Abstract
Latent class models are useful for classifying subjects by dietary patterns. Our goals were to use latent transition models to identify dietary patterns during pregnancy and postpartum, to estimate the prevalence of these dietary patterns, and to model transition probabilities between dietary patterns as a function of covariates. Women who were enrolled in the Pregnancy, Infection, and Nutrition Study (University of North Carolina, 2000–2005) were followed for 1 year postpartum, and their diets were assessed in the second trimester and at 3 and 12 months postpartum (n = 519, 484, and 374, respectively) by using a food frequency questionnaire. After adjusting for energy intake, parity, smoking status, race, and education, we identified 3 dietary patterns and named them “prudent,” “health conscious Western,” and “Western.” Nulliparas were 2.9 and 2.1 times more likely to be in the “prudent” class than the “health conscious Western” or the “Western” class, respectively. The 3 dietary patterns were very stable, with the “health conscious Western” class being the least stable; the probability for staying in the same class was 0.74 and 0.87 at 3 and 12 months postpartum, respectively. Breastfeeding mothers were more likely than nonbreastfeeding mothers to switch dietary pattern class (P = 0.0286). Except for breastfeeding mothers, most women did not switch dietary patterns from pregnancy to postpartum.
Keywords: dietary patterns, eating patterns, finite mixture models, hidden Markov models, latent class analysis, latent transition models, postpartum, pregnancy
Studies of dietary behavior over time as measured by empirically derived dietary patterns can be classified according to 2 related goals. The first goal is concerned with testing the stability of dietary patterns over time because the assessment of associations between diet and chronic disease often requires long periods of follow-up. One hypothesis is that a single dietary pattern structure can be identified at different time points, and that dietary pattern scores (or a subject's classification) are similar. In contrast, the second goal is to quantify changes in a person's dietary patterns over time. Some of the challenges of studying dietary patterns over time are measuring changes in variables that are not directly observed, determining the number of dietary patterns and their characterization, and identifying emerging and disappearing dietary patterns.
Few studies (1–9) have examined a person's changing of dietary patterns over time. Two studies (5, 9) assessed a person's change in dietary patterns on a categorical scale, but these studies first derived the dietary patterns on a continuous scale and later categorized them by using quintiles. Specifically, the authors estimated within-subject change in dietary patterns by classifying participants according to the cross-tabulation of scores’ quintiles at each time point. An alternative approach is to use a latent transition model (LTM) to directly classify subjects into mutually exclusive classes (i.e., dietary patterns) at each time point. An advantage of this latter approach is that it also allows estimation of the probabilities of changing classes over time.
The LTM (10–12) is 1 extension of latent class models (13, 14) for analyzing longitudinal data in which multiple indicators of the latent class variable are repeatedly measured over T equally spaced time points, and the main interest is to model transition probabilities between latent classes. Traditional LTMs involve categorical outcomes, and typical assumptions are that, 1) indicators at each time point are conditionally independent given the class; 2) conditional response probabilities are time-invariant so the characterization of the classes does not change over time; and 3) transition probabilities might need to be time-invariant for the model to have a unique solution. These assumptions may not be realistic when studying changes in dietary patterns from pregnancy to postpartum. First, for some food items the conditional response probabilities (e.g., the probability of consuming raw fish in a woman who belongs to the “health conscious” group) are not time-invariant because during pregnancy the consumption of certain foods is discouraged (e.g., raw fish, alcohol, and caffeine) or encouraged (e.g., foods that are rich in iron, calcium, and folate), and some food items may be craved (e.g., desserts and certain beverages). Second, the probability of switching dietary patterns from pregnancy to 3 months postpartum might not be the same as from 3 to 12 months postpartum, and it may depend on factors such as parity, breastfeeding practices, and gestational weight gain.
To date, the movement between categorical dietary patterns over time has not been studied by using LTMs. In this study, we used data from the Pregnancy, Infection, and Nutrition Study and a LTM to 1) directly classify women into mutually exclusive dietary pattern groups at pregnancy and at 3 and 12 months postpartum, and 2) estimate the probabilities of women changing dietary patterns over time.
MATERIALS AND METHODS
Participants
The data analyzed are from women from the third cohort of the Pregnancy, Infection, and Nutrition Study who were followed for 1 year after delivery. Between 2000 and 2005, pregnant women seeking services from prenatal clinics at University of North Carolina hospitals were recruited for enrollment. Of a total 2,006 pregnancies in 1,875 women enrolled in the study, 1,169 women were eligible for the postpartum recruitment. Of these, 938 women were invited to participate and 688 (73.3% of those invited) agreed to participate and completed a home interview at 3 months postpartum (15). There were no significant differences (P < 0.05) in pregravid body mass index (measured as weight (kg)/height (m)2), parity, bed rest, general health, and total physical activity between women who completed the interview at 3 months postpartum and those who were excluded or refused (15). There were 571, 545, and 424 pregnancies with complete dietary assessment at pregnancy and at 3 and 12 months postpartum, respectively.
Dietary data
Dietary intake was assessed through a self-administered semiquantitative 119–food item Block Food Frequency Questionnaire (16) to measure usual intakes in the past 3 months. The same dietary instrument was administered at 26–29 weeks' gestation and at 3 and 12 months postpartum. DietSys Plus, version 5.6, software (Block Dietary Data Systems, Berkeley, California), which uses an updated food composition table based on nutrient values from the National Health and Nutrition Examination Survey III and the US Department of Agriculture's 1998 nutrient databases, was used to calculate daily energy intake in kilocalories, nutrients, and grams. Pregnant women who had daily energy intakes below the 2.5th or above the 97.5th percentiles were excluded in an attempt to exclude implausible energy intakes. For this analysis, only 1 pregnancy per woman was selected by keeping the data on the pregnancy with the greatest number of completed food frequency questionnaires.
