Abstract
The genitourinary tract is a common extrapulmonary site of tuberculosis infection, yet remains a rare clinical entity in North America. We report the case of a 37-year-old man who presented for extracorporeal shock wave lithotripsy for a suspected ureteral stone on imaging. Further workup confirmed a diagnosis of genitourinary tuberculosis. Medical management was undertaken and, ultimately, nephrectomy performed. This case highlights the importance of maintaining a high index of clinical suspicion for genitourinary tuberculosis.
Case report
We report the case of an otherwise healthy 37-year-old male that was referred for extracorporeal shock wave lithotripsy (ESWL) of a presumed left ureteric stone. The patient reported a 2-month history of left flank pain and 5 months of intermittent hematuria. He also reported several months of feeling unwell with occasional fever. The patient was an immigrant from the Philippines, who works as a prison guard in Canada. Physical examination was unremarkable. A kidney, ureter, bladder (KUB) radiography pre-ESWL revealed a 5 × 4-mm oval calcified density within the left hemipelvis that was presumed to represent a ureteral calculus (Fig. 1). Based on history and imaging review, non-contrast and subsequently contrast-enhanced abdominal/pelvis computed tomography (CT) scan were obtained to further workup the etiology of the calcification. These studies demonstrated left hydroureteronephrosis, with renal parenchymal and calyceal calcifications, but no ureteric calculus (Fig. 2). There were innumerable septated cystic lesions throughout the left renal parenchyma that was suspicious for a chronic infection, such as genitourinary (GU) tuberculosis (TB).
Fig. 1.
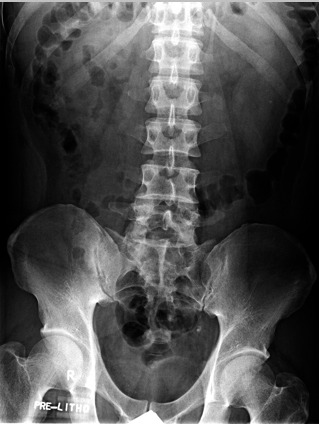
A kidney, ureter, bladder (KUB) radiograph demonstrating an oval density over the left hemipelvis.
Fig. 2.
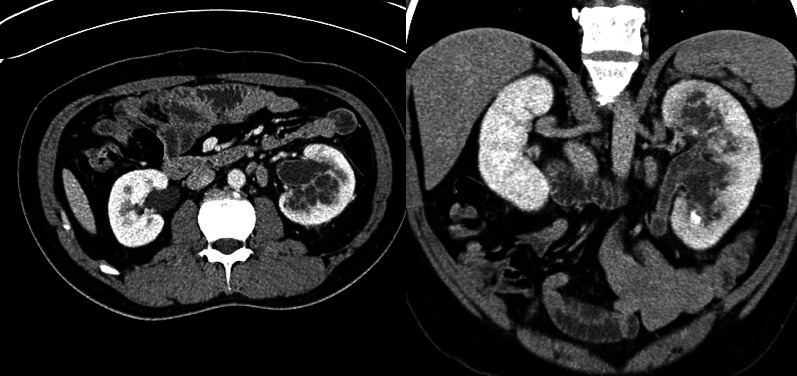
Axial (a) and coronal (b) abdominal computed tomography images showing small septated-appearing cystic spaces throughout the left renal parenchyma, left hydronephrosis, and calcifications.
Urinalysis demonstrated hematuria with sterile pyuria. The patient underwent full evaluation for a working diagnosis of GU TB; chest X-ray was normal. Sputum acid-fast bacillus (AFB) tests were negative. Urine AFB and polymerase chain reaction (PCR) were positive for TB infection. The patient was subsequently medically treated with TB quadruple therapy: Isoniazid, rifampin, ethambutol and pyrazinamide. Urine culture demonstrated growth of Mycobacterium tuberculosis.
Due to the presence of left-sided obstruction, cystoscopy was performed for attempted left ureteric stent insertion. Direct visualization revealed an erythematous and friable bladder mucosa with diffuse bleeding. The left ureteric orifice was unable to be visualized. A left percutaneous nephrostomy tube was placed. Antegrade nephrostogram demonstrated dilatation and distortion of the obstructed collecting system with pan-ureteral strictures (Fig. 3).
Fig. 3.
