Abstract
After a heart attack, patients often undergo a procedure to open up the clogged artery and install a tiny meshlike device called a stent to keep the artery propped open. In most cases, the body reacts to this foreign object with scar-tissue formation, and the artery narrows again. To combat this re-clogging process, National Institutes of Health inventors developed paclitaxel-coated stents and later licensed it to Angiotech. Approved by the Food and Drug Administration in March 2004, these stents are expected to substantially reduce the use of coronary artery bypass surgery, an expensive operation now performed annually on 350,000-plus Americans. This and three other examples of NIH licensing success stories are described in this paper: (a) Kepivance, which improves the quality of life for cancer patients by eliminating mouth sores, (b) AIDS drug ddI, an important component of many combination drug therapies, and (c) Vitravene, the first and only antisense drug to be approved by FDA. These four examples will illustrate the success not only of the NIH licensing program, but also the innovative approaches taken by NIH inventors and the persistence of its commercial partners. This paper also highlights the business and legal lessons learned from these four cases.
Introduction
In the last seventeen years (FY1988 to FY2004), the National Institutes of Health, Office of Technology Transfer (OTT), has executed more than 2,500 licenses resulting in more than 200 biomedical products. Of these, about twenty products belong to the category of lifesaving drugs and vaccines.1 From that smaller group, this paper highlights four cases. These four stories will not only illustrate the success of the NIH licensing program, but also the innovative approaches of the scientists making the discoveries and the persistence of the NIH commercial partners. Indeed, licensing success stories are rare because of the complexities, technical hurdles, expense of development, and other substantial obstacles that need to be overcome when converting an early-stage invention into a useful biomedical product.
At NIH, we have realized that there is a need to measure success in the same vocabulary as the mission, that is, to look at how many lives have been impacted, how many unmet medical needs have been met, how many research and clinical breakthroughs have been spawned, and how many other similar public-health goals were achieved. To ascertain the public-health impacts of the products resulting from the licensing of NIH intramural inventions, it is important that the measures used be not purely numerical, such as the number of lives saved, the number of doses used, or the reduction in mortality or morbidity, but also include qualitative metrics such as the unique or novel nature of the treatment or product, its first-of-a-kind nature, the improvement in quality of life afforded by the treatment, or the development of new research findings or products.
Choosing which four case studies to feature in this paper was difficult, but we hope they exemplify the nonmonetary, hard-to-measure metric of benefiting human health against which all nonprofit research institutions try to measure their work. As described below, these benefits include: reduction in hospitalization, mortality, overall health-care costs, and side-effects, and dramatic improvement in the quality of life for a group of patients for whom there was no alternative but to suffer the consequences of their current treatment. Also, the choice of these cases was deliberately made to capture the diversity of diseases that NIH addresses and medical products that find their way into the market through academic discoveries.
Paclitaxel-Coated Stents: A Way to Bypass Surgery
This particular case study is a unique example wherein the combination of two existing products, paclitaxel (drug) and stents (device), proved valuable to treating a disease—coronary disease.
For several years, stents (tiny mesh tubes made out of soft but sturdy metals) have been used in surgical procedures to prop open arteries after the blood vessels have been cleared of blockages by balloon angioplasty. Unfortunately, scar tissue often forms near the implanted stent that can cause the artery to re-clog or restenose in approximately 10 percent to 40 percent of patients.
In one of the latest surgical technology revolutions, cardiac stents are now being coated with paclitaxel to produce dramatically better outcomes for patients. Paclitaxel is perhaps best known in the form offered by Bristol-Myers Squibb (BMS) as the drug Taxol.
“Taxol embedded in a polymer on the stent itself modifies the healing process, so that scar tissue does not build,” explained John Groetelaars, vice president for Boston Scientific Canada, a leading developer of drug-eluting stents. The polymer has a time-release mechanism, so that the drug can be dispensed or eluted into the tissue nearby. This has dramatically reduced restenosis rates to just 3 percent to 6 percent, meaning far fewer return visits to the catheterization lab or operating room for cardiac patients.2 (See figure 1.)
Figure 1.

