Abstract
The emerging field of regenerative medicine will require a reliable source of stem cells in addition to biomaterial scaffolds and cytokine growth factors. Adipose tissue has proven to serve as an abundant, accessible and rich source of adult stem cells with multipotent properties suitable for tissue engineering and regenerative medical applications. There has been increased interest in Adipose-derived Stem Cells (ASCs) for tissue engineering applications. Here, methods for the isolation, expansion and differentiation of ASCs are presented and described in detail. While this article has focused on the isolation of ASCs from human adipose tissue, the procedure can be applied to adipose tissues from other species with minimal modifications.
Keywords: Adipose-derived stem cells (ASCs), Biopsy, Differentiation, Expansion, Isolation, Lipoaspirate, Mesenchymal Stem Cells (MSCs)
1. Introduction
By definition, a stem cell is characterized by its ability to undergo self-renewal and its ability to undergo multilineage differentiation and form terminally differentiated cells. Ideally, a stem cell for regenerative medicinal applications should meet the following set of criteria: (i) should be found in abundant quantities (millions to billions of cells); (ii) can be collected and harvested by a minimally invasive procedure; (iii) can be differentiated along multiple cell lineage pathways in a reproducible manner; (iv) can be safely and effectively transplanted to either an autologous or allogeneic host [1].
Tissue specific stem cells comprise the second group and are derived from specific organs, such as brain, gut, lung, liver, adipose tissue and bone marrow [2]. It has become evident that these stem cells persist in adult tissues, although they represent a rare population localized in small niches [3]. Postnatal (adult) stem cells are not totipotent; however, they are pluripotent. These cells retain a broad differentiation potential but their developmental potential is more restricted than embryonic stem cells. Adult stem cells were initially thought to have the differentiation capacity limited to their tissue of origin, however recent studies have demonstrated that stem cells have the capacity to differentiate into cells of mesodermal, endodermal and ectodermal origins [4–10]. The plasticity of MSCs most often refers to the inherent ability retained within stem cells to cross lineage barriers and to adopt the phenotypic, biochemical and functional properties of cells unique to other tissues. Adult mesenchymal stem cells can be isolated from bone marrow and adipose tissue. With the increased incidence of obesity in the U.S. and abroad, subcutaneous adipose tissue is abundant and readily accessible [11]. Approximately 400,000 liposuction surgeries are performed in the U.S. each year and these procedures yield anywhere from 100 ml to >3 liters of lipoaspirate tissue [12]. This material is routinely discarded. As discussed below, adipose-derived stem cells are multipotent and hold promise for a range of therapeutic applications.
Adipocytes develop from mesenchymal cells via a complex cascade of transcriptional and non-transcriptional events that occurs throughout human life. Stromal cells that have preadipocyte characteristics can be isolated from adipose tissue of adult subjects, propagated in vitro and induced to differentiate into adipocytes [13–16]. Adipocyte differentiation is a complex process accompanied by coordinated changes in cell morphology, hormone sensitivity and gene expression that have been studied primarily in murine preadipocyte cell lines rather than in human preadipocytes. This protocol describes primary in vitro culture of stromal cell isolated from either large or small quantities of human adipose tissue. While historically in the literature adipose-derived stromal cells have been termed “pre-adipocytes” [14, 15], there is a growing appreciation that they are multipotent, with chondrogenic, neurogenic and osteogenic capability [14, 15, 17–19].
A variety of names have been used to describe the plastic adherent cell population isolated from collagenase digests of adipose tissue. The following terms have been used to identify the same adipose tissue cell population: Adipose-derived Stem/Stromal Cells (ASCs); Adipose Derived Adult Stem (ADAS) Cells, Adipose Derived Adult Stromal Cells, Adipose Derived Stromal Cells (ADSC), Adipose Stromal Cells (ASC), Adipose Mesenchymal Stem Cells (AdMSC), Lipoblast, Pericyte, Pre-Adipocyte, Processed Lipoaspirate (PLA) Cells. The use of this diverse nomenclature has lead to significant confusion in the literature. To address this issue, the International Fat Applied Technology Society reached a consensus to adopt the term “Adipose-derived Stem Cells” (ASCs) to identify the isolated, plastic-adherent, multipotent cell population. The ASCs have been distinguished from the plastic adherent adult stem/progenitor cells from bone marrow originally referred to as fibroblastoid colony forming units, then in the hematological literature as marrow stromal, subsequently as mesenchymal stem cells, and most recently as multipotent mesenchymal stromal cells (MSCs).
