Abstract
Clinical-grade mesenchymal stromal cells (MSCs) are usually expanded from bone marrow (BMMSCs) or adipose tissue (ADSCs) using processes mainly differing in the use of fetal calf serum (FCS) or human platelet lysate (PL). We aimed to compare immune modulatory properties of clinical-grade MSCs using a combination of fully standardized in vitro assays. BMMSCs expanded with FCS (BMMSC-FCS) or PL (BMMSC-PL), and ADSC-PL were analyzed in quantitative phenotypic and functional experiments, including their capacity to inhibit the proliferation of T, B, and NK cells. The molecular mechanisms supporting T-cell inhibition were investigated. These parameters were also evaluated after pre-stimulation of MSCs with inflammatory cytokines. BMMSC-FCS, BMMSC-PL, and ADSC-PL displayed significant differences in expression of immunosuppressive and adhesion molecules. Standardized functional assays revealed that resting MSCs inhibited proliferation of T and NK cells, but not B cells. ADSC-PL were the most potent in inhibiting T-cell growth, a property ascribed to interferon-γ-dependent indoleamine 2,3-dioxygenase activity. MSCs did not stimulate allogeneic T cell proliferation but were efficiently lysed by activated NK cells. The systematic use of quantitative and reproducible validation techniques highlights differences in immunological properties of MSCs produced using various clinical-grade processes. ADSC-PL emerge as a promising candidate for future clinical trials.
Introduction
Adult mesenchymal stromal cells (MSCs) are considered a promising tool for cell therapy in regenerative medicine and for the prevention or treatment of severe inflammatory and autoimmune diseases [1]. Indeed, preliminary encouraging results have been recently reported in steroid-resistant graft-versus-host disease (GVHD), fistuling Crohn's disease, progressive multiple sclerosis, or kidney transplant rejection [2–5]. Despite intensive efforts, no specific MSC marker has been identified. The widely adopted MSC definition according to the International Society for Cellular Therapy relies on three main criteria: (i) their adhesion to plastic; (ii) their expression of a set of membrane molecules (CD73, CD90, and CD105), together with a lack of expression of HLA-DR and the hematopoietic and endothelial markers CD11b, CD14, CD34, CD31, and CD45; and (iii) their ability to differentiate along the adipogenic, osteogenic, and chondrogenic pathways [6]. However, even these minimal criteria designed to harmonize the identification of cultured MSCs are not definitive, and differences may exist depending on the tissue sources, culture conditions, and species. In agreement, several important issues should be taken into account to delineate efficient and safe clinical-grade cell culture conditions, including starting material, cell density, number of population doubling (PD), and culture media. First, the most reliable sources of MSCs for clinical application are bone marrow and adipose tissue that are widely available, easy to collect under standardized procedures, and give rise to high numbers of MSCs upon various ex vivo culture processes [7]. Several differences have been already reported between MSCs obtained from bone marrow (BMMSCs) and adipose tissue (ADSCs). In particular, ADSCs express CD34, especially in early stages of culture, and display a CD49dhiCD54hiCD106lo phenotype when compared to BMMSCs [8,9]. Moreover, even if ex vivo expanded MSCs share many biological features, some specific discrepancies have been reported between ADSCs and BMMSCs in their differentiation potential, gene expression and proteomic profiles, or immunological properties [9–13]. Finally, expression of HLA-DR is modulated depending on the starting material, that is, the use of unprocessed BM versus BM mononuclear cells obtained by density-gradient centrifugation, and the presence of fibroblast growth factor-2 (FGF-2) [14–16]. Concerning culture conditions, even if a consensus on the best medium for MSC culture is lacking, both fetal calf serum (FCS) and human platelet lysate (PL) contain the essential growth factors to sustain MSC expansion, whereas FGF-2 is the most common growth supplement capable of increasing the MSC growth rate and life span [17,18].