Cardiovascular Stents
Taxol, originally discovered in the 1960s, and its equivalents are currently the most successful anticancer drugs on the market.3 However, nobody thought of using paclitaxel to prevent arterial re-clogging until, over lunch, NIH inventors Steven Sollott, MD, and James Kinsella, MD, brainstormed this very idea. According to Dr. Kinsella, the idea was to use Taxol at a lower concentration than used for cancer treatment so that it did not kill cells but only modified their activity. The experiments were initiated, proof of concept was shown in rat models, and a patent application was filed. An exclusive license was awarded to Angiotech Pharmaceuticals Inc., a company involved in similar research. After the license agreement was signed between NIH and Angiotech, there were several instances where both parties had to show openness and flexibility. As a new class of products that combined multiple components, the actual royalty base was a matter of debate. Was this device a combination of two components, the drug and the stent, or did the polymer coating that aided in the slow release of the drug from the stent constitute a third component? Should the selling price of the combination product be split two ways or three ways?
Additionally, at the time the license was signed, the actual outcome of the patent prosecution had not yet been determined, and that had to be taken into account while managing the agreement. Also, as Angiotech moved forward with the product development, it had to acquire additional patent rights from third parties in an unexpected manner. At each step of the way, the chief executive officer of Angiotech, who himself was an inventor on some of the technologies that went into Taxus Express, acted as a product and business champion. During the time of product development, the company also entered into sublicensing deals with Boston Scientific and Cook Inc., among others. Boston Scientific later received exclusive worldwide rights for the Taxus stents in the field of coronary disease and developed it into a commercial product, while Cook has continued with the development of other paclitaxel-eluting products. Interestingly, later sublicensees actually opened up areas of product development that were not anticipated in the original discovery.
Subsequently, Angiotech established a cooperative research and development agreement with NIH to add additional collaborative strength to support the license agreement. Growing a single product into what became a platform, required enormous dedication on the part of Angiotech to meet all the postlicense challenges, and NIH showed its flexibility and dedication to the project by ensuring that the legal and business hurdles were solved amicably between the partners. Taxus Express was approved for sale in Europe in January 2003 and in the United States in March 2004. The Food and Drug Administration’s prior approval of paclitaxel as chemotherapy was critical in facilitating approval of the paclitaxel-stent combination product because the drug’s safety was already well-established. Market approval was made easier because the stent incorporated the active agent from an approved drug such as Taxol rather than an investigational drug.
These stents are expected to substantially reduce coronary artery bypass surgery, an expensive, highly invasive operation now performed on more than 350,000 Americans a year. In addition, paclitaxel-coated stents are finding use in peripheral organs such as the colon.
Keratinocyte Growth Factor: Reducing Costs and Increasing the Quality of Life for Cancer Patients
This is a story of how an intriguing development in a research laboratory many years ago ended up improving the quality of life for cancer patients undergoing treatment—often nearly as important as the therapy itself.
Oral mucositis (painful sores and ulcers in the lining of the mouth) is a common side effect of many types of cancer therapies. Chemotherapy and radiotherapy target and destroy rapidly dividing tumor cells, which also results in major damage to the rapidly dividing cells that comprise the tissues lining the mouth and throat. Oral mucositis can be extremely painful and have a devastating impact on patients. It can make patients’ everyday activities, such as eating, drinking, swallowing, and talking, difficult or impossible. Patients suffering from these debilitating mouth sores may require longer hospitalization, high doses of narcotics such as morphine, and intravenous feeding to receive nutrition and maintain hydration.
Until recently, there were no approved drugs available to prevent oral mucositis. Discovery of keratinocyte growth factor (KGF) led to a breakthrough in this field, a first of its kind demonstrating a clinically meaningful benefit in preventing or curing oral mucositis.4, 5 Palifermin, a manmade version of KGF, like the natural KGF, stimulates cell growth on the surface layer of the mouth. The theory is that this leads to faster replacement of these cells when killed by cancer treatments and speeds up the healing process of mouth ulcers.
The motivation that led to the discovery of KGF resided in the conventional wisdom that many cancers owe their origins and growth to hormones and growth factors. In 1989, NIH scientists discovered a growth factor that they named KGF.6 However, contrary to its hypothesized role, it soon became clear that KGF was not the villain promoting tumors. Its unique sequence and high degree of specificity to epithelial cells led the inventors to file an invention report. At that time, KGF seemed a very promising molecule with several possible medical applications. The scientists appreciated that publishing their results would assist public health at one level, but seeking patent protection was important because it would allow a company to develop the product commercially and, thus, open doors for eventual use in patients. Neither the inventors nor the licensees knew at that time in which direction the clinical development of the molecule would take them. It took almost sixteen years of commitment, hard work, persistence, and ingenuity from scientists at NIH and Amgen to convert this invention into a clinical application.
