Abstract
Peripheral neuropathy is underappreciated as a potential cause of functional limitations. In the present article, we assessed the cross-sectional association between peripheral neuropathy and physical functioning and how the longitudinal association between age and functioning differed by neuropathy status. Physical functioning was measured in 1996–2008 using timed performances on stair-climb, walking, sit-to-stand, and balance tests at the Michigan site of the Study of Women's Health Across the Nation, a population-based cohort study of women at midlife (n = 396). Peripheral neuropathy was measured in 2008 and defined as having an abnormal monofilament test result or 4 or more symptoms. We used linear mixed models to determine whether trajectories of physical functioning differed by prevalent neuropathy status. Overall, 27.8% of the women had neuropathy. Stair-climb time differed by neuropathy status (P = 0.04), and for every 1-year increase in age, women with neuropathy had a 1.82% (95% confidence interval: 1.42, 2.21) increase compared with a 0.95% (95% confidence interval: 0.71, 1.20) increase for women without neuropathy. Sit-to-stand time differed by neuropathy status (P = 0.01), but the rate of change did not differ. No differences between neuropathy groups were observed for the walk test. For some performance-based tasks, poor functioning was maintained or exacerbated for women who had prevalent neuropathy. Peripheral neuropathy may play a role in physical functioning limitations and future disability.
Keywords: peripheral nerve dysfunction, peripheral neuropathy, physical functioning
Understanding the determinants of health, disease, and disability are essential in creating “a society in which all people live long, healthy lives” (1, p. 1). Persisting knowledge gaps about the determinants of poor physical functioning limit our ability to achieve this goal. Many investigators have estimated the prevalence of disability or limitations in physical functioning in persons over the age of 65 years without appreciating the earlier initiation and progression of these problems. In 2009, the National Health Interview Survey found that almost 20% of midlife adults aged 45–64 years had some functional limitations or disability (2). Women were more likely to report functional limitations than were men (2, 3). Expanding assessments of physical limitations to include the midlife years among women is warranted because middle-aged women with limitations in physical functioning are at a high risk for future disability and decreased quality of life.
One seldom-explored cause of limitations in physical functioning is peripheral neuropathy, a disorder of the peripheral nerves. Common symptoms of peripheral neuropathy include numbness, pain, and tingling in the lower extremities. Overt signs of advanced disease, such as foot infection and ulceration, can be treated clinically; however, the adverse impact of neuropathy on physical functioning, often experienced before overt clinical manifestations, is underappreciated in the clinical setting (4).
Peripheral neuropathy has been well-described as a complication of diabetes mellitus, but other risk factors and sequelae of neuropathy have not been fully explored. Neuropathy has recently attracted attention as a health condition that occurs in persons without diabetes (5, 6). Many individuals with diabetes have peripheral neuropathy at the time of their diabetes diagnosis (7), and it has been speculated that episodic hyperglycemia or obesity may contribute to the etiology of neuropathy before the actual diagnosis of diabetes (8–10). The co-occurrence of peripheral neuropathy with diabetes, obesity, and limitations in physical functioning may help explain the high prevalence of disability reported among women in the United States. The purpose of the present investigation was to assess the association between peripheral neuropathy and performance-based physical functioning in a population-based bi-ethnic cohort of women at midlife in the United States. We evaluated whether baseline characteristics were associated with subsequent peripheral neuropathy status and how neuropathy was associated with previous and concurrent physical functioning. We hypothesized that peripheral neuropathy would be associated with poor physical functioning in a cross-sectional analysis and that women with neuropathy would have poorer age-related physical functioning trajectories than would women without neuropathy.
MATERIALS AND METHODS
Study population
The Study of Women's Health Across the Nation (SWAN) is an ongoing population-based longitudinal study of women from 7 sites in the United States. The recruitment and sampling of the SWAN cohort have been described in detail previously (11). In the present study, we utilized data from the Michigan cohort of SWAN, which consists of 543 women (325 African American and 218 Caucasian) from 2 communities near Detroit who at baseline in 1996 were 42–52 years of age, had an intact uterus and at least 1 ovary, reported no current use of hormones, and had had at least 1 menstrual period in the prior 3 months. Of the original sample, 418 (77%) were still active in study year 12 (2008) and eligible to participate in the peripheral neuropathy substudy. Of these, 371 (89%) underwent a foot examination and completed a symptom questionnaire to determine whether they had experienced peripheral neuropathy. Twenty-five (6%) completed only the symptom questionnaire administered via telephone or mail, and 22 (5%) refused participation in the substudy. The analytic sample included 396 women who participated in the neuropathy substudy and completed the examination and/or questionnaire in 2008. Women who participated in the neuropathy substudy did not differ by race/ethnicity or baseline age, weight, height, waist and hip circumferences, blood pressure, or fasting glucose levels from nonparticipants. The parent study and the substudy were approved by the University of Michigan Health Institutional Review Board, and all participants provided written informed consent.
