Abstract
Background.
There has been little research on the relative importance of frailty markers. The objective was to investigate the association among seven frailty domains (nutrition, physical activity, mobility, strength, energy, cognition, and mood) and their relative contribution in explaining differences among individuals in five samples of older persons.
Methods.
Data from five studies of aging were analyzed using multiple correspondence analysis. Aggregation of frailty markers was evaluated using graphical output. Decomposition of variability was used to assess the relative contribution of each marker in each sample. Results were combined across the samples to assess the average contribution.
Results.
Frailty markers were found to consistently aggregate in each sample, suggesting a possible underlying construct. Physical strength had the highest contribution on average in explaining differences among individuals. Mobility and energy also had large contributions. Nutrition and cognition had the smallest contributions.
Conclusions.
Our results provide further evidence supporting the notion that frailty domains may belong to a common construct. Physical strength may be the most important discriminating characteristic.
Key Words: Frailty, Multivariate analyses, Older adults
Frailty is acknowledged to be a state of decreased reserve and decline in multiple physiologic systems, resulting in an increased risk of adverse outcomes (1–7). Nevertheless, there remains debate on its characteristics (1). Understanding the relationship among proposed characteristics is necessary to elucidate whether these characteristics could form a construct of frailty and how such characteristics interrelate.
The International Database Inquiry on Frailty (FrData) is an initiative aimed at improving our understanding of seven frailty domains: nutrition, physical activity, mobility, strength, energy, cognition, and mood. Selection of domains was based on a literature review implemented by the Canadian Initiative on Frailty and Aging (8) and based on clinical and biological plausibility. Using the methodology developed in a previous publication (9), the aim of this study is to explore associations among domains using data from five samples of older persons from the United States, Netherlands, Mexico, and Canada.
The specific goals of this study are to investigate the aggregation of seven frailty markers as well as the relative importance of each marker in explaining differences among participants. Identifying frailty markers that are consistently more important in explaining differences among older persons, despite how these markers are measured across studies, could lead to considering the relative weight of frailty markers in future research studies and to developing a shorter clinical assessment tool.
METHODS
Data Selection
Baseline data were extracted from four studies on aging: Established Populations for Epidemiologic Studies of the Elderly (EPESE; 10), Longitudinal Aging Study Amsterdam (LASA; 11), Mexican Health and Aging Study (MHAS; 12), and NuAge (nutrition as a determinant of successful aging; 13). Data from the two EPESE sites were analyzed separately for a total of five samples. For consistency, analyses presented here were restricted to subsamples aged 65 years or older. Furthermore, participants with disability in activities of daily living (ADL) at baseline were excluded from the analysis because our interest in frailty is its usefulness in detecting vulnerable elders before the onset of disability; and to ensure that any relationships found were not artifacts of underlying disability. ADL disability was defined as being unable or needing help to eat, dress, transfer, bathe, or toilet (14). Data were provided by each study’s principal investigators. Ethics approval was obtained from the Research Ethics Committee, Jewish General Hospital, Montreal, Canada. Studies are presented in order of their initiation date.
EPESE started in 1981 and consisted of prospective epidemiologic studies of 14,000 persons aged 65 years or older in four locations: East Boston, Iowa, New Haven, and North Carolina (10). Based on measures available, the East Boston and Iowa sites were selected for this study. The Boston site comprised 3,809 working class Italian Americans. Of the 3,809, 3,210 (84.3%) participants without ADL disability were retained for analysis. The Iowa site consisted of 3,673 participants from a population of rural dwellers. Of the 3,673, the 3,447 (93.9%) participants without ADL disability were selected for analysis.
LASA started in 1992 and included 3,107 persons aged 55–85 years from three areas in the Netherlands with no inclusion or exclusion criteria other than age (11). The data from the second wave in 1995 were used because the interview included an assessment of grip strength. Of the 1,509 participants at the second wave, 1,436 (95.2%) without ADL disability were retained for analysis.
