Abstract
Objective:
Our objective was to systematically analyze the evidence for an association between serum level long chain omega-3 polyunsaturated fatty acid (n-3 PUFA) and prostate cancer risk from human epidemiological studies.
Study Procedures:
We searched biomedical literature databases up to November 2011 and included epidemiological studies with description of long chain n-3 PUFA and incidence of prostate cancer in humans. Critical appraisal was done by two independent reviewers. Data were pooled using the general variance-based method with random-effects model; effect estimates were expressed as risk ratio with 95% confidence interval (CI). Heterogeneity was assessed by Chi2 and quantified by I2, publication bias was also determined.
Results:
In total, 12 studies were included. Significant negative association was noted between high serum level of n-3 PUFA doc-osapentaenoic acid (DPA) and total prostate cancer risk (RR:0.756; 95% CI 0.599, 0.955; p = 0.019). Likewise, a positive association between high blood level of fish oil contents, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and high-grade prostate tumour incidence (RR:1.381; 95% CI 1.050, 1.817; p = 0.021) was noted; however, this finding was evident only after adjustment was done on interstudy variability through the removal of a lower quality study from the pool.
Conclusions:
High serum levels of long chain n-3 PUFA DPA is associated with reduced total prostate cancer risk. While high blood level of EPA and DHA is possibly associated with increased high-grade prostate tumour risk.
Introduction
Due to widespread use of prostate-specific antigen (PSA) screening, more prostate cancer is being detected. To find ways to prevent prostate cancer, several studies have tried to identify risk factors (i.e., lifestyle and diet). Researchers have studied the effects of long-chain omega-3 polyunsaturated fatty acids (n-3 PUFA), found in marine animals, on the prevalence of prostate cancer. These mechanisms of n-3 PUFA regulate inflammation via the eicosanoid pathway1–4 and modify androgen production.5 In particular, dietary intake of long-chain n-3 PUFA or its individual components (eicosa-pentaenoic acid [EPA], docosahexaenoic acid [DHA], doc-osapentaenoic acid [DPA]), have been proposed to have an association with prostate cancer risk; however, these results have been inconsistent, largely variable and heterogeneous.6–9 These inconsistent results were mainly due to research variations in dietary assessment techniques and under- or over-reporting of values, which decreased the accuracy of measuring individual’s fatty acid intake.10,11 Experts have suggested that levels of fatty acids in blood, tissue or erythrocyte membranes could provide a more reliable method of estimating fatty acid consumption.12–16 We conducted a meta-analysis to quantitatively estimate the correlation between blood levels of long chain n-3 PUFA and its derivatives with the incidence of prostate cancer in epidemiological studies.
Methods
We searched biomedical electronic databases, regardless of language. MEDLINE, UNBOUND MEDLINE, EMBASE, Science Direct, OVID, Proquest (database of dissertation and thesis) and the Cochrane Library were searched up to November 2011. MEDLINE Medical Subject Heading (MeSH) terms used were “omega 3 fatty acids” AND “prostate neoplasm.” Common keyword searches were “prostate cancer,” “carcinoma,” “neoplasm,” “tumor,” “omega,” “long chain fatty acids” and “polyunsaturated.” References from studies that met our inclusion criteria and review articles or textbooks were searched for potentially relevant titles. External peer reviewers were asked to identify additional relevant studies. Industry/nutrition experts were also inquired to obtain unpublished data.
We included prospective or retrospective case control studies of human population, where the blood level of long chain n-3 PUFA (DHA, DPA and EPA) was determined as exposure and incidence of prostate cancer was analyzed as outcome. All included studies provided effect estimates with corresponding confidence intervals pertaining to comparison between high long chain n-3 PUFA blood level and the reference group (lowest blood level). This unvarying method of comparison among the studies eliminated the differences of blood level n-3 PUFA source and ranges described in each study. Studies dealing with tissue n-3 PUFA levels were not included, since the sampling procedure was complex and usually done on high-risk patients, which could affect the reliability of effect estimates. Animal and in-vitro studies were excluded because correlation with in-vivo human physiologic outcome is uncertain. Cross-sectional and ecologic analyses were excluded, since these studies were unable to provide informative effect estimates.17
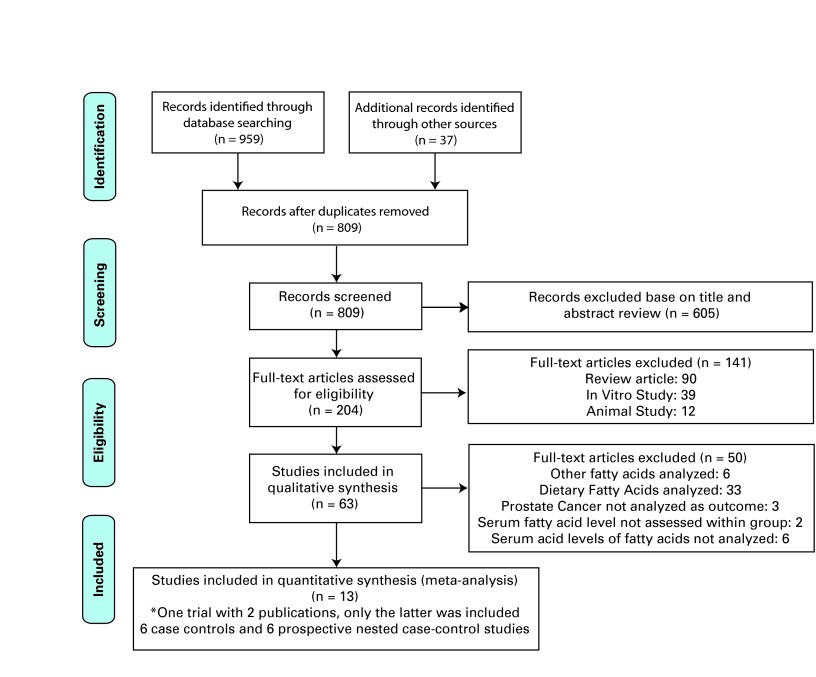
Two physician reviewers independently evaluated all citations and abstracts, and then they requested all the relevant full-text articles (Fig. 1). All articles obtained were independently reviewed by two reviewers knowledgeable in principles of critical appraisal. When discrepancy of evaluation arose, both reviewers resolved disagreements; a senior physician resolved unsettled issues. Articles retrieved were critically appraised and scored according to the National Health Service (NHS-UK) recommendation for review of qualitative studies.18 The maximum score was 11 points; studies that scored below 8 were excluded. Then, we used the Newcastle-Ottawa Quality Assessment Scale (NOQAS) of Cochrane Collaboration19 to rate each included study and enhance quality assessment, and to rank studies when heterogeneity was noted.
