Abstract
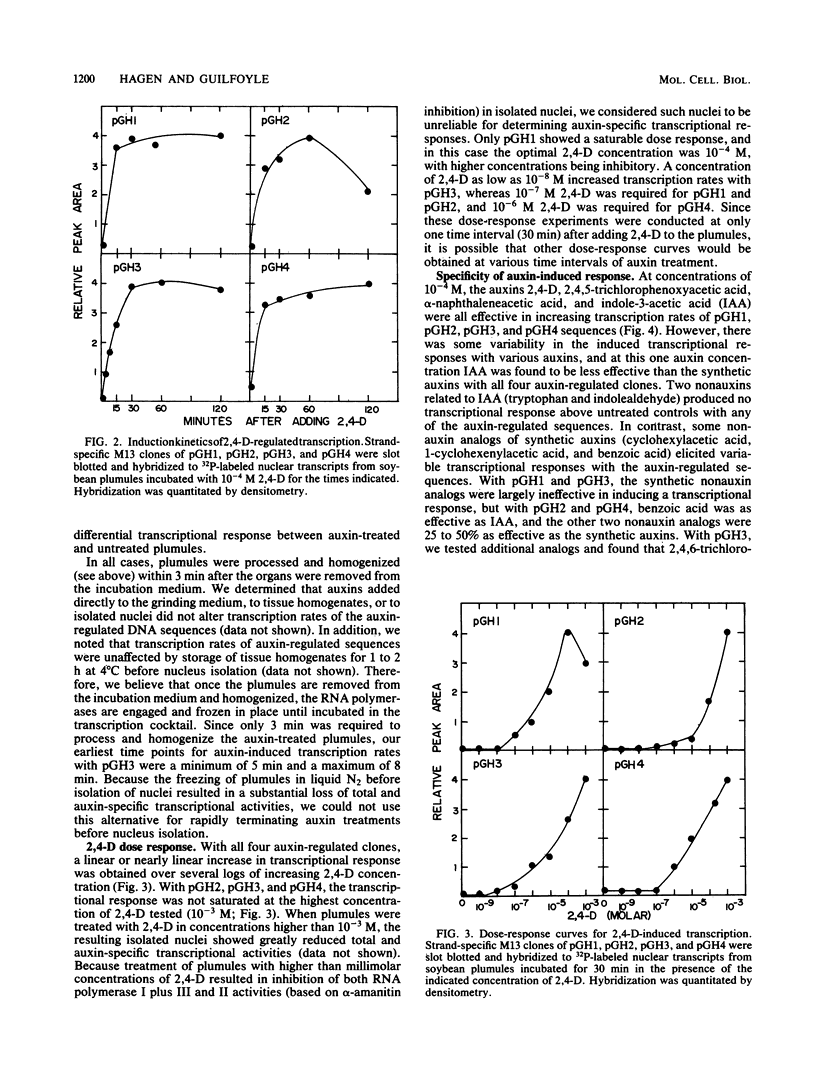
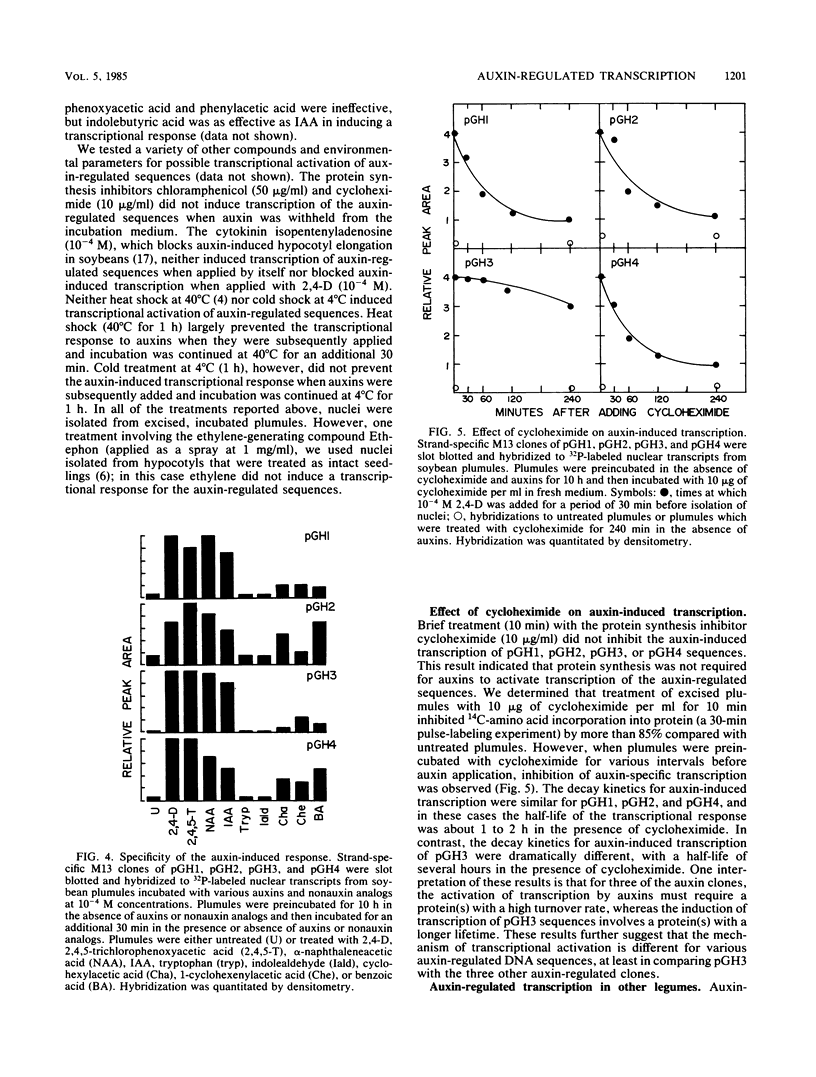
Nuclei isolated from excised soybean plumules that were treated with 2,4-dichlorophenoxyacetic acid (2,4-D) were active in transcription of four auxin-regulated genes or DNA sequences, which have been described previously (G. Hagen, A. Kleinschmidt, and T. Guilfoyle, Planta 162:147-153, 1984). The rates of transcription of the auxin-responsive sequences were 10- to 100-fold greater with nuclei isolated from auxin-treated plumules than with those from untreated plumules. The transcriptional response was also observed with hypocotyls of intact soybean seedlings and hypocotyl sections, as well as with green bean and mung bean plumules that were treated with 2,4-D. Other auxins, including 2,4,5-trichlorophenoxyacetic acid, alpha-naphthaleneacetic acid, and indole-3-acetic acid, also induced the transcriptional response. Increased transcription rates were observed within 5 min after application of auxins to excised plumules, and half-maximal to maximal transcription rates were achieved by 15 min after application of auxins. As little as 10(-7) to 10(-8) M 2,4-D induced a transcriptional response, but maximal transcription rates were achieved at 10(-3) M 2,4-D. Brief treatment with the protein synthesis inhibitor cycloheximide did not inhibit the induction of transcription by auxins. These results clearly demonstrated that auxin-regulated gene expression is under rapid transcriptional control.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Y. M., Lin C. Y., Chang H., Guilfoyle T. J., Key J. L. Isolation and Properties of Nuclei from Control and Auxin-treated Soybean Hypocotyl. Plant Physiol. 1975 Jul;56(1):78–82. doi: 10.1104/pp.56.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoyle T. J., Lin C. Y., Chen Y. M., Nagao R. T., Key J. L. Enhancement of soybean RNA polymerase I by auxin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):69–72. doi: 10.1073/pnas.72.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Samuels M., Sharp P. A. In vitro transcription: whole-cell extract. Methods Enzymol. 1983;101:568–582. doi: 10.1016/0076-6879(83)01038-1. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Olszewski N., Hagen G., Guilfoyle T. J. A transcriptionally active, covalently closed minichromosome of cauliflower mosaic virus DNA isolated from infected turnip leaves. Cell. 1982 Jun;29(2):395–402. doi: 10.1016/0092-8674(82)90156-8. [DOI] [PubMed] [Google Scholar]
