Abstract
Background and Aims: Here, we have investigated the role of miR-483 and miR-214 in the prognosis and multidrug resistance (MDR) of esophageal squamous cell carcinoma. Methods: The expression of miR-483 and miR-214 was detected in 104 cases of esophageal cancer tissues and matched adjacent benign esophageal tissues by quantitative real-time PCR. The relation of microRNA expression with survival was statistically analyzed. The roles of miR-483 and miR-214 in MDR of esophageal squamous cell cancer cells were further evaluated. Results: The expression of miR-483 and miR-214 was found significantly upregulated in esophageal squamous cell cancer tissues. The expression levels of miR-483 and miR-214 showed an inverse correlation with overall survival. High expression of miR-483 and miR-214 might predict less chemotherapy effect. Downregulation of miR-483 and miR-214 could confer sensitivity of both P-glycoprotein-related and P-glycoprotein-nonrelated drugs to esophageal cancer cells, and it might induce increased accumulation of adriamycin (ADR) and decreased amount of ADR released. Conclusions: miR-483 and miR-214 might play important roles in the pathogenesis of esophageal cancer and should be considered as potential targets for intervention in this malignancy.
Introduction
Esophageal squamous cell carcinoma (ESCC) is a major cause of mortality and morbidity in China (Hong et al., 2011). The biology of ESCC included aggressive local invasion, early metastasis, and multidrug resistance (MDR) to chemotherapy. So far, the mechanism underlying the MDR of esophageal cancer remains largely unknown.
MicroRNAs (miRNAs) are a class of 20–25 nucleotide noncoding RNAs, which post-transcriptionally modulate gene expression through canonical base pairing (Hong et al., 2010a). Single-stranded miRNAs can bind messenger RNAs of potentially hundreds of genes at the 3′ untranslated region (UTR) with perfect or near-perfect complementarity, resulting in degradation or inhibition of the target messenger RNA. Emerging data have revealed that the miRNAs are important in a wide range of normal physiologic and pathologic processes, including cell proliferation, apoptosis, and drug resistance (Landau and Slack, 2011; Majumder and Jacob, 2011). MiR-483 and miR-214 might play important roles in the process of tumorigenesis. MiR-483 was found overexpressed in adrenocortical carcinoma, colon, breast, and liver cancers and Wilms' tumors (Patterson et al., 2011). MiR-483 can promote the proliferation of hepatocellular carcinoma cells, and also act as an antiapoptotic oncogene involved in human tumorigenesis (Veronese et al., 2010). MiR-214 has been identified as aberrantly expressed in pancreatic cancer (Zhang et al., 2010b). Overexpression of miR-214 decreased the sensitivity of the cells to gemcitabine. MiR-214 could induce cell survival and cisplatin resistance through targeting the 3′-UTR of the PTEN (Yang et al., 2008). So far, the role of miR-483 and miR-214 in the process of esophageal carcinogenesis remains largely unknown.
In this report, miR-483 and miR-214 are identified as miRNAs whose rate of expression has predictive value for survival and chemotherapy effects in patients with esophageal cancer.
Materials and Methods
Tissue samples
Frozen specimens were obtained from 104 consecutive patients (76 male and 28female, from 36 to 74 year old), who had undergone resection for esophageal squamous cell cancer. All samples were obtained with the patient's informed consent, and studies were performed under the aegis of Institutional Review Board (IRB)-approved protocols. None of the patients received preoperative chemotherapy. Benign adjacent esophageal tissues were available from all esophageal cancer specimens. The specimens were collected from each patient immediately after surgical removal and snap-frozen in liquid nitrogen and maintained at −80°C until use. Routinely, the resected specimens were histologically examined by H&E staining and were reviewed by a pathologist and the diagnoses confirmed. Clinical and biologic information was available for all patients. Fifteen patients were treated with postoperative chemotherapy (the triple combination docetaxel-cisplatin-fluorouracil) four times.