Fig. 1.
Prisma chart literature search process and result.
The general variance-based method was used to analyze the cohort studies, because variance estimates were based on adjusted measures of effect with 95% confidence interval (CI) that account for confounding variables and known to be superior in pooling observational data.19 Relative risk (RR) or odds ratio (OR) and corresponding CI, with adjustments for confounding variables, were used to estimate the risk ratio of prostate cancer incidence and subcategories (advanced and high grade type prostate tumour) with highest blood level of long chain omega-3 fatty acids component (DPA, DHA, EPA) versus the reference group. Only the most recent and comprehensive data were included when a study was published at several times and on different dates.
We used Cochran’s chi-square test (Q) and I squared (I2) to assess inter-study heterogeneity and variance, respectively.20 In cases of heterogeneity (p < 0.1), the source was identified by performing subgroup analyses on the basis of important differences in study design (retrospective case control vs. nested case-control). Afterwards, sensitivity analysis was repeated by excluding the study with the lowest NOQAS from the pool to acquire homogeneous pool estimates.
The random effect model was used to determine pooled effect estimates, since this model is more conservative.21 For analyzing the summation effect of long chain n-3 PUFA (DPA+DHA+EPA) and commercially available fish oil n-3 PUFA content (DHA+EPA) with prostate cancer incidence and its subcategories, we used a mixed effect analysis-random effects model to combine studies within each subgroup of long chain n-3 PUFA. The Comprehensive Meta Analysis software version 2 (Biostat, Englewood, NJ)22 and RevMan523 were used for the statistical analysis of pooled data and construction of forest plots. Publication bias was examined using Egger’s regression intercept,24 Begg-Mazumdar rank correlation25 analysis and visual inspection of funnel plots.26
Results
In total, we included 12 articles for this meta-analysis: 6 case-control studies27–32 and 6 nested case control studies (Table 1).33–38 All studies uniformly compared prostate cancer risk with the groups of involved population with the highest blood level of long chain n-3 PUFA and the reference group (lowest blood level). Most studies analyzed risk of prostate cancer development as part of their studies’ outcome.27–37 Four studies included advanced stage (defined as extension of tumour through the capsule) prostate cancer.28,35–37 Five studies included high-grade tumour (defined as tumour Gleason score ≥7) in their analysis of outcome.30,35–38 The age range of the study population was 40 to 86 years old. Overall, we analyzed 4516 prostate cancer cases and 5728 matching controls.
Table 1.
Summary of Studies Characteristics Included in the Meta-Analysis
| Authors (Year) | Source | Study design | Age of study population (Case/control) | Years of follow-up | Ascertain of cases (Prostate Ca) | Blood omega-3 fatty acid level determination | Relative risk* (CI) omega-3 | Level of comparison used |
Quality score (NOQAS**) Selection (S) Comparability (C) Exposure (E)
|
Quality score (NHS++) |
Adjustment variables | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S (4) | C (2) | E (3) | |||||||||||
| Harvei 199733 | Norway | Nested Case Control | Ave. 50 yr (141 / 282) | 19.2 years (Ave 11.6) | Cancer registries | Serum fatty acids | Total Risk EPA 1.2 (0.6–2.1) DHA 1.0 (0.5–1.8) DPA 0.7 (0.3–1.3) |
Quartile | 3 | 2 | 3 | 9/11 | Age, area of residence |
| Mannisto 200334 | Finland | Nested Case Control | 50–69 yr (198/198) | 5–10 years | Cancer registry and histopathology review | Serum fatty acids | Total risk EPA 1.12 (0.61–2.04) DHA 0.71 (0.40–1.26) |
Quartile | 2 | 2 | 3 | 8/11 | Age, Area of residence (urban/rural), level of education, body mass index, alcohol consumption, and the number of years of smoking. |
| Chavarro 200735 | US | Nested Case Control | 40–84 yr (476/476) | 13 years | Hospital Record and histopathology review | Blood level fatty acids | Total risk DPA 0.6 (0.38–0.93) EPA 0.57 (0.36–0.92) DHA 0.60 (0.39–0.93) Advance DPA 0.72 (0.3–1.73) EPA 1.27 (0.49–3.29) DHA 0.98 (0.39–2.50) High grade DPA 0.30 (0.12–0.80) EPA 0.42 (0.15–1.14) DHA 0.53 (0.21–1.31) |
Quintile | 2 | 1 | 3 | 9/11 | Age, smoking status at baseline, and length of follow-up |