Although MSCs initially attracted the interest for their ability to differentiate into multiple cellular phenotypes, it is now widely accepted that their paracrine production of trophic factors together with their broad immune modulatory and anti-inflammatory functions are the most likely mechanisms for their therapeutic activity. MSCs profoundly affect the function of a large panel of effector cells of adaptative and innate immunity, including T-cells, B-cells, NK cells, monocytes/macrophages, dendritic cells, neutrophils, and mast cells [1,19]. Inhibition of immune cells relies on a combination of factors that are not constitutively expressed by MSCs, but are induced after MSC priming by inflammatory stimuli [20]. Interferon (IFN)-γ is the pivotal licensing agent for MSC suppressive function [21], whereas tumor necrosis factor (TNF)-α or interleukin (IL)-1α/β cooperates with IFN-γ to reinforce MSC-mediated inhibition of T-cell proliferation [22]. The specific molecular mechanisms involved in the immune regulatory properties of MSCs are still under evaluation and involve both cell contact-dependent mechanisms, such as the Jagged/Notch and PD-1/PD-L1 pathways [23,24], and soluble inducible factors, including indoleamine-2,3-dioxygenase (IDO), prostaglandin-E2 (PGE2), nitric oxide (NO), heme oxygenase, galectins, HLA-G5, transforming growth factor-β1, and TNF-α-induced protein 6 (TSG-6) [21,25–29]. Interestingly, besides the general concerns about the validity of mouse models, the major interspecies differences among the molecular pathways supporting the immune-regulating activity of MSCs have been reported. In particular, murine MSCs preferentially use inducible NO synthase (iNOS), whereas IDO is the most important T-cell inhibitory system in human MSCs [30]. Therefore, it is crucial to design fully standardized and reproducible in vitro assays, including phenotypic and functional experiments, to compare qualitatively and quantitatively the immunological properties of clinical-grade MSCs. So far, such an effort of standardization has not been undertaken, leading to inconstant, not comparable, and sometimes contradictory results.
In this study, we developed reproducible immunological assays to quantify the differences in immune-modulatory properties of MSCs produced according to a good manufacturing practice (GMP) following three procedures: BMMSCs expanded in a medium supplemented with clinical-grade PL (BMMSC-PL), BMMSCs expanded with FCS and FGF-2 (BMMSC-FCS), and ADSC-PL. This comprehensive work led to the identification of significant differences among these various GMP-grade MSC subsets that could be relevant for their further clinical use.
Materials and Methods
GMP-grade MSC production
Healthy donor recruitment followed the institutional review board approval and written informed content process according to the Declaration of Helsinki. Cryopreserved ex vivo expanded clinical-grade human MSCs at passage 1 (P1) were provided by the French Blood Bank of Toulouse (France) for ADSC-PL and Tours (France) for BMMSC-FCS, and by the Institute of Clinical Transfusion Medicine and Immunogenetics in Ulm (Germany) for BMMSC-PL. ADSC-PL were obtained from lipoaspirates after digestion with 0.4 U/mL NB6 collagenase (Roche Diagnostics, Mannheim, Germany) for 45 min at 37°C, filtration, and centrifugation to obtain the stromal vascular fraction (SVF) [31]. The SVF was seeded at 4×103 cells/cm2 onto CellSTACK closed cell culture chambers (Corning, Lowel, MA) in minimum essential medium α (αMEM) (Macopharma, Tourcoing, France) supplemented with 2% apheresis-derived clinical-grade PL produced as previously described [32], 1 IU/mL heparin, and 10 μg/mL ciprofloxacin (Ciflox; B.Braun, Boulogne, France). BMMSC-FCS were obtained as previously reported from unprocessed BM seeded at 5×104 cells/cm2 onto CellSTACK in an αMEM supplemented with 10% screened FCS (Hyclone, Logan, UT), 1 ng/mL FGF-2 (R&D Systems, Abington, United Kingdom), and 10 μg/mL ciprofloxacin [14]. Finally, BMMSC-PL were produced from unprocessed BM seeded at 1.5×104 cells/cm2 onto CellSTACK in an αMEM supplemented with 8% whole-blood-derived pooled clinical-grade PL, 2 IU/mL heparin (Braun, Melsungen, Germany), and 12 μg/mL ciprofloxacin (Fresenius Kabi, Bad Homburg, Germany) [33]. For all MSC cultures, the medium was renewed twice a week until the cells reached confluence (end of P0). Cells were then detached using trypsin (Trypzean; Lonza, Verviers, Belgium), reseeded at 1,000/cm2 (2,000/cm2 for ADSC-PL) until near the confluence (end of P1), and then frozen until use.
MSCs were thawed, seeded at 1,000 cells/cm2 in the same kind of culture medium used during the expansion step, and cultured until almost confluence to avoid any bias associated with the use of freshly thawed MSCs [34]. All phenotypic and functional experiments were then performed at the end of P2.