Of the 119 food and beverage items assessed in the food frequency questionnaire, 104 items were aggregated a priori into 29 food and beverage groups according to nutrient content and culinary usage; we excluded 9 items because of very low consumption (<10% of subjects), 4 condiments, and 2 unclassifiable foods (breakfast bars or power bars and meat substitutes) (Web Table 1, available at http://aje.oxfordjournals.org/). Even after collapsing the groups, many food and beverage groups' distributions (in g/day or mL/day) still had a lump at 0 because of nonconsumers and were right skewed. Hence, we categorized most food and beverage groups into 3-level variables according to tertiles of consumption among consumers to distinguish “low,” “medium,” and “high” consumption. Four foods (fruits rich in vitamin C; beef; pork; and fried chicken or fried fish) were not consumed by a large percent of women (>25%) and were categorized into 3-level variables: “no consumption,” “below the median,” or “above the median.” Coffee, alcohol, and diet soft drinks were treated as binary variables (consumed or not consumed) because they were consumed by less than 60% of the women. We used the same percentiles for all 3 time points to make categories comparable across time, and percentiles were estimated from 12-month postpartum data because this time point is the most likely of the 3 times available to represent usual diet.
Maternal weight, height, age, education level, race, smoking behavior during pregnancy, and parity were assessed at enrollment through a self-reported questionnaire. Breastfeeding status and duration were assessed at 3 and 12 months postpartum. Weight gain was calculated as the difference between pregravid weight and weight measured near the time of delivery.
The latent transition model
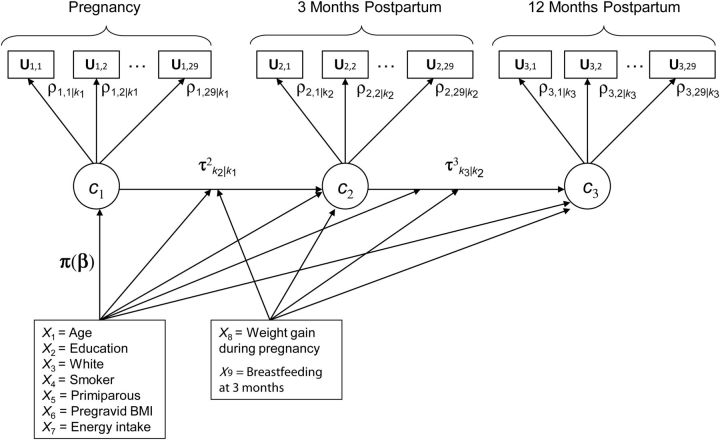
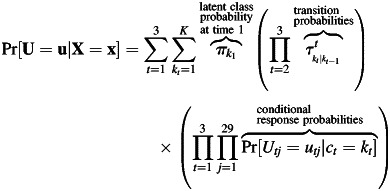
LTMs assume that time is a discrete process, and at each time point a latent class variable (with K classes) explains the associations among the observed outcomes through a statistical model. The number of classes, K, is assumed to be known, although in practice this might not be the case. These models are useful in studying the latent class at time t + 1 as a function of previous latent classes and typically just as a function of the previous one. The LTM without covariates is a special case of the hidden (latent) Markov model (17), which is an extension of a finite mixture model that allows dependent data. Although the LTM and the hidden Markov model most often use discrete outcomes and first-order transition probabilities, other scales and higher-order models can be accommodated. One difference between the LTM and the hidden Markov model is that the former is used when there are few time points, whereas the hidden Markov model can handle a large number of time points. Figure 1 shows a path diagram of a LTM to study the change of 1 dietary pattern to another from pregnancy to 3 and 12 months postpartum. A path diagram is a pictorial representation of a system of simultaneous equations (18). By convention, in path diagrams, circles represent latent (i.e., unobserved) variables, squares represent observed variables, and straight 1-headed arrows represent “causal” relationships. Let U be a vector of the subject's intake of the 29 food groups for all 3 time points; let ct be a latent class variable at time t = 1, 2, 3 for categorical dietary pattern at pregnancy and at 3 and 12 months postpartum; and let kt = 1, 2, …, K be the latent class (i.e., dietary pattern membership) at time t. Hence, women's dietary intakes (measured by using 29 food and beverage groups) are each classified into 1 of K dietary patterns at pregnancy and at 3 and 12 months postpartum (corresponding to latent class variables c1, c2, and c3, respectively). Dietary pattern memberships at pregnancy and postpartum are explained by covariates (e.g., age and parity) and are adjusted for energy intake. Arrows from c1 to c2 and from c2 to c3 represent movement from 1 dietary pattern at 1 time point to another dietary pattern at the next time point with corresponding vectors of transition probabilities τ2k2|k1 and τ3k3|k2. For example, for K = 3 classes, vector τ2k2|k1 has 9 probabilities: transition probability from class 1 in pregnancy to classes 1, 2, or 3 at 3 months postpartum and similarly for classes 2 and 3 at pregnancy. Note that only 6 of 9 transition probabilities need to be estimated because a woman in 1 particular class can change to only 1 of 3 classes, and these 3 probabilities sum to 1. Transition probabilities can also depend on other covariates that do not explain class membership at time point 1, such as weight gain and breastfeeding practices; these are represented in the path diagram by arrows from another box with these covariates to the postpartum latent classes and to the transition probabilities' arrows. Dietary pattern membership at postpartum time points can be explained both by covariates and by transition probabilities (19). The LTM is formulated in 2 parts that are estimated simultaneously. The first part explains how the observed outcomes (e.g., food and beverage group intakes) are related and is known as the measurement model in the latent variable model literature. As in latent class models, in LTM the 29 outcomes at each time point are typically assumed to be conditionally independent given class membership. This means that the observed correlation between any 2 food and beverage groups is attributable to the common latent class and does not depend directly on other food and beverage groups. The joint probability of dietary intake at 3 time points can be expressed by conditioning on time and class because the latent classes are mutually exclusive events. Mathematically, the measurement model is expressed as:
 |
The second part, known as the structural model, explains how the latent variables (e.g., dietary pattern classes) are related. In particular, in LTM the structural part specifies 1) a model for the class membership at the first time point; and 2) a model for transition probabilities from a latent class at 1 time point to a latent class at the next time point. Typically, the class membership at the first time point πk1 ≡ Pr[c1 = k1|X = x]) depends on covariates X, and it is modeled with a baseline-category logit model for nominal response (20) with the particularity that the class is not observed. Assuming 3 classes (i.e., K = 3) and choosing the third class arbitrarily as the referent, the first part of the structural model is as follows:
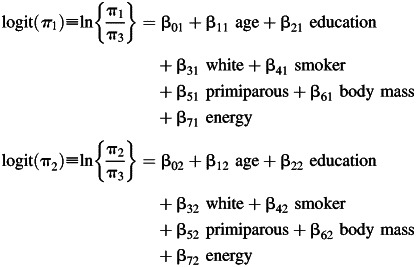 |
Similarly, transition probabilities from 1 dietary pattern at time t – 1 to another dietary pattern at time t (τ′kt|kt−1≡ Pr[ct = kt|ct−1 = kt−1, X = x]) are modeled by using a baseline-category logit model for nominal response.