| Crowe 200836 | Netherlands | Nested Case Control | 53–67 yr (962/1061) | 4.2 years | National & regional cancer registry | Blood phospholipid | Total risk EPA 1.31 (0.96–1.81) DHA 1.39 (1.02–1.90) DPA 0.95 (0.65–1.39) Advance EPA 0.99 (0.49–2.01) DHA 1.22 (0.62–2.40) DPA 0.91 (0.42–2.00) High grade EPA 2.00 (1.07–3.76) DHA 1.41 (0.76–2.62) DPA 0.71 (0.35–1.46) |
Quintile | 2 | 2 | 3 | 9/11 | Age, BMI, smoking, alcohol intake, level of education, marital status, and physical activity |
| Park 200937 | USA | Nested Case Control | 45–75 yr (376/729) | 10 years | Tumor registry | Erythrocyte membrane fatty acids | Total risk EPA 1.11 (0.73–1.67) DHA 1.11 (0.73–1.69) DPA 0.78 (0.43–1.41) Advance/high grade EPA 1.61 (0.79–3.25) DHA 1.05 (0.51–2.16) DPA 1.13 (0.33–3.82) |
Quartile and Tertile | 2 | 2 | 3 | 10/11 | Age, area of residence, race/ethnicity, family history of prostate cancer, BMI, level of education, hour of fasting, date and time of blood draws, |
| Brasky 201138 | USA | Nested Case Control | 55–84 yr (1658/1803) | 7 years | End study prostate biopsies | Serum fatty acids | Low Grade EPA 1.01 (0.83–1.24) DHA 1.18 (0.97–1.44) High Grade EPA 1.09 (0.63–1.86) DHA 2.50 (1.34–4.65) |
Quartile | 4 | 2 | 3 | 9/11 | Age, race, family history of prostate cancer, diabetes, BMI, alcohol, and treatment arm. |
| Norrish 199932 | New Zealand | Nested Case Control (from PCPT) | 40–80 yr (317/480) | N/A | Histopathology | Erythrocyte membrane fatty acids | Total Risk EPA 0.59 (0.37–0.95) DHA 0.62 (0.39–0.98) Advance EPA 0.54 (0.31–0.98) DHA 0.66 (0.39–1.13) |
Quartile | 3 | 2 | 3 | 9/11 | Age, height, total non-steroidal anti-inflammatory drug use, socio-economic status, and food frequency questionnaire-estimated intake of total polyunsaturated fat |
| Shannon 201030 | USA | Case Control | 50–86 yr (127/183) | N/A | Histopathology | Erythrocyte Fatty membrane fatty acids | Total risk EPA 1.12 (0.64–1.96) DHA 1.14 (0.62–2.09) High grade EPA 0.83 (0.39–1.75) DHA 1.06 (0.48–2.32) |
Tertile | 3 | 2 | 3 | 9/11 | Age, BMI, race, and family history of prostate cancer |
| Godley 199627 | USA | Case Control | >45 yr (89/38) | N/A | Histopathology | Erythrocyte membrane fatty acids | Total Risk EPA 0.74 (0.23–2.33) DHA 0.36 (0.10–1.27) |
Quartile | 3 | 2 | 2 | 8/11 | Age and Race |
| New-comer 200128 | USA | Case Control | 41–66 yr (67/156) | N/A | Histopathology | Erythrocyte membrane fatty acids | Total risk EPA 1.3 (0.6–3.0) DHA 1.0 (0.4–2.3) |
Quartile | 3 | 1 | 3 | 8/11 | Age |
| Ukoli 200929 | Nigeria | Case Control | >=45 (66/226) | N/A | Histopathology | Serum fatty acids | Total risk EPA 1.09 (0.4–2.96) DPA 0.44 (0.17–1.19) DHA 0.56 (0.22–1.40) |
Quartile | 3 | 2 | 3 | 9/11 | Age, level of education, family history of prostate cancer, and waist-hip ratio. |
| Ukoli 201031 | Nigeria | Case Control | >=45 (48/96) | N/A | Histopathology | Serum fatty acids | Total Risk EPA 0.82 (0.21–3.24) DPA 1.01 (0.31–3.33) DHA 1.35 (0.40–4.61) |
Quartile | 3 | 2 | 3 | 9/11 | Age, level of education, family history of prostate cancer, and waist-hip ratio. |
Relative risks with corresponding 95% confidence interval were derived comparing the prostate cancer incidence among the population group with highest blood level n-3 PUFA versus lowest blood level PUFA
Newcastle-Ottawa Quality Assessment Score;
National Health Service-UK recommended critical appraisal of Case-control; BMI: body mass index.
Blood level omega-3 PUFA and prostate cancer risk
Visual inspection of funnel plot showed publication bias less likely (Fig. 2). Results showed that the pooled estimates of long chain n-3 PUFA DPA have a significant association with total prostate cancer incidence (pooled RR: 0.756; CI 0.599, 0.955; p = 0.019) (Fig. 3). In the homogeneous studies (p = 0.566), there were no study variations (I2 = 0%), and no publication bias in the Begg (p = 1.0) and Egger’s regression intercept (p = 0.54) (Table 2.1). When subgroup analysis was done by method of study (retrospective vs. prospective), the significant finding was retained in the prospective studies (pooled RR: 0.773; CI 0.605, 0.988; p = 0.040). High blood levels of total n-3 PUFA or other derivatives (together and individually) had no significant association to total prostate cancer risk, advanced prostate cancer and high-grade prostate tumour (Table 3).
Fig. 2.
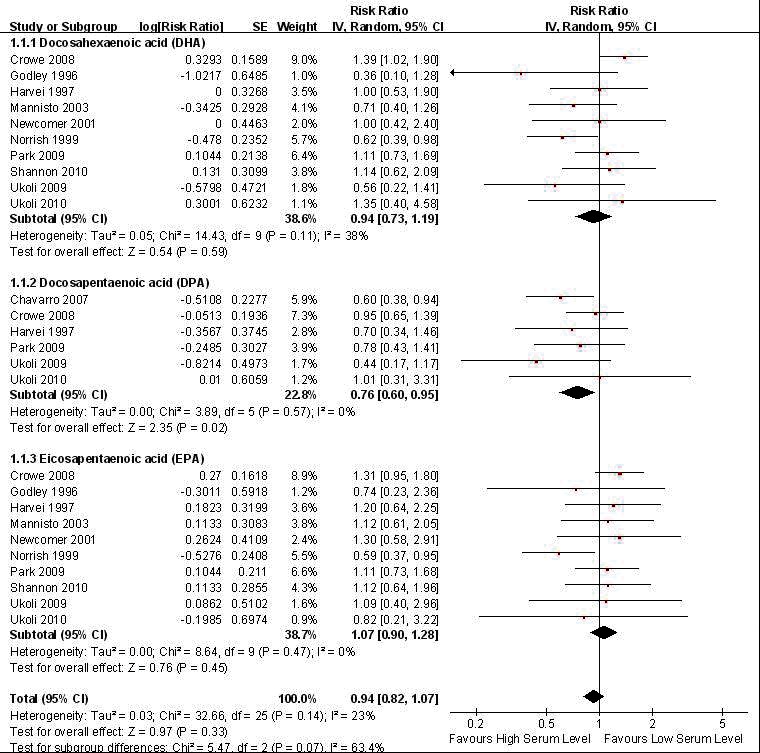
Forest plot of pooled effect of blood level omega-3 polyunsaturated fatty acid (PUFA) on total prostate cancer risk.
Fig. 3.