Immunophenotypic study
Thawed MSCs were stimulated or not for the last 40 h of culture by 100 IU/mL (10 ng/mL) IFN-γ and 15 ng/mL TNF-α (R&D Systems). The lack of cell cytotoxicity of this inflammatory stimulus was also checked [35]. Resting and primed MSCs (pMSCs) were then assessed for the expression of a panel of markers (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd). For each staining, 105 MSCs were incubated with the appropriate monoclonal antibody or appropriate isotypic control in phosphate buffer saline (PBS)–30% human serum for 30 min at 4°C. For ULBP-3 expression, cells were incubated with a specific primary unconjugated antibody, followed by staining with a conjugated goat anti-mouse IgG secondary antibody. According to the manufacturer's instruction, ULBP-1 expression was validated by intracellular staining using the Cytofix/Cytoperm kit (Becton-Dickinson, Le Pont de Claix, France). Data were expressed as the ratio of geometric mean fluorescence intensity obtained for each marker and its isotype-matched negative control.
Real-time quantitative polymerase chain reaction analysis
RNA was extracted from resting and pMSCs using a RNeasy Micro kit (Qiagen, Valencia, CA), and cDNA was generated using Superscript II reverse transcriptase (Invitrogen). For quantitative polymerase chain reaction (QPCR), assay-on-demand primers and probes, TaqMan Universal MasterMix, and ABI Prism 7000 apparatus were used (Applied Biosystems, Courtaboeuf, France). Gene expression was quantified based on the ΔΔCT calculation method. We identified CDKN1B and EIF2B1 as appropriate internal control genes with a low variability among three MSC and three pMSC samples using the TaqMan Express endogenous control plate (Applied Biosystems) and geNorm software (http://medgen.ugent.be/∼jvdesomp/genorm/). PCR data were normalized to the geometric mean of the two housekeeping genes. Results were then standardized by comparison to gene expression of a pool of five peripheral blood mononuclear cells (PBMCs).
Evaluation of immunosuppressive properties of MSCs
CD3pos T-cells, CD19pos B-cells, and CD56pos NK cells were purified from peripheral blood using appropriate negative selection kits (Miltenyi Biotec, Bergisch Gladbach, Germany) with at least 95% cell purity as evaluated by flow cytometry. Inhibition of immune cell proliferation by resting and pMSCs is described in detail in the Supplementary Design and Methods section.
Each immune cell batch was initially validated for its capacity to proliferate strongly without cell death in response to their specific stimuli: for T-cells, proliferation >70% and DAPIneg >60%; for B-cells, proliferation >50% and DAPIneg >80%; for NK cells, proliferation >70% and DAPIneg >50%. The same controls were repeated in all individual experiments.
MSC immunogenicity
Allogeneic purified T-cells were cultured in round-bottomed 96-well plates at 105 cells/well in RPMI–10% human AB serum with γ-irradiated resting MSCs at ratios ranging from 1:1 to 729:1 T:MSCs, 105 γ-irradiated autologous PBMCs (auto-MLR), 105 γ-irradiated allogeneic PBMCs (allo-MLR), or 0.5 μg/mL anti-CD3/anti-CD28 antibodies. Each experiment was performed in quadruplicate culture wells. Proliferation was assessed after 6 days of culture by incorporation of tritiated thymidine (3H-TdR) during the last 18 h and quantification of the radioactivity on a scintillation counter. The relative response index (RRI) was calculated as follows: RRI (%)=[cpm (T+MSCs) − cpm (auto-MLR)]/[cpm (allo-MLR) − cpm (auto-MLR)]×100. Any RRI >20% was considered positive.
Resting and pMSCs were also used as NK target cells in a nonradioactive cytotoxicity assay (Delfia Cytotoxicity kit; Perkin Elmer, Monza, Italy). Briefly, MSCs were loaded with a fluorescent dye and incubated for 3 h at various ratios of allogeneic NK cells preactivated during 48 h by 100 IU/mL rhIL-2. Cytotoxicity was quantified by assessing fluorescence release in coculture supernatants by a time-resolved fluorimeter (Victor™×4; Perkin Elmer).
IDO activity
Tryptophan and its catabolite kynurenine were measured in the supernatants of resting and pMSCs by high-performance liquid chromatography using added 3-nitro-l-tyrosine as an internal standard, as previously described [36]. IDO activity was expressed as the kynurenine/tryptophan ratio.
Statistical analysis
Data are expressed as median and ranges. Differences between groups were analyzed with Prism software (GraphPad, La Jolla, CA) using the Wilcoxon test for matched pairs for comparison of MSCs versus pMSCs or the Mann–Whitney nonparametric U test for comparison of ADSC-PL, BMMSC-PL, and BMMSC-FCS. The inhibition of T-cell proliferation and IDO activity were correlated using a two-tailed non-parametric Spearman's rank correlation test.