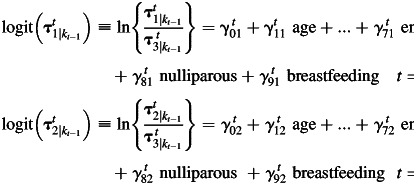 |
Figure 1.
Path diagram for the latent transition model to study women's changing of dietary patterns from pregnancy to 3 and 12 months postpartum, Pregnancy, Infection, and Nutrition Study, 2000–2005. Circles represent latent (i.e., unobserved) variables; squares represent observed variables; and arrows represent “causal” relationships. For explanations of statistical notations, see the Materials and Methods section. BMI, body mass index.
In summary, LTMs with categorical outcomes estimate 3 sets of parameters: 1) regression coefficients predicting class membership at the first time point; 2) conditional probabilities of the observed responses given the latent class; and 3) regression coefficients predicting transition probabilities of 1 latent class to another. LTMs are estimated iteratively by maximum likelihood by using the expectation-maximization algorithm (21) because the latent class variables are not observed. Model selection in LTMs often requires first determining the number of latent classes and then their characterization (e.g., testing whether item-response probabilities are time invariant), adjusting latent class membership and transition probabilities for covariates, and testing measurement invariance for transition probabilities. The order of decisions might have an impact on the results even if the number of classes is predetermined. There are 2 main reasons for testing and imposing measurement invariance across time points (12). The first reason is that the interpretation of class prevalences is easier when latent classes have the same meaning over time. The second reason is to guarantee the solution is unique (i.e., identified model).
Model selection
We considered only models with 3 latent classes on the basis of our previous work in which we identified 3 dietary patterns for the complete sample of pregnant women in the Pregnancy, Infection, and Nutrition Study (n = 1,285) (22). We interpreted and named the dietary patterns from the conditional response probabilities. We followed 4 general steps to select the “best” LTM. First, we tested measurement invariance across time for certain food and beverage groups, adjusting by energy intake. In other words, we tested whether the conditional response probabilities for these food and beverage groups changed over time. We guided our selection by choosing food and beverage groups that were significantly different over time by the correlation statistic test for categorical variables, which accounts for both outcome and time being ordinal. We adjusted P values for multiple comparisons by Bonferroni's method. Second, we adjusted the model for class membership at pregnancy by energy intake and covariates (maternal age, education level, race, parity, pregravid body mass index, and smoking behavior during pregnancy). Third, we added covariates to model latent transition probabilities and tested whether they were time invariant. Fourth, we fit a lag-2 LTM to assess whether dietary pattern class at pregnancy had an influence on dietary pattern class at 12 months postpartum (this would be reflected by an arrow (not shown) from c1 to c3 in Figure 1). Before adding covariates to the transition model, we constrained to 0 some transition probabilities that were extremely small (<0.05). We used Bayesian information criteria to compare models, and we used the χ2 test for change in log-likelihood for nested models. In latent class models, and hence in LTMs, subjects have a predicted probability for belonging to each class at any given time point. Women were classified into the class with the highest associated probability of class membership. Finally, we estimated the agreement between dietary patterns' classifications over time with the weighted К statistic for comparison with other studies. Statistical analyses were performed by using SAS/STAT, version 9.2, software (SAS Institute, Inc., Cary, North Carolina), and the procedure PROC LTA, version 1.2.7 alpha (23, 24), and Mplus, version 6.11 (25).
