Abstract
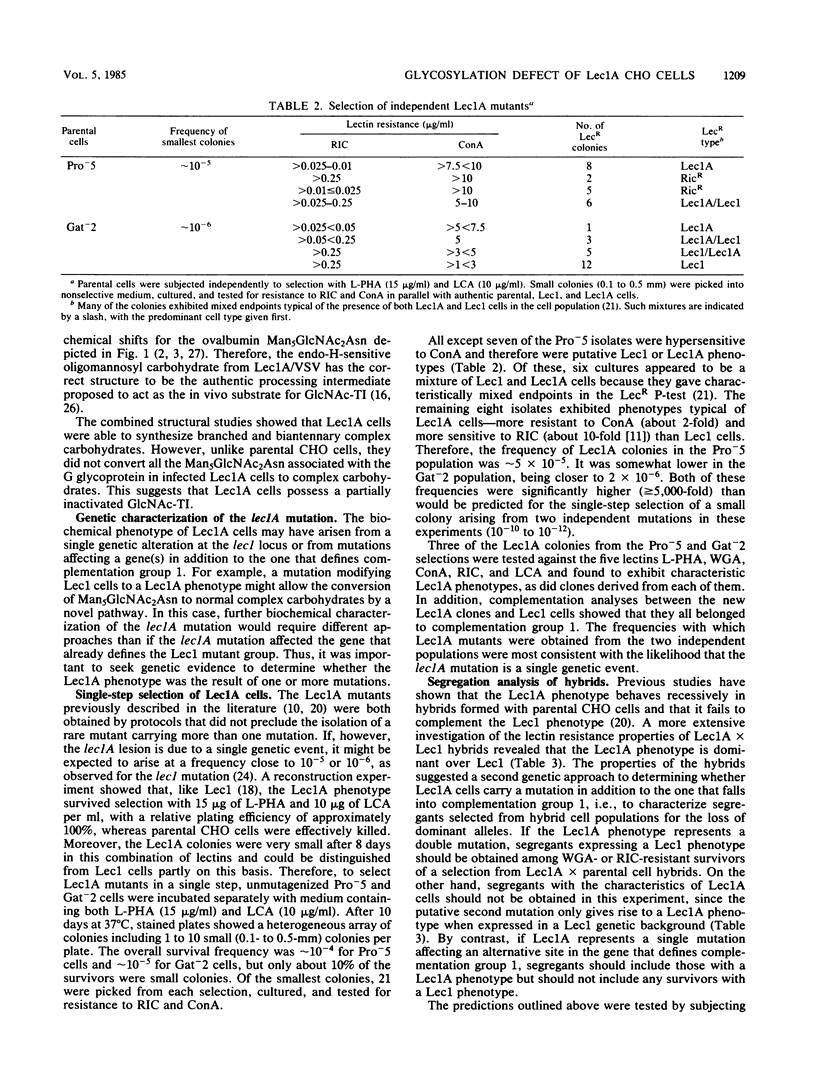
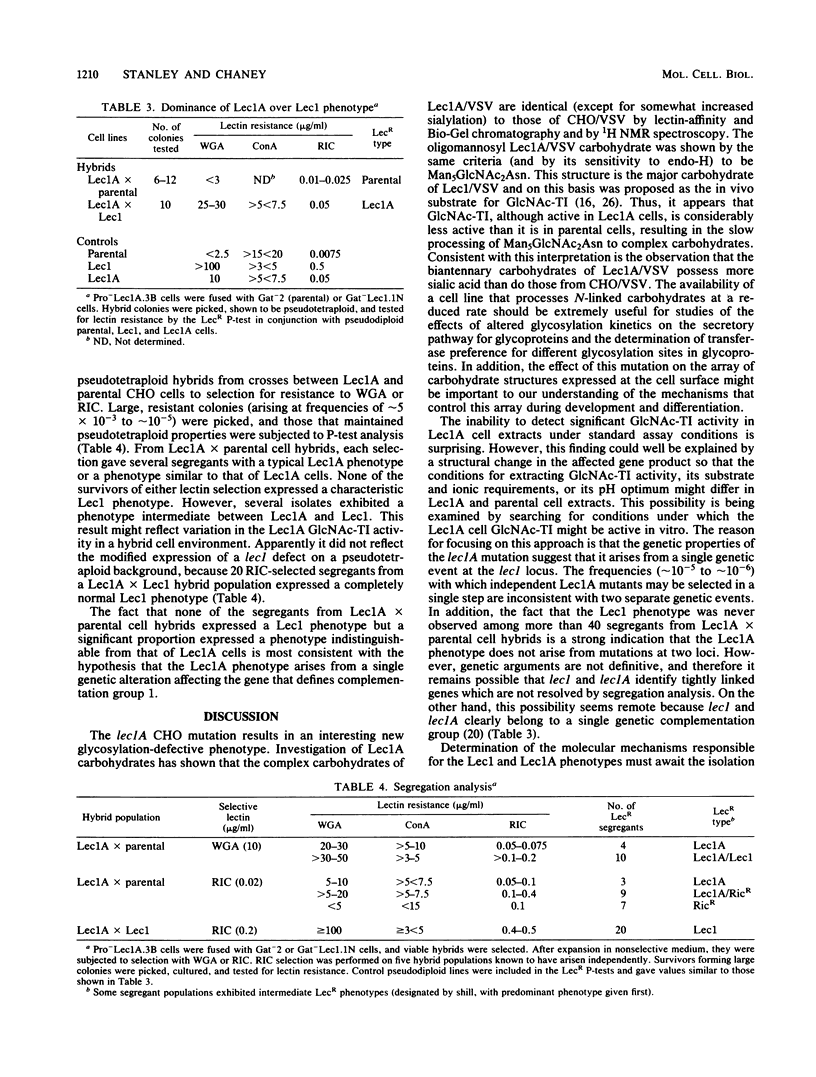
Lec1 CHO cell glycosylation mutants are defective in N-acetylglucosaminyltransferase I (GlcNAc-TI) activity and therefore cannot convert the oligomannosyl intermediate (Man5GlcNAc2Asn) into complex carbohydrates. Lec1A CHO cell mutants have been shown to belong to the same genetic complementation group but exhibit different phenotypic properties. Evidence is presented that lec1A represents a new mutation at the lec1 locus resulting in partial loss of GlcNAc-TI activity. Structural studies of the carbohydrates associated with vesicular stomatitis virus grown in Lec1A cells (Lec1A/VSV) revealed the presence of biantennary and branched complex carbohydrates as well as the processing intermediate Man5GlcNAc2Asn. By contrast, the glycopeptides from virus grown in CHO cells (CHO/VSV) possessed only fully processed complex carbohydrates, whereas those from Lec1/VSV were almost solely of the Man5GlcNAc2Asn intermediate type. Therefore, the Lec1A glycosylation phenotype appears to result from the partial processing of N-linked carbohydrates because of reduced GlcNAc-TI action on membrane glycoproteins. Genetic experiments provided evidence that lec1A is a single mutation affecting GlcNAc-TI activity. Lec1A mutants could be isolated at frequencies of 10(-5) to 10(-6) from unmutagenized CHO cell populations by single-step selection, a rate inconsistent with two mutations. In addition, segregants selected from Lec1A X parental cell hybrid populations expressed only Lec1A or related lectin-resistant phenotypes and did not include any with a Lec1 phenotype. The Lec1A mutant should be of interest for studies on the mechanisms that control carbohydrate processing in animal cells and the effects of reduced GlcNAc-TI activity on the glycosylation, translocation, and compartmentalization of cellular glycoproteins.