2. Isolation Of Mesenchymal Stem Cells From Adipose Tissue
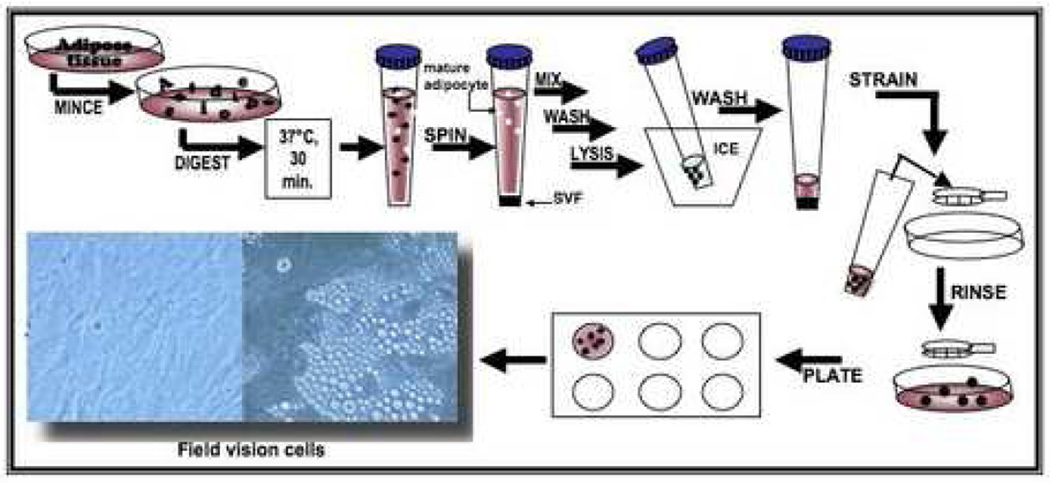
The initial methods to isolate cells from adipose tissue were pioneered by Rodbell and colleagues in the 1960s [20–22]. They minced rat fat pads, washed extensively to remove contaminating hematopoietic cells, incubated the tissue fragments with collagenase and centrifuged the digest, thereby separating the floating population of mature adipocytes from the pelleted stromal vascular fraction (SVF) (Figure 1). The SVF consisted of a heterogeneous cell population, including circulating blood cells, fibroblasts, pericytes and endothelial cells as well as “pre-adipocytes” or adipocyte progenitors [20–22]. The final isolation step selected for the plastic adherent population within the SVF cells, which enriched for the “pre-adipocytes”. Subsequently, this procedure has been modified for the isolation of cells from human adipose tissue specimens [23–27]. Initially, fragments of human tissue were minced by hand; however, with the development of liposuction surgery, this procedure has been simplified. During tumescent liposuction, plastic surgeons infuse the subcutaneous tissues with a saline solution containing anesthetic and/or epinephrine via a cannula and then remove both the liquid and tissue under suction [28]. The procedure generates finely minced tissue fragments whose size depends on the cannula’s dimensions. Independent studies have determined that liposuction aspiration alone does not significantly alter the viability of isolated SVF cells [29–31]. Indeed, adherent stromal cells with characteristics of adipocyte progenitors can be found directly within the liposuction aspiration fluid, as well as in SVF derived from the tissue fragment digests [32]. However, when ultrasound-assisted liposuction is performed, the number of cells recovered from tissue digests is reduced, as is their proliferative capacity [31]. The recovery of ASCs can be improved further by manipulating the centrifugation speed [33]. Investigators have achieved optimal cell recovery using a centrifugation speed of 1200 X g based on the subsequent formation of a human-derived adipose tissue depot following implantation in an immunodeficient murine model [33].
Figure 1.
Scheme for processing of adipose tissue and isolation of adipose-derived stem cells.