RESULTS
Identification of dietary patterns
Distributions of 8 food and beverage groups changed significantly (P < 0.002) from pregnancy to postpartum: fruits rich in vitamin C, other fruits, low-fat dairy, 100% juice, coffee, alcohol, diet soft drinks, and water (Table 1). During pregnancy, women consumed greater amounts of fruits, low-fat dairy, 100% juice, and water, and less alcohol, coffee, and diet soft drinks. Hence, we first assessed measurement invariance over time (Web Table 2 presents models that were compared for model selection). The reduced model (with only alcohol and coffee changing over time) was preferred by the Bayesian information criteria over the model with 8 food and beverage groups changing. However, the omnibus test was highly significant (P = 0.001) despite women's classifications being very similar (19, 11, and 9 women were classified differently by the 2 models compared at pregnancy and at 3 and 12 months postpartum, respectively). In light of these conflicting criteria, we chose the most parsimonious model. One class had higher probabilities for high consumption of fruits and vegetables, whole grains, beans, nuts, fish and chicken (not fried), water, and low-fat dairy; it was named “prudent” (Web Multipart Figure 1). A second class had high probabilities for consumption of high amounts of fast food, salty snacks, and sweets, but also for fruits and vegetables; it was named “health conscious Western.” The third class had lower probabilities for consumption of fruits and vegetables and high probabilities for consumption of fried fish and fried chicken and soft drinks; it was named “Western.”
Table 1.
Food and Beverage Group Percent Consumption and Tertiles or Medians Among Consumers at Pregnancy and at 3 and 12 Months Postpartuma, Pregnancy, Infection, and Nutrition Study, 2000–2005
| Food and Beverage Groups | Consumptionb, % |
First Tertilec, g/day for Foods and mL/day for Beverages |
Second Tertilec, g/day for Foods and mL/day for Beverages |
||||||
|---|---|---|---|---|---|---|---|---|---|
| During Pregnancy | 3 Months Postpartum | 12 Months Postpartum | During Pregnancy | 3 Months Postpartum | 12 Months Postpartum | During Pregnancy | 3 Months Postpartum | 12 Months Postpartum | |
| Other fruitsd | 100 | 99 | 100 | 90.3 | 68.1 | 61.0 | 181.1 | 142.8 | 135.3 |
| Vegetables | 100 | 100 | 100 | 63.5 | 61.1 | 62.8 | 112.5 | 116.2 | 111.0 |
| High-caratenoid vegetables | 98 | 98 | 99 | 30.9 | 33.5 | 32.9 | 70.0 | 78.5 | 71.6 |
| High-fat dairy | 99 | 96 | 98 | 15.4 | 9.1 | 12.0 | 30.8 | 30.8 | 30.8 |
| Low-fat dairyd | 93 | 93 | 94 | 228.0 | 157.5 | 137.1 | 456.1 | 355.6 | 304.8 |
| Nuts | 90 | 89 | 88 | 6.9 | 6.2 | 5.5 | 16.6 | 17.1 | 15.1 |
| Beans | 87 | 87 | 90 | 8.8 | 8.4 | 8.4 | 18.2 | 18.5 | 20.0 |
| Mixed dishes with meate | 100 | 100 | 99 | 81.6 | 72.3 | 74.4 | 143.4 | 138.3 | 125.6 |
| Eggs | 93 | 93 | 94 | 7.7 | 7.7 | 7.7 | 21.7 | 14.3 | 14.3 |
| Chicken (not fried) | 90 | 92 | 90 | 10.0 | 10.0 | 10.0 | 20.0 | 20.0 | 20.0 |
| Fish (not fried) | 83 | 87 | 89 | 6.0 | 7.9 | 7.8 | 14.3 | 17.1 | 16.7 |
| Processed meat | 91 | 93 | 93 | 8.3 | 9.5 | 9.5 | 18.2 | 20.8 | 22.6 |
| Refined grains | 100 | 100 | 100 | 71.8 | 63.9 | 64.0 | 108.5 | 109.1 | 105.7 |
| Whole grains | 84 | 80 | 86 | 16.0 | 16.0 | 14.0 | 41.1 | 35.7 | 33.0 |
| Salty snacks | 98 | 97 | 96 | 6.1 | 5.3 | 5.5 | 15.0 | 14.5 | 12.9 |
| Sweets | 100 | 100 | 99 | 34.6 | 31.7 | 31.2 | 64.0 | 62.9 | 52.8 |
| Waterd | 98 | 98 | 98 | 720.0 | 720.0 | 480.0 | 1200.0 | 1200.0 | 879.9 |
| 100% Fruit juiced | 97 | 95 | 91 | 218.0 | 182.6 | 182.6 | 407.9 | 310.6 | 303.2 |
| Coffeef | 52 | 56 | 63 | 85.7 | 171.4 | 220.0 | 280.0 | 300.0 | 439.9 |
| Alcoholf | 12 | 54 | 67 | 5.7 | 24.9 | 49.7 | 140.0 | 174.1 | 179.1 |
| Soft drinks | 96 | 97 | 98 | 335.7 | 355.0 | 336.0 | 591.4 | 699.3 | 710.0 |
| Diet soft drinksf | 34 | 43 | 50 | 50.7 | 153.8 | 153.8 | 331.3 | 355.0 | 355.0 |
| Fast food | 100 | 100 | 100 | 50.5 | 44.7 | 43.5 | 84.3 | 74.6 | 74.1 |
| Condiments with fat | 97 | 96 | 96 | 3.7 | 3.7 | 3.5 | 9.0 | 9.0 | 8.3 |
| Salad dressing | 94 | 94 | 94 | 6.9 | 4.6 | 4.6 | 13.7 | 13.9 | 13.7 |
| Median, g/day | |||||||||
| During Pregnancy | 3 Months Postpartum | 12 Months Postpartum | |||||||
| Rich vitamin C fruitsd,g | 77 | 68 | 66 | 19.2 | 10.1 | 10.1 | |||
| Beefg | 74 | 70 | 73 | 6.5 | 6.5 | 6.5 | |||
| Porkg | 72 | 75 | 74 | 6.8 | 6.8 | 8.2 | |||
| Fried chicken or fried fishg | 64 | 61 | 59 | 6.9 | 8.5 | 8.5 | |||
a Totals of 571, 545, and 424 pregnancies with complete dietary assessment at pregnancy and at 3 and 12 months postpartum, respectively.
b Consumption was defined as at least once per month.
c Values represent the upper cutpoints for the first and second tertiles of food and beverage consumption among consumers.
d Food and beverage group classifications changed significantly over time (P < 0.002).
e Category included vegetable stew with meat; spaghetti with tomato sauce and meat; vegetable soup with meat; other soups such as chicken noodle; dishes with beef or pork; pasta salad with meat; chicken stew; and pot pie.
f Coffee, alcohol, and soft drinks were treated as binary variables (consumed or not) because they were consumed by less than 60% of the women.
g Foods with a large percent (>25%) of nonconsumption were categorized into 3-level variables: no consumption, below the median, or above the median.
