Key Points
TP53 3′UTR variations demonstrate prognostic value in DLBCL.
Abstract
We identified multiple single nucleotide variants (SNVs) in the TP53 3′ untranslated region (3′UTR) in tumor specimens from 244 patients with diffuse large B-cell lymphoma (DLBCL). Patients carrying a wild-type TP53 coding sequence (CDS) and 1 or more 3′UTR SNVs had a better 5-year survival rate than patients carrying a wild-type CDS and the reference 3′UTR, yet there is no statistically significance difference in overall survival (OS). In contrast, 3′UTR variation predicted poorer OS for patients with a mutant TP53 CDS. We then sequenced TP53 3′UTR in 247 additional DLBCL patients as a validation set. Altogether, we identified 187 novel SNVs; 36 occurred at least twice. Most of the newly identified 3′UTR SNVs were located at sites that are complementary to seed sequences of microRNAs (miRNAs) that are predicted or experimentally known to target TP53. Three SNVs disrupt the seed match between miR-125b and the TP53 3′UTR, thereby impeding suppression of p53 by this miRNA. In addition, a germline SNV (rs78378222) located in the TP53 polyadenylation signal resulted in downregulation of both p53 messenger RNA and protein levels and reduction of cellular apoptosis. This study is the first to demonstrate the prognostic value of the TP53 3′UTR in cancer.
Introduction
The tumor suppressor p53, encoded by TP53, is recognized as the guardian of the human genome. p53 responds to diverse stressors by regulating many downstream target genes that promote cellular DNA repair, apoptosis, cell cycle arrest, and senescence. Restoring p53 function alone is sufficient to cause regression of several types of cancer in mice.1,2 This characteristic suggests that p53 itself or the p53 pathway must be derailed for tumor development, genetically or otherwise. Three distinct sets of nucleotide alterations can occur in the human TP53 gene: tumor-associated (somatic) mutations, germline Li-Fraumeni mutations, and germline p53 polymorphisms.3,4 Both tumor-associated mutations and Li-Fraumeni mutations occur in the p53 coding sequence (CDS) and produce mutant p53 proteins that lack most or all of the normal p53 functions and confer oncogenic properties.5 For instance, a missense base substitution at codon 273 of human TP53 (R273H) is frequently observed in somatic tumors and in tumors from Li-Fraumeni syndrome patients. Mouse models demonstrate that this mutant p53 allele has enhanced oncogenic potential that cannot be explained by simple loss of p53 function.6,7
In general, p53 CDS mutations contribute to oncogenesis by 2 distinct mechanisms: (1) mutant alleles can have dominant negative effects on wild-type (WT) p53 function; and (2) mutant p53 protein can actively promote tumorigenesis independently of WT p53 function. It is accepted that the WT TP53 CDS produces native p53 tumor suppressor, whereas most TP53 CDS mutations produce oncogenic mutant p53. By contrast, only a few of the >200 naturally occurring germline variants of TP53 in human populations cause measurable perturbation of p53 function.3 More than 90% of these p53 variants occur in 10 introns alleged to have no cancer-related biological consequences. None of the 20 variants in the TP53 CDS have been linked reproducibly to cancer predisposition.3,4 The best-studied TP53 CDS polymorphism is codon 72 (R72P), owing to an earlier finding that human papillomavirus causes more efficient degradation of p53R72 than p53P72.8 However, few associations between this polymorphism and various types of cancer have been found.3 Sequence variation in the untranslated regions (UTRs) of the TP53 gene has not been extensively studied.
Diffuse large B-cell lymphoma (DLBCL) is the most common type of lymphoma and is clinically heterogeneous with subsets of patients with better or worse prognosis.9,10 Biologically, DLBCL has been known to be heterogeneous with germinal center B-cell (GCB) and activated B-cell (ABC) subtypes. Recent advancements in next-generation sequencing have further broadened our understanding of genetic lesions in DLBCL. Three DLBCL-sequencing projects have provided a wealth of information on whole genomes, exomes, and/or transcriptomes for more than 100 DLBCL patient specimens, some with matched healthy tissues as controls.11-13 These studies have identified mutations in more than 100 genes not implicated previously in DLBCL pathogenesis, including genes involved in chromatin modification and immune regulation. The TP53 gene (the CDS) was found to be mutated in ∼20% of DLBCL cases in all 3 deep-sequencing projects. However, MLL2,11,13 CREBBP,11 EZH2,12 BCL2,12 GNA13,13 BTG1,13 PIM1,13 and PCLO13 were found to be mutated more frequently than the TP53 CDS in some of these studies. These findings challenge the notion that TP53 is the most commonly mutated gene in cancer14,15 at least within the context of DLBCL. In addition, noncoding genomic sequences have been largely overlooked in these sequencing projects.11,12
In a previous study, we found that ∼20% of DLBCL patients who received cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy had TP53 CDS mutations and that these mutations adversely affected prognosis.16 To provide an in-depth analysis of DLBCL patients treated with CHOP plus the monoclonal antibody rituximab (R-CHOP), we assembled a multicenter study designated as the DLBCL R-CHOP Collaborative Consortium. We reported that TP53 CDS mutations predicted poorer survival in 506 (largest-ever cohort) R-CHOP–treated DLBCL patients with either the GCB or the ABC subtype, and that TP53 monoallelic deletion and loss of heterozygosity did not confer worse survival.17 These findings suggested that assessment of TP53 CDS mutation status can be useful for stratifying patients receiving R-CHOP treatment into distinct prognostic subsets.
In this study, we retrospectively sequenced the TP53 5′UTR and 3′UTR in DLBCL patients to determine if TP53 3′UTR sequence alterations occur in cancer patients and to assess their potential prognostic importance. We also evaluated the potential impact of TP53 3′UTR sequence variation in combination with CDS mutations, and we determined the functional significance of 3′ UTR variants in p53 regulation.
Materials and methods
Patients
For the initial study group, we randomly selected 244 patients (training set) with DLBCL from a total of 506 de novo DLBCL patients treated with R-CHOP, whose TP53 CDSs had been sequenced in a previous study by our consortium.17 For the validation set, tumor specimens were also obtained from 247 DLBCL patients receiving R-CHOP treatment in the consortium program, none of whom were included in the 506 DLBCL patients in the previous p53 CDS study.17
All cases were reviewed by a group of hematopathologists (S.M.M., M.A.P., M.B.M., A.T., and K.H.Y.), and the diagnoses confirmed by World Health Organization classification criteria. The current study and material transfer agreement were approved by the institutional review board of each participating center. The overall collaboration was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center, Houston, Texas. This study was conducted in accordance with the Declaration of Helsinki.
All of these patients have undergone molecular and phenotypic classification with available clinical data. There are no statistical differences in regard to clinical parameters (age, gender, stage, lactic dehydrogenase level, tumor size, performance status, chemotherapy regimen, survival time, and recurrence rate), GCB/ABC classification and distribution, and survival among 3 groups (244 cases in the training set, 247 cases in the validation set, and unselected remaining 262 cases).
DNA sequencing and cell biology
Genomic DNA was extracted from formalin-fixed, paraffin-embedded tissue samples using the HighPure paraffin DNA extraction kit (Qiagen). The TP53 UTRs were sequenced bidirectionally using the Sanger method at Polymorphic DNA Inc. (Alameda, CA). All sequences were compared with the TP53 reference sequence (NM_000546.5). Single nucleotide variants (SNVs) in the 3′UTR were grouped into single nucleotide polymorphisms (SNPs) and novel SNVs (nSNVs). Any nucleotide change listed in the dbSNP database was considered an SNP; the remainder (none occurring in ≥1% of patients) were considered nSNVs.
The p53-null human lung adenocarcinoma cancer cell line H1299 was obtained from the American Type Culture Collection (Manassas, VA). For mutational analysis, the p53 expression vector pCMV-p53 (Clontech, Mountain View, CA) was modified to contain TP53 5′ and 3′UTRs as well as the 2.5-kb genomic DNA downstream of the 3′UTR. The resulting constructs were designated as TP53Ext, TP53ExtC1170T, and TP53ExtA1175C, respectively. MicroRNA (miRNA) expression vectors and Western blotting analysis were described previously18 and are included in the supplemental Materials and methods (see the Blood Web site).