Full text
PDF

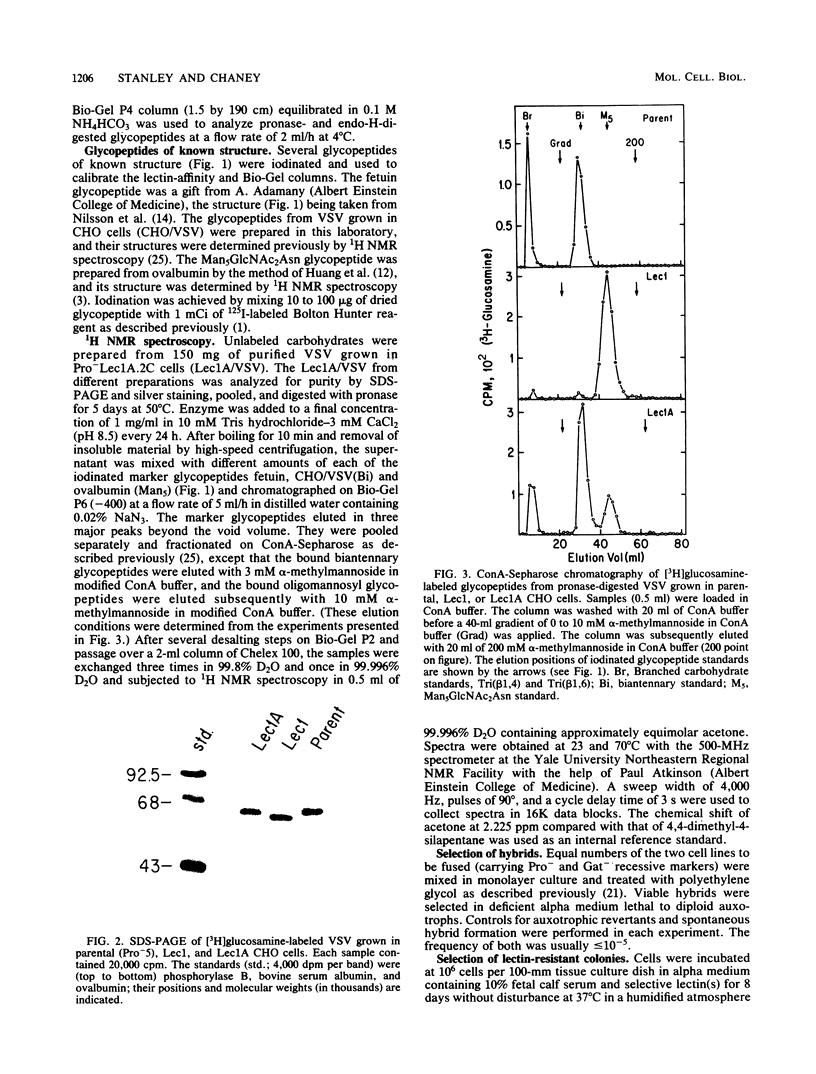
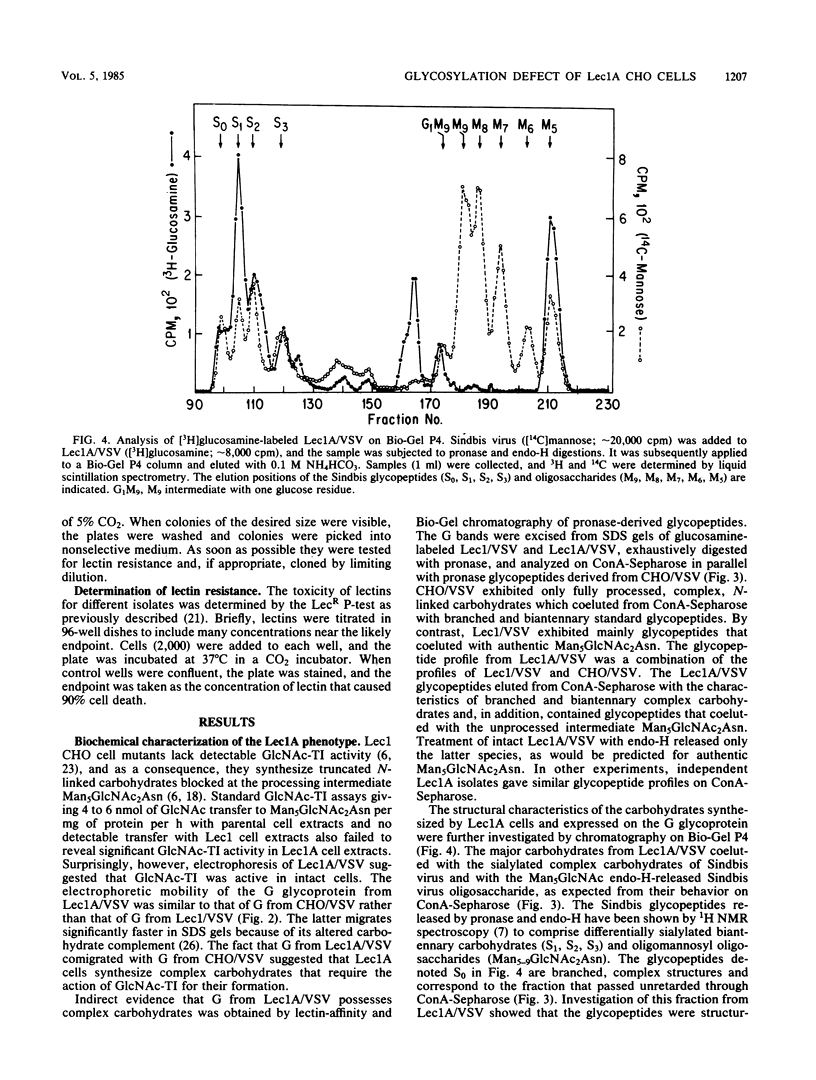
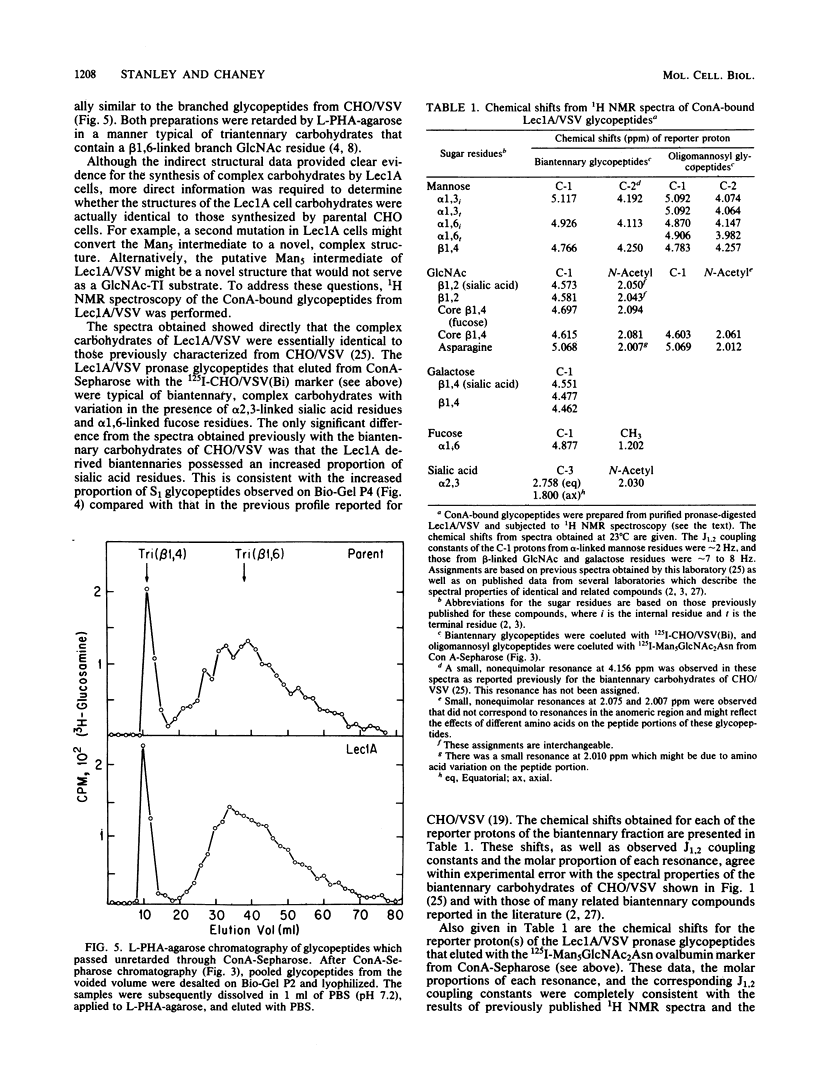

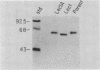
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell C., Stanley P. A dominant mutation to ricin resistance in Chinese hamster ovary cells induces UDP-GlcNAc:glycopeptide beta-4-N-acetylglucosaminyltransferase III activity. J Biol Chem. 1984 Nov 10;259(21):13370–13378. [PubMed] [Google Scholar]
- Carver J. P., Grey A. A. Determination of glycopeptide primary structure by 360-MHz proton magnetic resonance spectroscopy. Biochemistry. 1981 Nov 10;20(23):6607–6616. doi: 10.1021/bi00526a014. [DOI] [PubMed] [Google Scholar]
- Carver J. P., Grey A. A., Winnik F. M., Hakimi J., Ceccarini C., Atkinson P. H. Determination of the Structure of four glycopeptides from hen ovalbumin using 360-MHz proton magnetic resonance spectroscopy. Biochemistry. 1981 Nov 10;20(23):6600–6606. doi: 10.1021/bi00526a013. [DOI] [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J Biol Chem. 1982 Oct 10;257(19):11230–11234. [PubMed] [Google Scholar]
- Etchison J. R., Robertson J. S., Summers D. F. Host cell-dependent differences in the oligosaccharide moieties of the VSV G protein. J Gen Virol. 1981 Nov;57(Pt 1):43–52. doi: 10.1099/0022-1317-57-1-43. [DOI] [PubMed] [Google Scholar]
- Gottlieb C., Baenziger J., Kornfeld S. Deficient uridine diphosphate-N-acetylglucosamine:glycoprotein N-acetylglucosaminyltransferase activity in a clone of Chinese hamster ovary cells with altered surface glycoproteins. J Biol Chem. 1975 May 10;250(9):3303–3309. [PubMed] [Google Scholar]
