Abstract
Introduction:
Prostate biopsies incur the risk of being false-negative and this risk has not yet been evaluated for 12-core prostate biopsy. We calculated the false-negative rate of 12-core prostate biopsy and determined the patient characteristics which might affect detection rate.
Methods:
We included 90 prostate cancer patients (mean age of 64, range: 49–77) diagnosed with transrectal ultrasound guided 12-core prostate biopsy between December 2005 and April 2008. All patients underwent radical retropubic prostatectomy and the 12-core prostate biopsy procedure was repeated on surgical specimen ex-vivo. Results of preoperative and postoperative prostate biopsies were compared. We analyzed the influence of patient age, prostate weight, serum prostate-specific antigen (PSA) level, free/total PSA ratio, PSA density and Gleason score on detection rate.
Results:
In 67.8% of patients, prostate cancer was detected with repeated ex-vivo biopsies using the same mapping postoperatively. We found an increase in PSA level, PSA density and biopsy Gleason score; patient age, decreases in prostate weight and free/total PSA ratio yielded higher detection rates. All cores, except the left-lateral cores, showed mild-moderate or moderate internal consistency. Preoperative in-vivo biopsy Gleason scores remained the same, decreased and increased in 43.3%, 8.9% and 47.8% of patients, respectively, on final specimen pathology.
Conclusions:
The detection rate of prostate cancer with 12-core biopsy in patients (all of whom had prostate cancer) was considerably low. Effectively, repeat biopsies can still be negative despite the patient’s reality of having prostate cancer. The detection rate is higher if 12-core biopsies are repeated in younger patients, patients with high PSA levels, PSA density and Gleason scores, in addition in patients with smaller prostates, lower free/total PSA ratios.
Introduction
The most accurate way to detect cancer cells inside the prostate gland is the surgical removal and histopathological examination of the entire gland. As this approach is clinically inapplicable to each patient with suspicious findings, prostate biopsy is accepted as the best diagnostic technique to detect prostate cancer. Indeed, the introduction of transrectal ultrasound (TRUS)-guided systematic sextant biopsy method by Hodge and colleagues in 1989 revolutionized the early diagnosis of prostate cancer.1 However, there are two shortcomings of this technique. Firstly, the amount of tissue sampled during prostate biopsy is limited and cancer cells can be missed. Secondly, the way prostate biopsy accurately diagnoses prostate cancer is unclear and various prostate biopsy regimens were introduced to optimize the detection rate.2–4 Presumably, the most extensive and invasive regimens had better detection rates, compared to biopsy regimens with less biopsy cores. These regimens are introduced as “gold” standards, despite the inherent sampling error of needle-directed biopsies and false negative results.
The purpose of this study was to calculate the false negative rate of the 12-core prostate biopsy and correlate it to patient characteristics which might have affected this rate. In addition, we evaluated the internal consistency of the results of each core sampled ex-vivo and in-vivo.
Methods
We included consecutive prostate cancer patients who were diagnosed with TRUS-guided 12-core prostate biopsy due to elevated prostate-specific antigen (PSA) level or palpable nodule between December 2005 and April 2008. The biopsy was performed in our department. The patients’ characteristics, such as age, prostate weight, serum PSA level, free/total PSA ratio, PSA density, Gleason score and the region of the adenocarcinoma detected, were recorded. Patients were informed about the study and provided informed consent. The study was approved by the ethics committee at our institution.