Quantitative real-time polymerase chain reaction
Total RNAs from cells or tissues were extracted. Single-stranded cDNA was synthesized from 5 ng of total RNA using specific miRNA primers (TaqMan MicroRNA Assay) and the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems) (Yu et al., 2010). Comparative real-time PCR was performed in triplicate, including no-template controls. Relative expression was calculated using the comparative Ct method.
Kaplan–Meier survival curves
Survival curves were compared by log-rank analysis as described previously (Hong et al., 2010b). Significance was accepted with 95% confidence.
Cell culture
The human esophageal squamous cell line, ECA109, was routinely maintained in DMEM medium (GIBCO) as described previously (Zhang et al., 2010a). Throughout the experiment, the cells were used in logarithmic phase of growth.
miRNA transfection
Cells in exponential phase of growth were plated in 60 mm plates at 1×106 cells/plate and cultured for 16 h, and then transfected with the antagomirs of miR-48 and miR-214 as described previously (Hong et al., 2010b).
In vitro drug sensitivity assay
Adriamycin (ADR), cisplatin (CDDP), and 5-fludrouracil (5-flu) were all freshly prepared as described previously (Hong et al., 2007). Drug sensitivity was evaluated using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) assay.
Intracellular ADR concentration analysis
Fluorescence intensity of intracellular ADR was determined by flow cytometry (FCM) as described previously (Zhang et al., 2010a). Briefly, cells were seeded into six-well plates and cultured overnight. After addition of ADR to the final concentration of 5 μg/mL, cells continued to be cultured for 1 h. Cells were then harvested (for detection of ADR accumulation) or, alternatively, cultured in drug-free RPMI1640 for another 1 h followed by harvesting (for detection of ADR retention). The mean fluorescence intensity of intracellular ADR was detected using FCM. The experiment was independently performed three times.
Annexin V staining
Cells were washed twice with cold PBS and resuspended in 100 μL of binding buffer at a concentration of 1×106 cells/mL. Annexin V binds to those cells that express phosphatidylserine on the outer layer of the cell membrane, and propidium iodide stains the cellular DNA of those cells with a compromised cell membrane (Zhang et al., 2010a).
Statistical analysis
All the data were presented as the mean±SD, and were analyzed using the SPSS 13.0 program. The significance of differences from the control values was determined with Student's t-test or the χ2 test. p<0.05 was considered statistically significant.
Results
The expression of miR-483 and miR-214 in ESCC
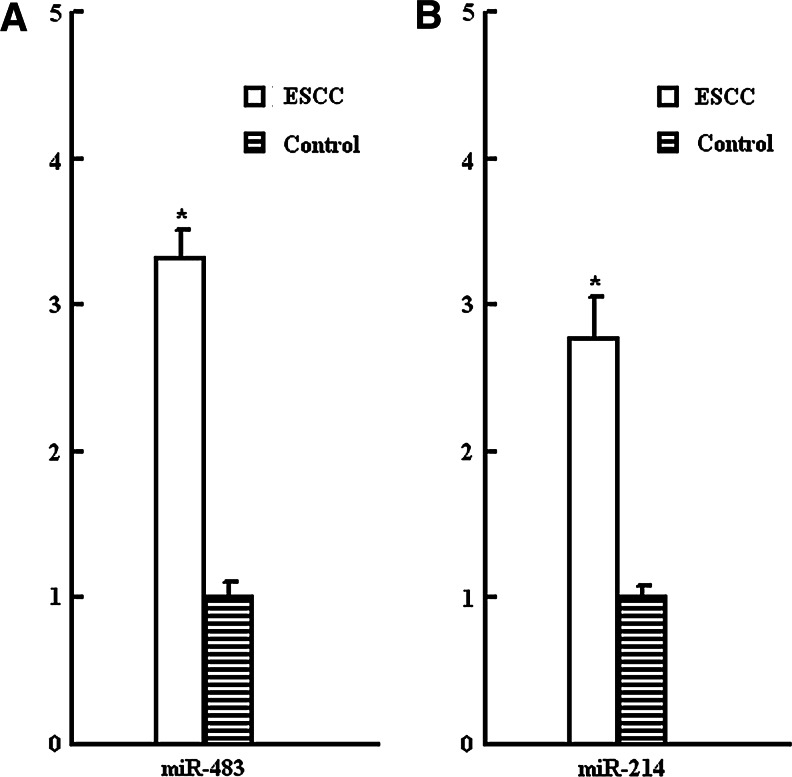
The expression of miRNAs in 104 cases of esophageal cancer tissues and matched adjacent benign esophageal tissues was detected by quantitative real-time PCR. miR-483 and miR-214 were found overexpressed in tumor samples relative to benign esophageal tissues (Fig. 1).