Amgen, a company working in the field of chemotherapy, approached NIH to license the technology. Knowing that Amgen had worked with other growth factors such as platelet-derived growth factor and granulocyte colony stimulating factor and that KGF would fit well in its portfolio, NIH granted the company an exclusive license in 1992. Once the license agreement was in place, NIH and Amgen scientists committed themselves to overcome the difficulties in the clinical development path of KGF. Amgen showed its commitment to the molecule in the persona of a dedicated clinical director who championed the development of KGF, also now known by its drug name Kepivance,7 throughout its life. In a larger company with multiple products and conflicting priorities, the importance of a product champion cannot be underestimated. This is especially true in a case such as Kepivance, where not only was the development journey long, but the target itself was not eminently clear until much later.
The choice of Amgen as a partner was based not only on its scientific capabilities and commitment, but also by the willingness to invest resources into an early-stage technology. While several other companies signed a commercial evaluation license with NIH, none was willing to make the material for testing purposes. This would have placed an enormous burden on the NIH lab to produce the required large quantities of the material. Therefore, Amgen’s commitment to scaling up production of the protein for all the downstream development made the company a clear winner for the exclusive commercial license to the technology. On the way to product development, while there were many technical challenges, there were also eureka moments and turning points that finally foreshadowed the development of KGF as a significant advance in cancer therapy. In December 2004, the FDA approved KGF/palifermin for reduction of the incidence and duration of severe oral mucositis in patients with hematological cancers undergoing bone marrow/blood cell transplantation.8 The use of Kepivance may significantly reduce medical costs through the prevention or reduction of oral mucositis in this patient population. Kepivance may also enable patients to undergo full doses of treatment, acquire fewer infections, and/or reduce their time in the hospital.
The strength of the collaboration between Amgen and NIH scientists also is illustrated by the fact that, despite the departure of two inventors from NIH during this period, the project moved forward without missing a beat. The scientific collaborations forged at the time of signing the license continue to this day as the researchers try to expand the use of this valuable drug to other cancers. Jeffrey Rubin, PhD, MD, the lead inventor, hopes that KGF will establish a place for itself in the cancer armamentarium. He also hopes that KGF finds a clinical use in many of the other settings where it is being tested. These include solid cancers, such as colorectal, head and neck, and lung, where substantial radiation damage to the oral cavity takes place. More than any other benefit, as improved treatments transform cancer from an acute life-threatening disease to a chronic disease, agents like KGF pave the way for new drugs that will allow patients to enjoy a better quality of life during their remaining years.
Videx: From Mono to Combination Therapy and beyond in the Fight against HIV
When AIDS was first identified in America in 1981, no one could predict that it would devastate millions of lives. And no one could foresee the enormous contributions that collaborations among industry, academia, and government laboratories would make in muzzling this killer disease. Highly active antiretroviral therapy (HAART) combination drug therapy has revolutionized HIV treatment, bringing hope to millions of sufferers. HAART has reduced the number of AIDS deaths by 70 percent in recent years.9 Today, there are four classes of anti-HIV drugs and nineteen different drugs that can be used in a HAART regimen.10 Videx, which initially was the only way to delay disease progression, has now become the backbone of many lifesaving HAART regimens. With HAART, the incidence of AIDS in the United States dropped dramatically from 33.4 per 100,000 people in 1994 to 17.2 in 2000, and the number of deaths each year has fallen dramatically. (See figure 2.)
Figure 2.