MHAS was initiated in 2001 and included a sample of Mexicans aged 50 years or older and their spouses regardless of age (12). Of the 4,869 participants aged 65 years or older, 4338 (89.1%) without ADL disability were retained.
NuAge began in 2004 and studied 1,793 community-dwelling persons in Quebec, Canada, aged 68–82 years, French or English speaking, willing to commit for a 5-year period, able to walk without help, free of disabilities in ADL, without cognitive impairment, and able to walk 100 m or climb 10 stairs without rest (13). This resulted in almost all participants having no ADL disability at baseline; 1,786 (99.6%) were included in our analysis.
Measures of Frailty Domains
Frailty domain measures were selected from each study and dichotomized into presence or absence of a frailty marker (Table 1). No measure of energy was available in LASA. Details on the methodology for selection and dichotomization of measures are presented elsewhere (9).
Table 1.
Measures and Cutoffs for the Frailty Markers in Each Sample
| Domain | Nutrition | Physical Activity | Energy | Mobility | Strength | Cognition | Mood |
| EPESE East Boston | BMI < 22 OR lost more than 10 pounds | Rarely or never takes walks or gardens AND physical activity < 3 times/wk | Often so sleepy during the day or evening that participant needs to take a nap | Unable, needing help, or having some or a lot of difficulty walking across a small room | Unable or having a lot of difficulty pulling or pushing large objects OR lifting or carrying over 10 pounds | ≥2 errors on the modified SPMSQ (15) | Modified CES-D ≥ 13 for men and ≥14 for women* (16) |
| EPESE Iowa | BMI < 22 OR lost more than 10 pounds and not on special diet | Once a week or less to taking walks, gardening, and jogging/biking/swimming/vigorous exercise | Often so sleepy during the day or evening that participant needs to take a nap | Unable, needing help, or having some or a lot of difficulty walking across small room | Unable or having a lot of difficulty pulling or pushing large objects OR lifting or carrying over 10 pounds | ≥2 errors on the modified SPMSQ (15) | Modified CES-D ≥ 16 for men and ≥16 for women (16) |
| LASA | ≥5% self-reported weight loss in last 6 mo OR BMI < 22 | <30 min of moderate intense physical activity per day based on the LASA Physical Activity Questionnaire (17) | Not available | 6-m gait speed test with turn: lowest 20% or those unable to complete the test | Grip strength: lowest 20% by sex: <31 kg for men; <19 kg for women† | MMSE ≤ 26 (18) | CES-D ≥ 16 (19) |
| MHAS | ≥5 kg self-reported weight loss OR BMI < 22 | Exercise or hard physical work ≤ 3 times/wk | Self-reported severe fatigue or exhaustion | “Yes” or “Can’t Do” to climbing several flights of stairs | “Yes” or “Can’t Do” to pulling/pushing or lifting/carrying | ≥2 failed subtests from the five subtests of the Cross-Cultural Cognitive Examination (20) | ≥5 of 9 depressive symptoms as established in the MHAS |
| NuAge | ≥ 5% self-reported weight loss in last 12 mo OR BMI < 22 | PASE (21) score < mean − 1 SD by age and sex (22) | Vitality subscale of the SF-36 (23) < mean − 1 SD by age and sex (24) | Gait speed < 1 m/s (25) | Grip strength < mean − 1 SD on by age, sex, and dominant hand (26),† | MMSE < 28 (18),‡ | GDS > 10 (27) |
Notes: CES-D = Center for Epidemiological Studies Depression; EPESE = Established Populations for Epidemiologic Studies of the Elderly; GDS = Geriatric Depression Scale; LASA = Longitudinal Aging Study Amsterdam; MHAS = Mexican Health and Aging Study; MMSE = Mini-Mental State Examination; NuAge = Nutrition as a determinant of successful aging; PASE = Physical Activity Scale for the Elderly; SF-36 = Short-Form (27) Health Survey; SPMSQ = Short Portable Mental Status Questionnaire.