Results
Modulation of GMP-grade MSC phenotype depending on culture procedures
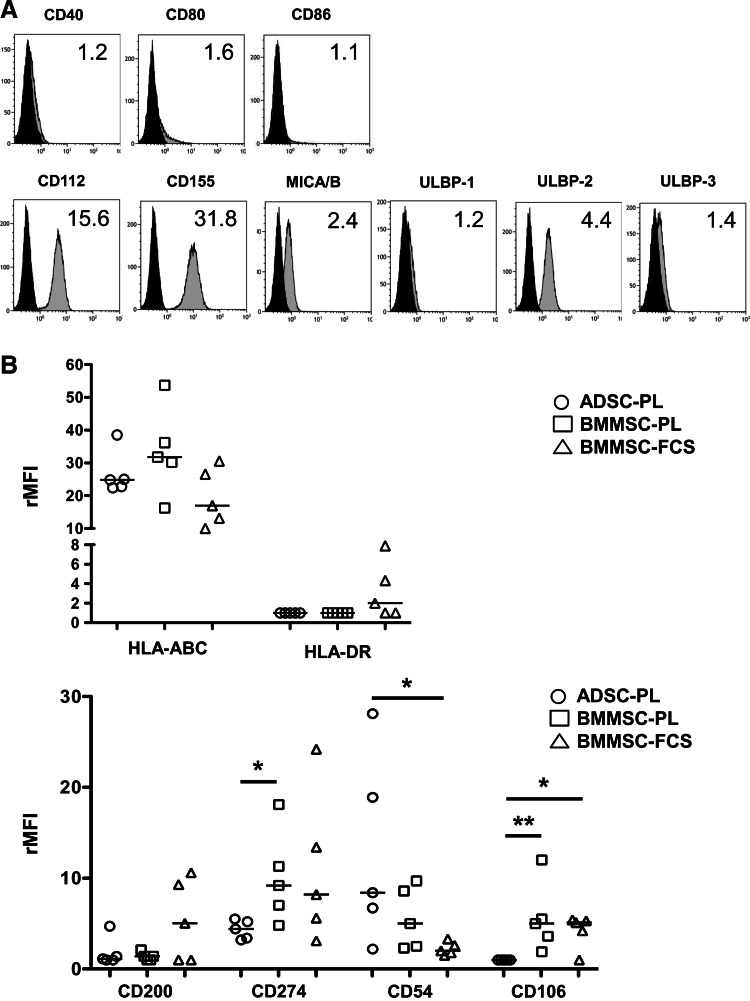
MSCs were produced and validated following the GMP procedures in three different cell therapy units (five batches/production process), cryopreserved to fit in with clinical applications that generally require repeated MSC infusions, and centralized for immunological evaluation. MSCs were then compared using standardized methods, including selected batches of antibodies, PCR reagents, culture supplements, standard operating procedures, and inclusion of internal controls for all functional experiments. Several markers were commonly expressed by ADSC-PL, BMMSC-PL, and BMMSC-FCS (Fig. 1A and Supplementary Table S2). In particular, all resting MSCs were negative for costimulatory molecules CD40, CD80, and CD86, and expressed a similar level of each NK-activating ligand. Among them, ULBP-2 was always detected at intermediate-to-high levels independently of the production process (4.6 [2.1–9.4]), whereas ULBP-1 and ULBP-3 were poorly expressed (ULBP1: 1.4 [1–2.1]; ULBP3: 1.4 [1–4.5]). Overall, the global expression of the NK-activating ligands was similar in all MSC subsets (Supplementary Fig. S1B). The balance between triggering of activating and inhibitory NK receptors determines NK cell functions. Resting MSCs displayed differential levels of HLA-ABC molecules with BMMSC-PL exhibiting the highest (31.8 [16.3–53.7]) and BMMSC-FCS the lowest (16.9 [10–30.6]), but this difference was not significant (Fig. 1B). Conversely, HLA-DR was expressed on three out of five BMMSC-FCS batches, as previously described [14], whereas ADSC-PL and BMMSC-PL never expressed this marker. Of note, the three HLA-DR-expressing BMMSC-FCS batches also expressed high levels of the CD200-immunosuppressive molecule, which remained very low on 9 out of 10 batches of MSCs produced in the PL (Fig. 1B and Supplementary Fig. S1A and Supplementary Table S2). Interestingly, ADSC-PL exhibited lower expression of CD274/PD-L1 than BMMSCs. Finally, we confirmed the overexpression of CD54/ICAM-1 and the low expression of CD106/VCAM-1 in ADSCs as compared to BMMSCs.