- Hakimi J., Carver J., Atkinson P. H. Application of high-field proton magnetic resonance spectroscopy in the structural determination of membrane-derived Sindbis virus glycopeptides. Biochemistry. 1981 Dec 8;20(25):7314–7319. doi: 10.1021/bi00528a041. [DOI] [PubMed] [Google Scholar]
- Hammarström S., Hammarström M. L., Sundblad G., Arnarp J., Lönngren J. Mitogenic leukoagglutinin from Phaseolus vulgaris binds to a pentasaccharide unit in N-acetyllactosamine-type glycoprotein glycans. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1611–1615. doi: 10.1073/pnas.79.5.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpaz N., Schachter H. Control of glycoprotein synthesis. Bovine colostrum UDP-N-acetylglucosamine:alpha-D-mannoside beta 2-N-acetylglucosaminyltransferase I. Separation from UDP-N-acetylglucosamine:alpha-D-mannoside beta 2-N-acetylglucosaminyltransferase II, partial purification, and substrate specificity. J Biol Chem. 1980 May 25;255(10):4885–4893. [PubMed] [Google Scholar]
- Hirschberg C. B., Baker R. M., Perez M., Spencer L. A., Watson D. Selection of mutant Chinese hamster ovary cells altered glycoproteins by means of tritiated fucose suicide. Mol Cell Biol. 1981 Oct;1(10):902–909. doi: 10.1128/mcb.1.10.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh P., Rosner M. R., Robbins P. W. Host-dependent variation of asparagine-linked oligosaccharides at individual glycosylation sites of Sindbis virus glycoproteins. J Biol Chem. 1983 Feb 25;258(4):2548–2554. [PubMed] [Google Scholar]