Statistical analysis
All statistical calculations were performed using GraphPad Prism 5. Overall survival (OS) was calculated from time of diagnosis to time of death from any cause or last follow-up. Progression-free survival (PFS) was calculated from time of diagnosis to time of progression or death from any cause. The actuarial probabilities of OS and PFS17 were determined using the Kaplan-Meier method, and differences were compared using the log-rank test. The 5-year survival and 5-year PFS rates describe the percentage of patients that are alive 5 years after their cancer is diagnosed; they were compared using Fisher’s exact test. All differences with P ≤ .05 were considered statistically significant.
Results
Distribution and frequency of SNVs in TP53 UTRs in 244 de novo DLBCL patients
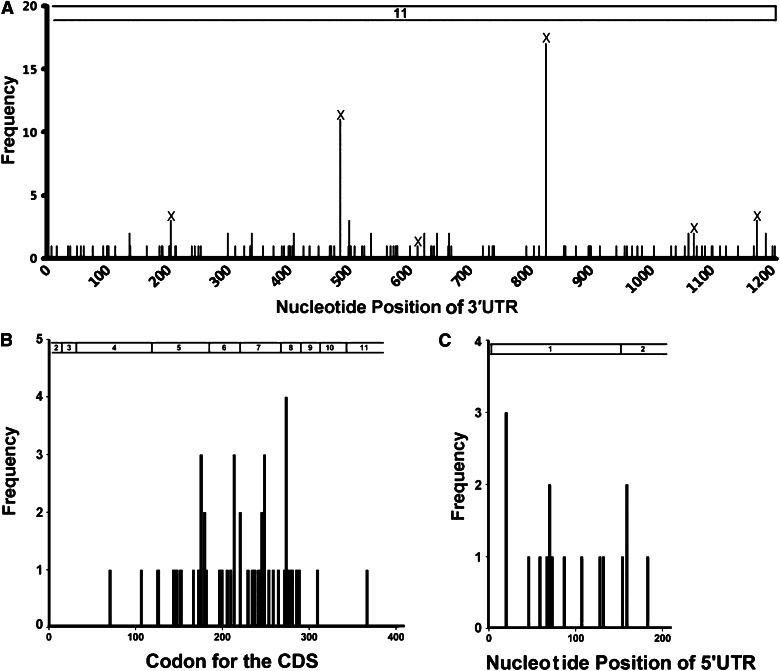
In the initial group of 244 DLBCL samples, we identified 128 SNVs in the UTRs of 107 patients, with 14 in the 5′UTR and 114 in the 3′UTR (Figure 1A; supplemental Table 1). Six 3′UTR SNVs have been reported as SNPs in dbSNP and occurred 37 times (Table 1). We designated the newly found noncoding SNVs in the TP53 gene as nSNVs. A total of 108 nSNVs were identified with 121 occurrences in the 3′UTR in 76 patients. More patients had at least 1 nSNV in the 3′UTR (n = 76, 31%) than they had nonsynonymous mutations in the CDS (n = 49, 20%). In the 76 patients with 3′UTR nSNVs, 32 (42%) had multiple lesions, compared with 2 of 49 (4%) patients with 2 or more CDS mutations. Ten of the TP53 3′UTR nSNVs occurred twice, and 1 occurred 3 times (Figure 1A). The 5′UTR SNVs occurred at a much lower frequency (n = 15 patients, 6%; supplemental Table 1; none of them reported in dbSNP) and were not further analyzed in the current study.
Figure 1.
Distribution of variation in the TP53 gene in 244 de novo DLBCL patients. (A) Distribution of SNVs in the 3′UTR (part of exon 11; nucleotides 1385-2591 of NM_000546.5 of the TP53 reference mRNA). (B) Distribution of CDS mutations in exons 3 to 10 and part of exons 2 and 11 (top bar; nucleotides 203-1384 of NM_000546.5). (C) Distribution of 5′UTR SNVs in exon 1 and part of exon 2, nucleotides 1-202 of NM_000546.5. Numbers on the top indicate exons.
Table 1.
Frequency of known TP53 3′UTR SNPs in DLBCL cohorts
| SNP ID | Position† | MAF‡ | Heterozygous frequency | Validation§ | SNP occurrence* | |
|---|---|---|---|---|---|---|
| First group (244) | Second group (247) | |||||
| rs8065799 | 9 A>C | N/A | N/A | — | 0 | 0 |
| rs1042526 | 37 A>T | N/A | N/A | Precious | 0 | 0 |
| rs35919705 | 105 C>T | 0.010 | 0.015 | Frequency | 0 | 0 |
| rs16956880 | 205 G>A | 0.032 | 0.024 | Cluster, Frequency, 1000Genome | 3 | 2 |
| rs79516338 | 309 G>A | N/A | 0.500 | Frequency | 0 | 0 |
| rs34486624 | 314 G>A | N/A | 0.020 | Frequency | 0 | 0 |
| rs17881366 | 328 G>A | 0.026 | 0.010 | Cluster, Frequency, 1000Genome | 0 | 0 |
| rs4968187 | 485 G>A | 0.035 | N/A | Cluster, Frequency, 1000Genome Precious | 11 | 5 |
| rs916131 | 491 G>A | N/A | N/A | Precious | 0 | 0 |
| rs916132 | 514 T>C | N/A | N/A | Precious | 0 | 0 |
| rs1811095 | 534 C>T | N/A | N/A | Cluster | 0 | 0 |
| rs35944977 | 534-535 TC>CT | N/A | N/A | — | 0 | 0 |
| rs1811096 | 535 C>T | N/A | N/A | — | 0 | 1 |
| rs17886358 | 569-570 GT>del | N/A | 0.435 | Cluster, Frequency | 0 | 0 |
| rs34607064 | 595 C>del | N/A | N/A | — | 0 | 0 |
| rs66759322 | 611 A>del | N/A | N/A | — | 0 | 0 |
| rs17879353 | 613 C>A | 0.007 | N/A | Cluster, Frequency | 1 | 1 |
| rs1042535 | 634 C>CG>G>GA | N/A | N/A | — | 0 | 0 |
| rs1794291 | 635 A>G | N/A | N/A | Cluster | 0 | 0 |
| rs34182553 | 674 C>del | N/A | N/A | — | 0 | 0 |
| rs77011241 | 773 C>T | N/A | 0.389 | Frequency, 1000Genome | 0 | 0 |
| rs35940853 | 798 T>del | N/A | N/A | — | 0 | 0 |
| rs17884306 | 826 G>A | 0.044 | N/A | Cluster, Frequency, 1000Genome, Precious | 17 | 24 |
| rs17884586 | 855 C>T | N/A | 0.012 | — | 0 | 0 |
| rs35659787 | 886 G>A | N/A | 0.10 | Frequency | 0 | 0 |
| rs55817367 | 925 C>T | 0.007 | N/A | 1000Genome | 0 | 0 |
| rs1794292 | 995 G>C | N/A | N/A | — | 0 | 0 |
| rs1794293 | 1057 C>G | N/A | N/A | — | 0 | 0 |
| rs114831472 | 1070 C>T | 0.032 | 0.065 | 1000Genome | 2 | 1 |
| rs78378222 | 1175 A>C | 0.010 | N/A | Cluster, Frequency, 1000Genome | 3 | 4 |
Minor allele found in the initiation set (244 cases) or the validation set (247 cases). Some patients possess more than 1 SNP. Underlining in column 2 indicates SNPs found in the cohort.
Counted from first nucleotide of the TP53 3′UTR; “del,” deletion.
MAF, minor allele frequency; N/A, not available.
Validation: “Cluster,” multiple submissions mapped to the same location and clustered into 1 refSNP cluster and assigned a reference SNP ID number; “Frequency,” validated by both population frequency data and genotype data; “1000Genome,” validated by the 1000 Genomes Project; “Precious,” cited as clinically relevant in the literature and belonging to a reserved set of clinically associated SNPs in dbSNP.