Study variables
Peripheral neuropathy was assessed at study year 12 using 2 independent approaches: the Michigan Neuropathy Screening Instrument symptom questionnaire (12) and monofilament testing (13). The Michigan Neuropathy Screening Instrument symptom questionnaire is a 15-item questionnaire used to acquire information about the presence (yes or no) of common neuropathy symptoms and signs, including numbness, pain, sensitivity, cramping, prickling feelings, problems with hot/cold differentiation, open sores, dry skin, weakness, and amputation. The number of positive symptoms and signs was calculated, with higher scores indicating an increased likelihood of neuropathy (14). Monofilament testing used a 10-gram prestressed filament briefly (<1 second) placed on the dorsal side of the great toe, midway between the nail fold and the distal interphalangeal joint, for 10 repetitions. The participant was asked to respond if she felt the filament after each repetition on both feet. An abnormal monofilament test result was defined as 80% or fewer correct responses to the brief sensation in either foot (15). In the present study, we combined results of the Michigan Neuropathy Screening Instrument symptom questionnaire and the monofilament testing to determine the presence of peripheral neuropathy. Neuropathy is considered a syndrome because different sizes of nerve fibers may be affected, so using more than 1 test for peripheral neuropathy increased the sensitivity of our assessment and allowed us to capture different types and sizes of nerve involvement (16). If the participant either reported 4 or more symptoms or signs on the questionnaire or had 80% or fewer correct responses in either foot from the monofilament testing, the participant was classified as having neuropathy.
Performance-based physical functioning on a variety of tests was measured by trained examiners. The stair climb, assessed at baseline and at each annual visit through study year 12, measured the time in seconds that elapsed while participants climbed 3 stairs in 3 repetitions. Participants could use the hand railings if needed. The 40-foot walk, assessed at each visit in years 4 through 12, measured the time in seconds that elapsed while participants walked 40 feet at a brisk, purposeful pace. If a participant typically used an assistive device, she could use it during the timed walk. The sit-to-stand test, assessed at each visit in years 4 through 12, measured the time in seconds that it took for participants to rise from a normal-height bench with their arms crossed over their chests to a standing position with both arms down at the sides. Balance was measured by the unipedal and tandem foot stands in year 12. The unipedal foot stand measured the time in seconds that participants were able to balance on only their right foot, up to a maximum of 30 seconds. The tandem foot stand measured the time in seconds that participants were able to balance with the right foot in front of the left foot in a tandem position, up to a maximum of 30 seconds. For regression analyses, balance stands were dichotomized as less than 30 seconds (i.e., failing the stand) versus 30 seconds and analyzed as dichotomous variables. The stair-climb, walk, and sit-to-stand tasks were measured and analyzed as continuous variables.
Age in years was calculated from the interval of birth to follow-up visit date. Race/ethnicity was self-identified at the 1996 baseline as African American or Caucasian. Annual anthropometric and blood pressure measurements were collected using a standardized protocol in 1996–2008. Weight in kilograms was measured using a balance beam scale and height in centimeters was measured using a stadiometer, with body mass index (BMI) calculated as weight (kg)/height (m)2. Blood pressure (mm Hg) was measured twice using a mercury sphygmomanometer after an initial minimum 5-minute resting period and a 2-minute resting period between each measure, and values were then averaged. Participants were classified as hypertensive if their average systolic blood pressure was 140 mm Hg or higher, their average diastolic blood pressure was 90 mm Hg or higher, or if they reported current use of hypertension treatment.
Fasting blood samples were collected from participants annually. For this substudy, samples were assayed for blood glucose levels at the Michigan Diabetes Research and Training Center in Ann Arbor, Michigan. The interassay coefficients of variation in study year 12 were 3.6% at 92 mg/dL and 2.8% at 310 mg/dL; the intraassay coefficient of variation was 2.0% at 84 mg/dL and 283 mg/dL. Participants were determined to have diabetes mellitus if they had a fasting glucose level of 126 mg/dL or higher, a report of a health care provider's diagnosis of diabetes, or affirmative responses to questions about current use of diabetes medication.
Statistical analysis
First, means and frequencies were calculated to quantify baseline study population characteristics. We used χ2 tests for categorical variables and Student's t tests for continuous variables to compare baseline population characteristics between women who did and did not have prevalent peripheral neuropathy in study year 12. Multivariable logistic regression was used to determine the association between baseline study population characteristics and prevalent neuropathy in study year 12.