Adipose tissue is collected by needle biopsy or liposuction aspiration. The adipose sample can be kept at room temperature for no more than 24 hours prior to use (Figure 1). ASCs can be isolated from adipose tissue by first washing the tissue sample extensively with phosphate-buffered saline (PBS) containing 5% Penicillin/Streptomycin (P/S). Upon removal of debris, the sample is placed in a sterile tissue culture plate with 0.075% Collagenase Type I prepared in PBS containing 2% P/S for tissue digestion. Mince the adipose tissue sample using two scalpels and pipette the sample up and down with a 25 or 50 ml pipette several times to further facilitate the digestion. Incubate the sample for 30 min at 37 °C, 5% CO2, and then neutralize the Collagenase Type I activity by adding 5 ml of α-MEM containing 20% heat inactivated fetal bovine serum (FBS, Atlanta Biological, Atlanta, GA),) to the tissue sample. Pipette the sample up and down several times to further disintegrate aggregates of the adipose tissue. Upon disintegration, transfer the sample to a 50 ml tube, avoiding the solid aggregates. The stromal vascular fraction (SVF), containing the ASCs, is obtained by centrifuging the sample at 2000 rpm for 5 min. Take the samples out of the centrifuge and shake them vigorously to thoroughly disrupt the pellet and to mix the cells. This step completes the separation of the stromal cells from the primary adipocytes. Repeat the centrifugation step. After spinning, aspirate all the collagenase solution above the pellet without disturbing the cells. The pellet is then resuspended in 1 ml lysis buffer, incubated for 10 min on ice, washed with 20 ml of PBS/2% P/S and centrifuged at 2000 rpm for 5 min. The supernatant is aspirated, the cell pellet is resuspended in a maximum of 3 ml of stromal medium (alpha-MEM, Mediatech, Herndon, VA) supplemented with 20% FBS, 1% L-glutamine (Mediatech), and 1% penicillin/streptomycin (Mediatech) and the cell suspension is filtered through 70 µm cell strainer. It is important to note that all fetal bovine serum should be pre-screened prior to purchase for its ability to support both cell proliferation and adipocyte differentiation. Wash the cell strainer with an additional 2 ml of stromal medium to obtain any additional cells. Plate the sample containing the cells in a lysine coated culture plate and incubate at 37 °C, 5% CO2. Inoculate the cells in a single well of a 12 well plate for an amount of about 500 mg of adipose tissue or in a single well of a 24 well plate for an amount of 250 to 150 mg of adipose tissue.
3. Culture and Expansion of Adipose-derived Stem Cells
Seventy-two hours after plating, aspirate the entire medium from the wells. Again it is essential to note that the percent of preadipocytes obtained from the stromal vascular fraction after digestion is patient-dependent. If the SVF does not expand well, increase the FBS contained in the stromal medium to 25%; however this may promote premature adipogenesis. Signs of deterioration such as granularity around the nucleus, cytoplasmic vacuolations and/or detachment of the cells from the plastic surface may indicate inadequate or toxic medium, microbial contamination or senescence of the primary cells.
Wash the cells with prewarmed PBS (1% antibiotic can be added to the solution). Pipette the solution over the cell layer several times in order to clean the cells thoroughly from any tissue fragments and/or blood cells. Add a volume of fresh stromal medium according to the well capacity of the culture plate. The cells are maintained in a humidified tissue culture incubator at 37 °C with 5% CO2. Medium is changed every second day until the cells reach 80–90% confluence, and then there are two options: either harvest the cells or directly induce the adipocyte differentiation. For harvesting viable ASCs, add a small volume (250–500 µl) of sterile warm PBS to the wells and allow PBS to remain on cells for 2 min. Replace the PBS with 500 µl of Trypsin/EDTA solution (0.5%). Incubate in incubator for 5–10 min. Verify under microscope that more than 90% of the cells have detached and then add 500 µl of stromal medium to allow the serum contained in the solution to neutralize the trypsin reaction. Transfer the medium containing the suspended cells from the well to a sterile 2 ml tube. Centrifuge at 1200 rpm for 5 min. Aspirate the supernatant and suspend the cells in a small volume of stromal medium (~250 µl). Proceed to cell counting by taking an aliquot of cells diluted in trypan blue (for a 1:8 dilution: add 12.5 µl of suspended cells to 87.5 µl of trypan blue). Count cells using the hemocytometer. After counting, cells can then be replated according to the well capacity in adequate cell culture plates.