Most TP53 CDS somatic mutations are clustered within exons 5 to 8.19 Although the nucleotide length (544 bp) of exons 5 to 8 accounts for only ∼46% of the coding exons (1182 bp), mutations in these exons account for 94% of all CDS somatic mutations.19 Our analysis of TP53 CDS in the 506 DLBCL patients confirmed the uneven codon distribution of single nucleotide missense substitutions: mutations in exons 5 to 8 account for 90% of all mutations in coding exons (exons 3-10 plus parts of exon 2 and exon 11).17 In the initial study group of 244 patients, 92% of all CDS mutations were found in exons 5 to 8 (45 of 49 cases, Figure 1B). In contrast, 3′UTR nSNVs were evenly dispersed across the 1207-bp 3′UTR in the 76 patients with nSNVs (Figure 1A). The distribution of SNVs in the 5′UTR in the 244 cases was similar to that in the 3′UTR (Figure 1C). These data suggest that UTR variants in the TP53 gene are distributed more evenly than are CDS mutations.
Survival in relation to clinical characteristics and TP53 3′UTR variation
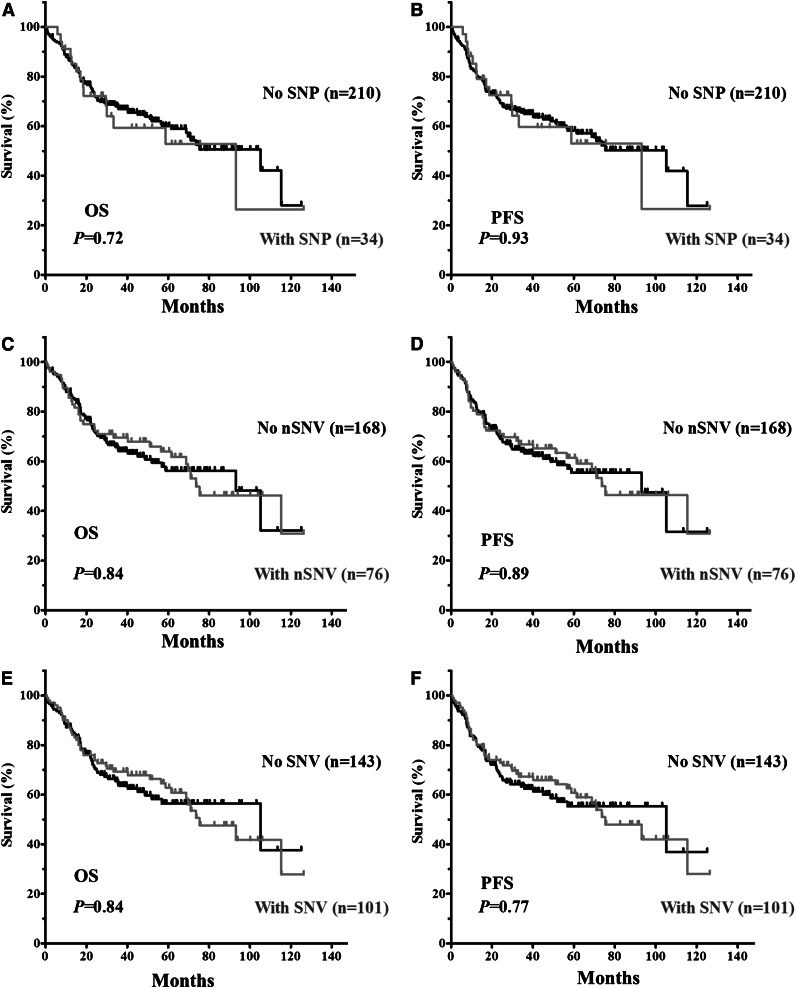
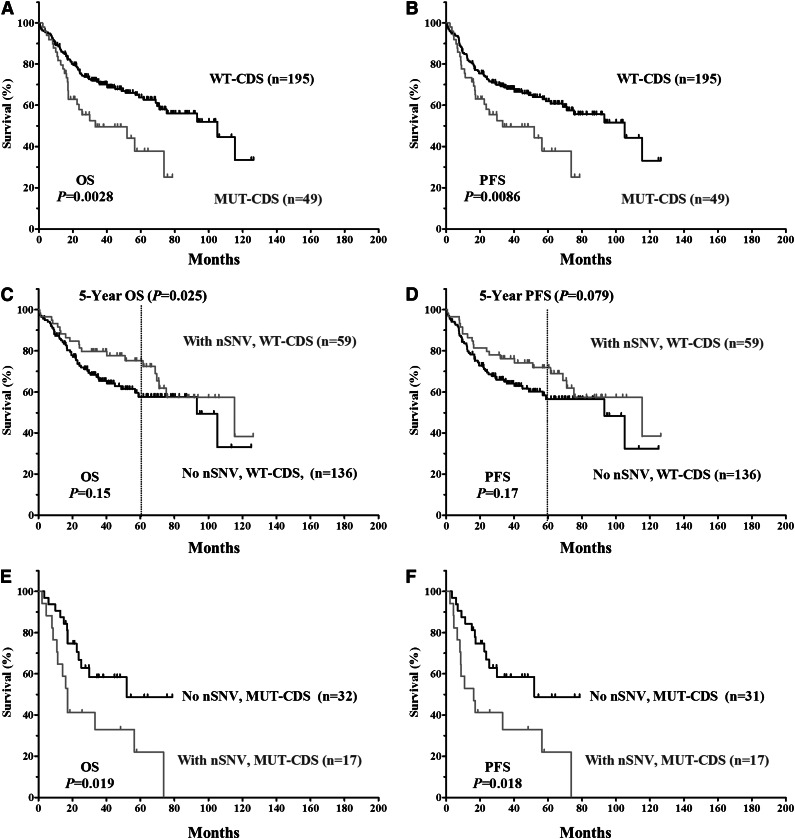
The clinical and biological characteristics of the 244 DLBCL patients and their impact on OS and PFS are summarized in supplemental Table 2. We first verified that no adverse clinical variables were associated with variation in the 3′UTR. When patients were divided based on the presence of TP53 3′UTR lesions (SNPs only, nSNVs only, or any SNVs [either SNP or nSNV]), no significant difference in OS or PFS was found (Figure 2). TP53 CDS mutations (MUT-CDS) predicted poorer OS (P = .0028) and PFS (P = .0086) in this 244-patient cohort (Figure 3A-B). We then divided patients into 2 groups according to the p53 CDS status and analyzed survival in relation to SNVs. We found that SNPs in the TP53 3′UTR had no impact on patient OS or PFS (supplemental Figure 1). Of the patients with WT-CDS only, those without nSNVs (n = 136) seemed to have poorer OS and PFS than those with nSNVs (n = 59), but the difference was not statistically significant (P = .15 for OS; P = .17 for PFS, respectively; Figure 3C-D). When 5-year survival rates were calculated, nSNVs conferred better survival in patients with WT-CDS (78% vs 59%, P = .025), and 5-year PFS also showed a trend (76% vs 58%, P = .079). In contrast, patients with MUT-CDS and nSNVs (n = 17) had poorer OS and PFS than did patients with MUT-CDS and no nSNVs (n = 32; P = .019 for OS; P = .018 for PFS; Figure 3E-F). Indeed, all patients with MUT-CDS and nSNVs died within 76 months after diagnosis (Figure 3E). We also combined SNPs and nSNVs as total SNVs; there were similar survival trends, but they were not statistically significant (supplemental Figure 2). After the cohort was divided into ABC- and GCB-DLBCL subsets, the prognostic impact of nSNVs in OS seemed to favor patients with WT-CDS and not favor patients with MUT-CDS in the GCB subset. Yet none were statistically significantly, most likely due to a smaller cohort (supplemental Figure 3). Again, nSNVs conferred better 5-year survival in GCB patients with WT-CDS (P = .0006). We did not find statistical differences in frequencies of subtypes, CDS mutation, and TP53 monoallelic deletion between patients with nSNVs and those without.
Figure 2.
Kaplan-Meier estimates of OS and PFS in DLBCL patients with SNVs in the TP53 3′UTR. (A) OS of patients with or without SNPs. (B) PFS of patients with or without SNPs. (C) OS of patients with or without nSNVs. (D) PFS of patients with or without nSNV. (E) OS of patients with or without SNVs (either SNP or nSNV). (F) PFS of patients with or without SNVs.
Figure 3.