EPESE East Boston used an abbreviated form of the CES-D. Previous work has shown that in that sample, using cutoffs of 13 (men) and 14 (women) is comparable to the more commonly used cutoff of 16.
In the absence of Dutch normative data on grip strength, cutoffs in LASA were defined using lowest sex-specific quintile. This was considered appropriate because the sample was representative random sample of the population. Cutoffs for grip strength in the NuAge study were based on normative data from a Canadian sample.
Due to the exclusion criteria for cognitive impairment in NuAge, a cutoff of 28 was used instead of 26 for the MMSE score in order to identify those participants with mild cognitive impairment.
Analysis
Multiple Correspondence Analysis (MCA) was used to graphically explore the relationships among all frailty markers simultaneously. A description of this method was presented elsewhere (28,29). Briefly, points on the graph represent the presence or absence of a frailty marker for each of the seven domains. The origin represents the “norm,” that is, the average profile under the assumption of independence between the markers. In general, the further away from the origin and closer to the axis a frailty marker is, the less prevalent this marker is in the sample; therefore, the greater its deviation from the norm. The degree of deviation from independence in the data is measured by total inertia, defined as the weighted average of squared chi-square distances between observed and expected distributions in a multiway contingency table. By decomposing the total inertia across the frailty markers, we can see which markers are the most important in explaining differences among individuals. Dimension 1 represents the most important deviation from independence, that is, the largest proportion of inertia, Dimension 2, the second most important, etc. The interpretation of dimensions is based on how the points representing the presence and absence of each frailty marker separate on the positive and negative side of each dimension. Moreover, markers closest to the axis of Dimension 1 and furthest from the origin would be most important in interpreting Dimension 1.
For our study, MCA was used to assess whether the presence of frailty markers in all domains aggregated on the graph, and the percent contribution of each frailty marker in explaining differences among participants.
The graphical output from MCA was used to assess the aggregation of frailty markers. Separation between presence and absence of frailty markers on the positive and negative side of Dimension 1 would indicate that frailty markers share an overall association, suggesting an underlying construct. To assess the dimensionality of the underlying construct, we retained the number of dimensions accounting for 70% or more of the total inertia (28).
Numerical output on the decomposition of the total inertia was used to determine the contribution of each frailty marker, expressed as a percentage of the total inertia, in explaining differences among participants. To differentiate between large and small contributions, we used the average contribution as a threshold (29). The threshold for samples with all seven frailty measures, each with two response categories—one for presence and one for absence of a frailty marker, is 100/14 = 7.1%. For LASA, where measures for six of the seven domains were available, the threshold becomes 100/12 = 8.3%. The percent contribution of frailty markers cannot be inferred from the graphical output of the MCA as it is calculated combining all dimensions and therefore independent of the number of dimensions chosen for display.
MCA was performed using PROC CORRESP (SAS software 9.2, Cary, NC; see Supplementary Material). The R software v.2.10.1 (30) was used to obtain coordinates for the graphs and for the numerical results on the percent contribution of the frailty markers. Multiple imputation was used for missing data (31) and was performed using Imputation and Variance Estimation Software (32).
In addition to the results for each of the five samples, we wanted to summarize the results on the relative contribution of the frailty markers to provide an overall impression of the findings. However, to our knowledge, no published literature exists on how to summarize results obtained from MCA across several studies. We undertook three separate approaches based on the ordering of the contributions for each of the markers. In a first intuitive approach, the seven markers were ranked from highest to lowest contribution. The number of times each marker was ranked first, second, third, and to last was counted across the studies. For example, mobility was ranked first once, second once, third once, and fourth twice; mood was ranked first once, second once, fourth twice, and fifth once. Therefore, mobility was ranked higher than mood. This approach, however, only considered the ordering of the contributions and not the relative difference in magnitude. A second approach was to order the frailty markers based on the average contribution for each marker defined as the sum of contributions divided by the number of samples. For markers, where measures were available in all five samples, the sum was divided by 5. For energy, where measures were available in only four of the five samples, the sum was divided by 4. A third approach ordered the frailty markers based on the weighted average of the contributions across the studies, where weights corresponded to individual study sample sizes.
