Abstract
Background
There is epidemiological evidence that cardiovascular risk factors (CVRF) also are risk factors for Alzheimer’s disease, but there is limited information on this from neuro-pathological studies, and even less from in vivo studies. Therefore, we examined the relationship between CVRF and amyloid-β (Aβ) brain burden measured by Pittsburgh Compound B-positron emission tomography (PiB-PET) studies in the Alzheimer’s Disease Neuroimaging Initiative.
Methods
Ninety-nine subjects from the Alzheimer’s Disease Neuroimaging Initiative cohort who had a PiB-PET study measure, apolipoprotein E genotyping data, and information available on CVRF (body mass index [BMI], systolic blood pressure, diastolic blood pressure [DBP1 and cholesterol and fasting glucose test results) were included. Eighty-one subjects also had plasma cortisol C-reactive protein, and superoxide dismutase 1 measurements. Stepwise regression models were used to assess the relation between the CVRF and the composite PiB-PET score.
Results
The first model included the following as baseline variables: age, clinical diagnosis, number of apolipoprotein ε4 alleles, BMI (P = .023), and DBP (P = .012). BMI showed an inverse relation with PiB-PET score, and DBP had a positive relation with PiB-PET score. In the second adjusted model, cortisol plasma levels were also associated with PiB-PET score (P = .004). Systolic blood pressure, cholesterol, or impaired fasting glucose were not found to be associated with PiB-PET values.
Conclusion
In this cross-sectional study, we found an association between Aβ brain burden measured in vivo and DBP and cortisol, indicating a possible link between these CVRF and Aβ burden measured by PiB-PET. These findings highlight the utility of biomarkers to explore potential pathways linking diverse Alzheimer’s disease risk factors.
Keywords: Alzheimer disease, Vascular risk factors, PiB, Amyloid-β, Cortisol, Blood pressure, Body mass index
1. Introduction
Dementia is the fourth highest cause of loss of disability-adjusted life years in high-income countries [1], with a projected 300% prevalence increase over the first half of this century [2]. Alzheimer’s disease (AD) is the most common cause of dementia, but it is frequently accompanied by vascular pathology, especially with increasing age, as shown by postmortem studies [3]. No preventive or disease-modifying treatment for AD is available presently, but if measures are undertaken to reduce the exposure to modifiable risk factors (RF), the incidence and prevalence of AD could, in theory, be reduced [2].
We now know that pathological hallmarks of AD begin to appear decades before symptom onset [4], and long before the dementia stage is reached, there is a preclinical phase of many years’ duration, during which the two signature lesions of AD—amyloid-β (Aβ) deposits and fibrillar tau lesions—progressively accumulate in the brain [5,6]. Therefore, many RF could exert their effects at stages in the life span when AD pathology progressively accumulates, but well before symptom onset. Many of these RF were previously acknowledged as cardiovascular risk factors (CVRF), but there is also evidence that hypertension [7,8], obesity [9], and diabetes [8,10,11] increase the risk of AD. Many conflicting reports of AD RF have been published, and the discrepancies may be a result of many methodological issues [12], including the fact that the effects of RF might differ based on the age of individuals [13]. For example, in the case of diastolic blood pressure (DBP), high levels at midlife [7] and low levels at advanced age both act as RF for dementia [14,15]. High cholesterol levels in midlife have also been associated with increased risk for AD, and a decrease in cholesterol levels after midlife has been described as a risk marker for dementia [16]. Further, it is important to consider that part of this effect could be attributed to genetic and early-life environmental factors that contribute to the linkage between RF and AD [17]. As CVRF can be treated with drugs that are already available, the incidence of AD could be reduced if treatments and adequate lifestyle changes are implemented and started at midlife [18], as pointed out by some observational studies [8,19]. These epidemiological findings have not been accompanied by studies of the association between CVRF and biomarker measurements that ascertain the burden of Aβ deposits or Aβ load. Further, cortisol levels have been related to worse cognitive scores and clinical outcomes [20,21], and they show an inverse correlation with hippocampal volume [21]. This has led to the hypothesis that increased glucocorticoid exposure promotes hippocampal damage and even AD neuropathology [22]. Therefore, we studied the relationship between body mass index (BMI), systolic blood pressure (SBP), DBP, altered fasting glucose, plasma levels of cortisol and acute-phase proteins, and Aβ burden, as measured by Pittsburgh Compound B-positron emission tomography (PiB-PET) studies, in subjects from the Alzheimer’s Disease Neuroimaging Initiative (ADNI).