Kaplan-Meier estimates of OS and PFS in DLBCL patients classified according to TP53 CDS status. (A) OS of patients with or without an MUT-CDS. (B) PFS of patients with or without an MUT-CDS. (C) OS of patients with a WT-CDS and nSNV. (D) PFS of patients with a WT-CDS and nSNVs. (E) OS of patients with an MUT-CDS and nSNV. (F) PFS of patients with MUT-CDS and nSNVs.
Validation of TP53 3′UTR variants
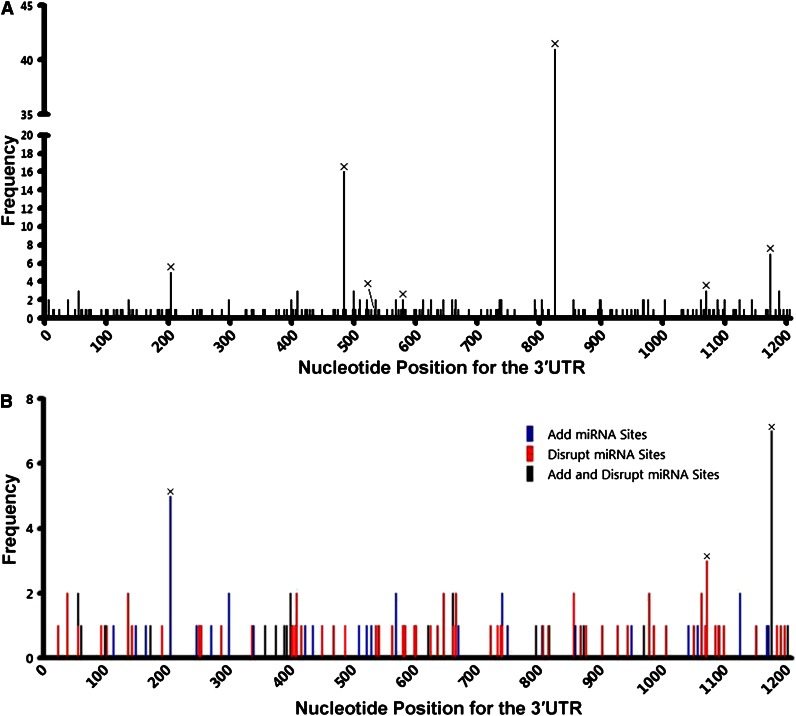
To determine whether nSNVs are common in DLBCL, we sequenced the TP53 3′UTR in tumor samples from an additional 247 DLBCL patients. We found 106 nSNVs in 70 (28%) patients with 5 occurring twice. All 6 SNPs identified in the initial set were found in this 247 patient group (Table 1; supplemental Table 3). For the combined 491 patients, 187 nSNVs were identified in the TP53 3′UTR: 151 occurred once, 31 occurred twice, and 5 occurred 3 times (supplemental Table 4). These data indicate that nSNVs in the TP53 3′UTR occur frequently in DLBCL. In addition, nSNVs were dispersed across the whole TP53 3′UTR (Figure 4A), providing additional evidence that 3′UTR nSNVs are distributed more evenly than are CDS mutations.
Figure 4.
Variation in the TP53 3′UTR in 491 de novo DLBCL patients. (A) Distribution of SNVs in the 3′UTR. (B) Potential effects of SNVs on the interaction of p53 mRNA and miRNAs that are experimentally validated or predicted (by TargetScan) to target the TP53 3′UTR. SNVs listed in dbSNP are marked with ×.
3′UTR variants in miRNA-binding sites
Eleven miRNAs are reported to target the human TP53 gene: miR-25, miR-30d,20 miR-125b,21 miR-504,22 miR-380-5p,23 miR-92a, miR-141,24 miR-200a,25 miR-1285,26 miR-15a, and miR-16.27 Among these miRNAs, 3 pairs have the same seed sequence: miR-25 and miR-92a, miR-141 and miR-200a, and miR-15a and miR-16. In addition, miR-504 and miR-1285 target 2 sites each. Therefore, the TP53 3′UTR has a total of 10 experimentally validated miRNA-targeting sites. Except for the miR-141 and miR-380-5p sites, 8 of 10 miRNA sites had 1 or more nSNVs occurring at least once in 491 patients (Table 2). We analyzed the potential of nSNVs to affect putative miRNA binding as predicted by TargetScan,28,29 in which 8 nucleotides in the target 3′UTR are critical to miRNA suppression: a sequence complementary to the 6-nucleotide seed sequence of the miRNAs and 2 flanking nucleotides (A1 and m8). Of the 146 patients carrying 1 or more nSNVs in the 3′UTR, 95 (65%) had nSNVs that disrupt and/or generate miRNA-binding sites as predicted by TargetScan; 78 of 95 patients carried nSNVs disrupting putative miRNA binding (Table 3; Figure 4B).
Table 2.
SNVs in the 3′UTR disrupting the validated seed match of TP53:miRNA
| TP53 3′UTR and miRNA† | Consequential pairing† | Seed match‡ | No. of patients* | |
|---|---|---|---|---|
| Seed + m8 + 1A | Other | |||
| Pos. 93-98 | 5′ CUUUGAACCCUUGCU––UGCAAUA | 7mer-1A | 1(1) | 0 |
| |::||||||||| | ||||
| hsa-miR-25/92a | 3′ AGUCUGGCUCUGUUCACGUUAC | |||
| |x|||| | ||||
| nSNV 93G>A | 5′ CUUUGAACCCUUGCU––UACAAUA | NO | ||
| Pos. 287-292 | 5′ UGCAGUUAAGGGUUAGUUUACA | 7mer-1A | 1(1) | 0 |
| |||| ||||||| | ||||
| hsa-miR-30d | 3′ GAAGGUCAGCCCCUA––CAAAUGU | |||
| |||| x|||||| | ||||
| nSNV 287G>A | 5′ UGCAGUUAAGGGUUAAUUUACA | NO | ||
| Pos.734-739 | 5′ AAGACUUGUUUUAUGCUCAGGGU | 7mer-m8 | 6(4) | 3(2) |
| ||| ||||||| | ||||
| hsa-miR-125b | 3′ AGUGUUCAAUCCCAGAGUCCCU | |||
| ||| x|||x|xx | ||||
| nSNV 733C>T | 5′ AAGACUUGUUUUAUGUUCAGGGU | NO | ||
| nSNV 737G>C | 5′ AAGACUUGUUUUAUGCUCACGGU | NO | ||
| nSNV 737G>A | 5′ AAGACUUGUUUUAUGCUCAAGGU | NO | ||
| nSNV 739G>A | 5′ AAGACUUGUUUUAUGCUCAGGAU | NO | ||
| nSNV 739G>C | 5′ AAGACUUGUUUUAUGCUCAGGCU | NO | ||
| nSNV 740U>A | 5′ AAGACUUGUUUUAUGCUCAGGGA | 8mer | ||
| Pos. 736-741 | 5′ GACUUGUUUUAUGCUCAGGGUCA | 8mer | 6(5) | 0 |
| ||||||| | ||||
| hsa-miR-504 | 3′ CUAUCUCACGUCUGGUCCCAGA | |||
| ||x|xx| | ||||
| nSNV 737G>C | 5′ GACUUGUUUUAUGCUCAAGGUCA | NO | ||
| nSNV 737G>A | 5′ GACUUGUUUUAUGCUCACGGUCA | NO | ||
| nSNV 739G>A | 5′ GACUUGUUUUAUGCUCAGGAUCA | NO | ||
| nSNV 739G>C | 5′ GACUUGUUUUAUGCUCAGGCUCA | NO | ||
| nSNV 740U>A | 5′ GACUUGUUUUAUGCUCAGGGCCA | NO | ||
| Pos. 804-809 | 5′ UGGGUCUCGCUUUGUUGCCCAGG | 7mer-m8 | 3(2) | 4(3) |
| :||||||:||||||||||||| | ||||
| hsa-miR-1285 | 3′ UCCAGAGUGAAACAACGGGUCU | |||
| :||||||:||||||||xx||| | ||||
| nSNV 805C>T | 5′ UGGGUCUCGCUUUGUUGTCCAGG | NO | ||
| nSNV 806C>T | 5′ UGGGUCUCGCUUUGUUGCTCAGG | NO | ||
| Pos. 967-972 | 5′ GGGGUCUCACAGUGUUGCCCAGG | 7mer-m8 | 5(2) | 2(2) |
| :|||||||| ||||||| | ||||
| hsa-miR-1285 | 3′ UCCAGAGUGAAACAACGGGUCU | |||
| :|||||||| ||||||| | ||||
| nSNV 968C>T | 5′ GGGGUCUCACAGUGUUGTCCAGG | NO | ||
| nSNV 969C>T | 5′ GGGGUCUCACAGUGUUGCTCAGG | NO | ||
| Pos. 1065-1070 | 5′ CCACGUCCAGCUGGAAGGGUCA | 7mer-1A | 4(3) | 1(1) |
| |||||| | ||||
| hsa-miR-504 | 3′ CUAUCUCACGUCUGGUCCCAGA | |||
| |x|x|x | ||||
| nSNV 1066G>A | 5′ CCACGUCCAGCUGGAAAGGUCA | NO | ||
| nSNV 1068G>A | 5′ CCACGUCCAGCUGGAAGGAUCA | NO | ||
| SNP 1070C>T | 5′ CCACGUCCAGCUGGAAGGGUUA | NO | ||
| nSNV 1071A>G | 5′ CCACGUCCAGCUGGAAGGGUCG | 6mer | ||
| Pos. 1084-1089 | 5′ GUCAACAUCUUUUACAUUCUGCAA | ? | 3(2) | 6(5) |
| || | | ||| | |||| | | ||||
| hsa-miR-15 | 3′ GUGUUUGGUAAUACACGACGAU | |||
| hsa-miR-16 | 3′ GCGGUUAUAAAUGCACGACGAU | |||
| | || ||||| | |||| | | ||||
| nSNV 1085C>T | 5′ GUCAACAUCUUUUACAUUCUGUAA | |||
| nSNV 1086A>G | 5′ GUCAACAUCUUUUACAUUCUGCGA | |||
Number of patients carrying SNVs affecting putative miRNA binding. Unique SNVs are indicated in parentheses. SNVs affecting TP53:miRNA complementarity other than the seed match are not included. Underlining in the column 2 indicates the altered nucleotides.