FIG. 1.
Comparative phenotype of ADSCs expanded with PL (ADSC-PL), BMMSCs expanded with PL (BMMSC-PL), and FCS (BMMSC-FCS). Thawed MSCs were collected at the end of P2 and stained with appropriate antibodies (gray histogram) or isotype-matched controls (black histogram). The ratio of mean fluorescence intensity (rMFI) is indicated on the top right of each panel. One example representative of the 15 MSC batches is shown for commonly expressed markers (A). For differentially expressed markers, data for all MSC batches were shown. Bars: median (B). ADSCs, mesenchymal stromal cells expanded from adipose tissue; BMMSCs, mesenchymal stromal cells expanded from bone marrow; PL, platelet lysate; FCS, fetal calf serum; P2, passage 2.
Overall, these results obtained on GMP-grade MSCs analyzed simultaneously using standardized methods confirmed and extended some previous data showing that ADSC-PL, BMMSC-PL, and BMMSC-FCS displayed reliable differences in their phenotypic profile.
Effect of inflammatory cytokines depending on MSC culture processes
Since tissue injury is usually associated with inflammation, we evaluated the modifications of MSC immunophenotype and gene expression after stimulation with IFN-γ and TNF-α. Among the 15 molecules tested, 8 were reproducibly induced by inflammatory stimuli (Fig. 2A and Supplementary Fig. S2 and Supplementary Table S3). The expression of these molecules was increased in the three MSC subsets, except for CD155, which was upregulated in BMMSCs, but slightly reduced in ADSCs. Concerning costimulatory molecules, while CD80 and CD86 expression remained negative, CD40 was strongly upregulated, especially in the pMSCs expanded in PL (primed ADSC-PL [pADSC-PL]: 8 [4.5–8.8]; primed BMMSC-PL [pBMMSC-PL]: 5 [2.7–8.7]), which significantly overexpressed CD40 compared to pBMMSC-FCS. Adhesion molecules were also upregulated in pMSCs, as expected. Interestingly, CD54 and CD106 induction inversely correlated with their level in resting MSCs (CD54: 120-fold for ADSC-PL, 200-fold for BMMSC-PL, and 400-fold for BMMSC-FCS; CD106: 10-fold for ADSC-PL, 5-fold for BMMSC-PL, and 3-fold for BMMSC-FCS); therefore, pMSCs reached similar expression of CD54 and CD106 regardless of their production process. HLA-DR induction was highly variable depending on the pMSC batches, without any link with the tissue origin or culture medium. Conversely, HLA-ABC was slightly less induced on pBMMSC-FCS and displayed a significantly lower expression on pBMMSC-FCS than on pMSCs obtained in the PL, in agreement with its lower level in this MSC subset in unprimed conditions (Fig. 1). CD112 and CD155, unlike ULBP and MICA/B, could be induced by inflammatory cytokines, except for pADSC-PL. This lack of induction of CD155 led to a global lower expression of NK-activating ligands by pADSC-PL as compared to pBMMSC-FCS (Fig. 2B). In relation to their lower HLA class I expression, pBMMSC-FCS exhibited a significantly higher ratio of activating/inhibitory NK ligands than the two other MSC subsets. Finally, pBMMSC-FCS also overexpressed CD274 as compared to pMSCs produced in PL.
FIG. 2.
Phenotypic modifications induced on MSCs by inflammatory stimuli. (A) Thawed MSCs were stimulated by IFN-γ and TNF-α for 40 h before staining with appropriate antibodies (gray histogram) or isotype-matched controls (black histogram). The ratio of rMFI is indicated on the top right of each panel. One example representative of the 15 MSC batches is shown. (B) The global expression of NK-activating ligands on pMSCs (n=15) was obtained by combining the individual rMFI from MICA/B, ULPB-1, ULBP-2, ULBP-3, CD112, and CD155. This activating profile was then analyzed compared to the level of expression of HLA-ABC, the main NK inhibitory ligand. *p<0.05; **p<0.01. IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α; pMSCs, primed MSCs.
