Abstract
Patent foramen ovale (PFO) is highly prevalent and associated with more than 150,000 strokes per year. Traditionally, it is thought that PFOs facilitate strokes by allowing venous clots to travel directly to the brain. However, only a small portion of PFO stroke patients have a known tendency to form blood clots, and the best treatment for this multi-organ disease is unclear. Therefore, mapping the changes in systemic circulation of PFO-related stroke is crucial in understanding the pathophysiology in order to individualize the best clinical treatment for each patient. We initiated a study using a novel quantitative, Two-Pass discovery workflow using high-resolution LC-MS/MS coupled with label-free analysis to track protein expression in PFO patients before and after endovascular closure of the PFO. Using this approach, we were able to demonstrate quantitative differences in protein expression between both PFO-related and non PFO-related ischemic stroke groups as well as before and after PFO closure. As an initial step in understanding the molecular landscape of PFO-related physiology, our methods have yielded biologically relevant information on the synergistic and functional redundancy of various cell-signaling molecules with respect to PFO circulatory physiology. The resulting protein expression patterns were related to canonical pathways including prothrombin activation, atherosclerosis signaling, acute phase response, LXR/RXR activation and coagulation system.
In particular, post PFO closure, numerous proteins demonstrated reduced expression in stroke-related canonical pathways such as acute inflammatory response and coagulation signaling. These findings demonstrate the feasibility and robustness of using a proteomic approach for biomarker discovery to help gauge therapeutic efficacy in stroke.
Keywords: proteomics, biomarker, discovery, stroke, cerebrovascular disease, ischemic stroke, patent foramen ovale, PFO, mass spectrometry
Introduction
Patent foramen ovale (PFO) is an independent stroke risk factor, but its best treatment is not clear (Figure 1) (1, 2). Highly prevalent (25-30% of the general population), PFO is often discovered only after a stroke and is associated with more than 150,000 strokes per year (3-25). Traditionally, it is thought that PFOs facilitate paradoxical embolism by allowing venous clots to travel directly to the brain. However, there is a significant disconnect between this simple mechanism and clinical data, as less than approximately 15% of PFO stroke patients have a known tendency to form venous clots (1, 2). Clinical trials to investigate treatment options are ongoing, but since individual risks vary and preferred treatment is likely not one-size-fits-all, controversies regarding PFO remain unresolved (26-35). In part, this is due to a poor understanding of the molecular landscape of PFO-related neurovascular injury (2, 36).
Figure 1.
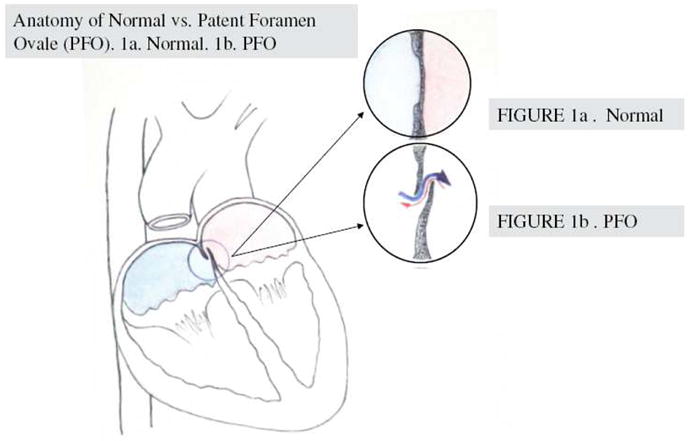
Anatomy of normal versus PFO. Normally, the septum separates the left and right chambers of the heart. PFO is a congenital condition whereby the opening between the left and right atrial chambers fails to close after birth. 1a. Normal septum; 1b. PFO
Since PFO is a complex, multi-organ disease involving the brain, heart and circulation, we initiated a study using mass spectrometry (MS) to follow blood protein expression in PFO patients before and after endovascular closure of the PFO. Clinical endovascular closure of PFO provides a rare bedside model in which to study the effects of a specific mechanical intervention on circulatory protein signaling, both immediately and over time (37).
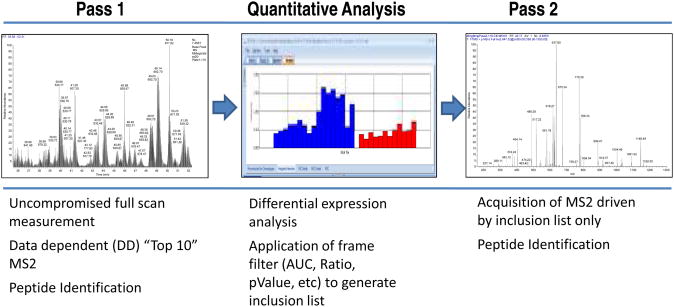
Although mass spectrometry has been applied to biomarker discovery for at least a decade, one of the most difficult problems has been the interpretation and ranking of putative biomarkers derived from differential expression LC-MS/MS experiments (38). Limitations of the classical approaches that depend on data-dependent MS acquisition from complex peptide mixtures include lack of rigorous quantification and independent parameters for evaluating the “usefulness” of a particular biomarker. In addition, the high dynamic range of clinical samples, such as plasma and serum, effectively limits protein identification of low-abundance proteins when data-dependent MS acquisition methods are used, since only the highest abundance proteins are identified repeatedly (39). Previous approaches have incorporated physical fractionation of protein samples to dig deeper into the proteome (40). However, albumin depletion and offline fractionation (such as cation exchange) often result in protein losses, greatly increased instrument run time and expense (41). In addition, rigorous quantification becomes increasingly difficult when sample preparation is so complex. As an improvement to these approaches, we have developed a Two-Pass, quantitative discovery workflow that includes application of expression trend ratios and ROC analyses for the efficient evaluation and scoring of putative biomarkers (Figure 2) (42). The Two-Pass approach couples very accurate, full-scan quantification with MS/MS acquisition driven by an inclusion list generated from analysis of the full scan data. Application of the inclusion list for MS/MS acquisition essentially uses the mass spectrometer to “fractionate” the sample, and results in the increased identification of lower abundance and clinically useful biomarkers. In addition, because multiple physical fractions do not need to be analyzed by MS, more clinical samples can be analyzed in the same amount of time, allowing for better statistics and evaluation of biological variability. Using this approach, we were able to demonstrate clear, quantitative differences in protein expression across the various sample groups and relate the expression patterns to relevant biological pathways. Here we utilize this Two-Pass, quantitative discovery workflow to explore circulatory protein expression in stroke patients before and after endovascular closure of the PFO.