Multivariable linear regression was used to determine the cross-sectional association between continuous physical functioning performance measures in study year 12 and prevalent peripheral neuropathy in study year 12, adjusted for other covariates measured in the same study year. Results from the timed stair-climb, walk, and sit-to-stand tasks were log-transformed to meet model assumptions of homoscedasticity and then back-transformed to their original scale for presentation as percent change with corresponding 95% confidence intervals. In a similar manner, multivariable logistic regression was used to determine the association between balance and prevalent peripheral neuropathy in study year 12. Results from the tandem stand and the unipedal stand tests were modeled as the log-odds of failing (i.e., standing for <30 seconds), and associations were presented as odds ratios with corresponding 95% confidence intervals. We tested for heterogeneity in the association of neuropathy with functioning by hypertension, race/ethnicity, BMI, and diabetes by evaluating the statistical significance of multiplicative interaction terms at the level of α = 0.05.
We next described the change in performance-based physical functioning over time by evaluating physical functioning measures (in seconds) for the study population at baseline and at study years 4, 8, and 12. Student's t tests were used to compare functioning measures in women who had prevalent peripheral neuropathy in study year 12 with those in women who did not have prevalent peripheral neuropathy.
Finally, linear mixed models (PROC MIXED) with random intercepts and slopes for age were used to determine whether trajectories of physical functioning differed between women who did and did not have prevalent peripheral neuropathy in year 12, with adjustment for baseline BMI and race/ethnicity. We adjusted for baseline BMI rather than time-varying BMI because BMI was conceptualized as a potential mediator of the relationship between neuropathy and poor physical functioning. Continuous outcome measurements (timed walk, timed stair climb, and timed sit-to-stand) were log-transformed to meet model assumptions of homoscedasticity and then back-transformed to the geometric mean of their original scale (seconds) for presentation. Predicted trajectories of the performance-based physical functioning measures with corresponding 95% confidence bands were graphed by peripheral neuropathy group using PROC SGPLOT. SAS, version 9.3 (SAS Institute, Inc., Cary, North Carolina) was used for all data management and analysis. Statistical tests were 2-sided, with the level of significance defined as P < 0.05.
RESULTS
The average baseline age of participants was 46.1 (standard deviation, 2.7) years; 8.5% were characterized as having diabetes at baseline, and 60% were African American. The prevalence of neuropathy in study year 12 was 27.8% (95% confidence interval (CI): 23.4, 32.3; n = 110). Women with prevalent neuropathy had bigger body sizes at baseline and were more likely to have diabetes at baseline than women without prevalent neuropathy (Table 1). In the multivariable model to determine the association between baseline characteristics and prevalent neuropathy (Table 2), baseline BMI remained a significant predictor of prevalent neuropathy at year 12.
Table 1.
Baseline (1996) Characteristics of Women With and Without Peripheral Neuropathy in 2008, Peripheral Neuropathy Substudy, Michigan Study of Women's Health Across the Nation
| Peripheral Neuropathy (n = 110) |
No Peripheral Neuropathy (n = 286) |
P Valuea | |||
|---|---|---|---|---|---|
| % | Mean (SD) | % | Mean (SD) | ||
| Age, years | 46.3 (2.6) | 46.1 (2.8) | 0.39 | ||
| Race | |||||
| African American | 59.1 | 59.8 | 0.9 | ||
| Caucasian | 40.9 | 40.2 | |||
| Body mass indexb | 34.9 (8.5) | 31.4 (7.7) | 0.0002 | ||
| Blood pressure | |||||
| Systolic, mm Hg | 120 (19.2) | 119 (19.3) | 0.51 | ||
| Diastolic, mm Hg | 71 (12.1) | 71 (10.4) | 0.7 | ||
| Hypertension | 30.9 | 24.8 | 0.22 | ||
| Glucose, mg/dL | 110 (46.5) | 101 (34.4) | 0.06 | ||
| Diabetes | 13.6 | 6.3 | 0.02 | ||
Abbreviation: SD, standard deviation.
a P values from χ2 tests for categorical variables and Student's t tests for continuous variables.
b Weight (kg)/height (m)2.
Table 2.
Multivariable Associationsa Between Baseline Characteristics (1996) and Peripheral Neuropathy in 2008, Peripheral Neuropathy Substudy, Michigan Study of Women's Health Across the Nation
| Characteristic | OR | 95% CI |
|---|---|---|
| Race/ethnicity (African American vs. Caucasian) | 0.96 | 0.60, 1.54 |
| Body mass indexb | 1.05 | 1.02, 1.08 |
| Hypertension (yes vs. no) | 1.07 | 0.63, 1.81 |
| Diabetes (yes vs. no) | 1.66 | 0.78, 3.57 |
Abbreviations: CI, confidence interval; OR, odds ratio.
a Models were also adjusted for age; body mass index was centered at mean 32.1.
b Weight (kg)/height (m)2.
In the cross-sectional multivariable analysis (Tables 3 and 4), peripheral neuropathy was significantly associated with poorer performance on the stair-climb and 40-foot walk tasks in year 12. African-American race/ethnicity was significantly associated with longer stair-climb and sit-to-stand times and failing the unipedal stand. BMI was significantly associated with poorer performance on the stair-climb, walk, sit-to-stand, and unipedal stand tasks. Diabetes was significantly associated with poorer performance for only the stair-climb assessment after adjustment for peripheral neuropathy.