Sensitivity analyses were performed including participants with ADL disability to examine the impact of excluding those with ADL disability.
RESULTS
Across the five samples, mean age ranged from 72.4 to 75.6, between 51.3% and 60.6% were women, and the mean number of chronic diseases was between 1.0 and 2.0 (Table 2). The prevalence of each frailty marker varied considerably across samples but was generally highest in MHAS.
Table 2.
Sample Characteristics
| EPESE East Boston (n = 3,210) | EPESE Iowa (n = 3,447) | LASA (n = 1,436) | MHAS (n = 4,338) | NuAge (n = 1,786) | |
| Age, mean (SD) | 72.9 (6.2) | 74.4 (6.5) | 75.6 (6.6) | 72.4 (6.3) | 74.4 (4.2) |
| Female, n (%) | 1,945 (60.6) | 2,117 (61.4) | 737 (51.3) | 2,240 (51.6) | 935 (52.4) |
| Number of chronic diseases, mean (SD) | 1.6 (1.3) | 2.0 (1.2) | 1.2 (1.1) | 1.0 (1.0) | 2.1 (1.5) |
| Missing (%) | 1.3 | 14.4 | 0 | 4.0 | 26.5 |
| Percentage with marker (%) | |||||
| Nutrition | 30.3 | 37.1 | 16.3 | 34.4 | 18.1 |
| Missing | 0.4 | 0.3 | 0.6 | 22.6 | 0.6 |
| Physical activity | 17.0 | 18.7 | 40.5 | 67.6 | 23.9 |
| Missing | 0.3 | 14.5 | 4.3 | 7.7 | 1.5 |
| Mobility | 5.3 | 2.5 | 27.7 | 43.9 | 21.7 |
| Missing | 1.9 | 10.0 | 2.7 | 20.1 | 0.7 |
| Strength | 30.7 | 15.6 | 25.3 | 32.1 | 13.6 |
| Missing | 2.7 | 11.9 | 1.1 | 10.1 | 0.6 |
| Energy | 17.7 | 27.0 | Not available | 26.7 | 9.5 |
| Missing | 2.7 | 11.6 | Not available | 7.3 | 0.5 |
| Cognition | 39.2 | 20.8 | 12.7 | 32.3 | 27.6 |
| Missing | 4.3 | 10.6 | 1.3 | 4.2 | 0.1 |
| Mood | 24.5 | 21.6 | 14.4 | 43.3 | 13.2 |
| Missing | 6.8 | 13.2 | 3.1 | 8.9 | 0.6 |
Note : EPESE = Established Populations for Epidemiologic Studies of the Elderly; LASA = Longitudinal Aging Study Amsterdam; MHAS = Mexican Health and Aging Study; NuAge = Nutrition as a determinant of successful aging.