- Narasimhan S. Control of glycoprotein synthesis. UDP-GlcNAc:glycopeptide beta 4-N-acetylglucosaminyltransferase III, an enzyme in hen oviduct which adds GlcNAc in beta 1-4 linkage to the beta-linked mannose of the trimannosyl core of N-glycosyl oligosaccharides. J Biol Chem. 1982 Sep 10;257(17):10235–10242. [PubMed] [Google Scholar]
- Nilsson B., Nordén N. E., Svensson S. Structural studies on the carbohydrate portion of fetuin. J Biol Chem. 1979 Jun 10;254(11):4545–4553. [PubMed] [Google Scholar]
- Oppenheimer C. L., Hill R. L. Purification and characterization of a rabbit liver alpha 1 goes to 3 mannoside beta 1 goes to 2 N-acetylglucosaminyltransferase. J Biol Chem. 1981 Jan 25;256(2):799–804. [PubMed] [Google Scholar]
- Robertson M. A., Etchison J. R., Robertson J. S., Summers D. F., Stanley P. Specific changes in the oligosaccharide moieties of VSV grown in different lectin-resistnat CHO cells. Cell. 1978 Mar;13(3):515–526. doi: 10.1016/0092-8674(78)90325-2. [DOI] [PubMed] [Google Scholar]
- Sefton B. M. Virus-dependent glycosylation. J Virol. 1975 Jan;17(1):85–93. doi: 10.1128/jvi.17.1.85-93.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P., Caillibot V., Siminovitch L. Selection and characterization of eight phenotypically distinct lines of lectin-resistant Chinese hamster ovary cell. Cell. 1975 Oct;6(2):121–128. doi: 10.1016/0092-8674(75)90002-1. [DOI] [PubMed] [Google Scholar]
- Stanley P. Carbohydrate heterogeneity of vesicular stomatitis virus G glycoprotein allows localization of the defect in a glycosylation mutant of CHO cells. Arch Biochem Biophys. 1982 Nov;219(1):128–139. doi: 10.1016/0003-9861(82)90141-2. [DOI] [PubMed] [Google Scholar]
- Stanley P. Lectin-resistant CHO cells: selection of new mutant phenotypes. Somatic Cell Genet. 1983 Sep;9(5):593–608. doi: 10.1007/BF01574260. [DOI] [PubMed] [Google Scholar]