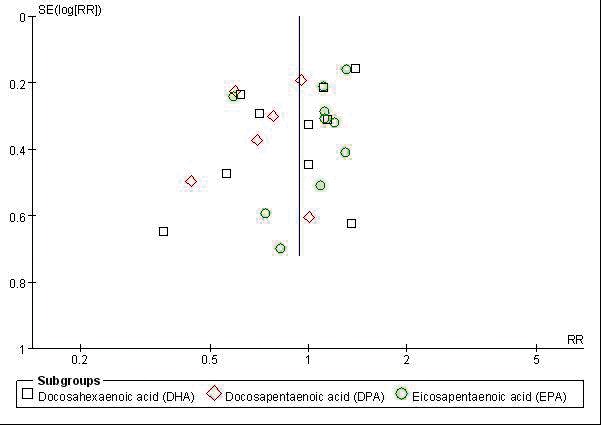
Publication bias determination using funnel plot.
Table 2.1.
Blood level omega-3 poly-unsaturated fatty acids vs. total prostate risk random effect analysis model
| Groups | Effect size and 95% interval | Test of null (2-Tail) | Heterogeneity^ | Tau-squared | Publication bias | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Omega-3 derivatives | # of Study | Point Estimates | Lower Limit | Upper Limit | Z-value | P-value | Q-value | df | p-value | I2 | Tau2 | Standard Error | Variance | Begg | Egger |
| DPA | 6 | 0.756 | 0.599 | 0.955 | −2.347 | 0.019 | 3.883 | 5 | 0.566 | 0.000 | 0.000 | 0.060 | 0.004 | 1.000 | 0.540 |
| DHA | 11 | 0.876 | 0.685 | 1.119 | −1.059 | 0.290 | 18.991 | 10 | 0.040 | 47.343 | 0.072 | 0.073 | 0.005 | 0.436 | 0.239 |
| DHA† | 10 | 0.935 | 0.733 | 1.194 | −0.538 | 0.591 | 14.450 | 9 | 0.107 | 37.716 | 0.053 | 0.069 | 0.005 | 0.211 | 0.127 |
| EPA | 11 | 0.971 | 0.784 | 1.204 | −0.264 | 0.792 | 14.741 | 10 | 0.142 | 32.162 | 0.039 | 0.056 | 0.003 | 0.533 | 0.671 |
| EPA† | 10 | 1.070 | 0.898 | 1.275 | 0.762 | 0.446 | 8.656 | 9 | 0.470 | 0.000 | 0.000 | 0.041 | 0.002 | 0.211 | 0.502 |
| (DHA+DPA+EPA)* | 0.942 | 0.834 | 1.064 | −0.962 | 0.336 | 32.676 | 25 | 0.139 | 23.492 | 0.026 | 0.031 | 0.001 | |||
| (DHA+EPA)* | 1.022 | 0.887 | 1.179 | 0.306 | 0.760 | 23.410 | 19 | 0.220 | 18.840 | 0.019 | 0.034 | 0.001 | |||
Inter-study heterogeneity was tested by Cochrane’s Q (Chi2) at a significance level of P<0.10 and quantified by I2, where I2 ≥50 % is considered to be evidence of substantial heterogeneity and ≥75%, considerable heterogeneity
Inter-study variation adjusted (heterogeneous study removed from the pool of effect estimates).
Generated from adjusted total effect estimates from each n-3 PUFA random effect analysis; DPA: docosapentaenoic acid; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid.
Table 3.
Blood level omega-3 polyunsaturated fatty acids vs. advanced prostate risk random effect analysis model
| Groups | Effect size and 95% interval | Test of null (2-Tail) | Heterogeneity^ | Tau-squared | Publication bias | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Omega-3 derivatives | No. studies | Point Estimates | Lower Limit | Upper Limit | Z-value | P-value | Q-value | df | p-value | I2 | Tau2 | Standard Error | Variance | Begg | Egger |
| DPA | 3 | 0.870 | 0.514 | 1.473 | −0.517 | 0.606 | 0.367 | 2 | 0.832 | 0.000 | 0.000 | 0.229 | 0.052 | 1.000 | 0.618 |
| DHA | 4 | 0.896 | 0.640 | 1.256 | −0.637 | 0.524 | 2.289 | 3 | 0.515 | 0.000 | 0.000 | 0.102 | 0.010 | 1.000 | 0.342 |
| EPA | 4 | 0.975 | 0.582 | 1.634 | −0.094 | 0.925 | 6.180 | 3 | 0.103 | 41.457 | 0.141 | 0.226 | 0.051 | 0.308 | 0.309 |
| (DHA+DPA+EPA)* | 0.908 | 0.708 | 1.164 | −0.760 | 0.447 | 8.870 | 10 | 0.545 | 0.000 | 0.000 | 0.064 | 0.004 | |||
| (DHA+EPA)* | 0.919 | 0.693 | 1.219 | −0.585 | 0.559 | 8.482 | 7 | 0.292 | 17.471 | 0.027 | 0.081 | 0.007 | |||
Generated from total effect estimates from each n-3 PUFA random effect analysis; DPA: docosapentaenoic acid; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid.
Significant heterogeneity was noted on the analysis of blood level n-3 PUFA DHA and EPA with total prostate cancer risk and high-grade prostate tumours (Table 2.2, Table 4); therefore the validity of the result was questioned. The inter-study variation ranged from 32% to 53%. Source of heterogeneity was identified (Table 2.2) and a nested case-control study34 was removed from the pooled estimate which resulted to reduced heterogeneity and variation (I2).
Table 2.2.