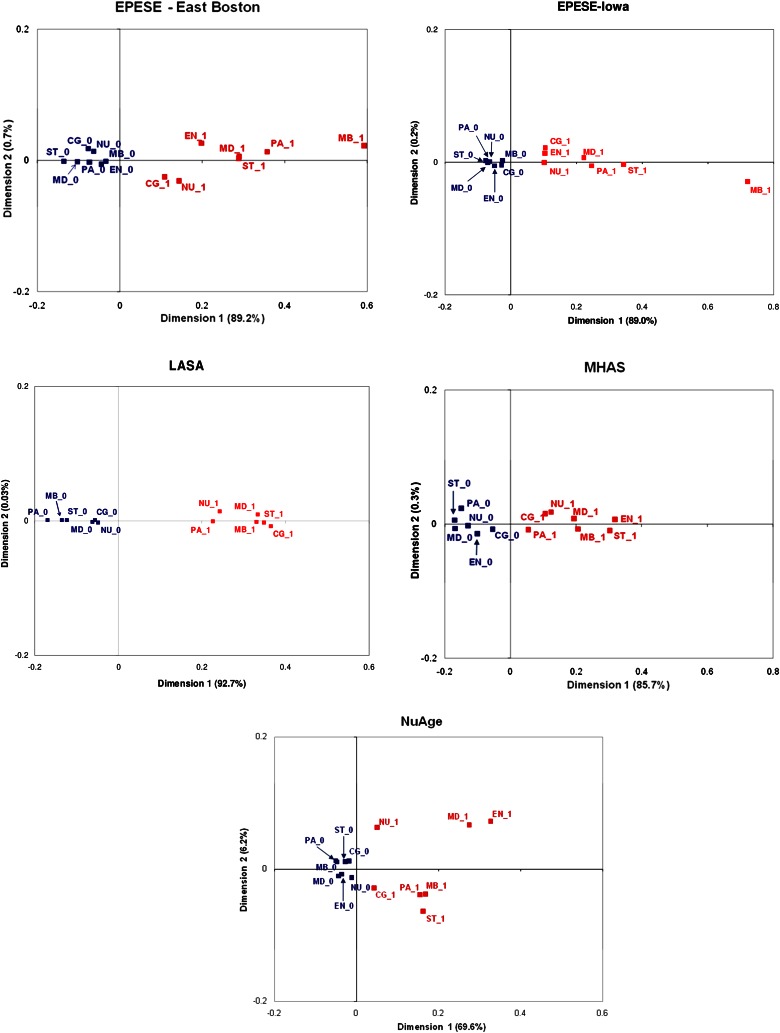
Figure 1 depicts the relationship between presence and absence of frailty markers across the seven frailty domains. The proportion of inertia explained by Dimension 1 ranged from 69.2% in NuAge to 92.7% in LASA. Given this high proportion, only Dimension 1 was retained for interpretation. Consistently across all samples, a clear separation was found between the presence of frailty markers on the positive side of Dimension 1 and the absence of frailty markers on the negative side. This separation along Dimension 1 indicates an overall aggregation among the frailty markers, consistent with the hypothesis of an underlying construct.
Figure 1.
Graphical results of the multiple correspondence analysis. Points in red (with suffix 1) correspond to the presence of a frailty marker; points in blue (with suffix 0) represent the absence of a frailty marker. Percentages for each dimension correspond to the proportion of explained inertia. Dimension 1 explains the largest proportion of inertia, Dimension 2, the second largest. CG = Cognition; EN = Energy; MB = Mobility; MD = Mood; NU = Nutrition; PA = Physical Activity; and ST = Strength.
Table 3 shows, in each sample and overall, the contribution and ranking of frailty markers in explaining the total inertia. The ordering of markers varied across the individual samples. However, strength, mobility, and mood consistently had a large contribution, above the threshold of 8.3% for LASA and 7.1% for the other samples, whereas nutrition and cognition were consistently below the threshold (29). On average, strength was found to have the largest contribution followed by mobility, mood, energy, and physical activity. Nutrition and cognition had the smallest contribution. The overall ordering of frailty markers presented based on the weighted average across the samples was equal to the ordering obtained using the unweighted average and the intuitive counting approach (results not shown).
Table 3.