Figure 2.
Diagram of the Two Pass workflow.
Left panel: full scan MS spectrum.
Center panel: Screen capture of SIEVE analysis illustrating differential expression of a frame.
Right panel: Fragmentation spectrum of a targeted mass from inclusion list generated from SIEVE differential analysis.
Materials and Methods
Clinical serum samples
A total of 64 plasma samples were obtained from stroke patients and healthy controls with similar risk factors from the Cardio-Neurology Clinic of Massachusetts General Hospital in accordance with IRB approval. PFO-related “cryptogenic ischemic strokes” were identified by two vascular board certified neurologists. Rigorous inclusion/exclusion criteria were applied to ensure proper diagnosis in each group, and all patients underwent the following testing to rule out other etiology related to ischemic embolic infarct: 1) physical exam by a vascular neurologist to document clinical syndrome consistent with ischemic infarct; 2) MRI/MRA and CTA to document ischemic infarct and to rule out other reasons of infarct such as intracranial stenosis, large vessel occlusion, dissection; 3) transthoracic and/or transesophageal echocardiogram to assess and document and presence of PFO and rule out atrial/ventricular thrombus or any valvular lesions that may be related to embolism; 4) extended cardiac monitoring to rule out atrial fibrillation or other cardiac arrhythmia. In addition other ischemic stroke subtypes such as lacunar infarct related to hypertension, vasculitis, endocarditis, or venous infarction were excluded. Also excluded were all patients with active infection, active pregnancy, or renal/liver failure, as these conditions may alter protein signaling profiles. Moreover, patients without baseline imaging such as CT or MRI needed to confirm clinical stroke syndromes, were excluded. Controls were recruited from subjects with similar baseline risk factors to match the study population. Subject demographics (N=14 PFO-related ischemic stroke, N=7 non PFO-related cryptogenic ischemic stroke and N= 18 control) are listed in Table 1. Patients were consecutively, prospectively enrolled post ischemic stroke in accordance with the approval of institutional IRB.
Table 1. Patient Clinical Characteristics and Sample Collection: PFO-related vs Non PFO- related vs Normal experiment.
| Characteristic | PFO-related (N=14) |
Non PFO-related (N=7) |
Normal (N=18) |
|---|---|---|---|
| Age (mean and range) | 46 (26-55) |
45 (25-53) |
44 (22-56) |
| Gender | 50%M | 57%M | 50%M |
| Race | 100% Caucasian | 100% Caucasian | 94% Caucasian |
| Hyperlipidemia | 14% | 14% | 11% |
| Hypertension | 7% | 0 | 5% |
| Diabetes | 0 | 0 | 0 |
| Residual right to left shunting post PFO closure on cardiac echo | 0 | N/A | N/A |
Standard operating procedures were strictly observed for all samples. All personnel were trained with SOP to process samples in the same fashion, as follows: blood from venous or atrial source was collected into EDTA-coated tubes and immediately processed (within 5 min) to obtain plasma by centrifugation at 3400 RPM for 15minutes at 20°C (to avoid platelet activation), removing the plasma supernatant without disturbing the clot, aliquoted immediately, and frozen at −80° C to ensure minimal protein degradation.
Samples were carefully transported frozen to be processed at the same time, in random order, by investigators blinded to the clinical data to avoid bias and batch variations.
Trypsin digestion, Reduction/Alkylation and Desalting
Plasma samples (25uL) were thawed on ice and processed as previously described (43).
High resolution LC-MS/MS
Liquid Chromatography and High-resolution Mass Spectrometry
As shown in Figure 1, the Two-Pass workflow strategy consists of the separate optimization of MS parameters and configuration for protein quantification and identification.
Pass 1. Plasma samples (500 ng of human samples), were prepared as described above and injected onto a Thermo Scientific Easy nLC system configured with a 10 cm × 100 um trap column and a 25 cm × 100 um ID resolving column. The sample load was optimized for optimum quantification (i.e. full scan data). Buffer A was 98% water, 2% methanol, and 0.2% formic acid. Buffer B was 10% water, 10% isopropanol, 80% acetonitrile, and 0.2% formic acid. Samples were loaded at 4 uL/min for 10 min, and a gradient from 0-45% B at 375 nL/min was run over 130 min, for a total run time of 150 min (including regeneration and sample loading). The Thermo Scientific LTQ Orbitrap Velos mass spectrometer was run in a standard Top-10 data-dependent configuration, except that a higher trigger-threshold (20K) was used to ensure that the MS2 did not interfere with the full-scan duty cycle. This ensured optimal full-scan data for quantification. MS2 fragmentation and analysis were performed in the ion trap mass analyzer.