Blood level omega-3 poly-unsaturated fatty acids vs. total prostate risk subgroup analysis model (prospective vs. retrospective)
| Groups | Effect size and 95% interval | Test of null (2-Tail) | Heterogeneity^ | Tau-squared | Publication bias | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Omega-3 derivatives | No. studies | Point Estimates | Lower Limit | Upper Limit | Z-value | P-value | Q-value | df | p-value | I2 | Tau2 | Standard Error | Variance | Begg | Egger |
| DPA | 6 | 0.756 | 0.599 | 0.955 | −2.347 | 0.019 | 3.883 | 5 | 0.566 | 0.000 | 0.000 | 0.060 | 0.004 | 1.000 | 0.540 |
| Retrospective | 2 | 0.620 | 0.278 | 1.382 | −1.168 | 0.243 | 1.126 | 1 | 0.289 | 11.180 | 0.039 | 0.488 | 0.238 | ||
| Prospective | 4 | 0.773 | 0.605 | 0.988 | −2.055 | 0.040 | 2.433 | 3 | 0.488 | 0.000 | 0.000 | 0.055 | 0.003 | ||
| DHA | 11 | 0.876 | 0.685 | 1.119 | −1.059 | 0.290 | 18.991 | 10 | 0.040 | 47.343 | 0.072 | 0.073 | 0.005 | 0.436 | 0.239 |
| Retrospective | 6 | 0.769 | 0.558 | 1.060 | −1.603 | 0.109 | 5.433 | 5 | 0.365 | 7.972 | 0.014 | 0.109 | 0.117 | ||
| Prospective | 5 | 0.942 | 0.670 | 1.325 | −0.342 | 0.733 | 11.213 | 4 | 0.024 | 64.327 | 0.094 | 0.107 | 0.011 | ||
| EPA | 11 | 0.971 | 0.784 | 1.204 | −0.264 | 0.792 | 14.741 | 10 | 0.142 | 32.162 | 0.039 | 0.056 | 0.003 | 0.533 | 0.671 |
| Retrospective | 6 | 0.851 | 0.634 | 1.143 | −1.069 | 0.285 | 4.603 | 5 | 0.466 | 0.000 | 0.000 | 0.097 | 0.009 | ||
| Prospective | 5 | 1.028 | 0.757 | 1.396 | 0.178 | 0.859 | 8.661 | 4 | 0.070 | 53.818 | 0.064 | 0.086 | 0.007 | ||
DPA: docosapentaenoic acid; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid.
Table 4.
Blood level omega-3 polyunsaturated fatty acids vs. high-grade prostate risk random effect analysis model
| Groups | Effect size and 95% interval | Test of null (2-Tail) | Heterogeneity^ | Tau-squared | Publication bias | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Omega-3 derivatives | No. of studies | Point estimates | Lower limit | Upper limit | Z-value | P-value | Q-value | df | p-value | I2 | Tau2 | Standard error | Variance | Begg | Egger |
| DPA | 3 | 0.597 | 0.299 | 1.193 | −1.460 | 0.144 | 3.291 | 2 | 0.193 | 39.231 | 0.149 | 0.381 | 0.145 | 1.000 | 0.930 |
| DHA | 5 | 1.233 | 0.769 | 1.978 | 0.869 | 0.385 | 8.593 | 4 | 0.072 | 53.449 | 0.154 | 0.205 | 0.042 | 0.221 | 0.051 |
| DHA† | 4 | 1.462 | 0.972 | 2.199 | 1.823 | 0.068 | 4.310 | 3 | 0.230 | 30.389 | 0.053 | 0.142 | 0.020 | 0.734 | 0.265 |
| EPA | 5 | 1.130 | 0.717 | 1.781 | 0.527 | 0.599 | 8.362 | 4 | 0.079 | 52.162 | 0.138 | 0.190 | 0.036 | 0.221 | 0.273 |
| EPA† | 4 | 1.317 | 0.910 | 1.908 | 1.458 | 0.145 | 3.931 | 3 | 0.269 | 23.675 | 0.034 | 0.117 | 0.014 | 0.734 | 0.952 |
| (DHA+DPA+EPA)* | 1.232 | 0.955 | 1.590 | 1.605 | 0.108 | 20.370 | 10 | 0.026 | 50.908 | 0.136 | 0.121 | 0.015 | |||
| (DHA+EPA)* | 1.390 | 1.070 | 1.80 | 2.500 | 0.010 | 8.498 | 7 | 0.291 | 17.629 | 0.024 | 0.074 | 0.005 | |||
Inter-study heterogeneity was tested by Cochrane’s Q (Chi2) at a significance level of P<0.10 and quantified by I2, where I2 ≥ 50 % is considered to be evidence of substantial heterogeneity and ≥75%, considerable heterogeneity;
Inter-study variation adjusted (heterogeneous study removed from the pool of effect estimates);
Generated from adjusted total effect estimates from each n-3 PUFA random effect analysis; DPA: docosapentaenoic acid; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid.
Reviewing the summation effect of fish oil content long chain n-3 PUFA (DHA+EPA) on prostate cancer development, we found a significant positive association (pooled RR: 1.39; CI 1.07, 1.80; p = 0.021) (Fig. 4) with high-grade prostate cancer. Adjusted inter-study heterogeneity was not significant (p = 0.291) with a small degree of inter-study variation (I2 = 17.6%). Publication bias of the respective n-3 PUFA subgroup analysis was not evident using Begg (p = 0.734), Egger’s (p = 0.265, 0.952) test (Table 4) and upon visual inspection of the funnel plot (data not shown).
Fig. 4.
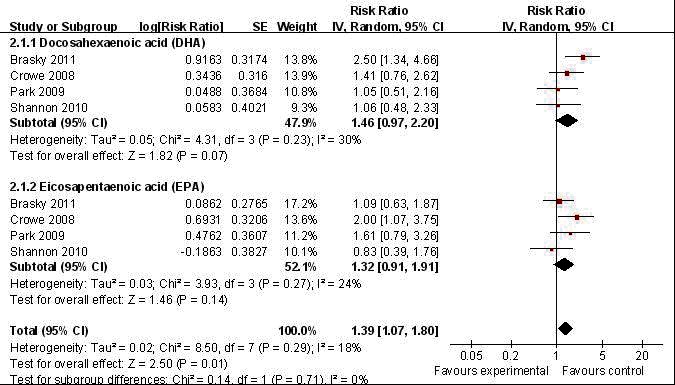
Forest plot of pooled effect of blood level omega-3 polyunsaturated fatty acid (PUFA) high-grade prostate tumour.
Discussion
Randomized clinical trials have not been done to clarify the role of n-3 PUFA in prostate cancer development due to ethical considerations and methodological limitations; as such, we investigated this relationship using the best credible epidemiological data available – case controls. Another important aspect in this meta-analysis is that all included studies were executed in the 1990s when PSA screening was utilized for early detection of prostate cancer.