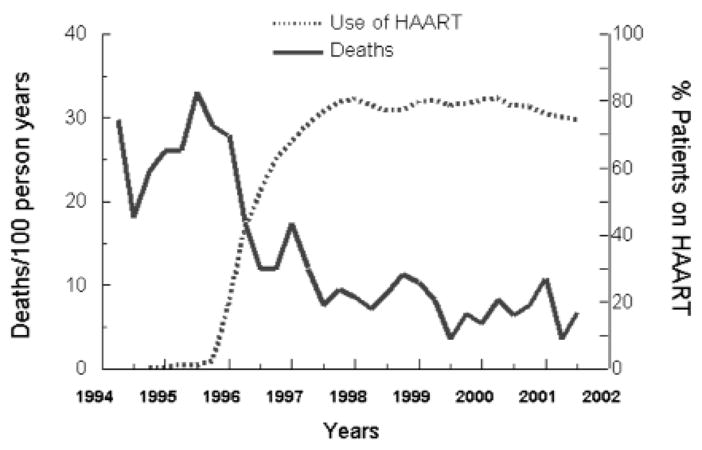
HAART Therapy and Mortality Rates
In early 1980, NIH scientists Robert Yarchoan, MD, Hiroaki Mitsuya, MD, PhD, and Samuel Broder, MD, ventured into this totally unknown arena by screening for compounds that could suppress HIV in tissue cultures. Suramin, the first drug tested to inhibit HIV activity, was followed by zidovudine (AZT). However, the initial hope brought by AZT disappeared as patients rapidly developed resistance to its therapeutic effect.11 As the epidemic progressed, new hope emerged in the laboratory, only to be dashed when experimental results were applied in the clinic. While the AZT studies were being performed, NIH scientists were also studying other compounds, including nucleoside analogs such as ddA, ddC, ddG, and ddI. Of all these compounds, ddI (didanosine) appeared most promising. It belongs to a class of compounds called nucleoside/tide reverse transcriptase inhibitors that interfere with the activity of an enzyme necessary for HIV to reproduce.
Lacking any drug company support, toxicity studies were never done, but the National Cancer Institute served as a pharmaceutical company and moved ddI rapidly to clinical testing in patients. Scientists soon realized that monotherapy was beset with problems of viral resistance, and the concept of combinational therapy was widely adopted. Although the concept of multiple-drug therapy had been used before in cancer and pain management, the HAART combination showed the sheer power of this concept most dramatically. Figure 2 shows the unmistakable decrease in mortality that correlated closely with an increase in use of combination therapy.
After much of ddI’s discovery and development had been done using federal funding, NIH sought to license its patents to a commercial entity that could market this drug to AIDS patients. The technology transfer challenge was to negotiate a license that would provide a strong incentive for a drug company to make the significant investment in getting this drug into the market while ensuring the long-term public health benefits of the NIH contributions. A balance was struck by offering a license that was initially exclusive, but which could become nonexclusive after ten years of commercial sales and prior to the expiration of the remaining terms of the NIH patents.
In 1988, NIH awarded Bristol-Myers Squibb (BMS) an exclusive license to these technologies. As a result of the substantial clinical development that had already been completed by NIH when the license with BMS was signed and NIH’s continued commitment to the project, the FDA approval of Videx (ddI) materialized fairly quickly. The rapid approval was also due to FDA’s willingness to fast track drug approval in urgent circumstances such as AIDS. In fact, the approval was so rapid that the therapeutic was approved before the United States Patent and Trademark Office allowed the patent claims for the underlying technology.
When the license was originally signed with BMS, the company received ten years of commercial exclusivity to the NIH technology. Due to the rapid FDA approval of the product, several more years of patent life remained on the product after the exclusivity period expired. Therefore, NIH was able to sign additional nonexclusive licenses with other drug manufacturers (mainly generic companies) that clearly resulted in lower prices and greater worldwide availability of this important AIDS drug. The timeline in the sidebar contains additional details about this case.
When the FDA approved Videx in 1991, it was highly atypical. First, it was unusual for a drug to be approved and launched simultaneously with both adult and pediatric indications and dosing. Secondly, Videx was an important test case for a new FDA approval process for speeding drugs to market in urgent circumstances, such as HIV. In partnership with BMS, the National Institute of Allergy and Infectious Diseases clinical trials group participated in the rapid testing of Videx. BMS provided supplemental funds for the key clinical trials used for FDA approval and operated an expanded access program that provided free drugs to more than 23,000 people prior to FDA approval.12 The safety data collected from the expanded access program helped speed FDA approval. FDA accepted early evidence of the effectiveness of Videx, without the full data set that it normally requires, and approved Videx before the clinical studies were complete. The Videx approval process now serves as a model for the FDA’s accelerated approval regulation, published the year after Videx was approved.