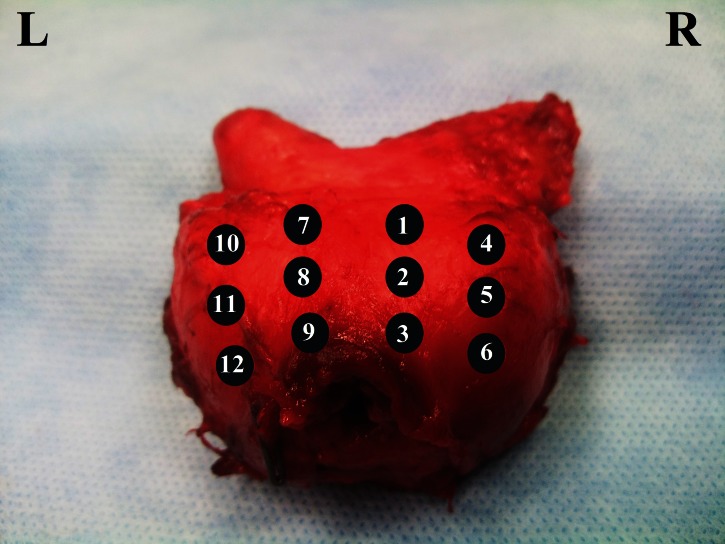
All patients underwent radical retropubic prostatectomy and 12-core prostate biopsy was taken from the same regions of the removed prostate ex-vivo (Fig. 1). The weights of the prostates were immediately measured after their removal, just before performing ex-vivo biopsies. Comparison of preoperative in-vivo and postoperative ex-vivo prostate biopsies was done regarding the detection rate; we also analyzed the influence of patient age, prostate weight, serum PSA level, free/total PSA ratio, PSA density and Gleason score on detection rate. Additionally, the internal consistency of the results of each core was evaluated.
Fig. 1.
Mapping of the prostate.
Continuous variables were summarized with mean, standard deviation, medium, minimum and maximum; categorical variables were presented with number and percentage. The internal consistency of the histopathological results of the preoperative and postoperative prostate biopsies was evaluated with Kappa value. A Kappa value of 0.0 to 0.2 was categorized as mild consistency, 0.2 to 0.4 as mild-moderate and 0.4 to 0.6 as moderate. The sensitivity value and 95% confidence intervals of sensitivity regarding patient characteristics were calculated. The p value was set to 0.05.
Results
A total of 90 prostate cancer patients (mean age of 64 range: 49–77) diagnosed with TRUS-guided 12-core prostate biopsy were included in the study. We tallied the patient characteristics (Table 1).
Table 1.
Patient characteristics
| Mean ± SD | Median | Min–Max | |
|---|---|---|---|
| Patient age (year) | 64 ± 7 | 65 | 49–77 |
| PSA (ng/mL) | 12.23 ± 9.22 | 9.38 | 2.10–46.00 |
| Prostate weight (gr) | 50.7 ± 24.9 | 45 | 10–45 |
| Gleason score | 6.6 ± 1.1 | 6 | 0–9 |
| Free/total PSA ratio | 0.14 ± 0.09 | 0.13 | 0.01–0.54 |
| PSA density | 0.28 ± 0.25 | 0.2 | 0.02–1.39 |
Patients were grouped according to their PSA levels. There were 5, 43 and 42 patients in groups of PSA level <4, 4–10 and >10 ng/mL, respectively. Free/total PSA ratio was <0.15 in 63, between 0.15 and 0.25 in 18 and >0.25 in 9 patients. PSA density was <15 mg/mL and ≥15 mg/mL in 28 and 62 patients, respectively. Gleason score was 5, 6, 7, 8 and 9 in 3, 42, 32, 13 patients, respectively (Table 2).
Table 2.
Sensitivity of ex-vivo 12-core prostate biopsy procedure in different patient groups.
| n | Sensitivity (%) | 95% CI* | ||
|---|---|---|---|---|
| PSA level (ng/mL) | <4 | 5 | 40.0 | 11.8–76.9 |
| 4–10 | 43 | 62.8 | 47.9–75.6 | |
| >10 | 42 | 76.2 | 61.5–86.5 | |
|
| ||||
| Free/total PSA ratio | <0.15 | 63 | 81.0 | 69.6–88.8 |
| 0.15–0.25 | 18 | 38.9 | 20.3–61.4 | |
| >0.25 | 9 | 33.3 | 12.6–64.0 | |
|
| ||||
| PSA density | <0.15 | 28 | 44.8 | 28.4–62.5 |
| ≥0.15 | 62 | 78.7 | 66.9–87.1 | |
|
| ||||
| Gleason score | 5 | 3 | 33.3 | 6.2–79.2 |
| 6 | 42 | 52.4 | 37.7–66.6 | |
| 7 | 32 | 84.4 | 68.3–93.1 | |
| 8–9 | 13 | 84.6 | 57.8–95.7 | |
|
| ||||
| Prostate weight (gr) | <30 | 9 | 100.0 | 70.0–100.0 |
| 30–60 | 57 | 75.4 | 62.9–84.7 | |
| >60 | 24 | 37.5 | 21.2–57.3 | |
|
| ||||
| Patient age (year) | <60 | 27 | 74.1 | 55.3–86.8 |
| 60–70 | 47 | 68.1 | 55.8–79.6 | |
| >70 | 16 | 56.3 | 33.2–76.9 | |
CI= confidence interval.