Data analysis and Pass 2
Proteomics data analysis was performed using Thermo Scientific SIEVE™ software version 2.0 that features chromatographic alignment, framing, differential ROC, ratio and trend analyses. Both Top-10 data-dependent scans and full-scan data were analyzed with the SIEVE™ software using chromatographic alignment followed by feature extraction using unsupervised statistical techniques including isotope deconvolution. Based upon various criteria including ROC AUC, low ratios, high ratios, high abundance, low abundance or trend ratios, an inclusion list was created for the best differentially expressed candidates. This inclusion list was used exclusively for MS2 acquisition in Pass 2. A larger sample load was used in the Pass 2 runs (600 ng to 800 ng), allowing for higher quality MS2 spectra. Because these full-scan spectra would not be used for quantification, peak shape and intensity reproducibility were not crucial. Fragmentation scans from Pass 2 were analyzed using SEQUEST™ and FDR analysis to make identifications. The fragmentation search results from Pass 2, and the quantitative information obtained from Pass 1, were combined and further analyzed using the SIEVE software.
Ingenuity Pathways (IPA) Data analysis
The differential expression results were uploaded and analyzed using IPA http://www.ingenuity.com/products/pathways_analysis.html, according to the manufacturer's instructions.
Results
Two Pass Workflow
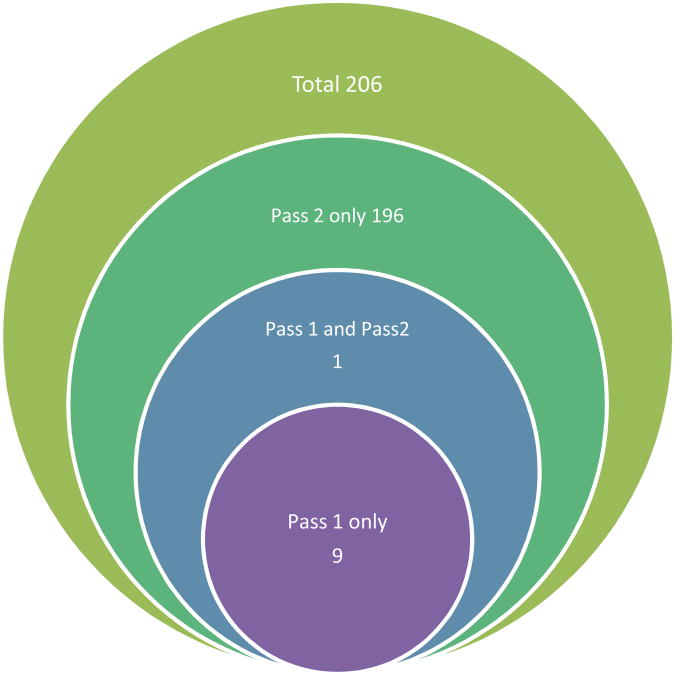
Figure 2 shows a graphical representation of the workflow that was applied to the clinical samples. This approach was recently developed in our laboratory to improve quantification by de-coupling it from protein/peptide identification(42). During Pass 1, parameters are optimized for acquisition of full-scan data with an emphasis on chromatographic reproducibility and robust spray. MS2 spectra are only acquired in a “Top 5 or 10” data-dependent manner so full scan measurements are not compromised. This approach makes high-quality relative quantification possible, even in complex samples such as plasma, but also provides a limited list of MS2 spectra for identification of high-abundance proteins. The full scan data are analyzed and differentially expressed features fulfilling a desired pattern filter are compiled into a target inclusion list for acquisition in Pass 2. Since the mass spectrometer only acquires data from masses that are on the target list (as opposed to default data-dependent acquisition), less intense (and therefore less abundant) masses may be triggered to produce fragmentation spectra for identification. Using this approach, no fractionation or depletion was necessary to identify proteins at intensities in the picomolar range (42). Figure 3 shows the distribution of protein IDs in Pass 1 and Pass 2 data that passed the filter criteria. As is evident in the figure, the majority of proteins, 95%, were uniquely identified in Pass 2 with 0.4% uniquely identified in Pass 1 data. Only one protein fulfilling the filtering criteria was identified in both Pass1 and Pass 2 data.
Figure 3.
Number of identified proteins using the selected frame filter for Pass 2.