Videx was a difficult drug to develop, in part because the active ingredient is destroyed by stomach acid. The original formula used chewable tablets that were not only large and fragile but also foul tasting and caused diarrhea. BMS did substantial work to develop a tablet formulation that would protect the active ingredient without causing side effects. BMS continued to work on this drug after FDA approval and made additional discoveries, including the development of an extended release formulation, Videx EC. This was the first once-a-day HIV medication used in combination HIV therapy.13
Today, there is no doubt that the NIH investment in Videx and other anti-HIV compounds has transformed HIV from a certain killer to a chronic, but serious, disease. This work also has encouraged the design of drugs that target different phases of the HIV lifecycle, and similar methods are now being applied to combat other viral diseases.
Vitravene: Antisense and the Emerging Area of Nucleic Acid Medicine
Antisense agents and RNA interference (RNAi) represent a new era of pharmacology, where the receptor is the RNA and the drug is the oligonucleotide. Antisense technology is the use of oligonucleotides (small nucleotide molecules similar to DNA) that bind to RNA sequences through Watson-Crick hybridization, resulting in blocking of target RNA and subsequent protein synthesis.14 Commercialization of Vitravene, the first and only (to date) FDA-approved antisense therapeutic, was a major advance.
Vitravene, a therapeutic that treats AIDS-related cytomegalovirus retinitis (CMV-R), is administered by periodic localized injections into the infected eye. The drug binds to a complementary CMV mRNA sequence and stops the production of the undesirable proteins. The resulting double-stranded complex is recognized by an enzyme, which leads to the degradation of the viral mRNA, but the Vitravene molecule remains intact and targets more CMV mRNA for destruction.
Scientists at NIH and FDA collaborated to create a totally new paradigm for oligonucleotide synthesis, the use of sulphur-substituted phosphorothioates as DNA backbones. This synthesis was rather difficult, but FDA researchers automated this process to achieve reliable, high yields of these oligonucleotides. With the AIDS epidemic gaining public attention, NIH and FDA scientists began trying several compounds to address AIDS and AIDS-related infections. The original phosphorothioate oligonucleotides had both specific and nonspecific effects, indicating more than one mechanism of action. This created a lot of excitement for two reasons. First, these new antisense oligonucleotides could be tailor-made for specific treatments, and, second, they were radically different from other types of therapeutics.
In the late 1980s and early 1990s, NIH filed for patents for the oligonucleotides and the sulphurization process. Commercial interest in the technology ebbed and flowed as antisense technology was beginning to mature and competing companies entered the field. Of the many companies in this field, only Isis Pharmaceuticals has been able to stay the course sufficiently to have a product approved by the FDA.
Isis received a nonexclusive license from NIH to commercialize the technology for CMV-R. From 1990 to 1996, Isis achieved significant milestones: the synthesis and screening of more than 10,000 phosphorothioate anti-sense oligo molecules, new methods of manufacturing and analysis, new animal models, and the completion of all three phases of clinical trials. Perhaps the most important overall milestone was accomplished by bringing Vitravene to market ahead of schedule.
In 1997, Isis entered a distribution agreement with Novartis, and, a year later, Vitravene received FDA approval. Since then, no other antisense drug has won FDA approval. From start to finish, Vitravene took only eight years to develop and reach the market, which is quite exceptional. Fortunately, the incidence of CMV-R has shown a dramatic reduction with the advent of HAART regimens. However, this eliminated the market for Vitravene. Isis has made substantial contributions to the antisense field with 1,500 issued patents for antisense technology (including RNAi) and more than 4,000 patients enrolled in clinical trials.
Since the days of Vitravene with the first-generation phosphorothioate oligonucleotides, the antisense field has continued to evolve with second-and third-generation technologies.15 NIH and FDA served as catalysts, but Isis paved the way for a new generation of antisense therapeutics. Currently, there are more than thirty clinical trials using antisense technology, with a new antisense therapeutic perhaps just around the corner.
Discussion
While these four case studies confirm that each product-development journey is unique with its own set of hurdles and challenges, several common themes emerge. Remarkably enough, all of the technologies described above have gone through license agreement amendments or re-negotiations, often multiple times, as the technology environment changed. To survive the long product-development journey associated with any successful biomedical product, the underlying license agreement truly must be a living document.
For instance, in case of Videx, when the ten-year exclusivity of the BMS license expired, NIH was able to sign additional nonexclusive licenses with other drug manufacturers that resulted in increased competition, lower prices, and greater worldwide availability of this lifesaving drug.