Nulliparas were 2.9 and 2.1 times more likely to be in the “prudent” than in the “health conscious Western” or the “Western” class, respectively (Table 2). Smokers were 4 times more likely to be in the “Western” class than the “prudent” class. White and more educated women were more likely to be in the “prudent” than the “Western” class. Women with higher energy intakes were more likely to be in the “health conscious Western” than the “prudent” class. Marginally, the prevalence of women in each of the 3 dietary patterns was approximately one-third at pregnancy and at postpartum, but at pregnancy the percent of women in the “health conscious Western” class was 10 percentage points higher than the percent of women in the “Western” class, and these percentages were reversed by 12 months postpartum (Table 3). The prevalence depended on parity, smoking status, education level, and race.
Table 2.
Odds Ratios for Predictors of Class Membership at Pregnancy, 3-Class Latent Transition Modela on 29 Food and Beverage Groups, Pregnancy, Infection, and Nutrition Study, 2000–2005
| Covariate | OR for “Prudent” | OR for “Health Conscious Western” | OR for “Western” | Change in 2 Log Likelihood | df | P Value |
|---|---|---|---|---|---|---|
| Nullipara | 1 | 0.34 | 0.47 | 8.1 | 2 | 0.0176 |
| Smoker | 1 | 1.66 | 4.02 | 12.2 | 2 | 0.0022 |
| White | 1 | 0.63 | 0.23 | 21.6 | 2 | 0.0001 |
| One or more years of graduate-level education | 1 | 0.98 | 0.23 | 30.7 | 2 | 0.0000 |
| Energy consumption during pregnancy | ||||||
| 2nd Quartile based on kcal at pregnancy | 1 | 4.98 | 1.35 | 9.3 | 2 | 0.0095 |
| 3rd Quartile based on kcal at pregnancy | 1 | 22.39 | 1.70 | 68.0 | 2 | 0.0001 |
| 4th Quartile based on kcal at pregnancy | 1 | 24.74 | 3.31 | 158.9 | 2 | 0.0001 |
Abbreviation: OR, odds ratio.
a The 3-class latent transition model has 2 parts that are estimated simultaneously. The first part, known as the measurement model, assumed that the 29 food and beverage groups at each point were conditionally independent given class membership and that food and beverage groups were time invariant except for coffee and alcohol. The second part, known as the structural model, specifies 2 models: 1 model for the class membership at the first time point and a second model for the transition probabilities. The first model is a baseline-category logit model for dietary pattern class membership at pregnancy adjusting for covariates (parity, smoking status, race, education level, and 3 dummy variables for quartiles of energy intake at baseline); estimates shown in this table. The second model is also a baseline-category logit model for transition probabilities from pregnancy to 3 months and from 3 to 12 months postpartum.
Table 3.
Class Prevalences and Transition Probabilities From 3-Class Latent Transition Modela on 29 Food and Beverage Groups, Pregnancy, Infection, and Nutrition Study, 2000–2005
| Dietary Pattern Class | Class Prevalence |
Transition Probabilities |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| During Pregnancy | 3 Months Postpartum | 12 Months Postpartum | Pregnancy to 3 Months Postpartum |
3–12 Months Postpartum |
|||||
| “Prudent” | “Health Conscious Western” | “Western” | “Prudent” | “Health Conscious Western” | “Western” | ||||
| “Prudent” | 33.7 | 33.6 | 34.3 | 0.93 | 0.07 | 0b | 0.97 | 0.03 | 0b |
| “Health conscious Western” | 37.9 | 31.4 | 28.4 | 0.06 | 0.74 | 0.20 | 0.05 | 0.87 | 0.08 |
| “Western” | 28.4 | 34.9 | 37.3 | 0b | 0.04 | 0.96 | 0b | 0.003 | 0.997 |
a The 3-class latent transition model has 2 parts that are estimated simultaneously. The first part, known as the measurement model, assumed that the 29 food and beverage groups at each point were conditionally independent given class membership and that food and beverage groups were time invariant except for coffee and alcohol. The second part, known as the structural model, specifies 2 models: 1 model for the class membership at the first time point and a second model for the transition probabilities. The first model is a baseline-category logit model for dietary pattern class membership at pregnancy adjusting for covariates (parity, smoking status, race, education level, and 3 dummy variables for quartiles for energy intake at baseline. The second model is also a baseline-category logit model for transition probabilities from pregnancy to 3 months and from 3 to 12 months postpartum; estimates shown in this table.
b Transition probability was constrained to 0 because it was negligible.