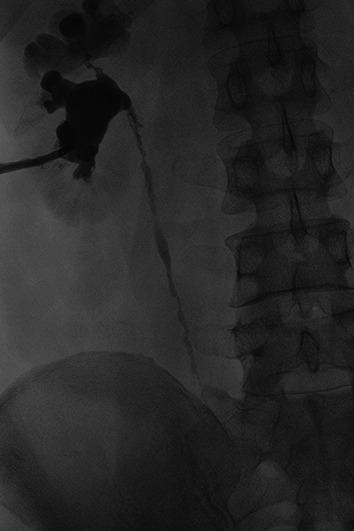
Left antegrade nephrostogram demonstrating scarring and dilatation of the upper pole with an abnormal infundibulum. The ureter demonstrates irregularity with a “beaded corkscrew” appearance.
Due to the extensive involvement of the kidney and ureter, reconstruction of the left-sided collecting system and ureter to alleviate the obstruction was not felt feasible upon consensus review. In the setting of a normal, functioning contralateral kidney on nuclear renogram, opinion favoured delayed laparoscopic nephrectomy. After several months of medical treatment and once the urine culture was negative for TB, the patient underwent laparoscopic nephrectomy without complication. Due to the inflammatory nature of the specimen, adrenal sparing technique was not possible.
Gross pathology of the left nephrectomy specimen showed an upper pole 3.5-cm cavitary lesion filled with inflammatory exudate (Fig. 4). Histology demonstrated active necrotizing granulomatous inflammation with a Ziehl-Neelsen stain positive for acid-fast bacilli, consistent with tuberculosis infection (Fig. 5). There was also acute on chronic pyelonephritis with areas of xanthogranulomatous pyelitis. Lymph nodes demonstrated reactive changes with no evidence of granulomatous inflammation.
Fig. 4.
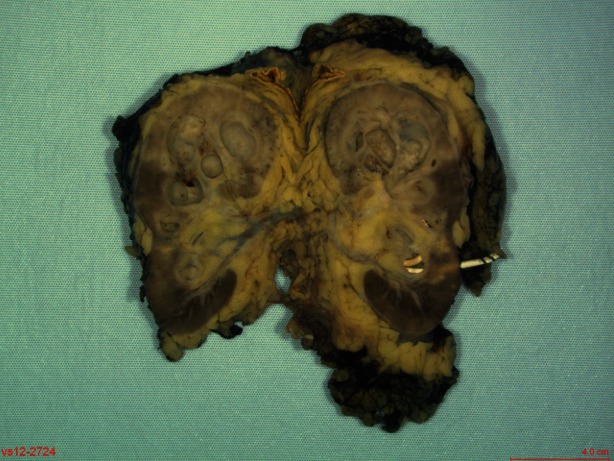
Gross pathology of the left nephrectomy specimen showing a 3.5-cm in diameter cavitary lesion filled with inflammatory exudate in the upper pole. The lesion involves the renal pelvis, which extended into the ureter distally occluding of the lumen.
Fig. 5.
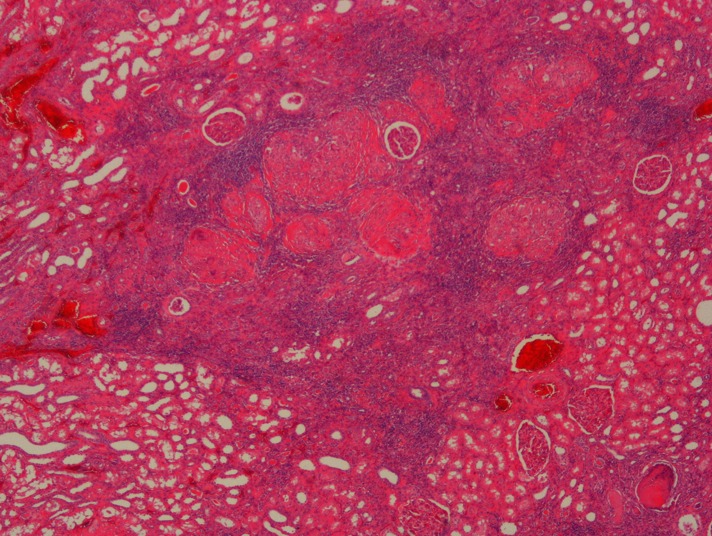
Left nephrectomy specimen micrograph showed numerous foci of necrotizing granulomatous inflammation in the renal cortex.
The patient was continued on his TB medications postoperatively with no complications and an uneventful recovery to date.