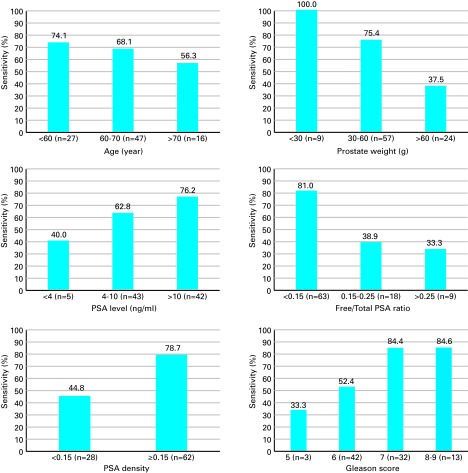
In 67.8% of patients, prostate cancer was detected histologically on samples obtained with repeat ex-vivo biopsies, using the same mapping postoperatively. Increase in PSA level, PSA density and biopsy Gleason score, whereas decrease in prostate weight, patient age and free/total PSA ratio yield higher prostate cancer detection rates on repeat ex-vivo 12-core prostate biopsies (Table 2) (Fig. 2).
Fig. 2.
Sensitivity change regarding patient’s age, prostate weight, prostate-specific antigen (PSA) levels, free/total PSA ratio, PSA density and Gleason scores.
The internal consistency of the histopathological results of the preoperative and postoperative prostate biopsies was evaluated with Kappa value. All but left-lateral core (#11 in Figure 1) showed mild-moderate or moderate internal consistency (Table 3).
Table 3.
Internal consistency between the prostate biopsies done in-vivo and ex-vivo
| Core | Kappa value* | P** |
|---|---|---|
| 1 | 0.28 | 0.007 |
| 2 | 0.29 | 0.006 |
| 3 | 0.32 | 0.001 |
| 4 | 0.22 | 0.033 |
| 5 | 0.31 | 0.003 |
| 6 | 0.52 | <0.001 |
| 7 | 0.42 | <0.001 |
| 8 | 0.40 | <0.001 |
| 9 | 0.36 | <0.001 |
| 10 | 0.30 | 0.004 |
| 11 | 0.18 | 0.085 |
| 12 | 0.39 | <0.001 |
Internal consistency was evaluated as kappa value with 0.0–0.2, 0.2–0.4 and 0.4–0.6 indicating mild, mild-moderate and moderate consistency, repectively.
P value for kappa value.
The mean Gleason scores of the biopsies done preoperatively and postoperatively were both 6.2 (range 4–9) and there was no statistically significant difference between them. However, they were both significantly different than the final Gleason score of the prostates resected, the mean of which was 6.7 (range 4–9) (p<0.001 for each). Compared to the preoperative in-vivo 12-core biopsies, the Gleason scores remained same, increased and decreased in 78.9%, 6.7% and 14.4%, respectively, on postoperative ex-vivo biopsies and 43.3%, 47.8% and 8.9% of patients on final specimen pathology (Table 4).
Table 4.
The changes in Gleason scores on postoperative ex-vivo biopsies and final specimen pathology compared to preoperative in-vivo biopsies.