FIG. 1.
The expression of miRNAs in esophageal cancer tissues. Relative expression of miR-483 (A) and miR-214 (B) in 104 ESCC tissues compared with matched adjacent benign esophageal tissues (Control) by real-time PCR. The error bar indicated standard deviation. *p<0.05 versus control. ESCC, esophageal squamous cell carcinoma; miRNA, microRNA.
The prognostic value of miR-483 and miR-214 in ESCC
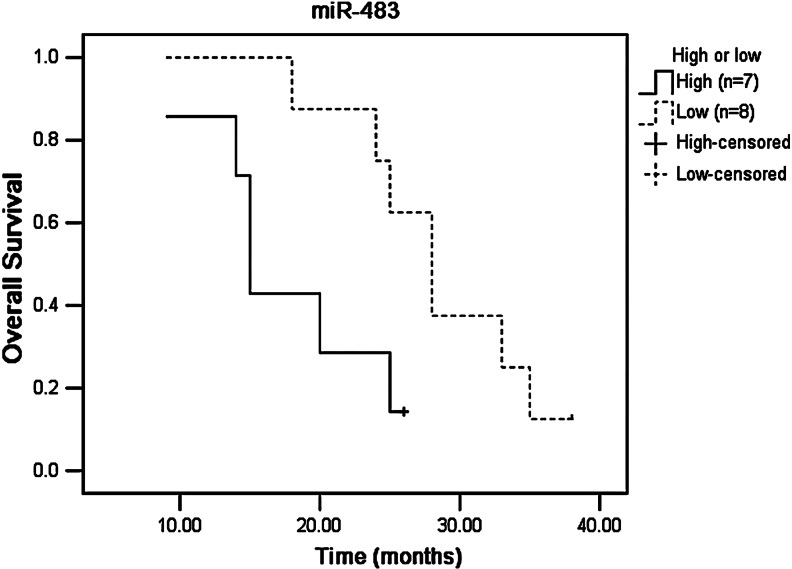
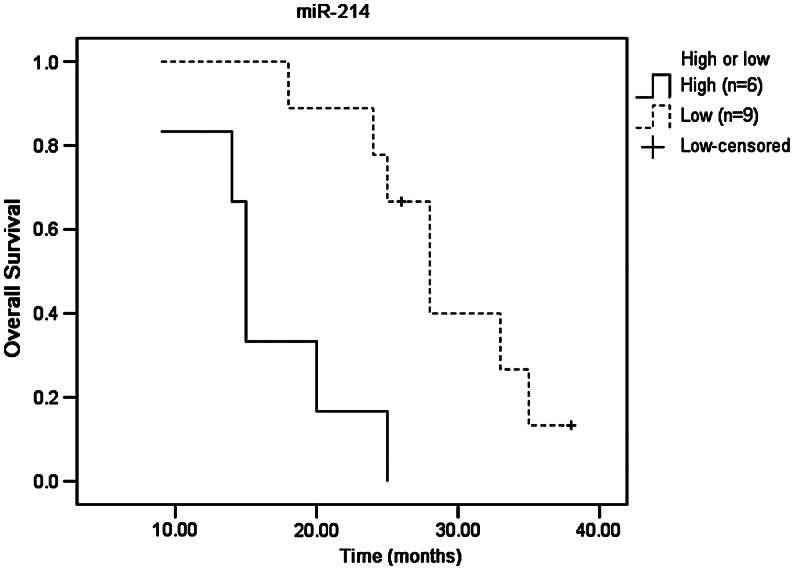
Kaplan–Meier survival curves were compared by log-rank analysis using the binomial variable of high or low expression relative to the mean expression of miR-483 and miR-214. Tumors with high expression of miR-214 and miR-483 had a poorer survival compared with those with low expression. Tumors with high expression of miR-483 had a median survival of 15.6 months (95% CI, 11.1–24.3) compared with 26.4 months (95% CI, 20.7–37.4) for those with low expression. Tumors with high expression of miR-214 had a median survival of 17.5 months (95% CI, 11.1–21.8) compared with 27.3 months (95% CI, 15.8–37.4) for those with low expression.