2. Methods
2.1. Subjects
The ADNI is a large, multicenter, longitudinal neuroimaging study that was launched in 2004 by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration, private pharmaceutical companies, and nonprofit organizations. ADNI 1 consists of 819 adult subjects— 229 cognitively normal (CN), 398 with mild cognitive impairment (MCI), and 192 with AD. Of these subjects, we included 22 CN, 51 MCI, and 26 AD subjects who had at least one PiB-PET measure. Participants in ADNI undergo baseline and periodic physical and neurologic examinations and standardized neuropsychological assessments, and they provide biological samples (blood, urine, and, in a subset, cerebrospinal fluid) throughout the study. Physical examination includes measurements of height, weight, SBP, and DBP. BMI was calculated as weight (kg) divided by the square of height (m). Imaging (magnetic resonance imaging and, for a subset, fluorodeoxyglucose PET and PiB-PET) is performed at baseline and at regular intervals thereafter, as reviewed previously [23,24] (http://www.adni-info.org/index). All AD subjects met National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association criteria for probable AD, with a Mini-Mental State Examination score between 20 and 26, a global clinical dementia rating of 0.5 or 1, and a sum of boxes clinical dementia rating of 1.0 to 9.0, and were therefore mildly impaired. Inclusion criteria for amnestic MCI subjects include a Mini-Mental State Examination score of 24 to 30 and a Memory Box score of at least 0.5. Further details on the ADNI cohort can be found at http://www.adni-info.org/index. Exclusion criteria include any serious neurological disease other than possible AD, history of brain lesions or head trauma, or psychoactive medication use (including antidepressants, neuroleptics, chronic anxiolytics, or sedative hypnotics). Subjects had to have a Hachinski Ischemic Score of ≤4 and good general health with no diseases precluding enrollment in ADNI. The selection criteria and methodology have been extensively described by Petersen et al [25] and are available at http://www.adni-info.org/index.
Subjects were classified based on fasting glucose levels as not altered (≤100 mg/dL) or impaired (≥100 mg/dL) according to criteria of the American Diabetes Association [26]. Fasting glucose measures were available for only 66.7% of the sample.
2.2. Apolipoprotein E genotyping
Apolipoprotein E (APOE) genotyping was performed using TaqMan polymerase chain reaction assays, as described previously [27].
2.3. Plasma measurements
Plasma was prepared from blood samples collected from each study subject, following an overnight fast, at each visit scheduled in the ADNI protocol. At each scheduled visit, blood samples were collected into two 10-mL ethylenedia-minctetraacetic acid Vacutainer tubes (BD, Franklin Lakes, NJ), followed by centrifugation, within 1 hour, at 1500 RCF at room temperature. The plasma was transferred into a labeled 14-mL polypropylene transfer tube, which was then capped, placed on dry ice, and shipped to the UPenn Bio-marker Core laboratory. Aliquots (0.5 mL), prepared from plasma samples after thawing at room temperature, were stored in labeled polypropylene aliquot tubes at −80°C until the day of testing. In the morning on the day of testing, the plasma samples were thawed at room temperature.
C-reactive protein (CRP), cortisol, and superoxide dismutase 1 (SODI) levels were measured in plasma samples taken at baseline and at 12 months by Rules-Based Medicine, Inc. (RBM, Austin, TX) using the multiplex Human DiscoveryMAP panel and a Luminex xMAP platform (for additional information, refer to http://www.rulesbasedmedicine.com).
2.4. Pittsburgh Compound B-positron emission tomography
ADNI PiB-PET studies were performed at 14 different sites, where the production and radiolabeling of PiB were performed as outlined previously by Mathis et al [28]. The ADNI PiB-PET images underwent several quality control and standardization steps. Regional assessment of the PiB-PET data involved sampling 13 different brain areas using an automated region-of-interest template method, and standardized uptake value ratios (SUVRs) were calculated as in Jagust et al [29]. A PiB retention summary measure was formed by combining anterior cingulated cortex, lateral temporal cortex, precuneus, parietal cortex, and frontal cortex region-of-interest values for each subject, obtaining the mean value.
2.5. Statistical analysis
The normal distribution of the variables was tested and, in case of non-normal distributions, logarithmic transformations were applied (in BMI, CRP, SODI and cortisol data sets). A baseline regression model that included age, ApoE genotype, clinical diagnostic category, BMI, SBP, DBP, and fasting glucose group as independent variables and PiB summary score as dependent variable was established and backward elimination was applied. In a stepwise fashion, nonsignificant CVRF variables were excluded until only significant variables were included in the model. Confounding effects were also taken into account. Owing to the presence of bivariate outliers on replot representation, an MM-estimator-based multivariate resistant regression was performed [30,31]. The relationship between cortisol and PiB summary score was studied in another multiple linear regression model adjusted for age, clinical diagnosis, and ApoE genotype. Spearman correlation was used to assess the correlation between CRP, SODI, and PiB summary score. Analysis of variance and χ2 tests were applied for comparisons between groups, as presented in Table 1.