| Gleason score difference | Postoperative | Final | ||
|---|---|---|---|---|
|
| ||||
| n | % | n | % | |
| Same | 71 | 78.9 | 39 | 43.3 |
| Increased | 6 | 6.7 | 43 | 47.8 |
| Decreased | 13 | 14.4 | 8 | 8.9 |
| Total | 90 | 100.0 | 90 | 100.0 |
Discussion
Although TRUS-guided systematic sextant biopsy method has been accepted as the “gold” standard technique for prostate biopsies, there is controversy regarding the efficiency of this technique; many studies have been conducted to improve the detection rate of this procedure. Several authors claim that sampling done with sextant biopsy is not enough and suggest increasing the number of cores. Levine and colleagues demonstrated that two consecutive sets of TRUS-guided sextant biopsies of the prostate performed in a single office visit is a cost-effective biopsy strategy, as it increased the number of cancers detected by 30%.5 In another study, Babaian and colleagues reported that classical sextant biopsy protocol could not detect 20% to 25% of prostate cancer; they introduced an 11-core biopsy technique which improved the detection rate.6 The current tendency is to increase the number of cores; the detection rates of 18-, 20- and 24-core prostate biopsies have been evaluated.2–4 Moreover, some authors have evaluated saturation biopsy as primary biopsy due to the increased rate of detection.7 However, most of these studies do not compare the risks and complications (such as bleeding, urinary obstruction, vasovagal reaction and infection) between the classical sextant biopsy protocol and protocols with more biopsy cores, besides the improved cancer detection rat.8
Similarly, the direction of the biopsies, the number of cores and the standard way of obtaining sextant biopsies have been replaced by laterally directed sextant biopsies to optimize the detection rate.9–10 Biopsy cores obtained this way include biopsies from the posterolateral aspect of the peripheral zone, the most common location for early prostate cancer. In their study, Eskicorapci and colleagues demonstrated that adding lateral peripheral biopsies to the conventional sextant biopsy technique increased the rate of cancer detection by 25.5%.11
As prostate cancer is multifocal and the sampled amount with sextant biopsy technique is around 90 mm3, the volume of the prostate plays a role in the detection rate.12 In their study, Eskicorapci and colleagues demonstrated that patients with a larger prostate had lower cancer detection rates; the authors determined the optimum number of cores per biopsy according to prostate volume in patients who experienced prostate biopsy for the first time.13 Similar concerns have been raised by Vashi and colleagues; they concluded that younger men and men with larger prostate glands require more than 6 cores to confirm the diagnosis of life-threatening prostate cancer.14 In addition to prostate volume, PSA level may be associated with biopsy outcomes. There are several studies demonstrating that the detection of prostate cancer with prostate biopsy increases as the PSA level increases.15–18 Although different uses of PSA, such as PSA density, free/total PSA rate or PSA velocity, have been suggested to improve the detection of prostate cancer, these indications are not yet used in routine practice.19–26
In spite of all these ancillary techniques and extensive sampling strategies, prostate biopsies still incur the risk of being false negative. Two studies provide more concrete data regarding the reliability of prostate biopsies. In 1998, Svetec and colleagues performed an ex-vivo sextant biopsy on 90 prostates removed for biopsyproven cancer and found that 45.6% were negative.27 Similar results have been reported by Fink and colleagues in 2001. They compared the cancer detection of two consecutive sets of ex-vivo prostate biopsies using either the sextant or the 10-core technique.28 The authors demonstrated that two consecutive sets of sextant biopsies detected 74.7% of prostate cancer, whereas the cumulative cancer detection rate was 90.1% for two sets of the 10-core technique.28 The authors concluded that 9.9% of all the cancers, most of which were clinically significant, were not diagnosed, even though 20-core biopsies were taken.28
In this study, the false negative rate of 12-core prostate biopsy technique was found to be more than 30%. To our knowledge, there are no studies evaluating the detection ability of this procedure in patients documented with prostate cancer. The detection rate increased with the increases in PSA level, PSA density and biopsy Gleason score. Similarly, decreases in prostate weight, patient age and free/total PSA ratio yielded higher prostate cancer detection rates on repeat 12-core prostate biopsies done ex-vivo (Table 2, Fig. 2). These findings support the results demonstrated by Leibovici and colleagues.29 They showed an association between positive biopsy results and age, serum PSA levels and prostate volume. Although Numao and colleagues demonstrated that most cancers missed by 12-core prostate biopsies are mostly low-grade and low-volume diseases, they also showed a risk of missing significant anterior cancers.30
Readers may wonder if repeat 12-core prostate biopsies done ex-vivo can represent the TRUS-guided 12-core prostate biopsies done in-vivo prior to surgery. Mild-moderate or moderate internal consistency was demonstrated in all of the sampled cores, except the left-lateral core (Kappa value = 0.18); this shows that the results obtained with ex-vivo and in-vivo biopsies are similar. On the other hand, we do not have any explanation as to why the biopsies taken from the left-lateral core was not similar. We believe further studies are needed to elucidate this finding.