- Stanley P., Narasimhan S., Siminovitch L., Schachter H. Chinese hamster ovary cells selected for resistance to the cytotoxicity of phytohemagglutinin are deficient in a UDP-N-acetylglucosamine--glycoprotein N-acetylglucosaminyltransferase activity. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3323–3327. doi: 10.1073/pnas.72.9.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. Selection of lectin-resistant mutants of animal cells. Methods Enzymol. 1983;96:157–184. doi: 10.1016/s0076-6879(83)96015-9. [DOI] [PubMed] [Google Scholar]
- Stanley P. Selection of specific wheat germ agglutinin-resistant (WgaR) phenotypes from Chinese hamster ovary cell populations containing numerous lecR genotypes. Mol Cell Biol. 1981 Aug;1(8):687–696. doi: 10.1128/mcb.1.8.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P., Siminovitch L. Selection and characterization of Chinese hamster ovary cells resistant to the cytotoxicity of lectins. In Vitro. 1976 Mar;12(3):208–215. doi: 10.1007/BF02796443. [DOI] [PubMed] [Google Scholar]
- Stanley P., Vivona G., Atkinson P. H. 1H NMR spectroscopy of carbohydrates from the G glycoprotein of vesicular stomatitis virus grown in parental and Lec4 Chinese hamster ovary cells. Arch Biochem Biophys. 1984 Apr;230(1):363–374. doi: 10.1016/0003-9861(84)90119-x. [DOI] [PubMed] [Google Scholar]
- Tabas I., Schlesinger S., Kornfeld S. Processing of high mannose oligosaccharides to form complex type oligosaccharides on the newly synthesized polypeptides of the vesicular stomatitis virus G protein and the IgG heavy chain. J Biol Chem. 1978 Feb 10;253(3):716–722. [PubMed] [Google Scholar]