Contribution (%) to the Total Inertia and Ranking of Each Frailty Marker
| Study | Strength | Mobility | Mood | Energy | Physical Activity | Nutrition | Cognition |
| EPESE East Boston | |||||||
| % Inertia | 20.0 | 12.5 | 15.4 | 4.6 | 15.0 | 4.5 | 3.6 |
| Ranking | 1 | 4 | 2 | 5 | 3 | 6 | 7 |
| EPESE Iowa | |||||||
| % Inertia | 25.9 | 18.2 | 12.0 | 3.5 | 15.1 | 3.7 | 2.5 |
| Ranking | 1 | 2 | 4 | 6 | 3 | 5 | 7 |
| LASA | |||||||
| % Inertia | 18.6 | 18.7 | 8.9 | Not available | 12.3 | 5.2 | 9.0 |
| Ranking | 2 | 1 | 5 | Not available | 3 | 6 | 4 |
| MHAS | |||||||
| % Inertia | 17.0 | 12.1 | 8.1 | 13.8 | 1.0 | 3.2 | 1.7 |
| Ranking | 1 | 3 | 4 | 2 | 7 | 5 | 6 |
| NuAge | |||||||
| % Inertia | 7.8 | 12.2 | 23.9 | 23.3 | 12.4 | 2.5 | 1.7 |
| Ranking | 5 | 4 | 1 | 2 | 3 | 6 | 7 |
| Weighted average (%) | 18.6 | 14.0 | 12.1 | 10.6 | 8.9 | 3.7 | 2.9 |
| Ranking | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
Note : EPESE = Established Populations for Epidemiologic Studies of the Elderly; LASA = Longitudinal Aging Study Amsterdam; MHAS = Mexican Health and Aging Study; NuAge = Nutrition as a determinant of successful aging.
Sensitivity analyses including those with ADL disability showed very similar graphical results and percent contributions of the frailty markers (results not shown).
DISCUSSION
Our results showed that the proposed frailty markers consistently aggregated in the five samples, revealing a possible underlying construct. Furthermore, strength had the highest contribution overall in explaining differences among participants across the samples. Mobility and energy followed as the next most discriminating markers. Nutrition and cognition appeared to be least discriminating. Results were similar even when participants with ADL disability were included.
In the absence of validated techniques in the literature, we utilized an ad hoc approach to averaging our results across the samples in order to obtain a summary of our findings. Results based on three different averaging approaches provided identical ordering of frailty markers, lending a degree of validation.
To our knowledge, apart from our work (9), only two other studies have explored how various characteristics of frailty aggregate (33,34). Consistent with Sourial and colleagues (9) and Bandeen-Roche and colleagues (33), our results show an aggregation of the proposed frailty markers. Bandeen-Roche concluded that there was aggregation of markers by identifying underlying classes of individuals using the Cardiovascular Health Study frailty criteria applied to latent class analysis. Our study showed an aggregation of markers by directly examining how frailty domains cluster together graphically using MCA. Although latent class analysis may provide a means of model validation, MCA provides insight on the relationships among the domains. Furthermore, similar to Sarkisian and colleagues (34), our results provide evidence to support that frailty markers should not be considered of equal weight and may have varying degrees of importance in characterizing older persons.
Strengths include the use of five diverse study populations, a common methodological approach and an innovative multivariate technique, MCA. Frailty domain measures were both self-report and performance-based. Self-report measures for strength such as the ability to pull or push objects and self-report measures of mobility such as the ability to climb stairs have also been used elsewhere as measures of function (35). A certain level of consistency across studies that used self-report measures and those that used performance-based tests lends credence to the use of self-report measures as valid measures of physical function. Finally, the variability observed in the ranking of markers across samples may have been in part due to differences in the measures used as well as differences in populations. For example, in NuAge, strength was ranked lower than in other studies (5th of 7th). Given NuAge selected highly functional older persons, it may be that in this population, strength does not discriminate as well as other frailty markers such as mood. The MHAS sample had the lowest prevalence of chronic disease but the highest prevalence of most frailty markers, probably due to underreporting of chronic diseases previously observed in this population (36). The absence of a measure of energy in LASA may have influenced the relative contribution of the other frailty markers. In the absence of a gold standard for the measurement of frailty domains, we selected, within each study, the most appropriate measures available. The measures used may have affected the findings to some degree. For example, the relatively low contribution of nutrition in explaining differences between individuals may have been potentially due to body mass index being a poor measure of frailty. The dichotomization of frailty measures while necessary to facilitate interpretation across samples may have resulted in some loss of information. Finally, while a rigorous imputation method was used for missing data, the results may have differed had complete data been available.