Filter syntax: Ratio of PFOstroke/Normal > 1.5* Ratio of NonPFOstroke/Normal AND Ratio of NonPFOstroke/Normal > Ratio of Normal/Normal
Differential protein expression in PFO-related stroke samples versus non-PFO-related stroke samples
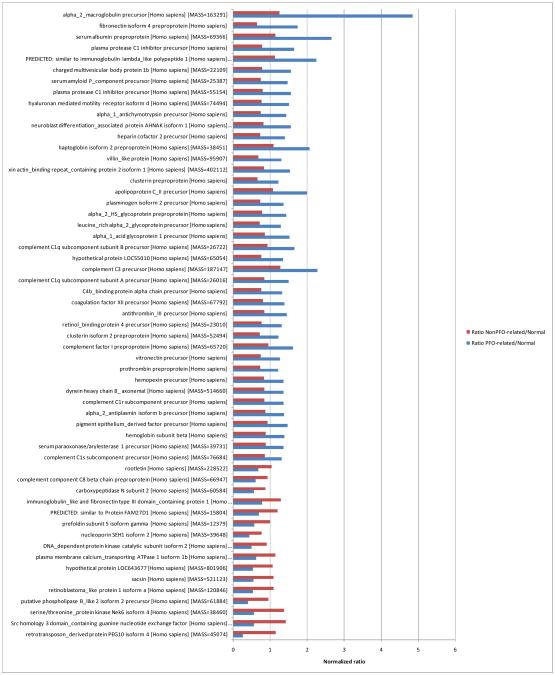
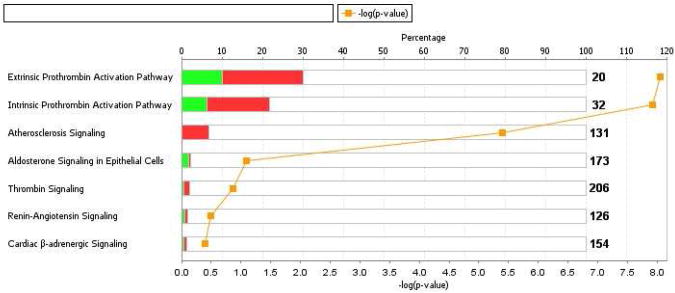
When the Two-Pass workflow was applied to the PFO-related stroke, non-PFO-related stroke and normal (non stroke) samples in Table 1 in a trend analysis, a striking differential expression pattern was evident (Figure 4). In order to identify which proteins were differentially expressed between PFO-related and Non-PFO-related stroke, we applied the following expression filter to the complete data set at the protein level [Normalized ratio PFO-related/Normal>1.2 AND Normalized ratio PFO-related/Normal<0.6]. The resulting data were further analyzed and only proteins with at least 2 peptides and the 1%FDR and P-value<0.01 stringency parameters were retained. The resulting collection of 57 proteins, shown in Figure 4, provides an initial list of biomarker candidates differentiating the PFO-related and non-PFO-related sample groups. The proteins with the highest expression ratios in PFO-related vs non-PFO-related samples, (ratio > 2.0) were alpha2 macroglobulin, serum albumin, immunoglobulin lambda, and complement C3 precursor. Conversely, the proteins with the highest expression ratios in Non PFO-related vs PFO-related samples were serine/threonine protein kinase and Src homology 3 domain containing guanine nucleotide exchange factor. We further analyzed this protein dataset with Ingenuity Pathways Analysis (IPA) to determine canonical pathways that were significantly associated with the dataset. Figure 5 shows that several pathways including extrinsic and intrinsic prothrombin activation, atherosclerosis signaling, aldosterone signaling, renin-angiotensin signaling, cardiac beta-adrenergic signaling and thrombin signaling pathways had highly significant (P-value <0.001) overlap with the PFO-related and non-PFO-related putative marker dataset. The highest overlap was with extrinsic prothrombin activation where 2 of 20 proteins had decreased and 4 of 20 proteins had increased expression versus the experimental dataset (Figure 5 and Supplementary Table 1).
Figure 4.
Differentially expressed proteins (Pvalue <0.01) in PFO stroke samples vs Non PFO. stroke samples. Protein search stringency parameters: FDR <1%, peptides>1.
Figure 5.
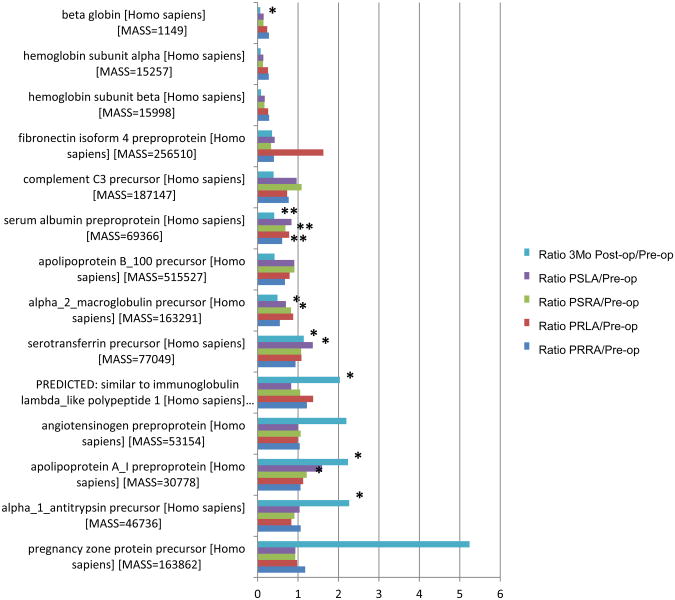
Protein expression patterns before, during and after PFO endovascular closure. As described in the methods, longitudinal blood samples were taken from a cohort of 4 patients upon admission, from left and right chambers immediately before and after closure and at 3 month follow up.
A. Proteins with increased expression levels (ratio >1.2, Pvalue <0.01) in 3 month follow up post-closure venous plasma (relative to pre-closure venous plasma) samples. Corresponding expression levels of PSLA, PSRA, PRLA and PRRA plasma for each patient are also shown.
B. Proteins with decreased expression levels (ratio <0.5, Pvalue<0.01) in 3 month follow up post-closure venous plasma (relative to pre-closure venous plasma) samples. Matched expression levels of PSLA, PSRA, PRLA and PRRA plasma for each patient are also shown.