When expanded from a frozen vial, the cells are rapidly thawed at 37 °C and immediately seeded in a 28 cm2 or a 175 cm2 plate with complete stromal medium. Medium is changed the day after seeding and then every second day.
4. Freezing and Long-term Storage of Adipose-derived Stem Cells
ASCs should be harvested at 80–90% confluence for freezing. To collect cells, remove the culture medium and replace with a small volume of sterile, warm PBS. After two minutes, remove the PBS and replace with trypsin-EDTA solution. Incubate the culture dish at 37 °C for at least 5 minutes, or until approximately 90% of the cells have detached from the bottom of the dish. Progress can be monitored under a microscope; the treated cells will be rounded and floating. Add an equal volume of stromal medium to inactivate the trypsin, and transfer the suspension to a conical centrifuge tube. Centrifuge the tube at 1200 rpm for 5 minutes to pellet the cells. Carefully remove the supernatant, and resuspend the cell pellet in 1–2 ml of room temperature cryopreservation medium. Cryopreservation medium consists of 80% fetal bovine serum, 10% dimethylsulfoxide (DMSO) and 10% DMEM/Ham’s F-12, and should be used within two weeks of its preparation. Determine the cell concentration, and then dilute to a final concentration of 2 million viable cells per milliliter of cryopreservation medium. Aliquot the suspension into labeled cryovials, 1 ml/vial. Place the vials in an alcohol freezing container, and store at −80 °C overnight. The freezing container will cool the vials slowly, at approximately 1°C every minute, until they reach −80 °C. The next day, the frozen vials can be transferred to a liquid nitrogen container for long-term storage. Vials of cells should always be stored in the vapor phase of the LN2 tank.
5. Mesenchymal Differentiation Assays for Adipose-derived Stem Cells
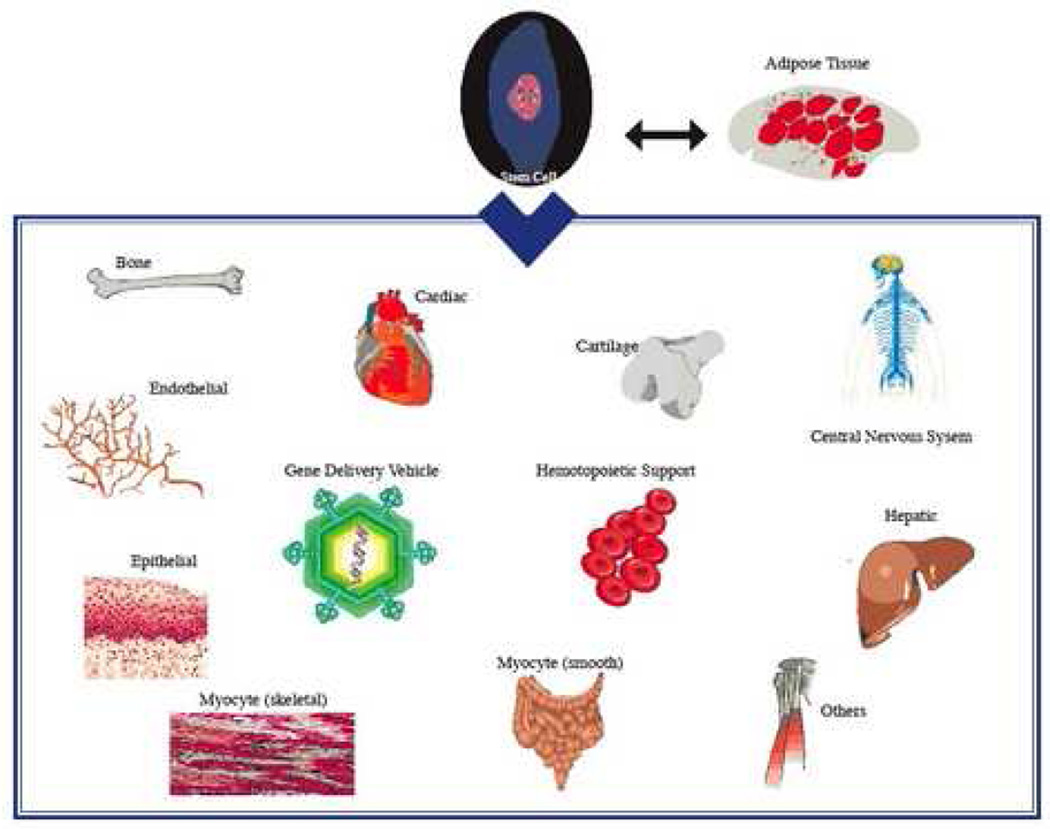