Table 3.
Mean and Percent Difference for Characteristics in 2008 by Performance-based Physical Functioning in 2008, Peripheral Neuropathy Substudy, Michigan Study of Women's Health Across the Nation
| Characteristic | Test |
|||||
|---|---|---|---|---|---|---|
| Stair Climb |
40-foota Walk |
Sit-to-Stand |
||||
| % Difference | 95% CI | % Difference | 95% CI | % Difference | 95% CI | |
| Mean time at age 46 years | 16.86b | 14.76, 19.25 | 10.34b | 9.38, 11.41 | 1.56b | 1.28, 1.91 |
| Peripheral neuropathy (yes vs. no) | 14.07 | 6.78, 21.87 | 10.79 | 5.55, 16.28 | 7.79 | −2.40, 19.03 |
| Age, years | 0.64 | −0.43, 1.72 | −0.09 | −0.87, 0.70 | −0.94 | −2.53, 0.67 |
| Race/ethnicity (African American vs. Caucasian) | 7.88 | 1.58, 14.58 | 4.04 | −0.53, 8.81 | 10.94 | 1.25, 21.57 |
| Body mass indexc | 1.21 | 0.81, 1.60 | 1.13 | 0.85, 1.42 | 0.84 | 0.25, 1.43 |
| Hypertension (yes vs. no) | 2.23 | −4.05, 8.92 | 1.37 | −3.32, 6.29 | −0.47 | −9.62, 9.60 |
| Diabetes (yes vs. no) | 7.37 | 0.23, 15.03 | 3.54 | −1.61, 8.95 | 5.04 | −5.32, 16.54 |
Abbreviation: CI, confidence interval.
a 12.2 m.
b Values are expressed as seconds rather than % difference.
c Weight (kg)/height (m)2.
Table 4.
Multivariable Associations Between Characteristics and Performance-based Physical Functioning Stands, Peripheral Neuropathy Substudy, Michigan Study of Women's Health Across the Nation, 2008
| Tandem Stand (<30 vs. 30 seconds) |
Unipedal Stand (<30 vs. 30 seconds) |
|||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Peripheral neuropathy (yes vs. no) | 1.38 | 0.85, 2.25 | 1.78 | 0.94, 3.35 |
| Age, years | 1.05 | 0.97, 1.14 | 1.05 | 0.95, 1.15 |
| Race (African American vs. Caucasian) | 1.47 | 0.93, 2.33 | 2.13 | 1.26, 3.59 |
| Body mass indexa | 1.01 | 0.98, 1.04 | 1.12 | 1.08, 1.17 |
| Hypertension (yes vs. no) | 1.28 | 0.79, 2.07 | 1.32 | 0.77, 2.26 |
| Diabetes (yes vs. no) | 1.61 | 0.97, 2.67 | 1.32 | 0.68, 2.55 |
Abbreviations: CI, confidence interval; OR, odds ratio.
a Weight (kg)/height (m)2.
On average, women had increasingly poor physical functioning between baseline and assessment of neuropathy (Table 5). Women with prevalent neuropathy at year 12 were significantly different from women without neuropathy on all physical functioning assessments, not only in year 12 but also at every prior study year. Women with neuropathy required significantly more time to complete the stair-climb, 40-foot walk, and sit-to-stand trials than did women without neuropathy. In addition, women with neuropathy were unable to maintain the balance stands for as long as women without neuropathy.
Table 5.
Performance-based Physical Functioning Measures by Selected Visit and Peripheral Neuropathy Status, Peripheral Neuropathy Substudy, Michigan Study of Women's Health Across the Nation, 1996–2008
| Measure and Visit | Time to Complete Measure, secondsa |
P Valueb | ||
|---|---|---|---|---|
| Total Substudy | Women With Peripheral Neuropathy | Women Without Peripheral Neuropathy | ||
| Stair climb | ||||
| Baseline | 19.6 (5.3) | 21.4 (7.3) | 18.9 (4.1) | 0.001 |
| Year 4 | 19.6 (7.8) | 21.7 (10.5) | 18.7 (6.3) | 0.01 |
| Year 8 | 20.6 (5.9) | 22.9 (7.7) | 19.7 (4.9) | 0.001 |
| Year 12 | 22.0 (9.1) | 26.3 (13.6) | 20.4 (5.9) | <0.0001 |
| 40-foot walk | ||||
| Year 4 | 9.2 (2.5) | 9.8 (3.5) | 8.9 (1.8) | 0.03 |
| Year 8 | 12.0 (4.6) | 13.3 (7.5) | 11.4 (2.6) | 0.02 |
| Year 12 | 11.6 (3.5) | 13.3 (5.4) | 10.9 (2.1) | <0.0001 |
| Sit-to-stand | ||||
| Year 4 | 1.3 (0.6) | 1.5 (0.7) | 1.3 (0.6) | 0.01 |
| Year 8 | 1.4 (0.6) | 1.6 (0.8) | 1.3 (0.5) | 0.001 |
| Year 12 | 1.8 (1.1) | 2.0 (1.3) | 1.7 (0.9) | 0.03 |
| Tandem stand | ||||
| Year 12 | 21.8 (10.9) | 20.0 (11.4) | 22.5 (10.6) | 0.05 |
| Unipedal stand | ||||
| Year 12 | 15.6 (11.5) | 11.9 (10.5) | 16.9 (11.6) | 0.0002 |
Abbreviation: PN, peripheral neuropathy.