- Rousseau G. G. Control of gene expression by glucocorticoid hormones. Biochem J. 1984 Nov 15;224(1):1–12. doi: 10.1042/bj2240001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Ray P. M. Early auxin-regulated polyadenylylated mRNA sequences in pea stem tissue. Proc Natl Acad Sci U S A. 1982 Jan;79(2):418–421. doi: 10.1073/pnas.79.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucker D. S., Yamamoto K. R. Early events in the stimulation of mammary tumor virus RNA synthesis by glucocorticoids. Novel assays of transcription rates. J Biol Chem. 1984 Jun 25;259(12):7416–7420. [PubMed] [Google Scholar]
- Vanderhoef L. N., Stahl C. A. Separation of two responses to auxin by means of cytokinin inhibition. Proc Natl Acad Sci U S A. 1975 May;72(5):1822–1825. doi: 10.1073/pnas.72.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. C., Key J. L. Isolation of cloned cDNAs to auxin-responsive poly(A)RNAs of elongating soybean hypocotyl. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7185–7189. doi: 10.1073/pnas.79.23.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M. Steroid receptors: elements for modulation of eukaryotic transcription. Annu Rev Biochem. 1976;45:721–746. doi: 10.1146/annurev.bi.45.070176.003445. [DOI] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin- and ethylene-induced changes in the population of translatable messenger RNA in Basal sections and intact soybean hypocotyl. Plant Physiol. 1982 Feb;69(2):338–340. doi: 10.1104/pp.69.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin-induced changes in the patterns of protein synthesis in soybean hypocotyl. Proc Natl Acad Sci U S A. 1980 Jan;77(1):357–361. doi: 10.1073/pnas.77.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin-induced changes in the population of translatable messenger RNA in elongating sections of soybean hypocotyl. Plant Physiol. 1982 Feb;69(2):332–337. doi: 10.1104/pp.69.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]