ASCs display multipotency, meaning they retain the ability to differentiate into cell types of multiple different lineages (Figures 2 and 3). The multipotency of these cells has generated interest in their potential therapeutic value for regenerative medicine. Differentiation of these cells can be directed by the addition of specific cocktails of chemical inducers or cytokines. The differentiation process is patient dependent. The age of the donor can be a factor, since some studies suggest that the differentiation capacity is higher in culture from younger subjects compared to older people.
Figure 2.
Multilineage differentiation potential for adipose-derived stem cells.
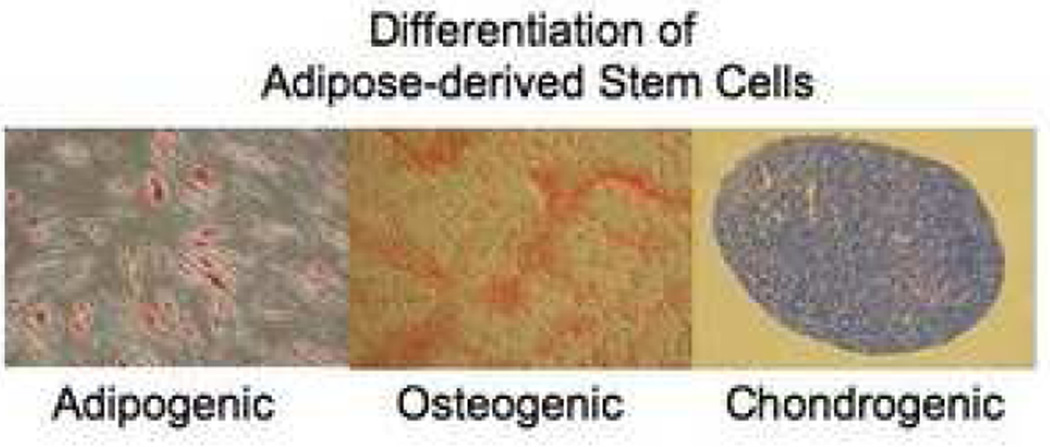
Figure 3.
Mesenchymal lineage differentiation assays for adipose-derived stem cells. Adipogenic differentiation: As the stem cells proliferate, some of these cells differentiate into preadipocytes. The preadipocytes undergo a second differentiation step and begin to fill with lipid. Lipid accumulates within the cell in small vacuoles, which appear as droplets. Osteogenic differentiation: As cells undergo osteogenic differentiation they proliferate rapidly and form tightly packed colonies. In some cases, these colonies give rise to dense nodules from which radiated highly elongated spindle-shaped cells with large nuclei. There are several methods to determine osteogenesis, such as demonstration of mineralization by staining with Alizarin red, measurement of alkaline phosphatase (AP) activity, level of calcium, and detection of lineage specific gene and protein regulations. Chondrogenic differentiation: Chondrogenesis occurs in three stages. Cartilage formation initiates when the dividing MSCs begin expressing extra cellular matrix proteins that tell them to condense into nodules. Cells in these nodules become chondrocytes and begin secreting the proteoglycans and collagen necessary for cartilage formation.