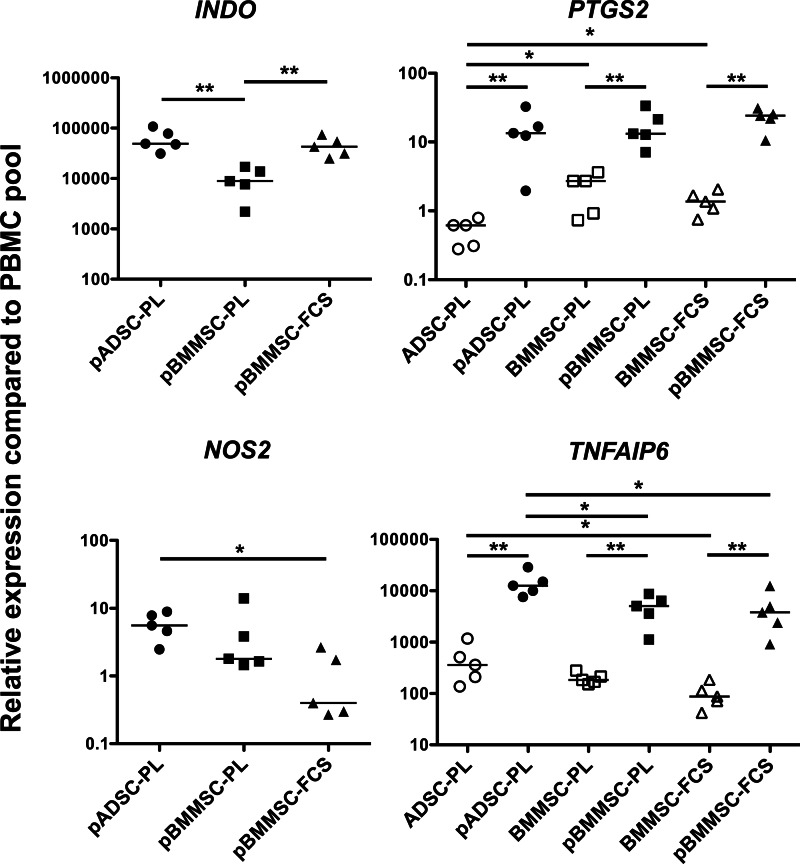
We next sought to evaluate the differences in the main molecular pathways that are supposed to be used by human MSCs to inhibit the immune response. Particular attention was focused on four factors reproducibly involved in the suppression of T-cells, NK cells, and cells of innate immunity, that is, IDO, PGE2, NO, and TSG-6 [20,27,29,30]. To this aim, we quantified in both resting and pMSCs the expression of genes encoding for IDO (INDO), the PGE2-producing enzyme Cox-2 (PTSG2), the NO-producing enzyme iNOS (NOS2), and TSG-6 (TNFAIP6). The four immunosuppressive molecules were upregulated by inflammatory cytokines. As previously described, INDO and NOS2 were not expressed by unstimulated MSCs (data not shown), but were strongly induced by IFN-γ and TNF-α exposure (Fig. 3). Notably, pBMMSC-PL significantly expressed INDO at lower levels, whereas pADSC-PL significantly expressed more NOS2. PTGS2 was constitutively expressed by MSCs, but with important differences among the different MSC subtypes: for instance, ADSC-PL expressed 3.6-fold less Cox-2 mRNA transcripts than BMMSC-PL and 3.2-fold less than BMMSC-FCS. However, Cox-2 expression was strongly upregulated after stimulation, and all pMSCs displayed similar expression of this immunosuppressive enzyme. More strikingly, TNFAIP6 was significantly overexpressed by both resting and pADSC-PL. Altogether, two opposite MSC subsets were evident, as pADSC-PL exhibited a CD40hiiNOS2hiTSG-6hi phenotype, whereas pBMMSC-FCS was HLA-ABCloPD-L1hiCD200dim and overexpressed NK-activating ligands.
FIG. 3.
Expression of immunosuppressive molecules on resting and pMSCs. Thawed MSCs were stimulated or not by IFN-γ and TNF-α for 40 h, and INDO, PTGS2, NOS2, and TNFAIP6 mRNA expression was measured by RQ-PCR in ADSC-PL (n=5), BMMSC-PL (n=5), and BMMSC-FCS (n=5) and their corresponding primed counterpart. The arbitrary value of 1 was assigned to a pool of PBMCs. INDO and NOS2 expression was undetectable in resting MSC samples. *p<0.05; **p<0.01. RQ-PCR, real-time quantitative polymerase chain reaction; PBMCs, peripheral blood mononuclear cells.
These results underline some major differences between the immunological profiles of clinical-grade pMSCs depending on their production processes, thus suggesting that they could display some differences in the immunosuppressive functions.