After an extensive review, we found a significant negative association between high blood n-3 PUFA DPA level and total risk of prostate cancer. DPA is found in whale meat, seal oil and, to a lesser extent, in marine fatty fish oil together with other long chain n-3 PUFA series (DHA and EPA).39 Currently, few studies have been conducted to examine the biophysiological effect of DPA because of production costs. Human studies are lacking; most studies are in-vitro or with animal subjects.40 In the study by Wang and colleagues, the finding of high serum level DPA is a result of in-vivo biochemical conversion rather than mere high dietary exposure, since the commercially available supplement of long chain n-3 PUFA DPA is not common or readily available.40 Moreover, in the subgroup analysis of the pooled prospective studies, the significant association was retained; this illustrates the association as an effect of long-term lipid metabolism rather than short-term dietary exposure. Studies have shown that humans are able to biosynthesize DPA mainly through bioconversion from EPA by enzymes fatty acid elongase-2 and 5, and could be retro-converted to EPA in the liver and kidney.41–43 The mechanism of the protective effect of DPA on prostate cancer may be explained by biochemical processes involving: reduced prostacyclin production, expression of inflammatory genes and TNF-induced necrotic cell death; competition with cycloxygenase 2 (COX2) enzymes resulting to anti-neoplastic activity via proapoptotic pathway; and inhibition of angiogenesis.44–49 Detection of such association may suggest that serum level DPA implicates individual genetic difference in biochemical characteristics of enzymatic activities, which may be further investigated as a probable new serum biomarker for prostate cancer risk assessment in the future.
Heterogeneity was noted in the analysis of association of blood level DHA and EPA with prostate cancer and high-grade prostate tumour. The source of heterogeneity was mainly from the nested case cohort of “The Physician’s Health Study.”3 The authors acknowledged that their subjects were more knowledgeable and provided more reliable information. However, this group may have a generally higher DHA and EPA intake which affected the study’s results because of their increased awareness towards healthy practices. The study also failed to present adjustments for confounding variables, such as family history, body mass index and racial ethnicity, which were established risk factors for prostate cancer. When this study was excluded, a significant positive association was noted on fish oil containing long chain n-3 PUFA (EPA+DHA) with high grade prostate cancer (Table 4). Factors to consider on this relative association is the healthier lifestyle of patients taking fish oil. These patients tend to be more health conscious, which may produce a co-founding factor of early detection via PSA screening due to better health follow-up and health care access. However, the detection of high-grade prostate tumour instead of indolent or total prostate cancer risk among this subgroup is presumed to be due to a biochemical process in the prostate tissue. Since, it is well-illustrated in epidemiological studies that increased prostate cancer incidence due to early detection by vast PSA screening is more significant for general risk or detection of indolent type of prostate cancery.50–52
The finding of an association between EPA+DHA with high-grade prostate tumour was quite similar with the findings by the Prostate Cancer Prevention Trial (PCPT).50 Nonetheless, there is still debate about whether finasteride induces the development of high-grade prostate tumour or results to a better detection rate by reducing prostate size.50 As mentioned earlier, there are inconsistencies regarding the effects of long-chain PUFA, particularly EPA and DHA, in the development of prostate cancer. Some studies have recognized the effects of n-3 PUFA via eicosanoid pathway in cancer prevention, while others have implicated the role of dietary fat in changing the androgen milieu as a causative factor for prostate cancer. The detection of high-grade prostate tumour instead of indolent or total prostate cancer risk was presumed to be due to a biochemical process in the prostate tissue. The since increased early detection of prostate cancer due to PSA screening is more frequent in general risk or indolent type of cancer rather than in the high-grade subtype only.51,52
Reports have also shown that marine fish contaminated with environmental toxins, such as polychlorinated biphenyls or methylmercury compounds, can disrupt androgen and estrogen balance and could be linked to high-grade prostate cancer.53–54 Furthermore, the presence of long chain n-3 PUFA (DHA and EPA) in the prostate cell’s beta-oxidative metabolic process leads to the formation of lipid hydroperoxides in the microenvironment of the cell; this can generate reactive species.55–56 With chronic exposure to these reactive molecules, the prostate cell can become dysplastic and develop into an aggressive cell.
In this aspect, the possible role of both EPA and DHA needs to be examined further for their use as biomarkers for aggressive disease and to see if a reduction of these n-3 PUFA can decrease the risk. Possible reasons why EPA and DHA, but not DPA, are implicated in aggressive prostate cancer remain to be determined. The association of serum DPA in prostate cancer development still needs to be examined further, since lipid metabolism is far more intricate and genetic variations in individuals may be involved.57
Conclusion
This meta-analysis provided evidence to show that high blood level n-3 PUFA DPA is associated with reduced risk of prostate cancer. While high blood level of EPA and DHA in combination is associated with increase high-grade prostate tumour risk. These results must be interpreted with caution, since the etiology of prostate cancer is multifactorial and the metabolism of long chain n-3 PUFA in human body is complex.
Footnotes
Competing interests: None declared.
This paper has been peer-reviewed.