The mean Gleason score of the biopsies done preoperatively and postoperatively was not statistically different in our study. However, they were both significantly different than the final Gleason score of the prostates resected. These findings support the results of the meta-analysis done by Cohen and colleagues. They reviewed the medical records of 14 839 patients and found that the biopsy Gleason score matched the Gleason score of the prostatectomy specimen only in 63% of patients.31 More importantly, our study revealed that biopsy underestimated Gleason scores in 47.8% of patients, confirming previous radical prostatectomy series.32–35 These findings are important. Gleason scores obtained from prostate biopsy play an important role in deciding the optimal treatment for prostate cancer patients and Gleason score upgrading is linked with adverse pathological outcomes with biochemical reccurence.32 Therefore, urologists and patients must consider that Gleason score of the prostate biopsy can be understaged in half of the patients with prostate cancer.
This study has its limitations. Firstly, the preoperative TRUS-guided 12-core prostate biopsies were not performed by the same physician. Although there is no evidence to support that the results of prostate biopsy differ among urologists who perform it, we believe this factor may have influenced our data. Moreover, since all patients had initial positive in-vivo biopsy, it is not possible to measure the effect of prostate size change on the sensitivity of these biopsy results. However, the results of the present study showed that a decrease in prostate weight yields higher prostate cancer detection rates on repeat ex-vivo 12-core prostate biopsies. Secondly, comparing the clinical significance of the detected and missed prostate cancers might provide further understanding on the risk of false-negative prostate biopsies. Thirdly, this study included men with previously positive prostate biopsy and excluded men with false-negative initial biopsy. Therefore, the actual risk of false-negative biopsy may be much higher and further studies are required to determine this risk.
Conclusion
The detection rate of prostate cancer with the 12-core biopsy technique in patients with proven prostate cancer on radical prostatectomy specimen was considerably low when repeated in the same way ex-vivo. Effectively, repeat biopsies done in patients with persistent PSA elevations and other biopsy indications can still demonstrate negative cancer findings despite the fact that these patients have prostate cancer. The detection rate of prostate cancer is higher if 12-core biopsies are repeated in patients with younger age, higher PSA levels, PSA density and Gleason scores and in patients with smaller prostates and lower free/total PSA ratios.
Footnotes
Competing interests: None declared.
This paper has been peer-reviewed.
References
- 1.Hodge KK, McNeal JE, Terris MK, et al. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol. 1989;142:71–4. doi: 10.1016/s0022-5347(17)38664-0. discussion 74–5. [DOI] [PubMed] [Google Scholar]
- 2.Ravery V, Dominique S, Panhard X, et al. The 20-core prostate biopsy protocol–a new gold standard? J Urol. 2008;179:504–7. doi: 10.1016/j.juro.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 3.Scattoni V, Raber M, Abdollah F, et al. Biopsy schemes with the fewest cores for detecting 95% of the prostate cancers detected by a 24-core biopsy. Eur Urol. 2010;57:1–8. doi: 10.1016/j.eururo.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Scattoni V, Roscigno M, Raber M, et al. Initial extended transrectal prostate biopsy–are more prostate cancers detected with 18 cores than with 12 cores? J Urol. 2008;179:1327–31. doi: 10.1016/j.juro.2007.11.052. discussion 1331. [DOI] [PubMed] [Google Scholar]