Standardized functional analyses reveal differences between ADSC-PL, BMMSC-PL, and BMMSC-FCS
Several culture conditions have been reported to evaluate immune properties of MSCs. To get reproducible and quantitative results, we decided to use purified immune cells obtained by negative selection and stimulated with fully standardized signals. In addition, considering crucial the antiapoptotic activity of MSCs, we evaluated the viability of responding cells and defined the minimal percentage of both viable cells and proliferating cells for each immune cell subset.
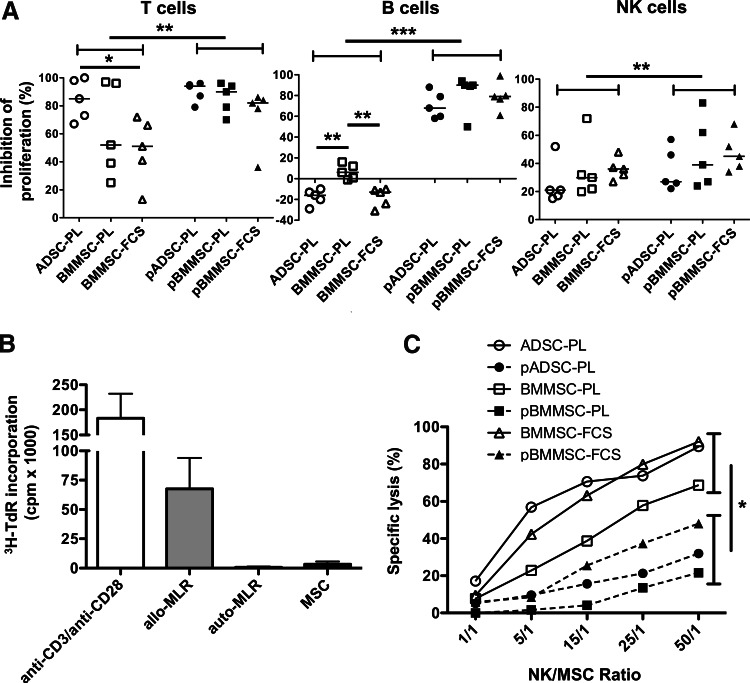
On the basis of these methods and criteria, we studied the capability of modulating T-cell, B-cell, and NK cell proliferation of the 15 MSC batches. Interestingly, while resting ADSCs always inhibited T-cell proliferation by more than 60%, the results were more heterogeneous for both BMMSC subsets, with two out of five batches inhibiting T-cell proliferation by <50% (Fig. 4A, left). Prestimulation of MSCs by inflammatory cytokines enhanced their T-cell-suppressive capacity, as expected, and leveled the variations among the MSC production processes. Regarding interactions between B-cells and MSCs, several studies reported confusing results claiming that MSCs favor or restrain B-cell growth and differentiation [37–39]. We confirmed here that resting MSCs did not inhibit B-cell proliferation, and that ADSC-PL and BMMSC-FCS, but not BMMSC-PL, could even increase the percentage of B-cells that have undergone more than one division (Fig. 4A, middle). However, priming by IFN-γ and TNF-α drove MSCs toward a B-cell inhibitory phenotype. Finally, the three MSC subsets similarly inhibited NK cell proliferation, although less efficiently than T-cell proliferation. MSC priming slightly, but significantly, increased their NK inhibitory potential. A trend for a better NK inhibitory potential by BMMSC-FCS was observed (Fig. 4A, right).
FIG. 4.
Immune properties of ADSC-PL, BMMSC-PL, and BMMSC-FCS. (A) Inhibition of T-, B-, and NK cell proliferation by primed and resting ADSC-PL (n=5), BMMSC-PL (n=5), and BMMSC-FCS (n=5) was assessed by the CFSE dilution method. Data are expressed as the percentage of inhibition of immune cell proliferation. (B) Responding T-cells were stimulated with irradiated resting allogeneic MSCs (five ADSC-PL, two BMMSC-PL, and three BMMSC-FCS batches all used at a 1:1 MSC:T ratio), with irradiated autologous PBMCs (auto-MLR) as negative control, and with irradiated allogeneic PBMCs (allo-MLR) and anti-CD3/anti-CD28 antibodies as positive controls. Each experiment was performed in sixplicate culture wells. Proliferation was assessed by the incorporation of tritiated thymidine (3H-TdR). Results represent the mean±SD of the 10 experiments. (C) Lysis of resting versus pMSCs (three ADSC-PL, two BMMSC-PL, and two BMMSC-FCS batches) by activated NK cells was assessed in a standard cytotoxicity assay. Results are represented as the mean for each MSC subtype. *p<0.05, **p<0.01, ***p<0.001. CFSE; carboxyfluorescein succinimidyl ester.