Nucleotides in the TP53 3′ UTR are numbered starting from the one after the stop codon. Nucleotides complementary to the seed sequence (positions 2-7) of miRNAs are indicated. Position (pos.) numbers correspond to nucleotides that are complementary to the miRNA seed sequence (in bold). rs114831472, affecting miR-504 binding, is “SNP 1070C.T.” One miR-504 site overlaps with the miR-125b site. “x” indicates that base pairing is disrupted.
The 7mer-m8 site comprises the seed match supplemented by a Watson-Crick match to miRNA nucleotide 8. The 7mer-A1 site comprises the seed match supplemented by an A across from miRNA nucleotide 1. The 8mer site comprises the seed match supplemented by both m8 and A1. “NO” indicates that the seed match was abolished. “?” indicates unconventional interaction.
Table 3.
Occurrence of nSNVs in the TP53 3′UTR potentially affecting miRNA binding in DLBCL
| Subgroup | No. of patients (%) | ||
|---|---|---|---|
| Training Set 76 of 244 | Validation Set 70 of 247 | Total 146 of 491 | |
| TP53 CDS status | |||
| MUT-CDS | 16 (21) | NA | NA |
| WT-CDS | 60 (79) | NA | NA |
| 3′UTR nSNV affecting putative miRNA binding* | |||
| Generating only | 12 | 5 | 17 |
| Disrupting only | 31 | 23 | 54 |
| Generating and disrupting | 15 | 9 | 24 |
| Others | 18 | 33 | 51 |
| No. of 3′UTR nucleotides altered | |||
| 1 | 42 (59.5) | 42 (60) | 84 (57.5) |
| 2 | 19 (23.8) | 18 (25.7) | 37 (25.3) |
| 3 | 8 (9.5) | 7 (10) | 15 (10.3) |
| 4 | 5 (4.8) | 3 (4.3) | 8 (5.5) |
| 5 | 1 (1.2) | 0 | 1 (0.7) |
| 6 | 1 (1.2) | 0 | 1 (0.7) |
Others, outside seed match or not in miRNA sites; NA, not applicable.
nSNVs predicted by TargetScan 5.0 to be targeted by miRNAs (based on the seed sequence of the miRNA plus A1 and m8). Some patients had both mutations generating miRNA binding and mutations disrupting it. Three SNPs listed in dbSNP that putatively affect miRNA binding (rs16956880 [205 G>A], rs11483 1472 [1070 G>A], and rs78378222 [1175 C>A]) were excluded.
p53 expression analysis of 3′UTR nSNVs
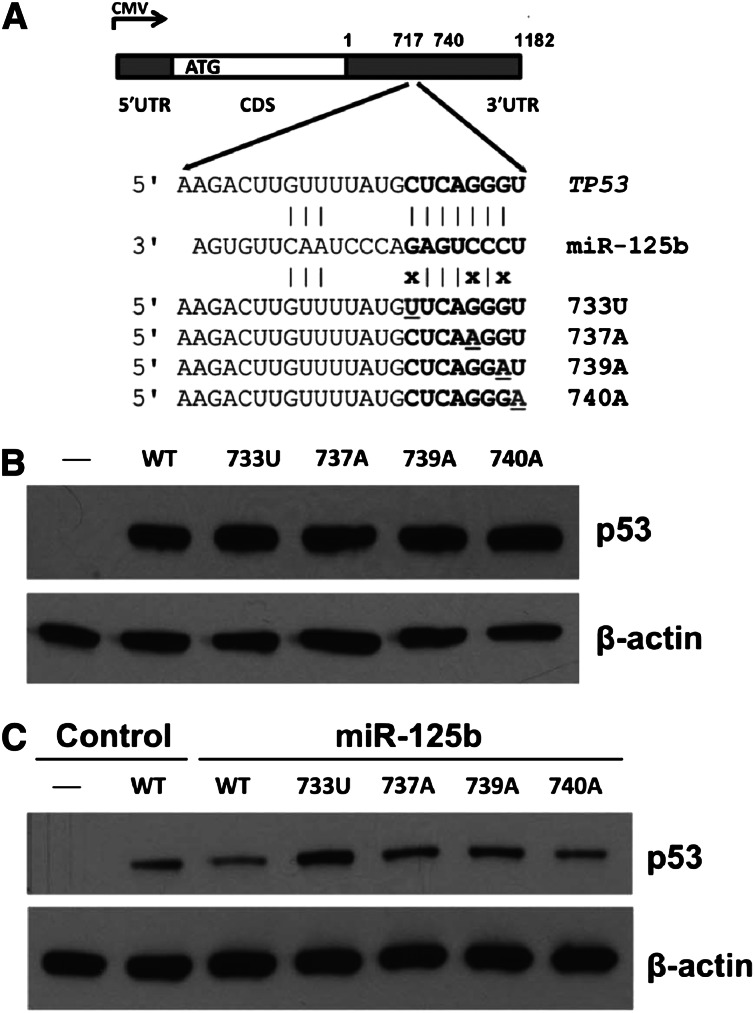
To test whether 3′UTR variation impacts p53 expression, we introduced gene expression cassettes carrying human TP53 complementary DNA (cDNA) with 3′UTR variants in the miR-125b–binding site (Figure 5A) into p53-null H1299 cells. As shown in Figure 5B, TP53 3′UTR nSNVs did not alter p53 expression compared with WT. However, when cells were cotransfected with miR-125b, 3 nSNVs (733U, 737A, and 739A) disrupting the seed match between miR-125b and the TP53 3′UTR released the negative regulation of p53 by miR-125b (Figure 5C). One variant (740A) that converts the seed match from 7mer to 8mer, which is predicted to have a greater effect on p53 downregulation, did not enhance p53 suppression by miR-125b. These results demonstrate that miRNAs and nSNVs in the TP53 3′UTR significantly alter p53 protein levels in vitro. We next analyzed the relationship between p53 protein expression with a 50% cutoff17 and 3′UTR nSNVs in the initiation set. We found that there was no correlation between nSNVs and p53 expression in the entire cohort, the WT-CDS group, or the MUT-CDS group (supplemental Table 5). There are at least 2 possibilities: (1) nSNVs may have either inhibitory or stimulatory effects on p53 regulation; and (2) the standard assay of p53 expression in tumors (percentage of cells with positive staining rather the signal strength)17 cannot detect the subtle modulation of p53 protein levels by nSNVs.