a Values represent the mean (standard deviation).
b P values from Student's t tests.
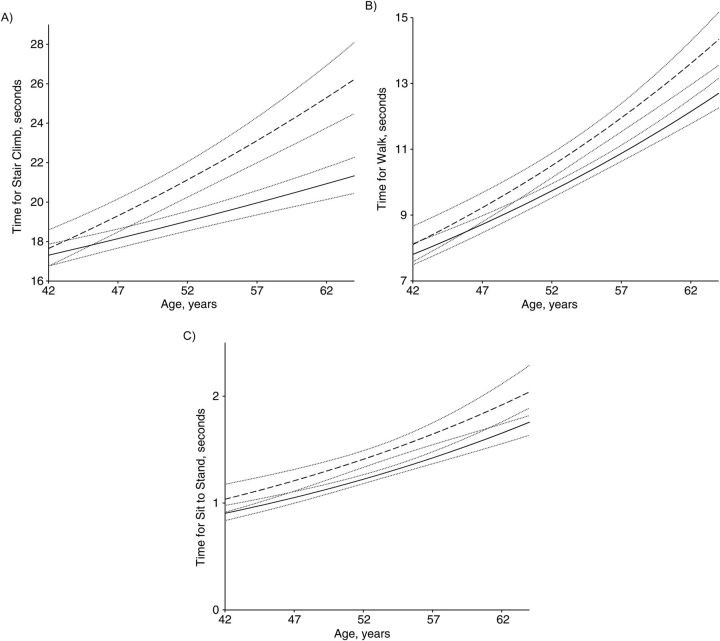
The longitudinal analysis characterized the trajectory of physical functioning over time by prevalent peripheral neuropathy status in year 12, adjusted for baseline BMI and race/ethnicity. Time to complete the stair climb at 46 years of age (mean baseline age) differed significantly by neuropathy status (P = 0.045) and in percent change over time (P < 0.001). For every 1-year increase in age, women found to have prevalent neuropathy in year 12 had a 1.82% (95% CI: 1.42, 2.21) average increase in time to complete the stair climb compared with a 0.95% (95% CI: 0.71, 1.20) average increase for women who did not have neuropathy. Time to complete the sit-to-stand test at 46 years of age also differed significantly by neuropathy status (P = 0.01). For every 1-year increase in age, women found to have prevalent neuropathy in year 12 had a 3.12% (95% CI: 2.11, 4.15) average increase in time to complete the sit-to-stand task compared with a 3.07% (95% CI: 2.44, 3.70) average increase for women who would not have neuropathy (P = 0.93). Time to complete the 40-foot walk at 46 years of age did not differ by neuropathy status (P = 0.09). For every 1-year increase in age, women found to have prevalent neuropathy in year 12 had a 2.63% (95% CI: 2.14, 3.11) average increase in time to complete the 40-foot walk compared with a 2.24% (95% CI: 1.94, 2.54) average increase for women who did not have neuropathy (P = 0.09). Figure 1 presents the trajectories of the physical functioning assessments over time by peripheral neuropathy status with corresponding 95% confidence bands, adjusted for baseline BMI and race/ethnicity.
Figure 1.
Previous trajectories of timed stair-climb (A), timed walk (B), and timed sit-to-stand (C) test results by peripheral neuropathy status in 2008 in the peripheral neuropathy substudy, Michigan Study of Women's Health Across the Nation. Results were adjusted for race/ethnicity and baseline body mass index. Dashed lines represent trajectories for women with peripheral neuropathy, solid lines represent trajectories for women without peripheral neuropathy, and dotted lines represent the 95% confidence intervals for trajectories.
DISCUSSION
To our knowledge, this is the first investigation of peripheral neuropathy and longitudinal physical functioning performance in community-dwelling women at midlife. Over one-quarter of Michigan SWAN participants had peripheral neuropathy in 2008. Even at baseline, which was 12 years before the neuropathy assessment, physical functioning as assessed by a variety of tests differed between women who did and did not have neuropathy 12 years later, and importantly, these differences persisted over time. Even after adjustment for BMI and race/ethnicity, peripheral neuropathy remained associated with diminished physical functioning. Notably, the association between peripheral neuropathy and functioning appeared to be independent of diabetes.