Table 1.
Baseline characteristics of participants according to their clinical status at baseline
| CN | MCI | AD | ||
|---|---|---|---|---|
| Features | PiB-PET (n = 22) | PiB-PET(n = 51) | PiB-PET (n = 26) | P value |
| Age (years) | 75.8 (6.1) | 74.8 (7.6) | 73.3 (8.9) | .516 |
| Gender (male %) | 63.6% | 66.7% | 65.4% | .969 |
| Ethnicity (% Caucasian) | 90.9% | 96.1% | 100% | .280 |
| MMSE | 28.8 (1.6) | 27.43 (1.5) | 25.27 (2.2) | <.001 |
| APOE ε4 (% with at least one copy) | 27.3% | 51% | 65.4% | .030 |
| BMI | 27.6 (3.5) | 25.8 (4.1) | 26.4 (4.4) | .180 |
| SBP (mmHg) | 134.7 (15.9) | 135.1 (17.7) | 136.9 (16.6) | .869 |
| DBP (mmHg) | 74.3 (8.2) | 73.7 (9.2) | 75.4 (9.8) | .725 |
| Cholesterol (mg/dL) | 189.0 (6.4) | 188.9 (5.7) | 204.5 (12.4) | .342 |
| Altered fasting glucose | 65% | 50% | 50% | .527 |
| cortisol (ng/mL) | 142 (114.5–189.7) | 160 (131.5–190) | 149 (132–182) | .314 |
| CRP (μg/mL) | 1.2 (0.42–1.7) | 1.3 (0.74–3.0) | 0.79 (0.33–1.9) | .541 |
| SODI (ng/mL) | 40.0 (30.0–73.0) | 44.5 (32.5–60.0) | 48.0 (38.0–74.0) | .264 |
Abbreviations: AD, Alzheimer’s disease; BMI. body mass index; CN, cognitive normal; DBP, diastolic blood pressure; CRP, C-reactive protein; MCI. mild cognitive impairment; MMSE, Mini-Mental State Examination; PiB-PET. Pittsburgh Compound B-positron emission tomography; SBP, systolic blood pressure; SODI, superoxide dismutase 1.
Mean (standard deviation), except in BMI, CRP, SODI, and cortisol: median (interquartile range).
3. Results
Baseline characteristics of the clinical groups are summarized in Table 1. The three groups only differed in cognitive scores and in the percentage of subjects with at least one copy of an APOE ε4 allele. There were differences neither in the values nor the distribution of the CVRF in the different diagnostic groups.
3.1. CVRF and PiB measurements
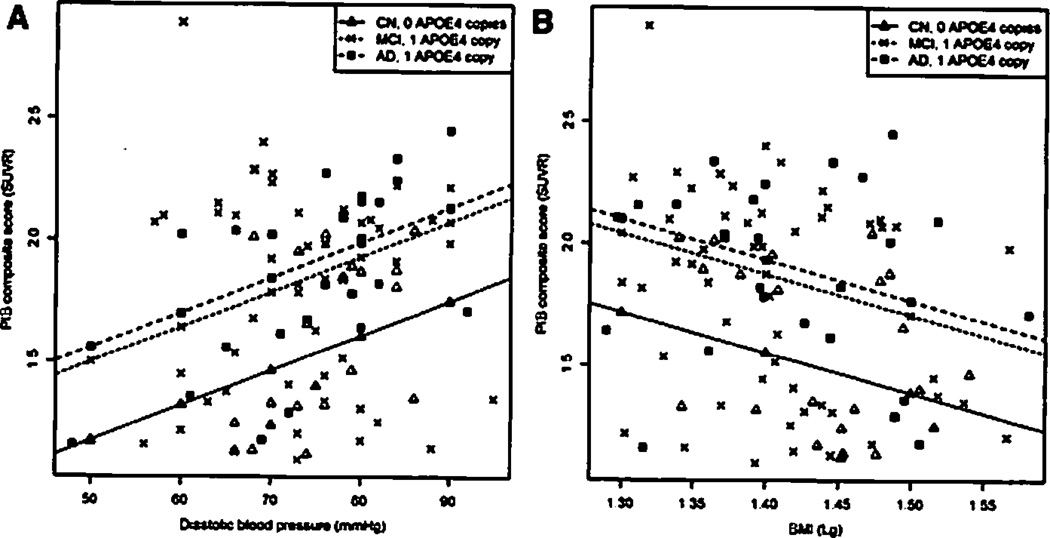
We established a model that included age, number of APOE ε4 alleles, clinical diagnosis, BMI, SBP, DBP, and fasting glucose group (R2 = 0.30). First fasting glucose group and thereafter SBP were excluded. In the final model (R2 = 0.36), there was an inverse association between the logarithm of BMI and PiB composite score (P = .023), with every 1 logarithmic unit change in BMI predicting −1.67 SUVR on the PiB summary score, whereas the association between DBP and PiB composite score was positive, with increases of 10 mmHg in DBP predicting a 0.144 SUVR higher PiB summary score (P = .012). There was no interaction between APOE ε4 alleles and the statistically significant CVRF. The predicted regression slopes of a CN subject with zero copies of APOE ε4, an MCI subject with one copy of APOE ε4, and an AD subject with one copy of APOE ε4 are represented in Figure 1A (DBP) and B (BMI).