Discussion
TB is a common disease worldwide, with about 8–10 million new cases each year.1–3 GU TB accounts for about 3% to 4% of all TB cases, with more than 90% of cases occurring in developing countries. The incidence of GU TB is also increasing, particularly in regions with a high prevalence of HIV. In GU TB, the kidneys are the most common target and are infected through hematogenous spread during the initial infection. However, signs and symptoms of renal TB may mimic other kidney abnormalities, and are often variable and non-specific.1
GU TB affects approximately twice as many males.4 About 50% of patients with GU TB present with storage symptoms, while 33% present with hematuria and flank pain.4,5 Renal colic is present in less than 10% of patients and is secondary to the passage of caseous material, necrotic renal papillary tissue, clots or stones.1,6 Constitutional symptoms are unusual and found in less that 20% of GU TB patients.6 The physical exam is usually unremarkable since signs typically develop later in the disease process.7
The diagnosis of GU TB should be considered with the findings of an abnormal urinalysis showing sterile pyuria and/or hematuria, which are present in more than 90% of patients.5,6 GU TB should be further suspected by the presence of a positive TB skin test and chest x-ray abnormalities that represent previous TB infections. These abnormalities should prompt an evaluation for GU TB with imaging studies and urine cultures.8,9
Plain abdominal radiography may demonstrate changes in kidney size and calcifications, which are present in 30–50% of cases.5,10 Kidney size may either be increased due to caseous lesions or decreased due to autonephrectomy.5 As in this case, the calcifications may mimic renal or ureteral calculi. In addition, obstructing calculi may be present in GU TB due to the formation of ureteral strictures.5
Intravenous pyelography has traditionally been used in determining structural involvement of GU TB. Findings include hydronephrosis and hydroureter secondary to ureteral strictures.11 A moth-eaten appearance due to calyceal erosion and cavitary lesions that communicate with the collecting system may be present. Other findings include scarring and angulation at the uretropelvic junction known as “Kerr’s kink.” The ureter may appear either as a “pipe-stem” or a beaded corkscrew ureter; the latter was seen in this case.5,6 A CT scan may show calcifications, renal parenchymal masses, thick urinary tract walls and extraurinary TB manifestations.11 CT is the most sensitive in detecting and characterizing renal calcifications.12
Confirmation of GU TB can be made by demonstrating Mycobacterium tuberculosis in the urine. A minimum of 3 to 5 first morning midstream samples are necessary for culture, as bacilli are intermittently shed and prolonged exposure to the urine acidity decreases mycobacterial growth.13 Culture requires approximately 6 weeks for reliable results. In addition, urine PCR may be performed and allows for more rapid detection of Mycobacterium tuberculosis.14
Management of GU TB is similar to other forms of TB infections. Treatment should be administered for a minimum of 6 months. Standard therapy includes isoniazid, rifampin, pyrazinamide and ethambutol for 2 months followed by isoniazid and rifampin for 4 months. These agents are able to eliminate active GU TB infections in most patients. However, additional drugs and prolonged treatment may be required if drug resistance, or when co-infection with HIV is present.15
About 55% of GU TB requires surgical management.4 Surgery should be deferred until medical therapy has been initiated for several weeks. A kidney that is unsalvageable or is nonfunctional despite adequate drainage and medical treatment may require nephrectomy.16 Surgery may be open or laparoscopic, as in this case. TB was once thought to be a contraindication to retroperitoneoscopic nephrectomy due concerns of a high conversion rate.17 More recently, laparoscopic nephrectomy had been shown to be a safe, effective and less invasive treatment modality for renal TB compared to open surgery.17–19 Although the laparoscopic approach may be technically difficult since the disease process is associated with severe inflammation and adhesions, prior studies have demonstrated there were no significant intraoperative and postoperative complications, such as local recurrence or disease dissemination.18 There was however, an increase in operating time compared to laparosopic treatment of other renal problems.17,19
Conclusion
Although TB is quite prevalent worldwide, GU TB remains an uncommon diagnosis in North America. This case highlights the potential pitfalls in the diagnosis of GU TB. It is important to have a high index of suspicion for tuberculosis in patients with urinary calcifications to facilitate proper treatment and to prevent any irreversible loss of kidney function.
Footnotes
Competing interests: None declared.
This paper has been peer-reviewed.