5.1. Adipogenic differentiation
When the cells reach between 80–90% confluence, the preadipocytes can be induced to differentiate. Aspirate the medium, add a small volume (about 1.5 ml for a 6 well plate) of prewarmed PBS + 1% antibiotic to wash the cells, and then remove the PBS by aspiration. Do not dry the well when changing the medium since adipocytes tend to float when new medium is added. Next, add the differentiation medium.
Adipogenic differentiation can be induced using culture medium supplemented with 0.5 mM isobutylmethylxanthine, 50 µM indomethacin and 0.5 µM dexamethasone. The adipocyte medium will be changed every 3 days until mature adipocytes are obtained After 12 days of differentiation, the cells can be fixed either using a 10% formalin solution or 70% ethanol. After removing the medium and washing the cells with PBS, immerse the cells in the fixative solution, either 10% formalin or 70% ethanol for 30 minutes or 1 hour respectively. Remove the fixative and air dry before staining or add water until performing the dye staining. The fixed cells can be stored at 4 °C or –20 °C for as long as several months. It is important to note that when using ethanol as the fixative, there is a risk that the lipids will be eluted from the cells.
The accumulation of neutral lipids can be detected by staining the cells in a solution of 0.5% Oil Red-O at room temperature for at least 60 min and can be performed outside of a biosafety hood. As an alternative, a two-step dye staining is performed by using a neutral lipid fluorescent dye (BODIPY, maximum fluorescence emission range is form 510 to 665 nm) and a nuclear fluorescent dye (DAPI, maximum excitation is about 360 nm and the emission maximum is 460 nm). All the procedures are performed at room temperature.
Immediately after drying the cells from the fixative solution or after removing the water from the wells, add 10 µg/ml BODIPY working solution and stain for 20 minutes. It is recommended to perform the staining in a semi-dark environment. After 20 minutes, remove all BODIPY solution and immediately wash with H2O several times. For the DAPI staining, remove all H2O from the wells and air dry the cells for 1–2 min. Add 300 nM DAPI working solution to the cells and counterstain for 20 min at room temperature.
The percentage of cells undergoing adipogenesis can be calculated by microscopic inspection. The number of cells in a field staining positive with BODIPY for lipid droplets can be determined as a percentage relative to the total number of cells in the field, i.e., the number of cells determined by positive staining with the DAPI nuclear stain.
If adipogenic differentiation does not occur efficiently you can further enhance adipogenesis using the following alternatives: (i) different PPARγ agonists (troglitazone, pioglitazone among others) can be added to the medium; (ii) 5% rabbit serum (RS) can be added to the differentiation medium to enhance differentiation (the ethyl acetate contained in the RS has been found 35-fold more abundant than in FBS); or (iii) another alternative would be to perform the addition of the differentiation medium multiple times after a three day rest period; i.e., 3 days on in the presence of the differentiation medium and three days off in the presence of the adipocyte medium [34]. Repeat this cycle until mature adipocytes are obtained.
5.2. Osteogenic differentiation
Osteogenesis can be induced using culture medium supplemented with 1 nM dexamethasone, 2 mM β-glycerolphosphate and 50 µM ascorbate-2-phosphate. The cells are induced in this medium for approximately 14 days and the osteogenic medium is replaced every 2–3 days. Mineralization is assessed by staining the cells with 40 mM Alizarin Red (pH 4.1) after fixation in 10% formalin.
5.3. Chondrogenic differentiation
For chondrogenic differentiation, the pellet culture system described by Sekiya et al. can be used [35]. The cell pellets are cultured in chondrogenic differentiation medium, which consists of high glucose DMEM supplemented with 500 ng/ml bone morphogenic protein-6, 10 ng/ml TGF-β3, 10−7 M dexamethasone, 50 µg/ml ascorbate 2-phosphate, 40 µg/ml proline, 100 µg/ml pyruvate and 50 mg/ml ITS + premix (Becton Dickinson: 6.25 µg/ml insulin, 6.25 µg/ml Transferrin, 6.25 ng/ml selenous acid, 1.25 mg/ml bovine serum albumin, 5.35 mg/ml linoleic acid). The medium is replaced every 2–3 days for 21 days. The pellets are then fixed in formalin, embedded in paraffin and sectioned. The sections can then be stained with Toluidine Blue.
