e31819f2330. [DOI] [PubMed] [Google Scholar]
- 35.Jallali N, et al. Vulnerability to ROS-induced cell death in ageing articular cartilage: the role of antioxidant enzyme activity. Osteoarthritis Cartilage. 2005;13:614–22. doi: 10.1016/j.joca.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz-Romero C, et al. Mitochondrial dysregulation of osteoarthritic human articular chondrocytes analyzed by proteomics: a decrease in mitochondrial superoxide dismutase points to a redox imbalance. Mol Cell Proteomics. 2009;8:172–89. doi: 10.1074/mcp.M800292-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji J, et al. Lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of brain injury. Nat Neurosci. 15:1407–13. doi: 10.1038/nn.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xun Z, et al. Targeting of XJB-5-131 to Mitochondria Suppresses Oxidative DNA Damage and Motor Decline in a Mouse Model of Huntington’s Disease. Cell Rep. 2:1137–42. doi: 10.1016/j.celrep.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson AR, et al. A mitochondrial-targeted radical scavenger delays aging in a mouse model of human progeria. JCI. 2012 Submitted. [Google Scholar]
- 40.Li X, et al. The action of resveratrol, a phytoestrogen found in grapes, on the intervertebral disc. Spine (Phila Pa 1976) 2008;33:2586–95. doi: 10.1097/BRS.0b013e3181883883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wuertz K, et al. The red wine polyphenol resveratrol shows promising potential for the treatment of nucleus pulposus-mediated pain in vitro and in vivo. Spine (Phila Pa 1976) 2011;36:E1373–84. doi: 10.1097/BRS.0b013e318221e655. [DOI] [PubMed] [Google Scholar]
- 42.Yang X, Wang D, Yang D, Shen F, XL Glutathione Protects human nucleus pulposus cells from hydrogen peroxide-induced cell death and impaired extracellular matrix metabolism. 58th Annual Meeting of the Orthopaedic Research Society; San Francisco, CA. February 2012; 2012. Abstract. [Google Scholar]