Moreover, the patent estate for the Videx technology was quite extensive and complicated. There were several formulation and methods-of-use patents, in addition to the composition-of-matter application. These myriad patents had been issued at different times, with some still pending ten years after FDA approval of the product, leading to some conflicting interpretations of the license language. Subsequently, NIH settled the dispute, but also revised the language in its model license agreements to be clear that product royalty obligations are based on the scope of a claim rather than its infringement.
Vitravene, based on a new concept in pharmacology that stimulated many potential applications, also had a similar story of license-agreement changes over its history. At one point early in its development, the technology had four co-exclusive licensees, each without the right to sublicense. NIH contemplated adding two more co-exclusive licensees, causing a dispute that had to be resolved by license amendment. In a co-exclusive license, if the maximum number of licensees is not defined, the transaction becomes a de facto nonexclusive license and its value to individual licensees greatly diminishes.
Another challenge was a direct result of carving out different co-exclusive licenses for the technology based on different application areas. For example, the right to contract manufacture antisense compounds with NIH’s technology was licensed to companies that never fully developed the capacity to do it in conjunction with other licensees, and that too created a problem. The product development was delayed and became more expensive due to the lack of economies of scale. Clearly, licenses for platform technologies that may have far-reaching consequences in multiple application areas must be drafted with care. There also must be enough flexibility built into the licensing agreement to provide intellectual property protection for new-product developers (either through new licenses or sublicenses), without stunting developments in other related fields.
Furthermore, depending on how fashionable the field is, the valuation of the technology can fluctuate wildly, which, in turn, can affect the licensing terms. In the case of Vitravene, where such a scenario clearly applied, the requirement placed upon NIH to have pre-set terms for new licensees turned out to be self-defeating, because then the valuation placed on the technology did not match the eventual market conditions. Clearly, successful license agreements are a result of active management by both parties. They cannot simply be treated as a piece of paper that is filed and forgotten once signed.
Even after product launch, licensees can face many problems that affect the ultimate success of the commercial product. Adverse drug reactions; product recalls; patent or legal problems, such as infringement, litigation costs, and interferences, are just some of the potential challenges.
Vitravene is a particularly telling example. The rise of HAART therapy using a combination of drugs such as Videx for treating AIDS patients reduced the market for the product to virtually nothing. While on one level Vitravene remains a medical and scientific success story, Isis has been unable to recover its cost of development.
For any technology transfer program to be successful, the value of developing and maintaining strong relationships with scientists cannot be underestimated. For instance, at the time of KGF’s discovery, it was not at all clear what the end product would be or which patient population would benefit. As a new protein that could potentially cause cancer, KGF was initially seen as a drug discovery target or tool, and NIH was not keen on the expenses of worldwide patent protection. This is where the inventors’ strength of conviction intervened, and they acquired the foreign rights from NIH and filed in a number of foreign countries. Amgen signed parallel licenses with NIH and the inventors, and it is clear now that there is a worldwide market for this drug.
This underscores the importance of a passionate inventor in converting inventions into successful biomedical products. Experienced scientists realize that, while they are very familiar with the requirements for publishing their results, the actual process of technology transfer is distinct and can sometimes be quite daunting. “Seeking patent protection, marketing the technology, negotiating licenses, and staying committed to the process of technology transfer and commercialization through the long road can be quite challenging,” said Rubin, one of the inventors of Kepivance. Rubin also remarked that savvy inventors recognize this and stay committed to the process. “At the end of it all, the process can be very rewarding, especially when you see the discovery being applied to the betterment of fellow humans,” he commented.
An ideal licensee is a true partner. Just like the inventors and technology transfer staff, licensees often do not know the final direction that clinical development of the technology will take. This highlights the importance of providing patent protection for early-stage inventions with diagnostic or therapeutic potential, even when this may be based upon limited insight. Unless there are players willing to commit and play the high-stakes technology investment game, many valuable inventions could go begging. In the case of Videx, for instance, no company was willing to step forward at the early stages, so NIH took it upon itself to pursue the early clinical trials. How frequently can that happen? Not often. Licensing is truly a team sport and all the players need to participate to have a successful game.