6. Neural Differentiation of ASCs
Neurospheres are generated as described in Kang et al. [36]. Undifferentiated adipose stem cells cultured at high densities will spontaneously form spherical clumps of cells that can be isolated in 0.25% trypsin/2.21 mM EDTA. Free-floating neurospheres released from the cell culture surface into the culture medium can also be collected. The spheres of cells are transferred to an Ultra Low Cluster culture dish and cultured in Neurobasal medium supplemented with B27 (1:50), 20 ng/ml bFGF and 20 ng/ml EGF for 4–7 days. The culture density of the spheroid bodies should be maintained at 10–20 cells/cm2 to prevent self aggregation.
Neurospheres derived from adipose stem cells are layered on a Nunc tissue culture dish and maintained in Neurobasal medium containing only the B27 supplement. During differentiation, 70% of the medium is replaced every 3–4 days.
The induction of neural differentiation can be confirmed using RT-PCR, immunohistochemistry or Western blotting for the expression of neuronal or glial antigens. The expression the neuronal associated markers such as nestin, NeuN, intermediate filament, MAP2, β-III tubulin and glutamate receptor subunits NR1 and NR2or the oligodendrocyte marker, S-100 or glial fibrillary acidic protein (GFAP).
7. Conclusion
In summary, ASCs provide unique opportunities for investigating novel treatments for a vast array of inherited and acquired diseases. In addition, ASCs may also provide an opportunity to identify new molecular targets for drug discovery. In this review article, a series of detailed protocols for the isolation, characterization, expansion and differentiation of ASCs has been provided. These protocols can readily be adapted to adjust for differences in the size of the adipose tissue sample. Many important scientific and medical questions remain before the clinical application of ASCs in humans. These will include the development of large scale manufacturing methods with appropriate quality assurance and quality control to generate cells in compliance with current Good Manufacturing Practices (cGMP).
Acknowledgments
Financial Support. The research was supported by the National Center for Research Resources, National Institutes of Health, grant number RR00164, and a grant from the State of Louisiana Millennium Health Excellence Fund and the Louisiana Gene Therapy Research Consortium.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gimble JM. Expert Opin Biol Ther. 2003;3:705–713. doi: 10.1517/14712598.3.5.705. [DOI] [PubMed] [Google Scholar]
- 2.Wei G, Schubiger G, Harder F, Muller AM. Stem Cells. 2000;18:409–414. doi: 10.1634/stemcells.18-6-409. [DOI] [PubMed] [Google Scholar]
- 3.Woodbury D, Reynolds K, Black IB. J. Neurosci. Res. 2002;96:908–917. doi: 10.1002/jnr.10365. [DOI] [PubMed] [Google Scholar]
- 4.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal Ginard B, Silvestri F, Leri A, Beltrami CA. New Engl. Jour. of Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 5.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 6.Kotton DN, Fine A. Cytotherapy. 2003;5:169–73. doi: 10.1080/14653240310001073. [DOI] [PubMed] [Google Scholar]
- 7.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 8.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 9.Prockop DJ. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 10.Terskikh AV, Easterday MC, Li L, Hood L, Kornblum MK, Geschwind DH, Weissman IL. Proc. ’l. Acad. Sci. U.S.A. 2001;98:7934–7939. doi: 10.1073/pnas.131200898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bray GA. J Clin Endocrinol Metab. 2004;89:2583–2589. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 12.Katz AJ, Llull R, Hedrick MH, Futrell JW. Clin. Plast. Surg. 1999;26:587–603. [PubMed] [Google Scholar]