Fig. 1.
Scatterplot with predicted robust regression slope for subjects with median age and vascular risk factor values, and representing subjects who are cog-nitively normal (CN) with no apolipoprotein E (APOE) ε4 alleles, mild cognitive impairment (MCI) subjects with one APOE ε4 allele, and Alzheimer’s disease (AD) subjects with one APOE ε4 allele. Values for CN subjects are represented with triangles, MCI subjects with crosses, and AD cases with squares. (A) Diastolic blood pressure and Pittsburgh Compound B (PiB)-positron emission tomography composite score. (B) Body mass index and PiB composite score.
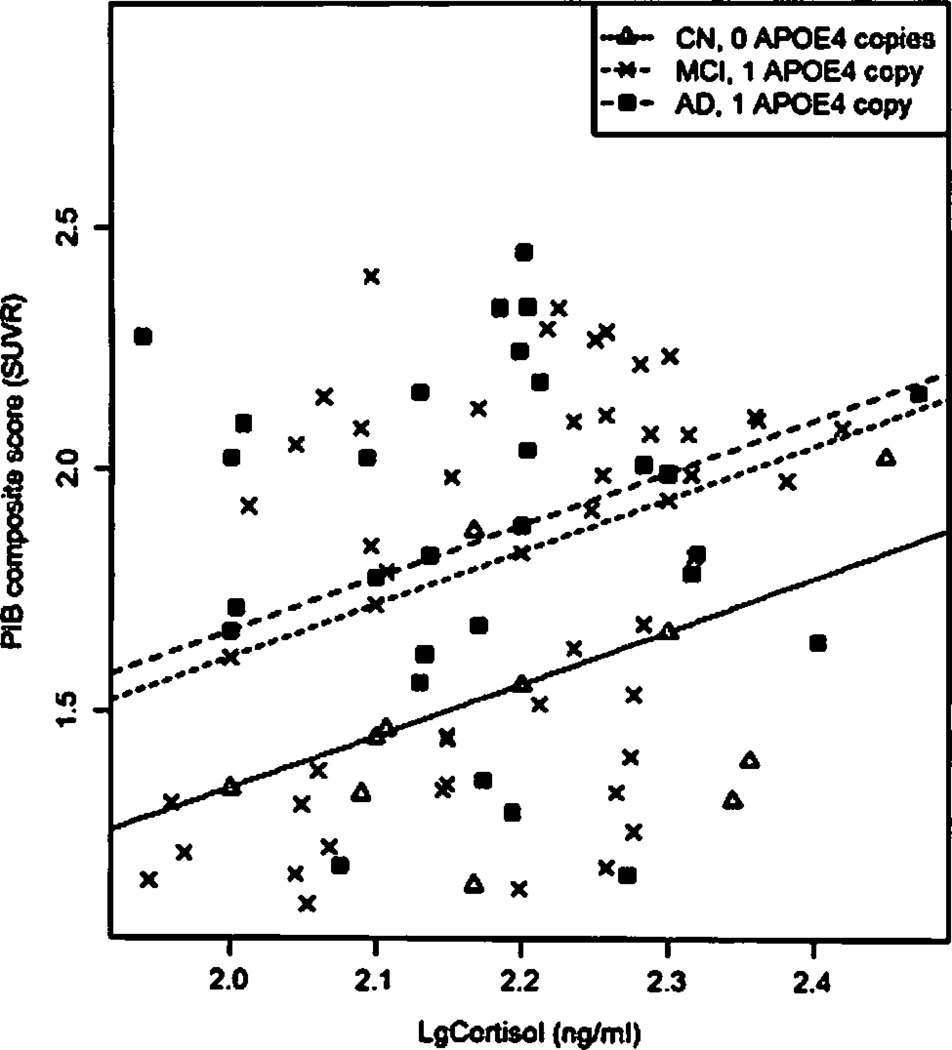
In the adjusted multivariate regression model, there was a positive association between plasma cortisol levels and PiB scores (P = .004), with every log increment of cortisol being associated with an increase of 1 SUVR PiB summary score (Figure 2).