Our results are consistent with studies in older adults. In a cross-sectional study of disabled women who were 65 years or older, more than half had some level of peripheral nerve dysfunction, and nerve dysfunction was significantly associated with functional limitations independent of diabetes (17). Women with nerve dysfunction were 2 times more likely to fail balance tests and have significantly slower walking speeds than were women with normal nerve function (17). Likewise, another study of older adults demonstrated that neuropathy was significantly associated with poorer lower extremity physical performance, independent of diabetes (18). In our population of women in their forties and fifties, neuropathy was significantly associated with the concurrent stair-climb and walk assessments even after adjustment for diabetes status.
Mobility is fundamental to the healthy aging process (19). Physical functioning is of great public health relevance because mobility impairments, such as inadequate walking speeds, compromise an individual's ability to safely negotiate his or her physical environment. National standards require the minimum pedestrian clearance velocity at a crosswalk to be 3.5 feet/second (1.1 m/second) (20). In other words, an individual must walk at a pace of at least 3.5 feet per second from curb to curb during the “walk” indication signal to safely use a crosswalk. In a previous investigation, Sowers et al. (21) reported that approximately one-third of Michigan SWAN participants walked at speeds slower than the federal standard for crossing a controlled intersection. In our investigation, we found that the ability to walk at the minimum pedestrian clearance velocity differed by peripheral neuropathy status. In 2008, women with peripheral neuropathy completed the 40-foot walk at a calculated velocity of 2.7 feet/second (0.8 m/second) on average, whereas women without neuropathy ambulated at a velocity of 3.6 feet/second (1.1 m/second). Although we did not assess each individual participant's environment, performance-based physical functioning measures determined in a controlled setting are highly predictive of future disability and loss of independence (22, 23).
To our knowledge, no similar references exist to evaluate the public health impact of decrements in the other physical functioning measures assessed here, such as stair climbing. Individuals with neuropathy might use compensatory mechanisms while walking, such as decreased speed and stride length, as well as increased time spent in double support (shuffling) compared with individuals without neuropathy (24). However, stair ascent and descent may be particularly hazardous because they are controlled from a single support limb with no ability to use these compensatory mechanisms. As such, it may be the “purest” physical functioning measure assessed in our study. We found significant baseline differences in stair-climb times between women found to have prevalent neuropathy at year 12 compared with women who did not, and these differences were exacerbated over time. Nevertheless, observed differences between neuropathy groups were relatively modest. Quantifying these differences in terms of clinical significance and incident disability will be important.
The association between peripheral neuropathy and poor physical functioning is not surprising. Nerve damage reduces the amount of perception feedback from receptors and causes impairments like reduced balance and position sense (17). In the lower extremities, impairments can cause instability while walking or standing and lead to falls and functional limitations (17). Limitations in physical functioning can lead to a loss of independence because activities like walking are critical for the maintenance of independence in a community (4, 17). Physical functioning limitations are risk factors for functional disability, defined as the inability to fulfill activities of daily living in an individual's environment or context (17, 25).
In our study, BMI was associated with neuropathy and physical functioning. Even at baseline, women who were and were not found to have peripheral neuropathy in study year 12 had significant differences in body size. Women with prevalent neuropathy in year 12 appeared to have a larger body size at baseline and to stay larger during the course of our study. Obesity is hypothesized to be a potential cause of peripheral neuropathy independent of diabetes (9, 10) because it co-occurs with abnormal cardiometabolic factors like hypertension, hyperlipidemia, hyperglycemia, and central adiposity (26), which increase the risk of incident neuropathy (27). It is also possible that obesity is a consequence of peripheral neuropathy. Individuals who experience numbness or pain in the lower extremities may be less physically active, and inactivity, particularly in the midlife years, may be a risk factor for obesity. The role of BMI clearly deserves greater attention in future research. Longitudinal studies should characterize the relationship between obesity and the onset of peripheral neuropathy and determine the role of physical activity in the relationship among obesity, neuropathy, and functioning.
The present study has some limitations. Notably, we measured peripheral neuropathy in study year 12 using a combined definition of monofilament testing result and a symptom score, and the study only included women who participated in the neuropathy substudy. A single measure of neuropathy precludes inferences of causality with respect to physical functioning, as it is unclear when the neuropathy developed. In general, defining neuropathy with only monofilament testing yielded similar results to our combined definition, with one exception: Monofilament test–defined neuropathy was associated with increased odds of failing the unipedal stand in the fully adjusted model (odds ratio = 3.07, 95% CI: 1.11, 8.48). The unipedal stand may be unique for individuals with insensate neuropathy because using only one foot may preclude normal compensatory mechanisms utilized to achieve full functional status. Women who did not participate in the neuropathy substudy had age-related trajectories of functioning similar to those of women with and without neuropathy (results not shown). Even though it appeared that the “missing” neuropathy group included women with and without neuropathy, it is possible that these women were not missing at random.