Differential protein expression before, during and after PFO endovascular closure in PFO-related stroke samples
In order to investigate the protein expression pattern related to endovascular closure of PFO in stroke, we analyzed the complete set of longitudinal samples from 14 patients described in Table 2. The matched samples were obtained from patient baseline pre-endovascularlar PFO closure venous blood (Preop), from the left (PRLA) and right (PRRA) atria immediately before endovascular closure, from the left (PSLA) and right (PSRA) atria immediately after endovascular closure and venous blood at 3 months follow up. We applied the following filter to the protein data obtained from the SIEVE trend analysis:
Table 2. Clinical Characteristics and Sample Collection: PFO endovascular closure longitudinal samples (N=14 patients).
| Code | Description |
|---|---|
| PreOp | Pre closure, venous |
| PRvenRA | Pre closure, right atrium |
| PRartLA | Pre closure, left atrium |
| PSvenRA | Post closure, right atrium |
| PSartLA | Post closure, left atrium |
| 3MoFU | 3 month Follow up, venous |
Ratio of 3 Month Follow up/Preop > 1.5 OR < 0.6.
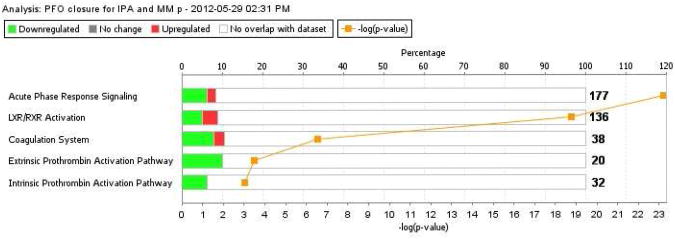
Only proteins represented by at least two peptides were allowed. The resulting protein expression pattern is shown in Figure 6. There was considerable variability in P-value across the matched samples but several proteins were significantly (P-value <0.05) differentially expressed, including beta globin, serum albumin, alpha 2 macroglobulin, serotransferrin, similar to immunoglobulin lambda like polypeptide 1, apolipoprotein AI and alpha 1 antitrypsin precursor. When we applied IPA canonical pathway analysis to the PFO closure dataset, several pathways were correlated with high significance (P-value < 0.0001) (Fig 7).The significant pathways included acute phase response signaling, LXR/RXR activation and extrinsic and intrinsic prothrombin activation pathways. Contrasting with the results from the previous (PFO-related stroke vs non-PFO-related stroke) IPA analysis, numerous proteins in the PFO closure dataset demonstrated reduced expression post-PFO closure with respect to the canonical pathways such as acute inflammatory response, cholesterol and coagulation signaling (Supplementary Table 2).
Figure 6.
Protein expression pattern before, during and after PFO endovascular closure.
* Pvalue < 0.05
** Pvalue < 0.001
Figure 7.
IPA canonical pathway analysis of PFO closure dataset.
P-value <0.0001
Discussion
As an initial step in understanding the molecular landscape of PFO-related physiology, our methods have yielded biologically relevant information on the synergistic and functional redundancy of various cell-signaling molecules with respect to PFO circulatory physiology. In particular, post-PFO closure, numerous proteins demonstrated reduced expression in stroke-related canonical pathways such as acute inflammatory response, LXR/RXR, and coagulation signaling. These findings demonstrate the feasibility and robustness of using the Two-Pass approach for biomarker discovery in a clinical model with patients as their own controls to gauge therapeutic efficacy of PFO endovascular closure.
PFO-related neurovascular injury certainly has a major impact on public health, and the high prevalence of PFO emphasizes the importance of proper prevention, treatment and management in at-risk individuals (1, 5-9, 11, 12, 20-25, 36, 44). In addition to paradoxical embolic strokes, PFOs are present in more than 60% of all migraine-with-aura patients, and 20-30% of the general population (45-51). But who should be screened to prevent future strokes? What therapeutic targets and options are best for stroke and migraine patients? This is likely a multi-organ disease dependent on interaction between the brain, heart and lungs – but with potential connection through circulatory signaling in blood. For example, we found that Serotonin (5-HT), a vasoactive prothrombotic substance that induces cardiac oxidative stress and a neurotransmitter carefully regulated and inactivated by the lung, is decreased post percutaneous closure of PFO – supporting the hypothesis that chronic right-to-left shunting may allow harmful vasoactive mediators to escape deactivation by the pulmonary vasculature (37, 52, 53). This increase in the brain vasculature's direct exposure to various vasoactive mediators may elevate the risk of cerebrovascular injury (37). Results of the current study support our earlier findings. In this study, the plasma signal change pre and post PFO closure suggest that PFO with large-venous-to arterial shunting may allow for harmful circulatory factors to travel directly from the venous (right atrial, deoxygenated) to the arterial (left atrial, oxygenated) side, bypassing important deactivation by the lung – potentially triggering a pro-inflammatory, procoagulant state. Conversely, obliterating this shunting by PFO closure seems to alter circulatory phenotype into a less procoagulant and proinflammatory state.
These data are hypothesis-generating and further studies in larger cohorts are needed to investigate and confirm individual factors. While this is a very small sample of patients, we made every effort to include patients with similar age, risk factor, and co-morbidities. PFO-related stroke patients are known to have fewer conventional vascular risk factors, which make them ideal for proteomic studies. The study design of obtaining pre and post procedure samples helps to minimize confounders by utilizing each individual patient's pre-op baseline as their own control. In addition, all patients in this cohort are on the same dose of 325mg of aspirin throughout the study. And all baseline blood and endovascular closure samples were remote from the time of stroke (>3 months), so that the changes we found in acute inflammatory factors over time were not related to decrease in acute inflammatory response over time. Migraine patients were excluded from this study so that the molecular mechanism of migraine would not confound findings in this study for PFO-related stroke. However, we are actively investigating this subpopulation, since migraine subtypes in specific patient populations are related to PFO and stroke. There were also no changes in any other medications throughout the longitudinal followup, such that no new confounders were introduced except for the PFO closure procedure itself. However, different treatment options, such as anticoagulation and antiplatelets, are needed to compare therapeutic efficacy in the future.