Fig. 2.
Scatterplot with predicted regression slopes for subjects with median age representing CN subjects with no APOE ε4 alleles. MCI subjects with one APOE ε4 allele, and AD subjects with one APOE ε4 allele. Values for CN subjects are represented with triangles, MCI subjects with crosses, and AD cases with squares.
3.2. PiB correlations with CRP and SODI
No correlation was found between the PiB summary score and CRP and SODI (PCRP = .158, rCRP = −.158; PSODI = .569 and rSODI = .064).
4. Discussion
This study confirms a relationship in vivo between one CVRF (DBP) and one marker of established disease (BMI) with brain Aβ burden measured by PiB-PET studies. Plasma cortisol levels also positively correlated with PiB scores. Our study shows that, in agreement with epidemiological evidence, there is a link between CVRF and a biomarker of Aβ deposition, namely PiB-PET scores. One previous study has reported a difference in weight based on the CSF signature according to previously reported epidemiological data [32]. To our knowledge, no other study has assessed the relationship of CVRF and AD in vivo, and there are only limited neuropathological studies reporting this association [33,34]. The inverse correlation between BMI and PiB-PET score measures could reflect the loss of weight that occurs in preclinical stages of AD [11,32,35,36].
The increasing trend of dementia incidence attributable to CVRF has been explained either by the additive effect of cerebrovascular pathology and AD pathology lowering the threshold for clinical symptoms [37,38] or by CVRF enhancing the underlying neurodegenerative process. Given the association between intracranial atherosclerosis and dementia in neuropathological studies [39], and the increase of Aβ deposition caused by oligemia and hypoxia, it is possible that these CVRF may contribute to mechanisms underlying AD [37–41]. Our study indicates that DBP may mediate cognitive deficits that are not only due to microvasculature damage but also due to Aβ deposition [13,42–46]. One explanation for finding only an association with DBP can be that the blood pressure decrease that occurs at presymptomatic stages of AD mainly affects SBP [13], and that DBP changes are not so affected by disease evolution [44], so that in the regression model SBP does not add any additional information to DBP. Another possible explanation is that the endothelial dysfunction is more dependent on DPB at older ages.
The absence of an increased burden of Aβ burden in subjects with fasting glucose impairment is consistent with previous neuropathological studies [47] and animal models that described increased cerebrovascular changes without an increase of amyloid burden [48].
Cortisol has been related to Aβ deposition and memory deficits in normal transgenic mice [49,50] and to hippocampal atrophy in human brain magnetic resonance imaging studies [21]. This study confirms the relation between cortisol and brain Aβ deposition in human subjects.
No correlation was found between acute-phase proteins and PiB scores. This is in agreement with a recent imaging and autopsy study that found no correlation between a marker of activated microglia (3H-PK11195) and PiB-PET scores [51], whereas the results of in vivo studies are conflicting [52–54]. In the autopsy study, a correlation with number of glial fibrillary acidic protein immunoreactive cells was found, indicating a relation with astrocytosis [51]. Larger studies with patients at different stages of AD might be necessary to assess whether inflammation is present only at certain stages of the disease.
We did not find an association between cholesterol levels and PiB-PET score. One reason is that at stages where the disease is already symptomatic, the presence of hypertension still predicts further greater cognitive decline, whereas there is no association with the presence of hypercholesterolemia [45]. Another reason can be the distribution of cholesterol levels among the different diagnostic groups. Although not statistically significant, cholesterol levels were higher in our AD group, which is in disagreement with cross-sectional and longitudinal studies performed at late life [55,56].
One of the difficulties in longitudinal epidemiological studies is to discern the underlying pathology or pathologies. Not all studies confirm the causal link between hypertension and AD [57]. With aging, mixed dementia increases in large autopsy series [3]. Therefore, the use of imaging techniques that distinguish the presence of the different substrates that can account for cognitive decline becomes crucial.
Our study has several strengths, such as the detailed clinical and biomarker characterization of the sample as well as the standardized protocols applied in ADNI. However, there are also some weaknesses inherent to this study, including the exclusion criteria used in ADNI, which prevented subjects with severe vascular disease from entering the study, and therefore subjects with serious vascular events are not represented in the ADNI cohort. Besides this, the skewed representation of the most impaired cases of MCI with lack of patients in early stages is a further limitation, because the complete spectrum of the continuum between subjects with no underlying neurodegenerative disease and subjects with AD neuropathology in a dementia stage could not be represented. Finally, this study represents a cross-sectional analysis that does not allow establishing whether the loss of weight represents an effect of the disease evolution and whether the DBP increases Aβ deposition in the brain.