Figure 5.
Expression analyses of TP53 3′UTR variation affecting miR-125b binding in p53-null cells. (A) Schematic representation of the p53 cDNA construct and 3′UTR variants. (B) Western blot of p53 expression in p53-null H1299 cells transfected with p53 constructs. (C) Western blot of p53 expression in p53-null H1299 cells transfected with p53 constructs with or without miR-125b. β-actin was used as a control.
Functional significance of a germline polymorphism in the TP53 3′UTR
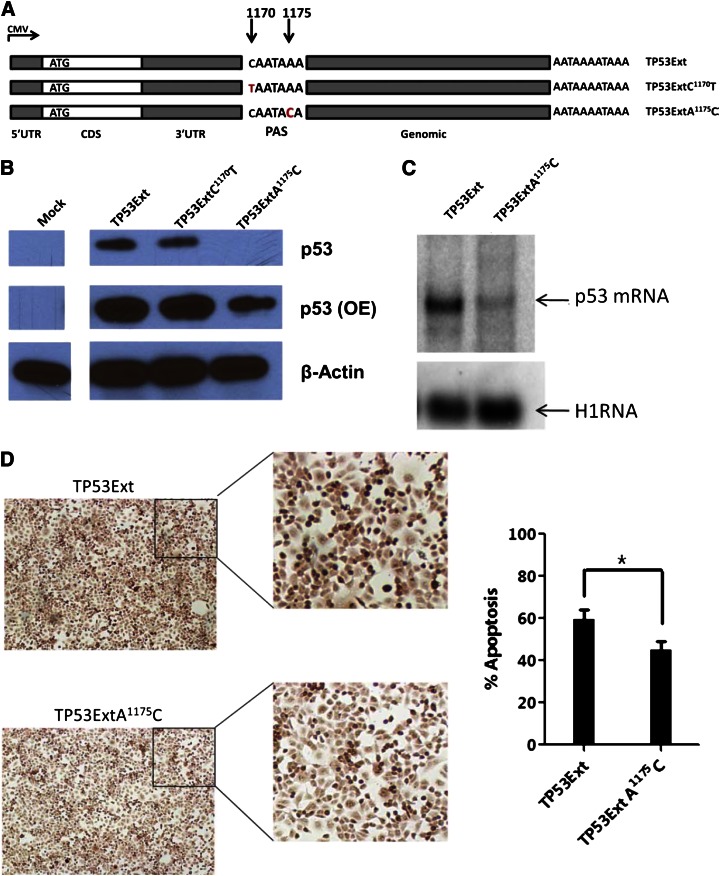
SNP rs78378222 (A1175C) is located in the polyadenylation signal (PAS) of TP53 and is strongly associated with basal cell carcinoma (BCC).30 This germline variant was found in 7 patients (6 A/C and 1 C/C). We predicted a decrease in p53 expression when the TP53 gene contains the PAS variant (AATAAA>AATACA). We introduced gene expression cassettes carrying human TP53 cDNA with this PAS variant (TP53ExtA1175C), or a control SNP rs114831472 (TP53ExtC1170T, not associated with BCC and other cancers) located 1 nucleotide upstream of the PAS, and the 2.5-kb genomic sequence downstream of the 3′UTR (Figure 6A) into p53-null H1299 cells. We found significantly lower p53 protein levels in cells with TP53ExtA1175C than in cells with WT control or TP53ExtC1170T (Figure 6B). Northern blot analysis confirmed that p53 messenger RNA (mRNA) levels were downregulated in cells with the p53 PAS variant (Figure 6C). We assessed the effect of the TP53 PAS variant on apoptosis by using the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay. In cells harboring the p53 PAS minor allele, cellular apoptosis was significantly lower than that in cells expressing the WT TP53 gene (Figure 6D). These data indicate that the TP53 variant rs78378222 hinders p53 expression and its downstream functions in apoptosis.
Figure 6.
p53 expression and function for the TP53 PAS variant in p53-null cells. (A) Schematic representation of the TP53 constructs. Each carries a WT 5′UTR, WT CDS, a WT or mutant 3′UTR, and a 2.5-kb native genomic sequence downstream of the TP53 PAS. (B) Western blot of p53 expression in p53-null H1299 cells transfected with p53 constructs. β-actin was used as a control. OE, overexposure. (C) Northern blot of p53 mRNAs in H1299 cells carrying exogenous p53 genes. A housekeeping RNA (H1RNA, the RNA subunit of human ribonuclease P) was used as a control. (D) Apoptosis of cells with p53 constructs; representative TUNEL images (left) and percentage of TUNEL-positive cells (right). *P ≤ .05.
Discussion
In this work, we found many previously unreported SNVs in the TP53 3′UTR in DLBCL tumor DNA samples. 3′UTR shortening31 and dysregulation of 3′UTR-binding proteins32 or miRNAs33 in cancer have been reported. This study, however, is the first to demonstrate that 3′UTR variation is widespread in the TP53 gene in cancer patients. Most DLBCLs originate from B cells in the germinal center, which have a high proliferation rate and are prone to genomic alterations.34 Germinal center B cells expand clonally and undergo physiological somatic hypermutation of their immunoglobulin variable-region genes and class-switch recombination to alter the immunoglobulin subtype mediated by activation-induced cytidine deaminase as part of the immune response. BCL2 point mutations in DLBCL are suspected to be caused by this somatic hypermutation mechanism.13 TP53 3′UTR nSNVs found in this study are predominantly G→A and C→T substitutions, and the frequency ratio of transition over transversion is 16.7, significantly higher than 2.1 (for all human variants in dbSNP), 2.0 (TP53 CDS mutation in DLBCL17), or 7.3 (known SNPs of TP53 3′UTR). However, only a small number of nSNVs are located in WRCY/RGYW sites (IUPAC degenerated codes; supplemental Table 6), hot spots for physiological hypermutations mediated by activation-induced cytidine deaminase. In addition, these nSNVs are not found in many projects that clearly sequence the full TP53 3′UTR from hundreds of individuals.35,36 Therefore, most nSNVs found in this study are likely cancer-specific mutations rather than germline polymorphisms or a result of a normally operative somatic hypermutation process. We acknowledge that the absence of patient-matched noncancerous tissue samples prevents us from completely ruling out the possibility of rare private germline variants in these patients. Counting CDS mutations and 3′UTR nSNVs, ∼45% of DLBCL patients (109 of 244) had alterations in TP53, making TP53 the most frequently mutated gene identified in DLBCL. A recent study reported an R-CHOP treatment-associated pattern of p53 and cell cycle dysregulation in DLBCL,37 further emphasizing the importance of p53 in DLBCL pathogenesis and prognosis.
Most of the TP53 3′UTR nSNVs are located in validated or putative miRNA-binding sites. Each specific mammalian cell expresses ∼70 miRNAs at an appreciable level,38 yet normal B-cell development and the initiation of malignancy and tumor progression are ordered and dynamic processes in which hundreds of miRNAs are differentially expressed in more than a dozen distinct cell populations.39-42 Thus, 3′UTR variation may have a direct biological impact on potential interactions between miRNAs and p53 in B-cell maturation or DLBCL pathogenesis. Because miRNAs repress gene expression, 3′UTR alterations that are predicted to disrupt miRNA-binding sites, as occurred in 78 of 146 patients with 1 or more nSNVs in this study (Table 3), could reduce the efficiency of miRNA suppression and subsequently increase p53 expression. This has been experimentally validated for a few nSNVs located at the miR-125b site. We reasoned that when the TP53 CDS is WT, the increased levels of tumor-suppressive p53 protein confer better 5-year survival in patients with 3′UTR nSNVs, as WT p53 is advantageous for patients undergoing R-CHOP chemotherapy17; in contrast, when the TP53 CDS is mutated concomitantly, the elevated levels of oncogenic p53 will worsen survival in patients with 3′UTR nSNVs. Our findings do show that variation in the TP53 3′UTR as a prognostic indicator of survival in DLBCL patients is dependent on the status of the CDS, yet there is no correlation between nSNV and p53 protein expression in the entire cohort, the WT-CDS group, or the MUT-CDS group. It should be noted that 3′UTR variation may also disrupt interactions between p53 mRNA and RNA-binding proteins, although the exact nucleotides have not been pinpointed.43,44 Additionally, 3′UTR variation could affect mRNA stability or translation efficiency due to other mechanisms. Further investigation is needed to ascertain the role of the nSNVs in p53 regulation and DLBCL prognosis.