Research and Development Timeline
1964
Synthesis of ddA reported
1980
Conversion of ddA to ddI reported
1985
NCI scientists identify activity of ddI against HIV
August 1985 – April 1993
NIH patents filed
1987
First meeting of National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group
January 1988
Bristol-Myers Squibb license to develop ddI signed
July 1988 – October 1989
Clinical trials begin phase I/II and phase II/III
August 1989 – April 1997
NIH patents 4,861,759, 5,026,687, 5,254,539, 5,376,642 and 5,616,566 issued
October 1991
FDA approves Videx
1991
First commercial sale
October 2001
License for NIH patents becomes nonexclusive
Figure 3.

Vitravene treats AIDS-related eye infections.
Acknowledgments
The authors are thankful to Bonny Harbinger for critical review of the manuscript and helpful discussions. We would also like to acknowledge Ansalan Stewart and Lisa Rovin for their work on developing some of the case studies presented here. Finally, we would like to acknowledge the numerous conversations we have had with NIH inventors, licensing specialists, and company personnel while preparing this review.
Contributor Information
Ruchika Nijhara, Postdoctoral fellow at the National Cancer Institute, however, she contributed to this work during a technology transfer fellowship at the National Institutes of Health in Bethesda, Maryland.
J. Lille Tidwell, Recently left a fellowship position at NIH to become a patent examiner at the U.S. Patent and Trademark Office in Arlington, Virginia.
Steven Ferguson, Director of the Division of Technology Development and Transfer, in Rockville, Maryland.
Krishna Balakrishnan, Marketing group leader at the NIH Office of Technology Transfer, in Rockville, Maryland.
Notes
- 1.National Institutes of Health Office of Technology Transfer. www.ott.nih.gov.
- 2.Gersohn K. New Developments in Cardiac Stents Lead to Improved ‘Keyhole’ Surgeries. Canadian Healthcare Technology. 2005 May; [Google Scholar]
- 3.U.S. Government Accounting Office. Technology Transfer: NIH-Private Sector Partnership in the Development of Taxol: GAO Report 03-829. Jun, 2004. http://www.gao.gov/new.items/d03829.pdf.
- 4.Garfunkel AA. Oral Mucositis—The Search for a Solution. New England Journal of Medicine. 2004:2649–2651. doi: 10.1056/NEJMe048239. [DOI] [PubMed] [Google Scholar]
- 5.Spielberger RP, et al. Palifermin for Oral Mucositis after Intensive Therapy for Hematologic Malignancies. New England Journal of Medicine. 2004;351:1590–1598. doi: 10.1056/NEJMoa040125. [DOI] [PubMed] [Google Scholar]
- 6.Rubin JS, et al. Purification and Characterization of a Newly Identified Growth Factor Specific for Epithelial Cells. Proceedings of the National Academy of Sciences USA. 1989;86:802–806. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amgen FDA Approves Kepivance for Severe Oral Mucositis in Cancer Patients Undergoing Bone Marrow Transplant; Pivotal Phase 3 Study Published in This Week’s. New England Journal of Medicine. 2004 Dec 15; press release. [Google Scholar]
- 8.Garfunkel Oral Mucositis—The Search for a Solution. doi: 10.1056/NEJMe048239. [DOI] [PubMed] [Google Scholar]
- 9.UNAIDS/World Health Organization. AIDS Epidemic Update. 2002 Dec; [Google Scholar]
- 10.“HIV Cases Climb Among Gay, Bisexual Men in US,” National News, The CDC HIV, STD, TB Prevention News Update, July 28, 2003.
- 11.HIV Trialists’ Collaborative Group. Zidovudine, Didanosine, and Zalcitabine in the Treatment of HIV Infection: Meta-Analyses of the Randomized Evidence. Lancet. 1999;353:2014–2025. [PubMed] [Google Scholar]
- 12.Bristol-Myers Squibb Co., Annual Report 1991, 2.
- 13.VidexEC. http://www.videxec.com.
- 14.Crooke ST. Progress in Antisense Technology. Annual Reviews in Medicine. 2004;55:61–95. doi: 10.1146/annurev.med.55.091902.104408. [DOI] [PubMed] [Google Scholar]
- 15.Kurreck J. Antisense Technologies: Improvement through Novel Chemical Modifications. European Journal of Biochemistry. 2003;270:1628–1644. doi: 10.1046/j.1432-1033.2003.03555.x. [DOI] [PubMed] [Google Scholar]