In conclusion, our study favors the hypotheses of an association between DBP and cortisol and the amyloid cascade, and it underlines the utility of biomarkers not only as a diagnostic tool but also as a means to study the course and etiology of AD in vivo.
Acknowledgments
The authors thank their ADNI colleagues for their contributions to the work summarized here, which has been supported mainly by the ADNI U01 AG024904. ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and the Foundation for the National Institutes of Health, and through generous contributions from the following companies and organizations: Pfizer Inc., Wyeth Research, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKIine, Merck & Co. Inc., AstraZeneca AB, Novartis Pharmaceuticals Corporation, the Alzheimer’s Association, Eisai Global Clinical Development, Elan Corporation plc, Forest Laboratories, and the Institute for the Study of Aging, with participation from the U.S. Food and Drug Administration. Other support has come from AG10124 and the Marian S. Ware Alzheimer Program. V.M.Y.L. is the John H. Ware 3rd Professor for Alzheimer’s Disease Research, and J.Q.T. is the William Maul Measey-Truman G. Schnabel, Jr., MD, Professor of Geriatric Medicine and Gerontology. The authors thank the ADNI Biomarker Core for the analyses. They also thank Donald Baldwin and the Molecular Diagnosis Genotyping Facility at the University of Pennsylvania Medical Center for provision of the APOE ε genotyping data. J.B.T.’s work was supported by a grant from the Alfonso Martín Escudero Foundation.
The ADNI: Data used here were produced by the ADNI Biomarker Core or obtained from the ADNI database (www.loni.ucla.edu/ADNI). Many ADNI investigators contributed to ADNI but did not participate in the analysis of the data presented here or in the writing of this report. ADNI investigators are listed at www.loni.ucla.edu/ADNI/Collaboration/ADNI_Manuscript_Citations.pdf.
L.M.S. is a member of the advisory board of Innogenetics Technical; M.W.W. is a member of the advisory board of Elan, Wyeth, Novartis, Banner, Lilly, Araclon, Biogen Idec Inc, and Pfizer, is consultant for Forest, Ipsen, Daiichi Sankyo, AstraZeneca, Medivation, TauRx, Bayer Healthcare, ExonHit Therapeutics, Servier, and Synarc, and obtained grants from DOD, Merck, Avid, and VA; C.R.J. is a shareholder of Synarc and consultant for GE, J and J, Janssen, Eisai, and Elan; W.J. is consultant for Synarc, Genentech, Merck & Co, Bayer, and TauRx.
References
- 1.WHO. The global burden of disease: 2004 update. 2008 http://www.who.int/healthinfo/g]obal_burden_disease/2004_report_update/en/index.html.
- 2.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 3.Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol. 2010;119:421–433. doi: 10.1007/s00401-010-0654-5. [DOI] [PubMed] [Google Scholar]
- 4.Braak H, Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011;121:171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- 5.Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 6.Acosta-Baena N, Sepulveda-Falla D, Lopera-Gomez CM, Jaramillo-Elorza MC, Moreno S, Aguirre-Acevedo DC, et al. Pre-dementia clinical stages in presenilin I E280A familial early-onset Alzheimer’s disease: a retrospective cohort study. Lancet Neurol. 2011;10:213–220. doi: 10.1016/S1474-4422(10)70323-9. [DOI] [PubMed] [Google Scholar]
- 7.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Wang YJ, Zhang M, Xu ZQ, Gao CY, Fang CQ, et al. Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology. 2011;76:1485–1491. doi: 10.1212/WNL.0b013e318217e7a4. [DOI] [PubMed] [Google Scholar]
- 9.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011;l2:e426–e437. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 10.Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001;154:635–641. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 11.Carlsson CM. Type 2 diabetes mellitus, dyslipidemia, and Alzheimer’s disease. J Alzheimers Dis. 2010;20:711–722. doi: 10.3233/JAD-2010-100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart R, Masaki K, Xue QL, Peila R, Petrovitch H, White LR, et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62:55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 13.Li G, Rhew IC, Shofer JB, Kukull WA, Breitner JC, Peskind E, et al. Age-varying association between blood pressure and risk of dementia in those aged 65 and older: a community-based prospective cohort study. J Am Geriatr Soc. 2007;55:1161–1167. doi: 10.1111/j.1532-5415.2007.01233.x. [DOI] [PubMed] [Google Scholar]
- 14.Verghese J, Upton RB, Hall CB, Kuslansky G, Katz MI. Low blood pressure and the risk of dementia in very old individuals. Neurology. 2003;61:1667–1672. doi: 10.1212/01.wnl.0000098934.18300.be. [DOI] [PubMed] [Google Scholar]