Proteomic profiling studies with respect to long term clinical outcome, such as recurrent TIA or stroke, comparison of various antiplatelet and anticoagulation treatments, and of patients with concurrent migraines are important and under way. In summary, we find that proteomic exploration can help to identify targets of PFO-related neurovascular disease in the context of a largely unexplored territory of circulatory signaling with the potential to triage and monitor therapeutic efficacy.
Acknowledgments
This work was supported by the NIH/NINDS: R01 NS067139 (MMN) and P01-NS55104 (EHL).
Abbreviations
- LC
liquid chromatography
- MS/MS
tandem mass spectrometry
- SPE
solid phase extraction
- ESI
electrospray ionization
- ACN
acetonitrile
- m/z
mass to charge ratio
- SRM
selective reaction monitoring
- apo
apolipoprotein
- LLOD
lower limit of detection
- LOQ
limit of quantitation
- FPR
false positive rate
- ROC
receiver operating characteristic
- AUC
area under the curve
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calvert PA, Rana BS, Kydd AC, Shapiro LM. Patent foramen ovale: anatomy, outcomes, and closure. Nat Rev Cardiol. 2011;8:148–160. doi: 10.1038/nrcardio.2010.224. [DOI] [PubMed] [Google Scholar]
- 2.Ning M, Lo EH, Inglessis I, Demirjian Z, Ning P, Dec G, Palacios I, Buonanno F. The Brain's Heart - Therapeutic Opportunities for Patent Foramen Ovale and Neurovascular Disease. Pharmacology and Therapeutics. 2012 doi: 10.1016/j.pharmthera.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kunitz SC, Gross CR, Heyman A, Kase CS, Mohr JP, Price TR, Wolf PA. The pilot Stroke Data Bank: definition, design, and data. Stroke. 1984;15:740–746. doi: 10.1161/01.str.15.4.740. [DOI] [PubMed] [Google Scholar]
- 4.Foulkes MA, Wolf PA, Price TR, Mohr JP, Hier DB. The Stroke Data Bank: design, methods, and baseline characteristics. Stroke. 1988;19:547–554. doi: 10.1161/01.str.19.5.547. [DOI] [PubMed] [Google Scholar]
- 5.Sacco RL, Ellenberg JH, Mohr JP, Tatemichi TK, Hier DB, Price TR, Wolf PA. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol. 1989;25:382–390. doi: 10.1002/ana.410250410. [DOI] [PubMed] [Google Scholar]
- 6.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 7.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke. 1999;30:2513–2516. doi: 10.1161/01.str.30.12.2513. [DOI] [PubMed] [Google Scholar]
- 8.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001;32:2735–2740. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 9.Lee BI, Nam HS, Heo JH, Kim DI. Yonsei Stroke Registry. Analysis of 1,000 patients with acute cerebral infarctions. Cerebrovasc Dis. 2001;12:145–151. doi: 10.1159/000047697. [DOI] [PubMed] [Google Scholar]
- 10.Grau AJ, Weimar C, Buggle F, Heinrich A, Goertler M, Neumaier S, Glahn J, Brandt T, Hacke W, Diener HC. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke. 2001;32:2559–2566. doi: 10.1161/hs1101.098524. [DOI] [PubMed] [Google Scholar]
- 11.Schulz UG, Rothwell PM. Differences in vascular risk factors between etiological subtypes of ischemic stroke: importance of population-based studies. Stroke. 2003;34:2050–2059. doi: 10.1161/01.STR.0000079818.08343.8C. [DOI] [PubMed] [Google Scholar]
- 12.Schneider AT, Kissela B, Woo D, Kleindorfer D, Alwell K, Miller R, Szaflarski J, Gebel J, Khoury J, Shukla R, Moomaw C, Pancioli A, Jauch E, Broderick J. Ischemic stroke subtypes: a population-based study of incidence rates among blacks and whites. Stroke. 2004;35:1552–1556. doi: 10.1161/01.STR.0000129335.28301.f5. [DOI] [PubMed] [Google Scholar]
- 13.Mohr J, Choi WC, Grotta JC, et al. Stroke: Pathophysiology, Diagnosis, and Management. Churchill Livingstone; New York: 2004. [Google Scholar]
- 14.Agmon Y, Khandheria BK, Meissner I, Gentile F, Whisnant JP, Sicks JD, O'Fallon WM, Covalt JL, Wiebers DO, Seward JB. Frequency of atrial septal aneurysms in patients with cerebral ischemic events. Circulation. 1999;99:1942–1944. doi: 10.1161/01.cir.99.15.1942. [DOI] [PubMed] [Google Scholar]
- 15.Pearson AC, Nagelhout D, Castello R, Gomez CR, Labovitz AJ. Atrial septal aneurysm and stroke: a transesophageal echocardiographic study. J Am Coll Cardiol. 1991;18:1223–1229. doi: 10.1016/0735-1097(91)90539-l. [DOI] [PubMed] [Google Scholar]
- 16.Mattioli AV, Aquilina M, Oldani A, Longhini C, Mattioli G. Atrial septal aneurysm as a cardioembolic source in adult patients with stroke and normal carotid arteries. A multicentre study. Eur Heart J. 2001;22:261–268. doi: 10.1053/euhj.2001.2293. [DOI] [PubMed] [Google Scholar]