References
- 1.De Marzo AM, Platz EA, Sutcliffe S, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–69. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan JM, Gann PH, Giovannucci EL. Role of diet in prostate cancer development and progression. J Clin Oncol. 2005;23:8152–60. doi: 10.1200/JCO.2005.03.1492. [DOI] [PubMed] [Google Scholar]
- 3.Chan JM, Stampfer MJ, Ma J, et al. Dairy products, calcium, and prostate cancer risk in the Physicians’ Health Study. Am J Clin Nutr. 2001;74:549–54. doi: 10.1093/ajcn/74.4.549. [DOI] [PubMed] [Google Scholar]
- 4.Chan JM, Weinberg V, Magbanua MJ, et al. Nutritional supplements, COX-2 and IGF-1 expression in men on active surveillance for prostate cancer. Cancer Causes Control. 2011;22:141–50. doi: 10.1007/s10552-010-9684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsson SC, Kumlin M, Ingelman-Sundberg M, et al. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79:935–45. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 6.Carayol M, Grosclaude P, Delpierre C. Prospective studies of dietary alpha-linolenic acid intake and prostate cancer risk: a meta-analysis. Cancer Causes Control. 2010;21:347–55. doi: 10.1007/s10552-009-9465-1. [DOI] [PubMed] [Google Scholar]
- 7.Ma RW-L, Chapman K. A systematic review of the effect of diet in prostate cancer prevention and treatment. J Hum Nutr Diet. 2009;22:187–99. doi: 10.1111/j.1365-277X.2009.00946.x. [DOI] [PubMed] [Google Scholar]
- 8.MacLean CH, Newberry SJ, Mojica WA, et al. Effects of omega-3 fatty acids on cancer risk. JAMA. 2006;295:403–15. doi: 10.1001/jama.295.4.403. [DOI] [PubMed] [Google Scholar]
- 9.Chua ME, Dy JS. Relationship of Dietary Intake of Omega-3 and Omega-6 Fatty Acids with Risk of Prostate Cancer Development. Philippine Journal of Urology. 2011. In Press. [DOI] [PMC free article] [PubMed]
- 10.Kohlmeier L. Future of dietary exposure assessment. Am J Clin Nutr. 1995;61:S702–9. doi: 10.1093/ajcn/61.3.702S. [DOI] [PubMed] [Google Scholar]
- 11.Byers T, Gieseker K. Issues in the design and interpretation of studies of fatty acids and cancer in humans. Am J Clin Nutr. 1997;66:S1541–7. doi: 10.1093/ajcn/66.6.1541S. [DOI] [PubMed] [Google Scholar]
- 12.Andersen LF, Solvoll K, Drevon CA. Very-long-chain n_3 fatty acids as biomarkers for intake of fish and n_3 fatty acid concentrates. Am J Clin Nutr. 1996;64:305–11. doi: 10.1093/ajcn/64.3.305. [DOI] [PubMed] [Google Scholar]
- 13.Wolk A, Furuheim M, Vessby B. Fatty acid composition of adipose tissue and serum lipids are valid biological markers of dairy fat intake in men. J Nutr. 2001;131:828–33. doi: 10.1093/jn/131.3.828. [DOI] [PubMed] [Google Scholar]
- 14.Zock PL, Mensink RP, Harryvan J, et al. Fatty acids in serum cholesteryl esters as quantitative biomark-ers of dietary intake in humans. Am J Epidemiol. 1997;145:1114–22. doi: 10.1093/oxfordjournals.aje.a009074. [DOI] [PubMed] [Google Scholar]
- 15.Arab L, Akbar J. Biomarkers and the measurement of fatty acids. Public Helath Nutr. 2002;5:865–71. doi: 10.1079/PHN2002391. [DOI] [PubMed] [Google Scholar]
- 16.Baylin A, Kim MK, Donovan-Palmer A, et al. Fasting whole blood as a biomarker of essential fatty acid intake in epidemiologic studies: comparison with adipose tissue and plasma. Am J Epidemiol. 2005;162:373–81. doi: 10.1093/aje/kwi213. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items nfor systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 18.Letts L, Wilkins S, Law M, et al. Guidelines for Critical Review Form: Qualitative Studies (Version 2.0) 2007 http://www.srs-mcmaster.ca/Portals/20/pdf/ebp/qualguidelines_version2.0.pdf. Accessed April 25, 2013. [Google Scholar]
- 19.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011. [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane-handbook.org. Accessed April 25, 2013.
- 20.Greenland S. Quantitative methods in review of epidemiologic literature. Epidemiol Rev. 1986;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 21.Hunter JE, Schmidt FL. Fixed effect vs. random effects meta-analysis models: implications for cumulative research knowledge. Int J Selection Assess. 2000;8:275–92. doi: 10.1111/1468-2389.00156. [DOI] [Google Scholar]
- 22.Borenstein M, Hedges L, Higgins J, et al. Comprehensive Meta Analysis Version 2. Biostat; Englewood, NJ: 2005. [Google Scholar]
- 23.Review manager (Revman) [Computer program]. Version 5.1. Copenhagen: The Nordic Cochrane Center, Cochrane Collaboration; 2011. [Google Scholar]
- 24.Egger M, Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 26.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 27.Godley PA, Campbell MK, Gallagher P, et al. Biomarkers of essential fatty acid consumption and risk of prostatic carcinoma. Cancer Epidemiol Biomarkers Prev. 1996;5:889–95. [PubMed] [Google Scholar]
- 28.Newcomer LM, King IB, Wicklund KG, et al. The association of fatty acids with prostate cancer risk. Prostate. 2001;47:262–8. doi: 10.1002/pros.1070. [DOI] [PubMed] [Google Scholar]
- 29.Ukoli FA, Akumabor PN, Oguike TC, et al. The association of plasma fatty acids with prostate cancer risk in Nigerians. Ethn Dis. 2009;19:454–61. [PubMed] [Google Scholar]
- 30.Shannon A, O’Malley J, Mori M, et al. Erythrocyte fatty acids and prostate cancer risk: A comparison of methods. Prostaglandins Leukot Essent Fatty Acids. 2010;83:161–9. doi: 10.1016/j.plefa.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ukoli FA, Fowke JH, Akumabor P, et al. The association of plasma fatty acids with prostate cancer risk in African Americans and Africans. J Health Care Poor Underserved. 2010;21(1 Suppl):127–47. doi: 10.1353/hpu.0.0242. [DOI] [PubMed] [Google Scholar]
- 32.Norrish AE, Skeaff CM, Arribas GLB, et al. Prostate cancer risk and consumption of fish oils: A dietary biomarker-based case-control study. Br J Cancer. 1999;81:1238–42. doi: 10.1038/sj.bjc.6690835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harvei S, Bjerve KS, Tretli S, et al. Prediagnostic level of fatty acids in serum phospholipids: omega-3 and omega-6 fatty acids and the risk of prostate cancer. Int J Cancer. 1997;71:545–51. doi: 10.1002/(SICI)1097-0215(19970516)71:4<545::AID-IJC7>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 34.Mannisto S, Pietinen P, Virtanen MJ, et al. Fatty acids and risk of prostate cancer in a nested case-control study in male smokers. Cancer Epidemiol Biomarkers Prev. 2003;12:1422–8. [PubMed] [Google Scholar]