Transition probabilities
Except for breastfeeding practice at 3 months postpartum, transition probabilities did not differ depending on covariates. Breastfeeding mothers were more likely to switch dietary pattern class than were nonbreastfeeding mothers (P = 0.0286). In particular, among women in the “health conscious Western” class, breastfeeding mothers were more likely to switch to the “prudent” class at both 3 and 12 months postpartum than were nonbreastfeeding mothers. However, among women in the “prudent” class, breastfeeding mothers were more likely to switch to the “health conscious Western” class at both 3 and 12 months postpartum than were nonbreastfeeding mothers (Table 4). The 3 dietary patterns were generally very stable, with probabilities of less than 0.1 of switching dietary patterns from 1 time point to the next except for women in the “health conscious Western” class who had a transition probability of 0.2 to switch to the “Western” class at 3 months postpartum. The “Western” class represented the most stable pattern, with a probability of 0.96 of women staying in the same dietary pattern at any given time. The least stable dietary pattern was “health conscious Western,” with a probability of 0.74 for remaining in that pattern over time. Agreement among dietary patterns' classification over time was high between pregnancy and 3 months postpartum (κ = 0.87, 95% confidence interval: 0.84, 0.90) and between dietary patterns at 3 and 12 months postpartum (κ = 0.97, 95% confidence interval: 0.95, 0.98).
Table 4.
Odds Ratio for the Association Between Breastfeeding at 3 Months Postpartum and Changing Dietary Pattern From 3-Class Latent Transition Modela on 29 Food and Beverage Groups, Pregnancy, Infection, and Nutrition Study, 2000–2005
| Dietary Pattern Class | Pregnancy to 3 Months Postpartum |
3–12 Months Postpartum |
||||
|---|---|---|---|---|---|---|
| OR for “Prudent” | OR for “Health Conscious Western” | OR for “Western” | OR for “Prudent” | OR for “Health Conscious Western” | OR for “Western” | |
| “Prudent” | Referent | 1.5 | 1b | Referent | 2.3 | 1b |
| “Health conscious Western” | 25.4 | Referent | 0.09 | 26.7 | Referent | 0.16 |
| “Western” | 1b | 0.0 | Referent | 1b | 0.0 | Referent |
Abbreviation: OR, odds ratio.
a The 3-class latent transition model has 2 parts that are estimated simultaneously. The first part, known as the measurement model, assumed that the 29 food and beverage groups at each point were conditionally independent given class membership and that food and beverage groups were time invariant except for coffee and alcohol. The second part, known as the structural model, specifies 2 models: 1 model for the class membership at the first time point and a second model for the transition probabilities. The odds ratios presented in this table are from the second model, which is a baseline-category logit model for transition probabilities from pregnancy to 3 months and from 3 to 12 months postpartum with breastfeeding as a covariate.
b Odds ratio is equal to 1 because corresponding transition probabilities were constrained to 0 because they were negligible.
DISCUSSION
In this article we reported how LTMs might be useful to study movement among discrete dietary patterns over time. In particular, women from the Pregnancy, Infection, and Nutrition Study were classified into 3 mutually exclusive dietary pattern classes (“prudent,” “health conscious Western,” and “Western”) at pregnancy and at 3 and 12 months postpartum. Probabilities for being in each dietary pattern class depended on energy intake, parity, smoking status, education level, and race. Nulliparas, nonsmokers, white women, and more educated women were more likely to be in the “prudent” class than the “Western” class. In general, the 3 dietary patterns were very stable with the “health conscious Western” class being the least stable (the probability for staying in the same class was 0.74 and 0.87 at 3 and 12 months postpartum, respectively). Women did not switch dietary patterns from pregnancy to postpartum except for breastfeeding women who were more likely to switch to a healthier dietary pattern class. The fact that more changes did not occur from pregnancy to the postpartum period is not surprising. Although it has been said that pregnancy is a time period in which women may be more motivated to make lifestyle changes (26), there have never been data to support this statement. Data from intervention studies related to smoking during pregnancy clearly show that light smokers are more likely to quit during pregnancy compared with heavy smokers (27). Similarly, there might be subgroups of women who are more likely to make changes as shown here in breastfeeding mothers, but in general, women tend to follow the same dietary patterns but eat more food. If pregnancy were indeed an easier time to make dietary behavior changes, we would see greater effects in intervention studies. Further, 1 study (28) recently showed that parenthood had no unfavorable effect on parents' diets nor did it lead to significant dietary improvements over a 7-year time period.
Most of the studies of changes in dietary patterns over time have used dietary patterns in a continuous scale derived by principal components or factor analysis and have followed 3 steps: 1) identifying the dietary patterns; 2) computing dietary pattern scores at each time point; and 3) comparing scores over time. Dietary patterns are often identified separately at each time point and verified by visual inspection to determine whether they have the same number of factors and similar factor loadings over time. Next, dietary pattern scores are calculated at each time point either by using the time-specific factor loadings (2, 3, 29, 30) or the same factor loadings for all time points (1, 6–9, 31, 32). When the goal is to study dietary patterns' stability, associations between factor scores over time by using time-specific loadings is important, but agreement between scores by using, for instance, Bland-Altman plots, is more appropriate. Further, when the goal is to study a person's changes in dietary patterns, using time-specific loadings is not appropriate because the dietary patterns would not be equally measured over time. Similarly, when subjects are classified into dietary patterns (i.e., considered in a categorical scale), the classification algorithm has to be the same over time to correctly measure a person's change in dietary patterns. We are aware of only 1 study (33) in which the authors directly classified subjects to examine the stability of dietary patterns; this study reported that half of the women maintained the same dietary patterns over a period of 5 years, and some patterns were more stable than others (κ = 0.5, suggesting moderate stability). However, the authors performed cluster analysis separately at baseline and 5 years later, and although clusters were compared visually for the 2 time points, this does not guarantee that the dietary patterns were equally measured over time as does the LTM with time-invariant food- and beverage-group conditional probabilities.