- 17.Cabanes L, Mas JL, Cohen A, Amarenco P, Cabanes PA, Oubary P, Chedru F, Guerin F, Bousser MG, de Recondo J. Atrial septal aneurysm and patent foramen ovale as risk factors for cryptogenic stroke in patients less than 55 years of age. A study using transesophageal echocardiography. Stroke. 1993;24:1865–1873. doi: 10.1161/01.str.24.12.1865. [DOI] [PubMed] [Google Scholar]
- 18.Webster MW, Chancellor AM, Smith HJ, Swift DL, Sharpe DN, Bass NM, Glasgow GL. Patent foramen ovale in young stroke patients. Lancet. 1988;2:11–12. doi: 10.1016/s0140-6736(88)92944-3. [DOI] [PubMed] [Google Scholar]
- 19.Lechat P, Mas JL, Lascault G, Loron P, Theard M, Klimczac M, Drobinski G, Thomas D, Grosgogeat Y. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148–1152. doi: 10.1056/NEJM198805053181802. [DOI] [PubMed] [Google Scholar]
- 20.Di Tullio M, Sacco RL, Gopal A, Mohr JP, Homma S. Patent foramen ovale as a risk factor for cryptogenic stroke. Ann Intern Med. 1992;117:461–465. doi: 10.7326/0003-4819-117-6-461. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics -- 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 22.Hara H, Virmani R, Ladich E, Mackey-Bojack S, Titus J, Reisman M, Gray W, Nakamura M, Mooney M, Poulose A, Schwartz RS. Patent foramen ovale: current pathology, pathophysiology, and clinical status. J Am Coll Cardiol. 2005;46:1768–1776. doi: 10.1016/j.jacc.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 23.Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59:17–20. doi: 10.1016/s0025-6196(12)60336-x. [DOI] [PubMed] [Google Scholar]
- 24.Meissner I, Whisnant JP, Khandheria BK, Spittell PC, O'Fallon WM, Pascoe RD, Enriquez-Sarano M, Seward JB, Covalt JL, Sicks JD, Wiebers DO. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. Stroke Prevention: Assessment of Risk in a Community. Mayo Clin Proc. 1999;74:862–869. doi: 10.4065/74.9.862. [DOI] [PubMed] [Google Scholar]
- 25.Pinto FJ. When and how to diagnose patent foramen ovale. Heart. 2005;91:438–440. doi: 10.1136/hrt.2004.052233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sacco RL, Di Tullio MR, Homma S. Treatment of patent foramen ovale and stroke: to close or not to close, that is not yet the question. Eur Neurol. 1997;37:205–206. doi: 10.1159/000117443. [DOI] [PubMed] [Google Scholar]
- 27.Nendaz MR, Sarasin FP, Junod AF, Bogousslavsky J. Preventing stroke recurrence in patients with patent foramen ovale: antithrombotic therapy, foramen closure, or therapeutic abstention? A decision analytic perspective. Am Heart J. 1998;135:532–541. doi: 10.1016/s0002-8703(98)70332-1. [DOI] [PubMed] [Google Scholar]
- 28.Donnan GA, Davis SM. Patent foramen ovale and stroke: closure by further randomized trial is required! Stroke. 2004;35:806. doi: 10.1161/01.STR.0000117965.15684.D3. [DOI] [PubMed] [Google Scholar]
- 29.Furlan AJ. Patent foramen ovale and recurrent stroke: closure is the best option: yes. Stroke. 2004;35:803–804. doi: 10.1161/01.STR.0000117963.58978.40. [DOI] [PubMed] [Google Scholar]
- 30.Tong DC, Becker KJ. Patent foramen ovale and recurrent stroke: closure is the best option: no. Stroke. 2004;35:804–805. doi: 10.1161/01.STR.0000117964.10781.BA. [DOI] [PubMed] [Google Scholar]
- 31.Ferguson T, Sansing LH, Herrmann H, Cucchiara B. To close or not to close: PFO, sex and cerebrovascular events. J Invasive Cardiol. 2006;18:E292–293. [PubMed] [Google Scholar]
- 32.Windecker S, Meier B. Is closure recommended for patent foramen ovale and cryptogenic stroke? Patent foramen ovale and cryptogenic stroke: to close or not to close? Closure: what else! Circulation. 2008;118:1989–1998. doi: 10.1161/CIRCULATIONAHA.107.757013. [DOI] [PubMed] [Google Scholar]
- 33.Kent DM, Thaler DE. The Risk of Paradoxical Embolism (RoPE) Study: developing risk models for application to ongoing randomized trials of percutaneous patent foramen ovale closure for cryptogenic stroke. Trials. 2011;12:185. doi: 10.1186/1745-6215-12-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, Felberg R, Herrmann H, Kar S, Landzberg M, Raizner A, Wechsler L. Closure or Medical Therapy for Cryptogenic Stroke with Patent Foramen Ovale. NEJM. 2012;366:991–999. doi: 10.1056/NEJMoa1009639. [DOI] [PubMed] [Google Scholar]
- 35.Johnston SC. Patent Foramen Ovale Closure. NEJM. 2012;366:1048–1050. doi: 10.1056/NEJMe1201173. [DOI] [PubMed] [Google Scholar]