High expression of miR-483 and miR-214 might predict less sensitivity to chemotherapy
Quantitative real-time PCR was used to detect the expression of miRNAs in 15 cases of patients with postoperative chemotherapy. Then, survival analysis was performed (Figs. 2 and 3). The patients with high expression of miR-483 and miR-214 might have a poor survival, indicating that miR-483 and miR-214 might predict poor sensitivity to chemotherapy.
FIG. 2.
The association of miR-483 expression with survival of ESCC patients with postoperational chemotherapy.
FIG. 3.
The association of miR-214 expression with survival of ESCC patients with postoperational chemotherapy.
Downregulation of miR-483 and miR-214 might reverse drug resistance of esophageal cancer cells
ECA109 cells were transfected with either the antagomirs of miR-483 and miR-214 or control RNA, and the viability of cells was measured using the MTT assay. As shown in Table 1, the IC50 values of miR-483 and miR-214 antagomir cells for ADR, 5-flu, and CDDP were significantly decreased compared with control cells. Taken together, miR-483 and miR-214 affected not only the sensitivity of cells to P-glycoprotein (P-gp-related) drug ADR, but also to P-gp-nonrelated drugs 5-flu and CDDP.
Table 1.
IC50 Values (μg/mL) of Anti-Cancer Drugs for Esophageal Cancer Cells
| ADR | 5-Flu | CDDP | |
|---|---|---|---|
| ECA109 | 5.31±0.11 | 4.09±0.05 | 4.11±0.18 |
| Control | 5.65±0.09 | 4.22±0.13 | 4.04±0.07 |
| miR-214 antagomir | 2.22±0.04a | 1.55±0.09a | 1.37±0.08a |
| miR-483 antagomir | 1.89±0.17b | 1.68±0.11b | 1.62±0.12a |
Survival rates of esophageal cancer cells to anticancer drugs were evaluated by MTT assay. The dose–effect curves of anticancer drugs were drawn on semi-logarithm coordinate paper and IC50 values were determined. Data were represented as mean±SD of three independent experiments. ap<0.05 versus Eca109 and control cells.
ADR, adriamycin; 5-flu, 5-fludrouracil; CDDP, cisplatin.