- 15.Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and the risk for dementia—a double edged sword. Ageing Res Rev. 2009;8:61–70. doi: 10.1016/j.arr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Solomon A, Kareholt I, Ngandu T, Winblad B, Nissinen A, Tuomilehto J, et al. Serum cholesterol changes after midlife and late-life cognition: twenty-one-year follow-up study. Neurology. 2007;68:751–756. doi: 10.1212/01.wnl.0000256368.57375.b7. [DOI] [PubMed] [Google Scholar]
- 17.Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Midlife overweight and obesity increase late-life dementia risk. Neurology. 2011;76:1568–1574. doi: 10.1212/WNL.0b013e3182190d09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu C, Xu W, Fratiglioni L. Vascular and psychosocial factors in Alzheimer’s disease: epidemiological evidence toward intervention. J Alzheimers Dis. 2010;20:689–697. doi: 10.3233/JAD-2010-091663. [DOI] [PubMed] [Google Scholar]
- 19.Launer LJ, Hughes T, Yu B, Masaki K, Petrovitch H, Ross GW, et al. Lowering midlife levels of systolic blood pressure as a public health strategy to reduce late-life dementia: perspective from the Honolulu Heart Program/Honolulu Asia Aging Study. Hypertension. 2010;55:1352–1359. doi: 10.1161/HYPERTENSIONAHA.109.147389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee BK, Glass TA, Wand GS, McAtee MJ, Bandeen-Roche K, Bolla KI, et al. Apolipoprotein E genotype, cortisol, and cognitive function in community-dwelling older adults. Am J Psychiatry. 2008;165:1456–1464. doi: 10.1176/appi.ajp.2008.07091532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang CW, Lui CC, Chang WN, Lu CH, Wang YL, Chang CC. Elevated basal cortisol level predicts lower hippocampal volume and cognitive decline in Alzheimer’s disease. J Clin Neurosci. 2009;16:1283–1286. doi: 10.1016/j.jocn.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 22.Landfield PW, Blalock EM, Chen KC, Porter NM. A new glucocorticoid hypothesis of brain aging: implications for Alzheimer’s disease. Curr Alzheimer Res. 2007;4:205–212. doi: 10.2174/156720507780362083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw LM, Korecka M, Clark CM, Lee VM, Trojonowski JQ. Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nat Rev Drug Discov. 2007;6:295–303. doi: 10.1038/nrd2176. [DOI] [PubMed] [Google Scholar]
- 24.Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack CR, Jagust W, et al. Ways toward an early diagnosis in Alzheimer’s disease: the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Alzheimers Dement. 2005;1:55–66. doi: 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 201l;34(Suppl 1):S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark C, Aisen P, Petersen R, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. Synthesis and evaluation of HC-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46:2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- 29.Jagust WJ, Bandy D, Chen K, Foster NL, Landau SM, Mathis CA, et al. The Alzheimer’s Disease Neuroimaging Initiative positron emission tomography core. Alzheimers Dement. 2010;6:221–229. doi: 10.1016/j.jalz.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yohoi V, Stahel WA, Zamar RH. A procedure for robust estimation and inference in linear regression. In: Stahel WA, Weisberg SW, editors. Directions in Robust Statistics and Diagnostics PI. New York, NY: Springer-Verlag; 1991. [Google Scholar]
- 31.Venables WN, Ripley BD. Modem applied statistics with S. Fourth. New York, NY: Springer Verlag; 2002. [Google Scholar]
- 32.Ewers M, Schmitz S, Hansson O, Walsh C, Fitzpatrick A, Bennett D, et al. Body mass index is associated with biological CSF markers of core brain pathology of Alzheimer’s disease. Neurobiol Aging. in press doi: 10.1016/j.neurobiolaging.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 34.Hoffman LB, Schmeidler J, Lesser GT, Been MS, Purohit DP, Grossman HT, et al. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology. 2009;72:1720–1726. doi: 10.1212/01.wnl.0000345881.82856.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luchsinger JA, Patel B, Tang MX, Schupf N, Mayeux R. Measures of adiposity and dementia risk in elderly persons. Arch Neurol. 2007;64:392–398. doi: 10.1001/archneur.64.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Custafson DR, Backman KM, Waern MM, Ostling SM, Guo XM, Zandi PP, et al. Adiposity indicators and dementia over 32 years in Sweden. Neurology. 2009;73:1559–1566. doi: 10.1212/WNL.0b013e3181c0d4b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA. Subcortical infarcts, Alzheimer’s disease pathology, and memory function in older persons. Ann Neurol. 2007;62:59–66. doi: 10.1002/ana.21142. [DOI] [PubMed] [Google Scholar]