- 36.Ning M, Palacios I, Demirjian Z, Inglessis I, Dec G, Ning P, Lo EH, Buonanno F. The Brain's Heart - Therapeutic Opportunities for Patent Foramen Ovale and Neurovascular Disease. Pharmacology and Therapeutics. 2012 doi: 10.1016/j.pharmthera.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ning M, Navaratna D, Demirjian Z, Inglessis-Azuaje I, McMullin D, Dec GW, Palacios I, Buonanno F, Lo EH. How the Heart Whispers to the Brain: Serotonin as Neurovascular Mediator in Patent Foramen Ovale Related Stroke. Stroke. 2011;42:e108. [Google Scholar]
- 38.Yates JR, Ruse CI, Nakorchevsky A. Proteomics by mass spectrometry: approaches, advances, and applications. Annu Rev Biomed Eng. 2009;11:49–79. doi: 10.1146/annurev-bioeng-061008-124934. [DOI] [PubMed] [Google Scholar]
- 39.Becker CH, Bern M. Recent developments in quantitative proteomics. Mutat Res. 722:171–182. doi: 10.1016/j.mrgentox.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brewis IA, Brennan P. Proteomics technologies for the global identification and quantification of proteins. Adv Protein Chem Struct Biol. 80:1–44. doi: 10.1016/B978-0-12-381264-3.00001-1. [DOI] [PubMed] [Google Scholar]
- 41.Yadav AK, Bhardwaj G, Basak T, Kumar D, Ahmad S, Priyadarshini R, Singh AK, Dash D, Sengupta S. A systematic analysis of eluted fraction of plasma post immunoaffinity depletion: implications in biomarker discovery. PLoS One. 6:e24442. doi: 10.1371/journal.pone.0024442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vogelsang M, A M, Sarracino DA, Prakash A, Krastins B, Garces A, Vadali G, Sutton JN, Peterman S, Lopez MF. A quantitative, Two Pass workflow for biomarker discovery that can identify pictogram amounts of proteins and peptides in biofluids without prior depletion or fractionation. Nat Methods In press. [Google Scholar]
- 43.Lopez MF, Sarracino DA, Prakash A, Athanas M, Krastins B, Rezai T, Sutton JN, Peterman S, Gvozdyak O, Chou S, Lo E, Buonanno F, Ning M. Discrimination of ischemic and hemorrhagic strokes using a multiplexed, mass spectrometry-based assay for serum apolipoproteins coupled to multi-marker ROC algorithm. Proteomics Clin Appl. 2012;6:190–200. doi: 10.1002/prca.201100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ning M, Gonzalez G. A 69-Year-Old Man with Dizziness and Vomiting - Diagnosis, Treatment and Prevention of Neurovascular Disease. N Engl J Med 2012 [Google Scholar]
- 45.Lidegaard O. Oral contraceptives, pregnancy and the risk of cerebral thromboembolism: the influence of diabetes, hypertension, migraine and previous thrombotic disease. Br J Obstet Gynaecol. 1995;102:153–159. doi: 10.1111/j.1471-0528.1995.tb09070.x. [DOI] [PubMed] [Google Scholar]
- 46.Musolino R, La Spina P, Granata A, Gallitto G, Leggiadro N, Carerj S, Manganaro A, Tripodi F, Epifanio A, Gangemi S, Di Perri R. Ischaemic stroke in young people: a prospective and long-term follow-up study. Cerebrovasc Dis. 2003;15:121–128. doi: 10.1159/000067139. [DOI] [PubMed] [Google Scholar]
- 47.Tzourio C, Tehindrazanarivelo A, Iglesias S, Alperovitch A, Chedru F, d'Anglejan-Chatillon J, Bousser MG. Case-control study of migraine and risk of ischaemic stroke in young women. Bmj. 1995;310:830–833. doi: 10.1136/bmj.310.6983.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carolei A, Marini C, De Matteis G. History of migraine and risk of cerebral ischaemia in young adults. The Italian National Research Council Study Group on Stroke in the Young. Lancet. 1996;347:1503–1506. doi: 10.1016/s0140-6736(96)90669-8. [DOI] [PubMed] [Google Scholar]
- 49.Henrich JB, Horwitz RI. A controlled study of ischemic stroke risk in migraine patients. J Clin Epidemiol. 1989;42:773–780. doi: 10.1016/0895-4356(89)90075-9. [DOI] [PubMed] [Google Scholar]
- 50.Merikangas KR, Fenton BT, Cheng SH, Stolar MJ, Risch N. Association between migraine and stroke in a large-scale epidemiological study of the United States. Arch Neurol. 1997;54:362–368. doi: 10.1001/archneur.1997.00550160012009. [DOI] [PubMed] [Google Scholar]
- 51.Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischaemic stroke in people with migraine: systematic review and meta-analysis of observational studies. Bmj. 2005;330:63. doi: 10.1136/bmj.38302.504063.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fishman AP, Pietra GG. Handling of bioactive materials by the lung (second of two parts) N Engl J Med. 1974;291:953–959. doi: 10.1056/NEJM197410312911808. [DOI] [PubMed] [Google Scholar]
- 53.Fishman AP, Pietra GG. Handling of bioactive materials by the lung (first of two parts) N Engl J Med. 1974;291:884–889. doi: 10.1056/NEJM197410242911706. [DOI] [PubMed] [Google Scholar]