Furthermore, residual confounding may exist in the relationship between peripheral neuropathy and physical functioning. BMI has been proposed as both a cause and a consequence of neuropathy. We adjusted only for baseline BMI in our longitudinal models so as not to artificially dilute the impact of neuropathy on functioning, but poorer physical functioning at baseline may be due to obesity and not neuropathy. Thus, unmeasured confounding by BMI is possible. Additionally, this study population may have lacked sufficient heterogeneity (e.g., 63% of participants had a BMI >30 in 2008) to detect interactions between obesity and neuropathy.
Studies of physical functioning do not typically evaluate neuropathy. Our results suggest that peripheral neuropathy is a prevalent but underappreciated condition in the general population and is associated with decreased physical functioning. Clinicians may wish to implement neuropathy testing for individuals in the general population who exhibit diminished functional capacity, and neuropathy assessment should be considered for inclusion in future studies of functional status and disability.
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, Michigan (Kelly R. Ylitalo, William H. Herman, Siobán D. Harlow); and Department of Internal Medicine, School of Medicine, University of Michigan, Ann Arbor, Michigan (Kelly R. Ylitalo, William H. Herman).
The Study of Women's Health Across the Nation (SWAN) received grant support from the National Institutes of Health, Department of Health and Human Services, through the National Institute on Aging, the National Institute of Nursing Research, and the National Institutes of Health Office of Research on Women's Health (grants NR004061, AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495, as well as grant AG017719 to the SWAN Repository).
We thank the study staff at each site.
Clinical Centers: University of Michigan, Ann Arbor, Michigan—Siobán Harlow, principal investigator (PI), 2011–present, MaryFran Sowers, PI, 1994–2011; Massachusetts General Hospital, Boston, Massachusetts —Joel Finkelstein, PI, 1999—present; Robert Neer, PI, 1994–1999; Rush University, Rush University Medical Center, Chicago, Illinois—Howard Kravitz, PI, 2009–present; Lynda Powell, PI, 1994–2009; University of California, Davis/Kaiser, California —Ellen Gold, PI; University of California, Los Angeles, Los Angeles, California—Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, New York—Carol Derby, PI ,2011–present, Rachel Wildman, PI, 2010–2011; Nanette Santoro, PI, 2004–2010; University of Medicine and Dentistry—New Jersey Medical School, Newark, New Jersey—Gerson Weiss, PI, 1994–2004; and the University of Pittsburgh, Pittsburgh, Pennsylvania—Karen Matthews, PI. NIH Program Office: National Institute on Aging, Bethesda, Maryland—Winifred Rossi, 2012–present; Sherry Sherman, 1994–2012; Marcia Ory, 1994–2001; National Institute of Nursing Research, Bethesda, Maryland—Program Officers. Central Laboratory: University of Michigan, Ann Arbor, Michigan—Daniel McConnell (Central Ligand Assay Satellite Services). SWAN Repository: University of Michigan, Ann Arbor, Michigan—Dan McConnell, 2011–present; MaryFran Sowers, 2000–2011. Coordinating Center: University of Pittsburgh, Pittsburgh, Pennsylvania—Maria Mori Brooks, PI 2012–present; Kim Sutton-Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, Massachusetts—Sonja McKinlay, PI 1995–2001. Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, National Institute of Nursing Research, Office of Research on Women's Health or the National Institutes of Health.
Conflict of interest: none declared.