To date, 3 studies have analyzed dietary patterns longitudinally during pregnancy (7) and postpartum (2, 9) to assess the stability of dietary patterns and to study their associations with predictors and other health behaviors. Cuco et al. (2) and Northstone and Emmett (9) derived the dietary patterns separately at each time point by using principal component analysis. Then, they visually assessed whether some principal components (dietary patterns) were similar over time, and they found 1 dietary pattern fewer at postpartum. Specifically, Cuco et al. (2) identified 2 dietary patterns (“sweetened beverages and sugars” and “vegetables and meat”) at preconception and at 4 time points during pregnancy, but only identified “sweetened beverages and sugars” at 6 months postpartum. Northstone and Emmett (9) found 4 dietary patterns both at pregnancy and at 4 years postpartum: “health conscious,” “processed,” “confectionery,” and “vegetarian,” but found only the “traditional” dietary pattern at pregnancy. In contrast to our approach in which women were directly classified into mutually exclusive dietary patterns, these 2 studies did not investigate the subjects’ overall dietary patterns as a combination of the derived factors, so women were not classified into a single dietary pattern. On the other hand, Crozier et al. (7) found that dietary patterns changed little from before pregnancy to early and late pregnancy. By using principal component scores based on dietary patterns defined before pregnancy, they showed that women had a very small decrease in the “prudent” score and little increase in the “high-energy” score from before to during pregnancy. Similarly, 1 study (34) examining individuals’ changes in dietary intake also showed little change from the first to the second trimester for most nutrients and food items.
There are several advantages of using the LTM to study dietary patterns over time. First, LTMs provide a direct classification of the subjects into mutually exclusive dietary patterns. Second, the meaning of the dietary patterns is the same over time because constraining for measurement invariance guarantees that the same dietary patterns are measured over time. Third, the LTM allows for estimating transition probabilities and for testing which covariates explain transitions over time. Fourth, the LTM allows the comparison of multiple groups easily; for example, we could compare the prevalence of dietary pattern classes and transition probabilities between genders. In general, 1 limitation for studying dietary patterns by using latent class models is the requirement of a large sample size, because for each class there are many parameters being estimated. For example, in this application with 27 time-invariant 3-level ordinal outcomes and 2 noninvariant outcomes over 3 time points, there were 66 parameters per class (2 parameters for each of the 27 time-invariant outcomes plus 6 parameters for each of the 2 noninvariant outcomes). Finally, the conditional independence assumption might not be true because correlated errors are expected among foods and beverages because of the nature of the food frequency questionnaire (e.g., multipass probing and foods and beverages organized in sections of food and beverage groups and meal patterns) and because of self-report bias for groups of foods and beverages that are perceived as healthy (35).
Dietary patterns can change over time for reasons such as nutritional advice, changes in food supply, or major life events like pregnancy and motherhood. Understanding the different dietary patterns during pregnancy and the first year postpartum and what factors influence the transition between dietary patterns could help create more effective interventions during pregnancy, which could be an important period in which to modify or improve health behaviors that would ideally be maintained over time.
ACKNOWLEDGMENTS
Author affiliations: Department of Biostatistics, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Daniela Sotres-Alvarez, Amy H. Herring); Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Anna-Maria Siega-Riz); and Department of Nutrition, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Anna-Maria Siega-Riz).
This work was supported by the National Institute of Child Health and Human Development, National Institutes of Health (grants HD37584 and HD39373), the National Institute of Diabetes and Digestive and Kidney Diseases (grants DK61981 and DK56350), and the Carolina Population Center.
This paper is derived from a chapter of the Doctor of Public Health dissertation of the first author at the Biostatistics Department of the University of North Carolina at Chapel Hill.
Preliminary findings were presented in a contributed oral presentation at the Spring Meeting of the Eastern North American Region of the International Biometric Society (March 17, 2009, San Antonio, Texas).
Conflict of interest: none declared.
REFERENCES
- 1.Crozier SR, Inskip HM, Godfrey KM, et al. Dietary patterns in pregnant women: a comparison of food-frequency questionnaires and 4-day prospective diaries. Br J Nutr. 2008;99(4):869–875. doi: 10.1017/S0007114507831746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuco G, Fernandez-Ballart J, Sala J, et al. Dietary patterns and associated lifestyles in preconception, pregnancy and postpartum. Eur J Clin Nutr. 2006;60(3):364–371. doi: 10.1038/sj.ejcn.1602324. [DOI] [PubMed] [Google Scholar]
- 3.Newby PK, Weismayer C, Akesson A, et al. Longitudinal changes in food patterns predict changes in weight and body mass index and the effects are greatest in obese women. J Nutr. 2006;136(10):2580–2587. doi: 10.1093/jn/136.10.2580. [DOI] [PubMed] [Google Scholar]
- 4.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298(7):754–764. doi: 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- 5.Schulze MB, Fung TT, Manson JE, et al. Dietary patterns and changes in body weight in women. Obesity (Silver Spring) 2006;14(8):1444–1453. doi: 10.1038/oby.2006.164. [DOI] [PubMed] [Google Scholar]