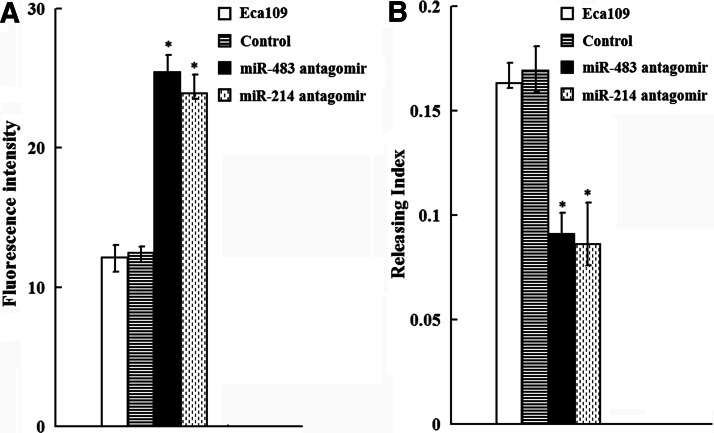
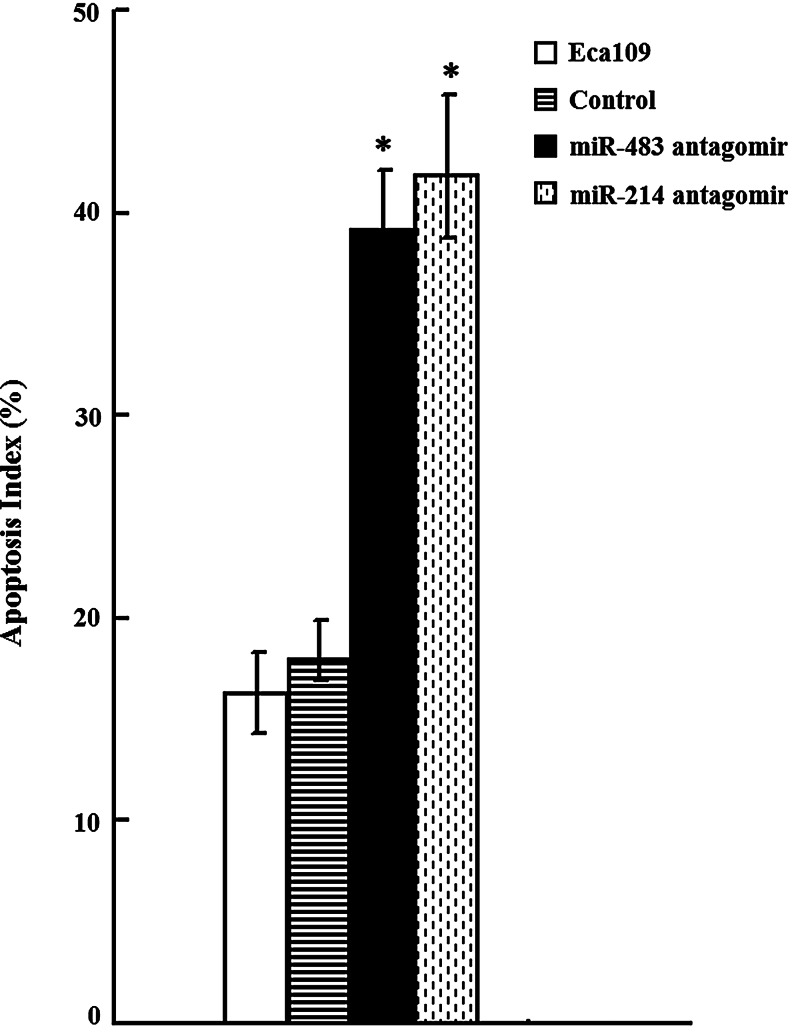
Since, MDR of cancer was mainly due to alterations of drug influx and efflux, ADR intracellular accumulation and releasing were explored. As shown in Figure 4, increased accumulation of ADR of miR-483 and miR-214 antagomir cells was observed compared with that of controls (p<0.01). Consistent with this, miR-483 and miR-214 antagomir cells showed a decreased releasing index. Further, we detected the capacity of miR-483 and miR-214 antagomir cells to undergo ADR-induced apoptosis using Annexin V staining. As shown in Figure 5, downregulation of miR-483 and miR-214 could promote ADR-induced apoptosis compared with that of control cells.
FIG. 4.
Effect of antagomirs of miR-483 and miR-214 on intracellular accumulation and releasing of ADR and ADR-induced apoptosis in esophageal cancer cells. (A) ADR was added to cells, and fluorescence intensity analysis of intracellular ADR in esophageal cancer cells. (B) ADR releasing index of esophageal cancer cells. *p<0.05 versus Eca109 and control cells. ADR, adriamycin.
FIG. 5.
Effect of antagomirs of miR-483 and miR-214 on ADR-induced apoptosis in ESCC cells. ECA109 cells were incubated with 1.5 μg/mL of ADR for 36 h. Annexin V/propidium iodide binding analyses of cells were presented. Results were representative of three independent experiments. *p<0.05 versus control cells.