REFERENCES
- 1.US Department of Health and Human Services. Healthy People 2020 Framework. Washington, DC: Department of Health and Human Services; 2010. http://healthypeople.gov/2020/Consortium/HP2020Framework.pdf. (Accessed April 28, 2010) [Google Scholar]
- 2.Pleis JR, Ward BW, Lucas JW. Summary health statistics for U.S. adults: National Health Interview Survey, 2009. Vital Health Stat 10. 2010;249:1–207. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Prevalence and most common causes of disability among adults–United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58(16):421–426. [PubMed] [Google Scholar]
- 4.van Schie CH. Neuropathy: mobility and quality of life. Diabetes Metab Res Rev. 2008;24(supp 1):S45–S51. doi: 10.1002/dmrr.856. [DOI] [PubMed] [Google Scholar]
- 5.Gregg EW, Sorlie P, Paulose-Ram R, et al. Prevalence of lower-extremity disease in the U.S. adult population [greater than or equal to] 40 years of age with and without diabetes. Diabetes Care. 2004;27(7):1591–1597. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 6.Ylitalo K, Sowers MF, Heeringa S. Peripheral vascular disease and peripheral neuropathy in individuals with cardiometabolic clustering and obesity. Diabetes Care. 2011;34(7):1642–1647. doi: 10.2337/dc10-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tesfaye S, Boulton AJ, editors. Diabetic Neuropathy. Oxford, United Kingdom: Oxford University Press; 2009. [Google Scholar]
- 8.Dyck PJ, Dyck PJB, Klein CJ, et al. Does impaired glucose metabolism cause polyneuropathy? Review of previous studies and design of a prospective controlled population-based study. Muscle Nerve. 2007;36(4):536–541. doi: 10.1002/mus.20846. [DOI] [PubMed] [Google Scholar]
- 9.Ziegler D, Rathman W, Dickhaus T, et al. Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy. Diabetes Care. 2008;31(3):464–469. doi: 10.2337/dc07-1796. [DOI] [PubMed] [Google Scholar]
- 10.Gordon Smith A, Robinson Singleton J. Idiopathic neuropathy, prediabetes and the metabolic syndrome. J Neurol Sci. 2006;242(1–2):9–14. doi: 10.1016/j.jns.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 11.Sowers MF, Crawford S, Sternfeld B, et al. SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopause. In: Lobo R, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. San Diego, CA: Academic Press; 2000. pp. 175–178. [Google Scholar]
- 12.Feldman EL, Stevens MJ. Clinical testing in diabetic peripheral neuropathy. Can J Neurol Sci. 1994;21(4):S3–S7. doi: 10.1017/s0317167100040671. [DOI] [PubMed] [Google Scholar]
- 13.Mueller MJ. Identifying patients with diabetes mellitus who are at risk for lower-extremity complications: use of Semmes-Weinstein monofilaments. Phys Ther. 1996;76(1):68–71. doi: 10.1093/ptj/76.1.68. [DOI] [PubMed] [Google Scholar]
- 14.Herman WH, Pop-Busui R, Braffett BH, et al. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med. 2012;29(7):937–944. doi: 10.1111/j.1464-5491.2012.03644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nang EE, Khoo CM, Tai ES, et al. Is there a clear threshold for fasting plasma glucose that differentiates between those with and without neuropathy and chronic kidney disease? The Singapore Prospective Study Program. Am J Epidemiol. 2009;169(12):1454–1462. doi: 10.1093/aje/kwp076. [DOI] [PubMed] [Google Scholar]
- 16.Dyck PJ, Karnes JL, Daube J, et al. Clinical and neuropathological criteria for the diagnosis and staging of diabetic polyneuropathy. Brain. 1985;108(Pt 4):861–880. doi: 10.1093/brain/108.4.861. [DOI] [PubMed] [Google Scholar]
- 17.Resnick HE, Vinik AI, Schwartz AV, et al. Independent effects of peripheral nerve dysfunction on lower-extremity physical function in old age: the Women's Health and Aging Study. Diabetes Care. 2000;23(11):1642–1647. doi: 10.2337/diacare.23.11.1642. [DOI] [PubMed] [Google Scholar]
- 18.Strotmeyer ES, de Rekeneire N, Schwartz AV, et al. The relationship between reduced peripheral nerve function and diabetes with physical performance in older white and black adults. Diabetes Care. 2008;31(9):1767–1772. doi: 10.2337/dc08-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webber SC, Porter MM, Menec VH. Mobility in older adults: a comprehensive framework. Gerontologist. 2010;50(4):443–450. doi: 10.1093/geront/gnq013. [DOI] [PubMed] [Google Scholar]
- 20.US Department of Transportation Federal Highway Administration. Manual on Uniform Traffic Control Devices for Streets and Highways. Washington, DC: US Department of Transportation Federal Highway Administration; 2009. http://mutcd.fhwa.dot.gov/pdfs/2009/coverintrotoc.pdf. ). (Accessed August 19, 2011) [Google Scholar]
- 21.Sowers M, Jannausch ML, Gross M, et al. Performance-based physical functioning in African-American and Caucasian women at midlife: considering body composition, quadriceps strength, and knee osteoparthritis. Am J Epidemiol. 2006;163(10):950–958. doi: 10.1093/aje/kwj109. [DOI] [PubMed] [Google Scholar]
- 22.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guralnik JM, Ferrucci L, Peiper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance batter. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 24.Courtemanche R, Teasdale N, Boucher P, et al. Gait problems in diabetic neuropathic patients. Arch Phys Med Rehabil. 1996;77(9):849–855. doi: 10.1016/s0003-9993(96)90269-5. [DOI] [PubMed] [Google Scholar]
- 25.Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38(1):1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher EJ, LeRoith D, Karnieli E. The metabolic syndrome- from insulin resistance to obesity and diabetes. Med Clin N Am. 2011;95(5):855–873. doi: 10.1016/j.mcna.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Tesfaye S, Selvarajah D. The Eurodiab study: what has this taught us about diabetic peripheral neuropathy? Curr Diab Rep. 2009;9(6):432–434. doi: 10.1007/s11892-009-0070-1. [DOI] [PubMed] [Google Scholar]