References
- 1.Eastwood JB, Corbishley CM, Grange JM. Tuberculosis and the kidney. JASN. 2001;12:1307–14. doi: 10.1681/ASN.V1261307. [DOI] [PubMed] [Google Scholar]
- 2.Gibson MS, Puckett ML, Shelly ME. Renal tuberculosis. Radiographics. 2004;24:251–6. doi: 10.1148/rg.241035071. [DOI] [PubMed] [Google Scholar]
- 3.Golden MP, Vikran HR. Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005;72:1761–8. [PubMed] [Google Scholar]
- 4.Figueiredo AA, Lucon AM, Junior RF, et al. Epidemiology of urogenital tuberculosis worldwide. Int J Urol. 2008;15:827–32. doi: 10.1111/j.1442-2042.2008.02099.x. [DOI] [PubMed] [Google Scholar]
- 5.Kollins SA, Hartman GW, Carr DT, et al. Roentgenographic findings in urinary tract tuberculosis: A 10 year review. AJR. 1974;121:487–99. doi: 10.2214/ajr.121.3.487. [DOI] [PubMed] [Google Scholar]
- 6.Simon HB, Weinstein AJ, Pasternak MS, et al. Genitourinary tuberculosis. Clinical features in a general hospital population. Am J Med. 1977;63:410–20. doi: 10.1016/0002-9343(77)90279-0. [DOI] [PubMed] [Google Scholar]
- 7.Altintepe L, Tonbul HZ, Ozbey I, et al. Urinary tuberculosis: ten years’ experience. Ren Fail. 2005;27:657–61. doi: 10.1080/08860220500234857. [DOI] [PubMed] [Google Scholar]
- 8.Christensen WI. Genitourinary tuberculosis: review of 102 cases. Medicine (Baltimore) 1974;53:377–90. doi: 10.1097/00005792-197409000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Weir MR, Thornton GF. Extrapulmonary tuberculosis. Experience of a community hospital and review of the literature. Am J Med. 1985;79:467–78. doi: 10.1016/0002-9343(85)90034-8. [DOI] [PubMed] [Google Scholar]
- 10.Roylance J, Penry JB, Davies ER, et al. The radiology of tuberculosis of the urinary tract. Clin Radiol. 1970;21:163–70. doi: 10.1016/S0009-9260(70)80109-X. [DOI] [PubMed] [Google Scholar]
- 11.Wang LJ, Wu CF, Wong YC, et al. Imaging findings of urinary tuberculosis on excretory urography and computerized tomography. J Urol. 2003;169:524–8. doi: 10.1016/S0022-5347(05)63947-X. [DOI] [PubMed] [Google Scholar]
- 12.Premkumar A, Lattimer J, Newhouse JH. CT and sonography of advanced urinary tract tuberculosis. AJR. 1987;148:65–9. doi: 10.2214/ajr.148.1.65. [DOI] [PubMed] [Google Scholar]
- 13.Sommers HM. The laboratory diagnosis of mycobacterial disease. In: Youmans GP, editor. Tuberculosis. Philadelphia, PA: Saunders; 1979. p. 511. [Google Scholar]
- 14.Barnard M, Albert H, Coetzee G, et al. Rapid molecular screening for multidrug-resistant tuberculosis in a high-volume public health laboratory in South Africa. Am J Respir Crit Care Med. 2008;177:787–92. doi: 10.1164/rccm.200709-1436OC. [DOI] [PubMed] [Google Scholar]
- 15.Abbara A, Davidson RN. Etiology and management of genitourinary tuberculosis. Nat Rev Urol. 2011;8:678–88. doi: 10.1038/nrurol.2011.172. [DOI] [PubMed] [Google Scholar]
- 16.Flechner SM, Gow JG. Role of nephrectomy in the treatment of non-functioning or very poorly functioning unilateral tuberculous kidney. J Urol. 1980;123:822–5. doi: 10.1016/s0022-5347(17)56149-2. [DOI] [PubMed] [Google Scholar]
- 17.Hemal AK, Gupta NP, Kumar R. Comparison of retroperitoneoscopic nephrectomy with open surgery for tuberculous nonfunctioning kidneys. J Urol. 2000;164:32–5. doi: 10.1016/S0022-5347(05)67442-3. [DOI] [PubMed] [Google Scholar]
- 18.Kim HH, Lee KS, Park K, et al. Laparoscopic nephrectomy for nonfunctioning tuberculous kidney. J Endourology. 2000;14:433–7. doi: 10.1089/end.2000.14.433. [DOI] [PubMed] [Google Scholar]
- 19.Lee KS, Kim HH, Byun SS, et al. Laproscopic nephrectomy for tuberculous nonfunctioning kidney: comparison with laparoscopic simple nephrectomy for other diseases. Urology. 2002;60:411–4. doi: 10.1016/S0090-4295(02)01759-4. [DOI] [PubMed] [Google Scholar]