- 6.Borland SE, Robinson SM, Crozier SR, et al. Stability of dietary patterns in young women over a 2-year period. Eur J Clin Nutr. 2007;62(1):119–126. doi: 10.1038/sj.ejcn.1602684. [DOI] [PubMed] [Google Scholar]
- 7.Crozier SR, Robinson SM, Godfrey KM, et al. Women's dietary patterns change little from before to during pregnancy. J Nutr. 2009;139(10):1956–1963. doi: 10.3945/jn.109.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Togo P, Osler M, Sorensen TI, et al. A longitudinal study of food intake patterns and obesity in adult Danish men and women. Int J Obes Relat Metab Disord. 2004;28(4):583–593. doi: 10.1038/sj.ijo.0802598. [DOI] [PubMed] [Google Scholar]
- 9.Northstone K, Emmett PM. A comparison of methods to assess changes in dietary patterns from pregnancy to 4 years post-partum obtained using principal components analysis. Br J Nutr. 2008;99(05):1099–1106. doi: 10.1017/S0007114507842802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins LM, Wugalter SE. Latent class models for stage-sequential dynamic latent variables. Multivariate Behav Res. 1992;27(1):131–157. [Google Scholar]
- 11.Chung H, Walls TA, Park Y. A latent transition model with logistic regression. Psychometrika. 2007;72(3):413–435. [Google Scholar]
- 12.Collins LM, Lanza ST. Latent Class and Latent Transition Analysis: With Applications in the Social, Behavioral, and Health Sciences. Hoboken, NJ: Wiley; 2010. [Google Scholar]
- 13.Rabe-Hesketh S, Skrondal A. Classical latent variable models for medical research. Stat Methods Med Res. 2007;17(1):5–32. doi: 10.1177/0962280207081236. [DOI] [PubMed] [Google Scholar]
- 14.Fahey MT, Thane CW, Bramwell GD, et al. Conditional Gaussian mixture modelling for dietary pattern analysis. J R Stat Soc (A) 2007;170(1):149–166. [Google Scholar]
- 15.Siega-Riz AM, Herring AH, Carrier K, et al. Sociodemographic, perinatal, behavioral, and psychosocial predictors of weight retention at 3 and 12 months postpartum. Obesity. 2009;18(10):1996–2003. doi: 10.1038/oby.2009.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Block G, Thompson FE, Hartman AM, et al. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc. 1992;92(6):686–693. [PubMed] [Google Scholar]
- 17.McLachlan GJ, Peel D. Finite Mixture Models. New York, NY: Wiley; 2000. [Google Scholar]
- 18.Bollen KA. Structural Equations with Latent Variables. New York, NY: Wiley; 1989. [Google Scholar]
- 19.Muthén B, Asparouhov T. LTA in Mplus: Transition Probabilities Influenced by Covariates. Mplus Web Notes: No.13 http://www.statmodel.com/examples/LTAwebnote.pdf . [Google Scholar]
- 20.Agresti A. Analysis of Ordinal Categorical Data. Hoboken, NJ: John Wiley & Sons, Inc.; 2010. [Google Scholar]
- 21.Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm. J R Stat Soc (B) 1977;39(1):1–38. [Google Scholar]
- 22.Sotres-Alvarez D, Herring AH, Siega-Riz AM. Latent class analysis is useful to classify pregnant women into dietary patterns. J Nutr. 2010;140(12):2253–2259. doi: 10.3945/jn.110.124909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanza ST, Collins LM, Lemmon DR, et al. PROC LCA: A SAS procedure for latent class analysis. Struct Equ Modeling. 2007;14(4):671–694. doi: 10.1080/10705510701575602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanza ST, Dziak JJ, Huang L, et al. Proc LCA & Proc LTA Users’ Guide (Version 1.2.7) State College, PA: Pennsylvania State University; 2011. [Google Scholar]
- 25.Muthén LK,, Muthén BO. Mplus Software Version 6.11 and Mplus User's Guide. 6th. Los Angeles, CA: Muthén & Muthén; 2010. [Google Scholar]
- 26.Phelan S. Pregnancy: a “teachable moment” for weight control and obesity prevention. Obstet Gynecol. 2010;202(2):135.e1–135.e8. doi: 10.1016/j.ajog.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stotts AL, Groff JY, Velasquez MM, et al. Ultrasound feedback and motivational interviewing targeting smoking cessation in the second and third trimesters of pregnancy. Nicotine Tob Res. 2009;11(8):961–968. doi: 10.1093/ntr/ntp095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laroche HH, Wallace RB, Snetselaar L, et al. Changes in diet behavior when adults become parents. J Acad Nutr Diet. 2012;112(6):832–839. doi: 10.1016/j.jand.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newby PK, Weismayer C, Akesson A, et al. Long-term stability of food patterns identified by use of factor analysis among Swedish women. J Nutr. 2006;136(3):626–633. doi: 10.1093/jn/136.3.626. [DOI] [PubMed] [Google Scholar]
- 30.Weismayer C, Anderson JG, Wolk A. Changes in the stability of dietary patterns in a study of middle-aged Swedish women. J Nutr. 2006;136(6):1582–1587. doi: 10.1093/jn/136.6.1582. [DOI] [PubMed] [Google Scholar]
- 31.Mishra GD, McNaughton SA, Bramwell GD, et al. Longitudinal changes in dietary patterns during adult life. Br J Nutr. 2006;96(4):735–744. [PubMed] [Google Scholar]
- 32.McNaughton SA, Mishra GD, Stephen AM, et al. Dietary patterns throughout adult life are associated with body mass index, waist circumference, blood pressure, and red cell folate. J Nutr. 2007;137(1):99–105. doi: 10.1093/jn/137.1.99. [DOI] [PubMed] [Google Scholar]
- 33.Greenwood DC, Gilthorpe MS, Golding C, et al. Stability over time of dietary patterns in the UK Women's Cohort Study. Proc Nutr Soc. 2003;62:89A. [Google Scholar]
- 34.Rifas-Shiman SL, Rich-Edwards JW, Willett WC, et al. Changes in dietary intake from the first to the second trimester of pregnancy. Paediatr Perinat Epidemiol. 2006;20(1):35–42. doi: 10.1111/j.1365-3016.2006.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kipnis V, Carroll RJ, Freedman LS, et al. Implications of a new dietary measurement error model for estimation of relative risk: application to four calibration studies. Am J Epidemiol. 1999;150(6):642–651. doi: 10.1093/oxfordjournals.aje.a010063. [DOI] [PubMed] [Google Scholar]