- 5.Levine MA, Ittman M, Melamed J, et al. Two consecutive sets of transrectal ultrasound guided sextant biopsies of the prostate for the detection of prostate cancer. J Urol. 1998;159:471–5. doi: 10.1016/S0022-5347(01)63951-X. discussion 475–6. [DOI] [PubMed] [Google Scholar]
- 6.Babaian RJ, Toi A, Kamoi K, et al. A comparative analysis of sextant and an extended 11-core multisite directed biopsy strategy. J Urol. 2000;163:152–7. doi: 10.1016/S0022-5347(05)67993-1. [DOI] [PubMed] [Google Scholar]
- 7.Pepe P, Aragona F. Saturation prostate needle biopsy and prostate cancer detection at initial and repeat evaluation. Urology. 2007;70:1131–5. doi: 10.1016/j.urology.2007.07.068. [DOI] [PubMed] [Google Scholar]
- 8.Serefoglu EC, Ozdemir AT, Balbay MD. Re: The 20-core prostate biopsy protocol–a new gold standard?: V. Ravery, S. Dominique, X. Panhard, M. Toublanc, L. Boccon-Gibod and L. Boccon-Gibod. J Urol 2008;179:504–7. J Urol. 2008;180:2256–7. doi: 10.1016/j.juro.2008.07.077. [DOI] [PubMed] [Google Scholar]
- 9.Stamey TA. Making the most out of six systematic sextant biopsies. Urology. 1995;45:2–12. doi: 10.1016/S0090-4295(95)96168-2. [DOI] [PubMed] [Google Scholar]
- 10.Aus G, Bergdahl S, Hugosson J, et al. Outcome of laterally directed sextant biopsies of the prostate in screened males aged 50–66 years. Implications for sampling order. Eur Urol. 2001;39:655–60. doi: 10.1159/000052523. discussion 661. [DOI] [PubMed] [Google Scholar]
- 11.Eskicorapci SY, Baydar DE, Akbal C, et al. An extended 10-core transrectal ultrasonography guided prostate biopsy protocol improves the detection of prostate cancer. Eur Urol. 2004;45:444–8. doi: 10.1016/j.eururo.2003.11.024. discussion 448–9. [DOI] [PubMed] [Google Scholar]
- 12.Letran JL, Meyer GE, Loberiza FR, et al. The effect of prostate volume on the yield of needle biopsy. J Urol. 1998;160:1718–21. doi: 10.1016/S0022-5347(01)62392-9. [DOI] [PubMed] [Google Scholar]
- 13.Eskicorapci SY, Guliyev F, Akdogan B, et al. Individualization of the biopsy protocol according to the prostate gland volume for prostate cancer detection. J Urol. 2005;173:1536–40. doi: 10.1097/01.ju.0000154242.60413.3d. [DOI] [PubMed] [Google Scholar]
- 14.Vashi AR, Wojno KJ, Gillespie B, et al. A model for the number of cores per prostate biopsy based on patient age and prostate gland volume. J Urol. 1998;159:920–4. doi: 10.1016/S0022-5347(01)63771-6. [DOI] [PubMed] [Google Scholar]
- 15.Aus G, Becker C, Franzen S, et al. Cumulative prostate cancer risk assessment with the aid of the free-to-total prostate specific antigen ratio. Eur Urol. 2004;45:160–5. doi: 10.1016/j.eururo.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Lodding P, Aus G, Bergdahl S, et al. Characteristics of screening detected prostate cancer in men 50 to 66 years old with 3 to 4 ng./ml. Prostate specific antigen. J Urol. 1998;159:899–903. doi: 10.1016/S0022-5347(01)63766-2. [DOI] [PubMed] [Google Scholar]
- 17.Horninger W, Reissigl A, Rogatsch H, et al. Prostate cancer screening in the Tyrol, Austria: experience and results. Eur J Cancer. 2000;36:1322–35. doi: 10.1016/S0959-8049(00)00113-1. [DOI] [PubMed] [Google Scholar]
- 18.Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350:2239–46. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 19.Huber PR, Schmid HP, Mattarelli G, et al. Serum free prostate specific antigen: isoenzymes in benign hyperplasia and cancer of the prostate. Prostate. 1995;27:212–9. doi: 10.1002/pros.2990270406. [DOI] [PubMed] [Google Scholar]
- 20.Benson MC, Whang IS, Pantuck A, et al. Prostate specific antigen density: a means of distinguishing benign prostatic hypertrophy and prostate cancer. J Urol. 1992;147:815–6. doi: 10.1016/s0022-5347(17)37393-7. [DOI] [PubMed] [Google Scholar]
- 21.Zlotta AR, Djavan B, Marberger M, et al. Prostate specific antigen density of the transition zone: a new effective parameter for prostate cancer prediction. J Urol. 1997;157:1315–21. doi: 10.1016/S0022-5347(01)64961-9. [DOI] [PubMed] [Google Scholar]