- 13.Rangwala SM, Lazar MA. Annu. Rev. Nutr. 2000;20:535–559. doi: 10.1146/annurev.nutr.20.1.535. [DOI] [PubMed] [Google Scholar]
- 14.Deslex S, Negrel R, Vannier C, Etienne J, Ailhaud G. Int.J.Obes. 1987;11:19–27. [PubMed] [Google Scholar]
- 15.Hauner H, Entenmann G, Wabitsch M, Gaillard D, Ailhaud G, R Negrel R, Pfeiffer EF. J. Clin. Invest. 1989;84:1663–1670. doi: 10.1172/JCI114345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halvorsen YD, Bond A, Sen A, Franklin DM, Lea-Currie YR, Sujkowski D, Ellis PN, Wilkison WO, Gimble JM. Metabolism. 2001;50:407–413. doi: 10.1053/meta.2001.21690. [DOI] [PubMed] [Google Scholar]
- 17.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Mol. Biol. Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guilak F, Lott KE, Awad HA, Cao Q, Hicok KC, Fermor B, Gimble JM. J. Cell. Physiol. 2006;206:229–237. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- 19.Gimble JM, Guilak F. Curr. Top. Dev. Biol. 2003;58:137–160. doi: 10.1016/s0070-2153(03)58005-x. [DOI] [PubMed] [Google Scholar]
- 20.Rodbell M. J Biol Chem. 1966;241:130–139. [PubMed] [Google Scholar]
- 21.Rodbell M. J Biol Chem. 1966;241:3909–3917. [PubMed] [Google Scholar]
- 22.Rodbell M, Jones AB. J Biol Chem. 1966;241:140–142. [PubMed] [Google Scholar]
- 23.Van RL, Bayliss CE, Roncari DA. J Clin Invest. 1976;58:699–704. doi: 10.1172/JCI108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjorntorp P, Karlsson M, Pertoft H, Pettersson P, Sjostrom L, U Smith U. J Lipid Res. 1978;19:316–324. [PubMed] [Google Scholar]
- 25.Deslex S, Negrel R, Vannier C, Etienne J, Ailhaud G. Int. J. Obes. 1987;11:19–27. [PubMed] [Google Scholar]
- 26.Hauner H, Entenmann G, Wabitsch M, Gaillard D, Ailhaud G, Negrel R, Pfeiffer EF. J. Clin. Invest. 1989;84:1663–1670. doi: 10.1172/JCI114345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hauner H, Wabitsch M, Pfeiffer EF. Horm Metab Res Suppl. 1988;19:35–39. [PubMed] [Google Scholar]
- 28.Illouz YG. Plast Reconstr Surg. 1983;72:591–597. doi: 10.1097/00006534-198311000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Moore JH, Jr, Kolaczynski JW, Morales LM, Considine RV, Pietrzkowski Z, Noto PF, Caro JF. Aesthetic Plast Surg. 1995;19:335–339. doi: 10.1007/BF00451659. [DOI] [PubMed] [Google Scholar]
- 30.Lalikos JF, Li YQ, Roth TP, Doyle JW, Matory WE, Lawrence WT. J. Surg. Res. 1997;1997:95–100. doi: 10.1006/jsre.1997.5090. [DOI] [PubMed] [Google Scholar]
- 31.Oedayrajsingh-Varma MJ, van Ham SM, Knippenberg M, Helder MN, Klein- Nulend J, Schouten TE, Ritt MJ, van Milligen FJ. Cytotherapy. 2006;8:166–177. doi: 10.1080/14653240600621125. [DOI] [PubMed] [Google Scholar]
- 32.Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki Y, Aiba-Kojima E, Sato K, Inoue K, Nagase T, Koshima I, Gonda K. J Cell Physiol. 2006;208:64–76. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- 33.Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki Y, Aiba-Kojima E, Sato K, Inoue K, Nagase T, Koshima I, Gonda K. J Cell Physiol. 2006;208:64–76. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- 34.Diascro DD, Vogel RL, Johnson TE, Witherup KM, Pitzenberger SM, Rutledge SJ, Prescott DJ, Rodan GA, Schmidt A. J Bone Miner Res. 1998;13:96–106. doi: 10.1359/jbmr.1998.13.1.96. [DOI] [PubMed] [Google Scholar]
- 35.Sekiya I, Colter DC, Prockop DJ. Biochem Biophys Res Comm. 2001;284:411–418. doi: 10.1006/bbrc.2001.4898. [DOI] [PubMed] [Google Scholar]
- 36.Kang SK, Putnam LA, Ylostalo J, Popescu IR, Dufour J, Belousov A. J. Cell Sci. 2004;117:4289–4299. doi: 10.1242/jcs.01264. [DOI] [PubMed] [Google Scholar]