Discussion
MiRNAs are small noncoding RNAs that modulate gene expression by base pairing to target messenger RNAs. Particular miRNA expression signatures could be useful for molecular diagnosis and/or prognosis. To our knowledge, we are the first to investigate the role of miR-483 and miR-214 in the prognosis and MDR of ESCC.
MiR-483 was widely expressed in cancer cells. MiR-483 might support cell survival by protecting cells from apoptosis. MiR-483 could inhibit Puma, which might promote apoptosis by interacting and inhibiting the antiapoptotic factors BCL2 and BCLXL (David and Meltzer, 2011). MiR-214 was frequently downregulated in cervical cancer and liver cancer, and its expression reduced the proliferation, migration, and invasiveness of cervical cancer cells (Tsuchiya et al., 2011). MiR-214 has diagnostic potential in breast cancer as an indicator of malignant disease and metastatic spread to regional lymph nodes (Wang et al., 2012). MiR-214 might inhibit angiogenesis by targeting Quaking and reducing angiogenic growth factor release (van Mil et al., 2012). MiR-214 might also regulate the acquired resistance to gefitinib in cancer cells via the PTEN/AKT signaling pathway. The reports were consistent with our findings that miR-483 and miR-214 might play important roles in the carcinogenesis and drug resistance.
In this study, both miR-483 and miR-214 were found to be inversely correlated with overall survival of patients with ESCC. Huang et al. (2012) have reported that miR-214 expression was significantly lower in ESCC tissues than in matched normal tissues. They also showed that miR-214 was inversely correlated to EZH2 protein expression and the clinical features such as pathological grade, tumor stage, and lymph node metastasis in ESCC. Thus, we assumed that miR-214 might play roles in the development of ESCC by regulation of EZH2. And miR-483 was assumed to be related with the prognosis of ESCC by mediating the PTEN/AKT signaling pathway and DPC4/Smad4 protein (Hao et al., 2011).
We further compared the expression of miRNAs in 15 cases of patients receiving postoperative chemotherapy. Although miR-483 and miR-214 might predict poor survival of patients with postoperative chemotherapy, it was hard to draw a conclusion that miR-483 and miR-214 might predict poor sensitivity to chemotherapy drugs. Thus, MTT assay was done. The data revealed that both miR-483 and miR-214 antagomir cells showed increased sensitivity to chemotherapeutic drugs.
ADR was used as probe to evaluate drug accumulation and retention in cancer cells. Both miR-483 and miR-214 antagomir cells showed increased ADR accumulation and decreased ADR releasing index, suggesting that miR-483 and miR-214 might have a direct or indirect function of pumping drug out of cells. MiR-483 and miR-214 antagomir cells also displayed a higher proportion of apoptosing cells after ADR treatment as compared with control cells, indicating that they might mediate the killing functions of anticancer drugs by regulation of apoptosis.
In conclusion, miR-483 and miR-214 could differentiate between patients with better or worse prognoses. The expression of them might help to guide the clinician when determining who should or should not receive aggressive therapy. Further analysis of the mechanism of miR-483 and miR-214 in drug resistance might help to generate a new approach to reverse MDR.
Acknowledgments
This study was supported in part by grants from the National Natural Scientific Foundation of China (81100714 and 81171923), the Foundation of Shaanxi Province Science and Technology research (2012KJXX-20), and the Top Ph.D. Foundation of China (201075).
Author Disclosure Statement
No competing financial interests exist.