- 38.Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O’Brien RJ. Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Ann Neurol. 2008;64:168–176. doi: 10.1002/ana.21413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB, Obrien RJ. Atherosclerosis, dementia, and Alzheimer disease in the Baltimore Longitudinal Study of Aging cohort. Ann Neurol. 2010;68:231–240. doi: 10.1002/ana.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koike MA, Green KN, Blurton-Jones M, LaFerla FM. Oligemic hypoperfusion differentially affects tau and amyloid-[beta] Am J Pathol. 2010;177:300–310. doi: 10.2353/ajpath.2010.090750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, Zhou K, Wang R, Cui J, Lipton SA, Liao FF, et al. Hypoxia-inducible factor lalpha (HlF-lalpha)-mediated hypoxia increases BACEI expression and beta-amyloid generation. J Biol Chem. 2007;282:10873–10880. doi: 10.1074/jbc.M608856200. [DOI] [PubMed] [Google Scholar]
- 42.Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gentile MT, Poulet R, Pardo AD, Cifelli G, Maffei A, Vecchione C, et al. [beta]-Amyloid deposition in brain is enhanced in mouse models of arterial hypertension. Neurobiol Aging. 2009;30:222–228. doi: 10.1016/j.neurobiolaging.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Skoog I, Lemfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, et al. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- 45.Li J, Zhang M, Xu ZQ, Gao CY, Fang CQ, Deng J, et al. Vascular risk aggravates the progression of Alzheimer’s disease in a Chinese cohort. J Alzheimers Dis. 2010;20:491–500. doi: 10.3233/JAD-2010-1383. [DOI] [PubMed] [Google Scholar]
- 46.Bellew KM, Pigeon JG, Stang PE, Fleischman W, Gardner RM, Baker WW. Hypertension and the rate of cognitive decline in patients with dementia of the Alzheimer type. Alzheimer Dis Assoc Disord. 2004;18:208–213. [PubMed] [Google Scholar]
- 47.Arvanitakis Z, Schneider JA, Wilson RS, Li Y, Arnold SE, Wang Z, et al. Diabetes is related to cerebral infarction but not to AD pathology in older persons. Neurology. 2006;67:1960–1965. doi: 10.1212/01.wnl.0000247053.45483.4e. [DOI] [PubMed] [Google Scholar]
- 48.Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, et al. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci U S A. 2010;107:7036–7041. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-β and tau pathology in a mouse model of Alzheimer’s disease. J Neurosci. 2006;26:9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Li M, Tang J, Song M, Xu X, Xiong J, et al. Glucocorticoids facilitate astrocytic amyloid-{beta} peptide deposition by increasing the expression of APP and BACEI and decreasing the expression of amyloid-{beta}-degrading proteases. Endocrinology. 2011;152:2704–2715. doi: 10.1210/en.2011-0145. [DOI] [PubMed] [Google Scholar]
- 51.Kadir A, Marutle A, Gonzalez D, Schöll M, Almkvist O, Mousavi M, et al. Positron emission tomography imaging and clinical progression in relation to molecular pathology in the first Pittsburgh Compound B positron emission tomography patient with Alzheimer’s disease. Brain. 2011;134:301–317. doi: 10.1093/brain/awq349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edison P, Archer HA, Gerhard A, Hinz R, Pavese N, Turkheimer FE, et al. Microglia, amyloid, and cognition in Alzheimer’s disease: an [1IC](R)PK11195-PET and [IIC]PIB-PET study. Neurobiol Dis. 2008;32:412–419. doi: 10.1016/j.nbd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 53.Okello A, Edison P, Archer HA, Turkheimer FE, Kennedy J, Bullock R, et al. Microglial activation and amyloid deposition in mild cognitive impairment: a PET study. Neurology. 2009;72:56–62. doi: 10.1212/01.wnl.0000338622.27876.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiley CA, Lopresti BJ, Venneti S, Price J, Klunk WE, DeKosky ST, et al. Carbon II-labeled Pittsburgh Compound B and carbon II-labeled (R)-PK11195 positron emission tomographic imaging in Alzheimer disease. Arch Neurol. 2009;66:60–67. doi: 10.1001/archneurol.2008.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reitz C, Tang MX, Luchsinger J, Mayeux R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch Neurol. 2004;61:705–714. doi: 10.1001/archneur.61.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stewart R, White LR, Xue QL, Launer LJ. Twenty-six-year change in total cholesterol levels and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2007;64:103–107. doi: 10.1001/archneur.64.1.103. [DOI] [PubMed] [Google Scholar]
- 57.Ninomiya T, Ohara T, Hirakawa Y, Yoshida D, Doi Y, Hata J, et al. Midlife and late-life blood pressure and dementia in Japanese elderly: the Hisayama Study. Hypertension. 2011;58:22–28. doi: 10.1161/HYPERTENSIONAHA.110.163055. [DOI] [PubMed] [Google Scholar]