- 22.Oesterling JE, Jacobsen SJ, Chute CG, et al. Serum prostate-specific antigen in a community-based population of healthy men. Establishment of age-specific reference ranges. JAMA. 1993;270:860–4. doi: 10.1001/jama.1993.03510070082041. [DOI] [PubMed] [Google Scholar]
- 23.Catalona WJ, Smith DS, Wolfert RL, et al. Evaluation of percentage of free serum prostate-specific antigen to improve specificity of prostate cancer screening. JAMA. 1995;274:1214–20. doi: 10.1001/jama.1995.03530150038031. [DOI] [PubMed] [Google Scholar]
- 24.Okihara K, Cheli CD, Partin AW, et al. Comparative analysis of complexed prostate specific antigen, free prostate specific antigen and their ratio in detecting prostate cancer. J Urol. 2002;167:2017–23. doi: 10.1016/S0022-5347(05)65075-6. discussion 2023–4. [DOI] [PubMed] [Google Scholar]
- 25.Carter HB, Pearson JD, Metter EJ, et al. Longitudinal evaluation of prostate-specific antigen levels in men with and without prostate disease. JAMA. 1992;267:2215–20. doi: 10.1001/jama.1992.03480160073037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmid HP, McNeal JE, Stamey TA. Observations on the doubling time of prostate cancer. The use of serial prostate-specific antigen in patients with untreated disease as a measure of increasing cancer volume. Cancer. 1993;71:2031–40. doi: 10.1002/1097-0142(19930315)71:6<2031:AID-CNCR2820710618>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 27.Svetec D, McCabe K, Peretsman S, et al. Prostate rebiopsy is a poor surrogate of treatment efficacy in localized prostate cancer. J Urol. 1998;159:1606–8. doi: 10.1097/00005392-199805000-00052. [DOI] [PubMed] [Google Scholar]
- 28.Fink KG, Hutarew G, Lumper W, et al. Prostate cancer detection with two sets of ten-core compared with two sets of sextant biopsies. Urology. 2001;58:735–9. doi: 10.1016/S0090-4295(01)01352-8. [DOI] [PubMed] [Google Scholar]
- 29.Leibovici D, Shilo Y, Raz O, et al. Is the diagnostic yield of prostate needle biopsies affected by prostate volume? Urol Oncol. 2011 Sep 14; doi: 10.1016/j.urolonc.2011.08.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Numao N, Kawakami S, Sakura M, et al. Characteristics and clinical significance of prostate cancers missed by initial transrectal 12-core biopsy. BJU Int. 2012;109:665–71. doi: 10.1111/j.1464-410X.2011.10427.x. . Epub 2011 Sep 21. [DOI] [PubMed] [Google Scholar]
- 31.Cohen MS, Hanley RS, Kurteva T, et al. Comparing the Gleason prostate biopsy and Gleason prostatectomy grading system: the Lahey Clinic Medical Center experience and an international meta-analysis. Eur Urol. 2008;54:371–81. doi: 10.1016/j.eururo.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 32.Davies JD, Aghazadeh MA, Phillips S, et al. Prostate Size as a Predictor of Gleason Score Upgrading in Patients With Low Risk Prostate Cancer. J Urol. 2011;186:2221–7. doi: 10.1016/j.juro.2011.07.104. Epub 2011 Oct 19. [DOI] [PubMed] [Google Scholar]
- 33.D’Amico AV, Renshaw AA, Arsenault L, et al. Clinical predictors of upgrading to Gleason grade 4 or 5 disease at radical prostatectomy: potential implications for patient selection for radiation and androgen suppression therapy. Int J Radiat Oncol Biol Phys. 1999;45:841–6. doi: 10.1016/S0360-3016(99)00260-6. [DOI] [PubMed] [Google Scholar]
- 34.Chun FK, Steuber T, Erbersdobler A, et al. Development and internal validation of a nomogram predicting the probability of prostate cancer Gleason sum upgrading between biopsy and radical prostatectomy pathology. Eur Urol. 2006;49:820–6. doi: 10.1016/j.eururo.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Fukagai T, Namiki T, Namiki H, et al. Discrepancies between Gleason scores of needle biopsy and radical prostatectomy specimens. Pathol Int. 2001;51:364–70. doi: 10.1046/j.1440-1827.2001.01207.x. [DOI] [PubMed] [Google Scholar]