The disappearance of the survival advantage at ∼10 years for DLBCL patients with a WT-CDS and nSNVs is noteworthy. The frequency of TP53 CDS mutations may be higher in relapsed DLBCL patients, and this may partially override the impact of 3′UTR variation on survival beyond 5 years. In patients with multiple myeloma, the frequency of TP53 CDS mutations in de novo disease is only ∼3%, yet this number rises up to 30% in patients with relapsed disease.45 Relapsed DLBCL is generally diagnosed by imaging and limited sampling such as fine-needle aspiration biopsy rather than by excisional biopsy; the lack of adequate specimens for genetic analysis precluded our testing for TP53 CDS mutations in DLBCL patients at relapse and is a limitation of this study.
TP53 germline variants have been investigated for cancer susceptibility using genome-wide association studies, in which a type I error rate (P-value threshold for significance) of 10−7 is generally regarded as indicating genome-wide significance.3,46 Until 2011, no genome-wide association study reported a significant association between any germline TP53 polymorphism (other than Li-Fraumeni Syndrome variants) and any cancer with a P value ≤ 10−7. In 2011, however, rs78378222 was found to be strongly associated with BCC, with an odds ratio (OR) = 2.16 (P = 2.2 × 10−20).36 rs78378222 is located in the fifth nucleotide of the TP53 PAS that is required for polyadenylation machinery recognition and subsequent cleavage, polyadenylation, and export of mature mRNAs to the cytoplasm. The risk-associated allele is a “C,” resulting in an alternative PAS (AATACA) replacing the canonical PAS (AATAAA). Among millions of SNPs in the human genome, rs78378222 has the strongest association with BCC.36 This SNP is also associated with an increased risk of prostate cancer (OR = 1.44; P = 2.4 × 10−6), glioma (OR = 2.35; P = 1.0 × 10−5), and colorectal adenoma (OR = 1.39; P = 1.6 × 10−4),36 as well as esophageal squamous cell carcinoma (OR = 3.22; P = 1.34 × 10−4).47 In an independent confirmation study, rs78378222 was found to be associated with a 3.5 times higher risk of glioma (P = 1.34 × 10−4).48 Stefansson and colleagues showed that rs78378222[A/C] heterozygotes expressed “somewhat less” p53 transcript levels than WT homozygotes in human blood specimens (P = .041).36 In the present study, we tested the role of the alternative PAS in p53 regulation using a p53-null cell line with exogenous p53 constructs. We found that the C allele, as compared with the A allele, dramatically lowers p53 mRNA levels and results in reduced p53 protein expression and cellular apoptosis. Our data are in agreement with a previous report that simian virus 40 mRNA with AATACA as PAS exhibits an eightfold decrease in cleavage efficiency compared with the canonical site.49,50 These findings provide direct evidence that rs78378222 negatively impacts p53 expression and function. p53 stabilization and function rely on various posttranslational modifications. As rs78378222 occurs in the PAS signal of TP53, none of the downstream p53 protein modifications are affected. Thus, this variant presents a unique instance of TP53 alleles with unambiguous reduced function, in contrast with most CDS mutants that are associated with a “loss of function” and/or a “gain of function.”5
In summary, in this study we have shown that sequence variation in the TP53 3′UTR is frequent in DLBCL and that the role of this sequence variation in patient survival depends on the status of the TP53 CDS. To our knowledge, this study is the first to demonstrate the prognostic importance of the TP53 3′UTR in cancer or any other disease. The full extent of the pathobiology of 3′UTR variants remains to be determined.
Supplementary Material
Acknowledgments
The authors thank the consortium program team of pathologists, hematologists, and clinicians; each of the contributing center principal physicians; their patients; and former and current hematopathology and hematology/oncology fellows for their support.
This work was supported by grants from the National Cancer Institute/National Institutes of Health (R01CA138688, 1RC1CA146299, P50CA136411, and P50CA142509 to Y.L. and K.H.Y.); the Shannon Timmins Leukemia Fellowship Award at The University of Texas MD Anderson Cancer Center (Z.Y.X.-M.); and The University of Texas MD Anderson Cancer Center Institutional Research and Development Fund, Institutional Research Grant Award, an MD Anderson Cancer Center Lymphoma Specialized Programs of Research Excellence Research Development Program Award, an MD Anderson Cancer Center Myeloma Specialized Programs of Research Excellence Research Development Program Award, a Gundersen Lutheran Medical Foundation Award, MD Anderson Cancer Center Collaborative Funds with Roche Molecular System, HTG Molecular Diagnostics, and Daiichi Sankyo Pharmaceutical (K.H.Y.).
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Y.L., K.H.Y., Z.Y.X.-M., and M.W.G. designed and conducted the research, performed and supported the statistical analysis, and wrote the manuscript; Y.L., Z.Y.X.-M., L.W., C.V., A.T., D.Z., L.Q., S.M.-M., K.D., A.C., Y.Z., G.B., K.L.R., E.D.H., W.W.L.C., J.H.v.K., Q.H., W.A., M.P., A.J.M.F., J.N.W., R.S.G., M.A.P., M.B.M., L.W., M.W., K.S.R., L.J.M., and K.H.Y. contributed vital new reagents, resources, and analytical tools under approval by the institutional review boards and the material transfer agreement; Z.Y.X.-M., C.V., A.T., S.M.-M., K.D., A.O., Y.Z., G.B., K.L.R., E.D.H., W.W.L.C., J.H.v.K., Q.H., W.A., M.P., A.J.M.F., J.N.W., R.S.G., M.A.P., M.B.M., M.W., L.J.M., and K.H.Y. collected clinical and follow-up data under approval by the institutional review boards and the material transfer agreement; Y.L., Z.Y.X.-M., C.V., and K.H.Y. analyzed the data and performed the statistical analysis; and all authors contributed vital strategies, participated in discussions, and provided scientific input.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yong Li, Department of Biochemistry and Molecular Biology, School of Medicine, University of Louisville, 319 Abraham Flexner Way, Louisville, KY 40202; e-mail: yong.li@louisville.edu; and Ken H. Young, Department of Hematopathology, Unit 72, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77230-1439; e-mail: khyoung@mdanderson.org.