- 35.Chavarro JE, Stampfer MJ, Li H, et al. A prospective study of polyunsaturated fatty acid levels in blood and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16:1364–70. doi: 10.1158/1055-9965.EPI-06-1033. [DOI] [PubMed] [Google Scholar]
- 36.Crowe FL, Allen NE, Appleby PN, et al. Fatty acid composition of plasma phospholipids and risk of prostate cancer in a case-control analysis nested within the European prospective investigation into cancer and nutrition. Am J Clin Nutr. 2008;88:1353–63. doi: 10.3945/ajcn.2008.26369. [DOI] [PubMed] [Google Scholar]
- 37.Park SY, Wilken LR, Henning SM, et al. Circulating fatty acids and prostate cancer risk in a nested case–control study: the Multiethnic Cohort. Cancer Causes Control. 2009;20:211–23. doi: 10.1007/s10552-008-9236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brasky TM, Till C, White E, et al. Serum phospholipid fatty acids and prostate cancer risk: results from the prostate cancer prevention trial. Am J Epidemiol. 2011;173:1429–39. doi: 10.1093/aje/kwr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer BJ, Lane AE, Mann NJ. Comparison of seal oil to tuna oil on plasma lipid levels and blood pressure in hypertriglyceridaemic subjects. Lipids. 2009;44:827–35. doi: 10.1007/s11745-009-3333-3. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Botolin D, Christian B, et al. Tissue-specific, nutritional, and developmental regulation of rat fatty acid elongases. J Lipid Res. 2005;46:706–15. doi: 10.1194/jlr.M400335-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaur G, Begg DP, Barr B, et al. Short-term docosapentaenoic acid (22:5n–3) supplementation increases tissue docosapentaenoic acid, DHA and EPA concentrations in rats. Br J Nutr. 2009;103:32–7. doi: 10.1017/S0007114509991334. [DOI] [PubMed] [Google Scholar]
- 42.Holub BJ, Swidinsky P, Park E. Oral Docosapentaenoic Acid (22:5n-3) Is Differentially Incorporated into Phospholipid Pools and Differentially Metabolized to Eicosapentaenoic Acid in Tissues from Young Rats. Lipids. 2011;46:399–407. doi: 10.1007/s11745-011-3535-3. [DOI] [PubMed] [Google Scholar]
- 43.Henderson RA, Jensen RG, Lammi-Keefe CJ, et al. Effect of fish oil on the fatty acid composition of human milk and maternal and infant erythrocytes. Lipids. 1992;27:863–9. doi: 10.1007/BF02535865. [DOI] [PubMed] [Google Scholar]
- 44.Kishida E, Tajiri M, Masuzawa Y. Docosahexaenoic acid enrichment can reduce L929 cell necrosis induced by tumor necrosis factor. Biochim Biophys Acta. 2006;1761:454–62. doi: 10.1016/j.bbalip.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 45.Careaga MM, Sprecher H. Synthesis of two hydroxy fatty acids from 7,10,13,16,19-docosapentaenoic acid by human platelets. J Biol Chem. 1984;259:14413–7. [PubMed] [Google Scholar]
- 46.Benistant C, Achard F, Ben Slama S, et al. Docosapentaenoic acid (22:5, n-3): metabolism and effect on prostacyclin production in endothelial cells. Prostag Leukotr Essent Fatty Acids. 1996;55:287–92. doi: 10.1016/S0952-3278(96)90010-1. [DOI] [PubMed] [Google Scholar]
- 47.Kelly L, Grehan B, Chiesa AD, et al. The polyunsaturated fatty acids, EPA and DPA exert a protective effect in the hippocampus of the aged rat. Neurobiol Aging. 2011;32:2318.e1–2318.e15. doi: 10.1016/j.neurobiolaging.2010.04.001. http://dx.doi/10.1016/j.neurobiolaging.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Tsuji M, Murota SI, Morita I. Docosapentaenoic acid (22:5, n-3) suppressed tube-forming activity in endothelial cells induced by vascular endothelial growth factor. Prostag Leukotr Essent Fatty Acids. 2003;68:337–42. doi: 10.1016/S0952-3278(03)00025-5. [DOI] [PubMed] [Google Scholar]
- 49.Solakivi T, Jaakkola O, Kalela A, et al. Lipoprotein docosapentaenoic acid is associated with serum matrix metalloproteinase-9 concentration. Lipids Health Dis. 2005;4:8. doi: 10.1186/1476-511X-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andriole GL, Humphrey PA, Serfling RJ, et al. High-grade prostate cancer in the Prostate Cancer Prevention Trial: fact or artifact? J Natl Cancer Inst. 2007;99:1355–6. doi: 10.1093/jnci/djm151. [DOI] [PubMed] [Google Scholar]
- 51.Legler JM, Feuer EJ, Potosky AL, et al. The role of prostate-specific antigen (PSA) testing patterns in the recent prostate cancer incidence decline in the United States. Cancer Causes Control. 1998;9:519–27. doi: 10.1023/A:1008805718310. [DOI] [PubMed] [Google Scholar]
- 52.Giovannucci E. Commentary: serum lycopene and prostate cancer progression: a re-consideration of findings from the prostate cancer prevention trial. Cancer Causes Control. 2011;22:1055–9. doi: 10.1007/s10552-011-9776-x. [DOI] [PubMed] [Google Scholar]
- 53.Brouwer A, Longnecker MP, Birnbaum LS, et al. Characterization of potential endocrine-related health effects at low-dose levels of exposure to PCBS. Environ Health Perspect. 1999;107(Suppl 4):639–49. doi: 10.1289/ehp.99107s4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ritchie JM, Vial SL, Fuortes LJ, et al. Comparison of proposed frameworks for grouping polychlorinated biphenyl congener data applied to a case-control pilot study of prostate cancer. Environ Res. 2005;98:104–13. doi: 10.1016/j.envres.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate ancer. Prostate Cancer Prostatic Dis. 2006;9:230–4. doi: 10.1038/sj.pcan.4500879. [DOI] [PubMed] [Google Scholar]
- 56.Federico A, Morgillo F, Tuccillo C, et al. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121:2381–6. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 57.Lemaitre RN, Tanaka T, Tang W, et al. Genetic Loci Associated with Plasma Phospholipid n-3 Fatty Acids: A Meta-Analysis of Genome-Wide Association Studies from the CHARGE Consortium. PLoS Genet. 2011;7:e1002193. doi: 10.1371/journal.pgen.1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]