In conclusion, our study provides further evidence that the proposed frailty domains may be part of a common underlying construct. Frailty markers may have varying degrees of importance in explaining differences among older persons. Strength, followed by mobility and energy, may be the most important discriminating characteristics, whereas nutrition and cognition may be least discriminating. Further studies are necessary to confirm these findings.
FUNDING
This work was supported by the Solidage Research Group and Dr. Joseph Kaufmann Chair in Geriatric Medicine, McGill University; the Canadian Initiative on Frailty and Aging; the Canadian Institutes of Health Research (CIHR) International Opportunity Program Development grant 68739; the CIHR team grant in frailty and aging 82945 and the Fonds de la recherche en santé du Québec; Netherlands Ministry of Welfare, Health and Sports of the Netherlands for LASA; the Mexican Red Temática de Investigación en Envejecimiento Salud y Desarrollo Social (Aging and Health Research Network) Consejo Nacional de Ciencia y Tecnología Mexico for Mexican Health and Aging Study and the CIHR grant (MOP 62842) for NuAge.
SUPPLEMENTARY MATERIAL
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Acknowledgments
We would like to thank the data managers for their assistance: Caroline Phillips for EPESE, Silvia Mejía Arango for MHAS, and Véronique Boutier and N’Deye Rokhaya Gueye for NuAge. We are grateful to Muriel Guériton for library assistance.
References
- 1.Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Hogan DB, MacKnight C, Bergman H. Models, definitions, and criteria of frailty. Aging Clin Exp Res. 2003;15:1–29. [PubMed] [Google Scholar]
- 4.Gill TM, Gahbauer EA, Han L, Allore HG. The relationship between intervening hospitalizations and transitions between frailty states. J Gerontol A Biol Sci Med Sci. 2011;66:1238–1243. doi: 10.1093/gerona/glr142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho YY, Matteini AM, Beamer B, et al. Exploring biologically relevant pathways in frailty. J Gerontol A Biol Sci Med Sci. 2011;66:975–979. doi: 10.1093/gerona/glr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalyani RR, Varadhan R, Weiss CO, et al. Frailty status and altered glucose-insulin dynamics. J Gerontol A Biol Sci Med Sci. doi: 10.1093/gerona/glr141. doi:10.1093/gerona/GLR141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hubbard RE, Lang IA, Llewellyn DJ, et al. Frailty, body mass index, and abdominal obesity in older people. J Gerontol A Biol Sci Med Sci. 2011;65:377–381. doi: 10.1093/gerona/glp186. [DOI] [PubMed] [Google Scholar]
- 8.Bergman H. The Canadian initiative on frailty and aging. Aging Clin Exp Res. 2003;15:1–2. [Google Scholar]
- 9.Sourial N, Wolfson C, Bergman H, et al. A correspondence analysis revealed frailty deficits aggregate and are multidimensional. J Clin Epidemiol. 2010;63:647–654. doi: 10.1016/j.jclinepi.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornoni-Huntley J, Ostfeld AM, Taylor JO, et al. Established populations for epidemiologic studies of the elderly: study design and methodology. Aging (Milano) 1993;5:27–37. doi: 10.1007/BF03324123. [DOI] [PubMed] [Google Scholar]
- 11.Deeg DJ, van Tilburg T, Smit JH, de Leeuw ED. Attrition in the Longitudinal Aging Study Amsterdam. The effect of differential inclusion in side studies. J Clin Epidemiol. 2002;55:319–328. doi: 10.1016/s0895-4356(01)00475-9. [DOI] [PubMed] [Google Scholar]
- 12.Wong R, Espinoza M, Palloni A. [Mexican older adults with a wide socioeconomic perspective: health and aging] Salud Publica Mex. 2007;49(suppl 4):S436–S447. doi: 10.1590/s0036-36342007001000002. [DOI] [PubMed] [Google Scholar]