References
- David S. Meltzer SJ. MicroRNA involvement in esophageal carcinogenesis. Curr Opin Pharmacol. 2011;11:612–616. doi: 10.1016/j.coph.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J. Zhang S. Zhou Y, et al. MicroRNA 483-3p suppresses the expression of DPC4/Smad4 in pancreatic cancer. FEBS Lett. 2011;585:207–213. doi: 10.1016/j.febslet.2010.11.039. [DOI] [PubMed] [Google Scholar]
- Hong L. Han Y. Li S, et al. The malignant phenotype-associated microRNA in gastroenteric, hepatobiliary and pancreatic carcinomas. Expert Opin Biol Ther. 2010a;10:1693–1701. doi: 10.1517/14712598.2010.532482. [DOI] [PubMed] [Google Scholar]
- Hong L. Han Y. Zhang H, et al. The prognostic and chemotherapeutic value of miR-296 in esophageal squamous cell carcinoma. Ann Surg. 2010b;251:1056–1063. doi: 10.1097/SLA.0b013e3181dd4ea9. [DOI] [PubMed] [Google Scholar]
- Hong L. Li S. Han Y, et al. Angiogenesis-related molecular targets in esophageal cancer. Expert Opin Investig Drugs. 2011;20:637–644. doi: 10.1517/13543784.2011.571203. [DOI] [PubMed] [Google Scholar]
- Hong L. Wang J. Han Y, et al. Reversal of multidrug resistance of vincristine-resistant gastric adenocarcinoma cells through up-regulation of DARPP-32. Cell Biol Int. 2007;31:1010–1015. doi: 10.1016/j.cellbi.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Huang SD. Yuan Y. Zhuang CW, et al. MicroRNA-98 and microRNA-214 post-transcriptionally regulate enhancer of zeste homolog 2 and inhibit migration and invasion in human esophageal squamous cell carcinoma. Mol Cancer. 2012;11:51. doi: 10.1186/1476-4598-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau DA. Slack FJ. MicroRNAs in mutagenesis, genomic instability, and DNA repair. Semin Oncol. 2011;38:743–751. doi: 10.1053/j.seminoncol.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder S. Jacob ST. Emerging role of microRNAs in drug-resistant breast cancer. Gene Expr. 2011;15:141–151. doi: 10.3727/105221611x13176664479287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson EE. Holloway AK. Weng J, et al. MicroRNA profiling of adrenocortical tumors reveals miR-483 as a marker of malignancy. Cancer. 2011;117:1630–1639. doi: 10.1002/cncr.25724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya S. Fujiwara T. Sato F, et al. MicroRNA-210 regulates cancer cell proliferation through targeting fibroblast growth factor receptor-like 1 (FGFRL1) J Biol Chem. 2011;286:420–428. doi: 10.1074/jbc.M110.170852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mil A. Grundmann S. Goumans MJ, et al. MicroRNA-214 inhibits angiogenesis by targeting Quaking and reducing angiogenic growth factor release. Cardiovasc Res. 2012;93:655–665. doi: 10.1093/cvr/cvs003. [DOI] [PubMed] [Google Scholar]
- Veronese A. Lupini L. Consiglio J, et al. Oncogenic role of miR-483-3p at the IGF2/483 locus. Cancer Res. 2010;70:3140–3149. doi: 10.1158/0008-5472.CAN-09-4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YS. Wang YH. Xia HP, et al. MicroRNA-214 regulates the acquired resistance to gefitinib via the PTEN/AKT pathway in EGFR-mutant cell lines. Asian Pac J Cancer Prev. 2012;13:255–260. doi: 10.7314/apjcp.2012.13.1.255. [DOI] [PubMed] [Google Scholar]
- Yang H. Kong W. He L, et al. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–433. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- Yu ZW. Zhong LP. Ji T. Zhang P, et al. MicroRNAs contribute to the chemoresistance of cisplatin in tongue squamous cell carcinoma lines. Oral Oncol. 2010;46:317–322. doi: 10.1016/j.oraloncology.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Zhang H. Li M. Han Y, et al. Down-regulation of miR-27a might reverse multidrug resistance of esophageal squamous cell carcinoma. Dig Dis Sci. 2010a;55:2545–2551. doi: 10.1007/s10620-009-1051-6. [DOI] [PubMed] [Google Scholar]
- Zhang XJ. Ye H. Zeng CW, et al. Dysregulation of miR-15a and miR-214 in human pancreatic cancer. J Hematol Oncol. 2010b;3:46. doi: 10.1186/1756-8722-3-46. [DOI] [PMC free article] [PubMed] [Google Scholar]