References
- 1.Ventura A, Kirsch DG, McLaughlin ME, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445(7128):661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 2.Xue W, Zender L, Miething C, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445(7128):656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whibley C, Pharoah PDP, Hollstein M. p53 polymorphisms: cancer implications. Nat Rev Cancer. 2009;9(2):95–107. doi: 10.1038/nrc2584. [DOI] [PubMed] [Google Scholar]
- 4.Palmero EI, Achatz MI, Ashton-Prolla P, Olivier M, Hainaut P. Tumor protein 53 mutations and inherited cancer: beyond Li-Fraumeni syndrome. Curr Opin Oncol. 2010;22(1):64–69. doi: 10.1097/CCO.0b013e328333bf00. [DOI] [PubMed] [Google Scholar]
- 5.Oren M, Rotter V. Mutant p53 gain-of-function in cancer. Cold Spring Harb Perspect Biol. 2010;2(2):a001107. doi: 10.1101/cshperspect.a001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olive KP, Tuveson DA, Ruhe ZC, et al. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell. 2004;119(6):847–860. doi: 10.1016/j.cell.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Lang GA, Iwakuma T, Suh YA, et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell. 2004;119(6):861–872. doi: 10.1016/j.cell.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Storey A, Thomas M, Kalita A, et al. Role of a p53 polymorphism in the development of human papillomavirus-associated cancer. Nature. 1998;393(6682):229–234. doi: 10.1038/30400. [DOI] [PubMed] [Google Scholar]
- 9.Lenz G, Staudt LM. Aggressive lymphomas. N Engl J Med. 2010;362(15):1417–1429. doi: 10.1056/NEJMra0807082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nogai H, Dörken B, Lenz G. Pathogenesis of non-Hodgkin’s lymphoma. J Clin Oncol. 2011;29(14):1803–1811. doi: 10.1200/JCO.2010.33.3252. [DOI] [PubMed] [Google Scholar]
- 11.Pasqualucci L, Trifonov V, Fabbri G, et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet. 2011;43(9):830–837. doi: 10.1038/ng.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morin RD, Mendez-Lago M, Mungall AJ, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476(7360):298–303. doi: 10.1038/nature10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lohr JG, Stojanov P, Lawrence MS, et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc Natl Acad Sci USA. 2012;109(10):3879–3884. doi: 10.1073/pnas.1121343109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408(6810):307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 15.Vogelstein B, Sur S, Prives C. p53: the most frequently altered gene in human cancers. Nature Education. 2010;3(9):6. [Google Scholar]
- 16.Young KH, Leroy K, Møller MB, et al. Structural profiles of TP53 gene mutations predict clinical outcome in diffuse large B-cell lymphoma: an international collaborative study. Blood. 2008;112(8):3088–3098. doi: 10.1182/blood-2008-01-129783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu-Monette ZY, Wu L, Visco C, et al. Mutational profile and prognostic significance of TP53 in diffuse large B-cell lymphoma patients treated with R-CHOP: report from an International DLBCL Rituximab-CHOP Consortium Program Study. Blood. 2012;120(19):3986–3996. doi: 10.1182/blood-2012-05-433334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Z, Li Y, Takwi A, et al. miR-301a as an NF-κB activator in pancreatic cancer cells. EMBO J. 2011;30(1):57–67. doi: 10.1038/emboj.2010.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petitjean A, Mathe E, Kato S, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28(6):622–629. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- 20.Kumar M, Lu Z, Takwi AAL, et al. Negative regulation of the tumor suppressor p53 gene by microRNAs. Oncogene. 2011;30(7):843–853. doi: 10.1038/onc.2010.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le MTN, Teh C, Shyh-Chang N, et al. MicroRNA-125b is a novel negative regulator of p53. Genes Dev. 2009;23(7):862–876. doi: 10.1101/gad.1767609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu W, Chan CS, Wu R, et al. Negative regulation of tumor suppressor p53 by microRNA miR-504. Mol Cell. 2010;38(5):689–699. doi: 10.1016/j.molcel.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swarbrick A, Woods SL, Shaw A, et al. miR-380-5p represses p53 to control cellular survival and is associated with poor outcome in MYCN-amplified neuroblastoma. Nat Med. 2010;16(10):1134–1140. doi: 10.1038/nm.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neveu P, Kye MJ, Qi S, et al. MicroRNA profiling reveals two distinct p53-related human pluripotent stem cell states. Cell Stem Cell. 2010;7(6):671–681. doi: 10.1016/j.stem.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Becker LE, Lu Z, Chen W, Xiong W, Kong M, Li Y. A systematic screen reveals MicroRNA clusters that significantly regulate four major signaling pathways. PLoS ONE. 2012;7(11):e48474. doi: 10.1371/journal.pone.0048474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian S, Huang S, Wu S, Guo W, Li J, He X. MicroRNA-1285 inhibits the expression of p53 by directly targeting its 3′ untranslated region. Biochem Biophys Res Commun. 2010;396(2):435–439. doi: 10.1016/j.bbrc.2010.04.112. [DOI] [PubMed] [Google Scholar]
- 27.Fabbri M, Bottoni A, Shimizu M, et al. Association of a microRNA/TP53 feedback circuitry with pathogenesis and outcome of B-cell chronic lymphocytic leukemia. JAMA. 2011;305(1):59–67. doi: 10.1001/jama.2010.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman RC, Farh KK-H, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stacey SN, Sulem P, Jonasdottir A, et al. A germline variant in the TP53 polyadenylation signal confers cancer susceptibility. Nat Genet. 2011;43(11):1098–1103. doi: 10.1038/ng.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayr C, Bartel DP. Widespread shortening of 3’UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell. 2009;138(4):673–684. doi: 10.1016/j.cell.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Topisirovic I, Siddiqui N, Orolicki S, et al. Stability of eukaryotic translation initiation factor 4E mRNA is regulated by HuR, and this activity is dysregulated in cancer. Mol Cell Biol. 2009;29(5):1152–1162. doi: 10.1128/MCB.01532-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10(10):704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8(1):22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 35.Packer BR, Yeager M, Burdett L, et al. SNP500Cancer: a public resource for sequence validation, assay development, and frequency analysis for genetic variation in candidate genes. Nucleic Acids Res. 2006;34(Database issue suppl 1):D617–D621. doi: 10.1093/nar/gkj151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stacey SN, Sulem P, Jonasdottir A, et al. Swedish Low-risk Colorectal Cancer Study Group. A germline variant in the TP53 polyadenylation signal confers cancer susceptibility. Nat Genet. 2011;43(11):1098–1103. doi: 10.1038/ng.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monti S, Chapuy B, Takeyama K, et al. Integrative analysis reveals an outcome-associated and targetable pattern of p53 and cell cycle deregulation in diffuse large B cell lymphoma. Cancer Cell. 2012;22(3):359–372. doi: 10.1016/j.ccr.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landgraf P, Rusu M, Sheridan R, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129(7):1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petriv OI, Kuchenbauer F, Delaney AD, et al. Comprehensive microRNA expression profiling of the hematopoietic hierarchy. Proc Natl Acad Sci USA. 2010;107(35):15443–15448. doi: 10.1073/pnas.1009320107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spierings DC, McGoldrick D, Hamilton-Easton AM, et al. Ordered progression of stage-specific miRNA profiles in the mouse B2 B-cell lineage. Blood. 2011;117(20):5340–5349. doi: 10.1182/blood-2010-10-316034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J, Jima DD, Jacobs C, et al. Patterns of microRNA expression characterize stages of human B-cell differentiation. Blood. 2009;113(19):4586–4594. doi: 10.1182/blood-2008-09-178186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malumbres R, Sarosiek KA, Cubedo E, et al. Differentiation stage-specific expression of microRNAs in B lymphocytes and diffuse large B-cell lymphomas. Blood. 2009;113(16):3754–3764. doi: 10.1182/blood-2008-10-184077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takagi M, Absalon MJ, McLure KG, Kastan MB. Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell. 2005;123(1):49–63. doi: 10.1016/j.cell.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 44.Mazan-Mamczarz K, Galbán S, López de Silanes I, et al. RNA-binding protein HuR enhances p53 translation in response to ultraviolet light irradiation. Proc Natl Acad Sci USA. 2003;100(14):8354–8359. doi: 10.1073/pnas.1432104100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chng WJ. Genetic abnormalities in multiple myeloma. In: Kumar S, Abraham J, editors. Multiple Myeloma. New York, NY: Demos Medical Publishing; 2010. pp. 215–236. [Google Scholar]
- 46.Thomas DC, Haile RW, Duggan D. Recent developments in genomewide association scans: a workshop summary and review. Am J Hum Genet. 2005;77(3):337–345. doi: 10.1086/432962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou L, Yuan Q, Yang M. A functional germline variant in the P53 polyadenylation signal and risk of esophageal squamous cell carcinoma. Gene. 2012;506(2):295–297. doi: 10.1016/j.gene.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 48.Egan KM, Nabors LB, Olson JJ, et al. Rare TP53 genetic variant associated with glioma risk and outcome. J Med Genet. 2012;49(7):420–421. doi: 10.1136/jmedgenet-2012-100941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wickens M, Stephenson P. Role of the conserved AAUAAA sequence: four AAUAAA point mutants prevent messenger RNA 3′ end formation. Science. 1984;226(4678):1045–1051. doi: 10.1126/science.6208611. [DOI] [PubMed] [Google Scholar]
- 50.Zarkower D, Stephenson P, Sheets M, Wickens M. The AAUAAA sequence is required both for cleavage and for polyadenylation of simian virus 40 pre-mRNA in vitro. Mol Cell Biol. 1986;6(7):2317–2323. doi: 10.1128/mcb.6.7.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.