- 13.Gaudreau P, Morais JA, Shatenstein B, et al. Nutrition as a determinant of successful aging: description of the Quebec longitudinal study Nuage and results from cross-sectional pilot studies. Rejuvenation Res. 2007;10:377–386. doi: 10.1089/rej.2007.0596. [DOI] [PubMed] [Google Scholar]
- 14.Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 15.Feil D, Marmon T, Unutzer J. Cognitive impairment, chronic medical illness, and risk of mortality in an elderly cohort. Am J Geriatr Psychiatry. 2003;11:551–560. [PubMed] [Google Scholar]
- 16.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 17.Stel VS, Smit JH, Pluijm SM, Visser M, Deeg DJ, Lips P. Comparison of the LASA Physical Activity Questionnaire with a 7-day diary and pedometer. J Clin Epidemiol. 2004;57:252–258. doi: 10.1016/j.jclinepi.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Radloff LS. The CES-D scale. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 20.Glosser G, Wolfe N, Albert ML, et al. Cross-cultural cognitive examination: validation of a dementia screening instrument for neuroepidemiological research. J Am Geriatr Soc. 1993;41:931–939. doi: 10.1111/j.1532-5415.1993.tb06758.x. [DOI] [PubMed] [Google Scholar]
- 21.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 22.Chad KE, Reeder BA, Harrison EL, et al. Profile of physical activity levels in community-dwelling older adults. Med Sci Sports Exerc. 2005;37:1774–1784. doi: 10.1249/01.mss.0000181303.51937.9c. [DOI] [PubMed] [Google Scholar]
- 23.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 24.Hopman WM, Towheed T, Anastassiades T, et al. Canadian normative data for the SF-36 health survey. Canadian Multicentre Osteoporosis Study Research Group. Can Med Assoc J. 2000;163:265–271. [PMC free article] [PubMed] [Google Scholar]
- 25.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 26.Desrosiers J, Bravo G, Hebert R, Dutil E. Normative data for grip strength of elderly men and women. Am J Occup Ther. 1995;49:637–644. doi: 10.5014/ajot.49.7.637. [DOI] [PubMed] [Google Scholar]
- 27.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 28.Sourial N, Wolfson C, Zhu B, et al. Correspondence analysis is a useful tool to uncover the relationships among categorical variables. J Clin Epidemiol. 2010;63:638–646. doi: 10.1016/j.jclinepi.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenacre MJ. Correspondence Analysis in Practice. New York, NY: Chapman & Hall \ CRC; 2007. [Google Scholar]
- 30.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 31.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 32.Raghunathan TE, Lepkowski JM, van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;27:85–95. [Google Scholar]
- 33.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women's health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 34.Sarkisian CA, Gruenewald TL, John Boscardin W, Seeman TE. Preliminary evidence for subdimensions of geriatric frailty: the MacArthur study of successful aging. J Am Geriatr Soc. 2008;56:2292–2297. doi: 10.1111/j.1532-5415.2008.02041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verbrugge LM. Flies without wings. In: Carey J, Robine JM, Michel JP, Christen Y, editors. Longevity and Frailty. Heidelberg, Germany: Springer Verlag; 2005. pp. 67–81. [Google Scholar]
- 36.Aguilar-Salinas CA, Gomez-Perez FJ, Rull J, Villalpando S, Barquera S, Rojas R. Prevalence of dyslipidemias in the Mexican National Health and Nutrition Survey 2006. Salud Publica Mex. 2010;52(suppl 1):S44–S53. doi: 10.1590/s0036-36342010000700008. [DOI] [PubMed] [Google Scholar]