Despite their immunosuppressive properties, MSCs should not be considered as intrinsically immunoprivileged cells, in agreement with their capacity to behave as antigen-presenting cells under specific conditions and to be recognized and killed by NK cells [21,40]. We first explored their capacity to activate purified allogeneic T-cells. Regardless of the production processes, MSCs were poorly immunogenic in vitro (Fig. 4B), as confirmed by the evaluation of the RRI at 1:1 ratio (3.6% [−0.3 to 10.4]).
Activated NK cells may efficiently lyse resting and not IFN-γ-exposed BMMSCs, but this kind of information is currently lacking for ADSCs [41]. Thus, a classical cytotoxicity assay was performed using ADSC-PL, BMMSC-PL, and BMMSC-FCS as target cells (Fig. 4C). These experiments revealed a significantly lower susceptibility of pMSCs to NK-mediated lysis; in particular, even if the low number of samples precluded any statistical analysis, our data suggested that resting BMMSC-PL were more resistant than the two other cell subsets, in agreement with their higher expression of HLA class I (Fig. 1). Among pMSCs, pBMMSC-FCS were killed more efficiently by NK cells, consistently with their higher expression of NK-activating ligands.
IDO activity correlates with the T-cell inhibitory potential of MSCs
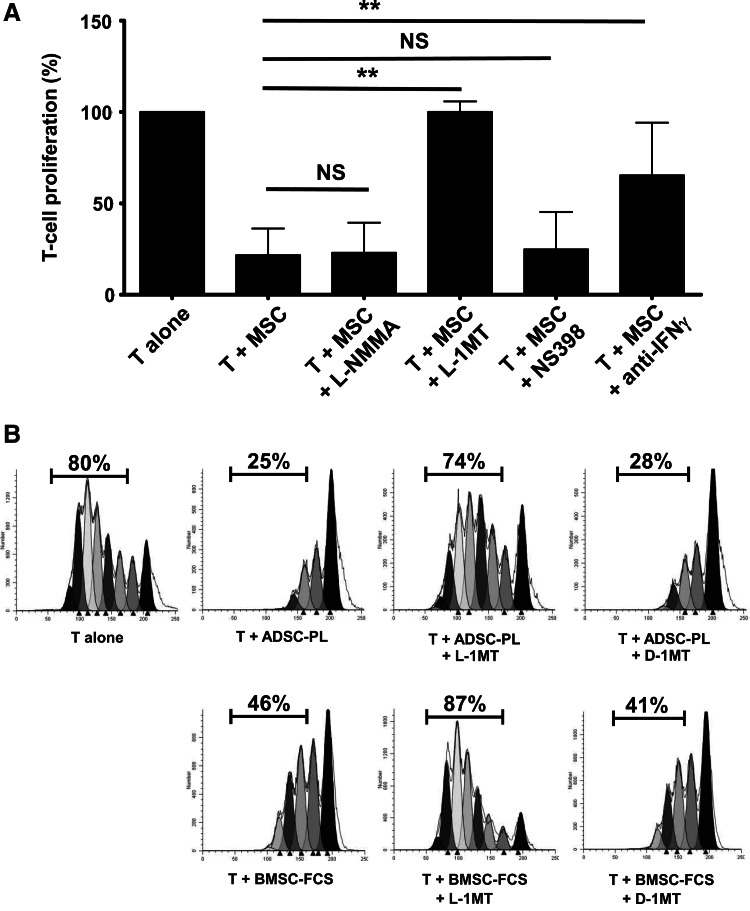
As functional analyses revealed some differences in the T-cell immunosuppressive potential between MSCs expanded in various culture systems, we next focused our attention on the mechanisms involved in the inhibition of T-cell proliferation that could explain these differences. On the basis of the QPCR results, we evaluated specific inhibitors of iNOS (L-NMMA), IDO (L-1MT), and Cox-2 (NS398), as well as IFN-γ blockade in the proliferation assay (Fig. 5A). L-1MT, unlike iNOS and Cox-2 inhibitors, abolished the T-cell inhibitory activity of resting BMMSC-PL, BMMSC-FCS, and ADSC-PL, and IFN-γ blockade partially restored T cell proliferation, as previously described [20]. Similar results were obtained with pMSCs (Supplementary Fig. S3). To further confirm the role of IDO-1, we compared the effect of L-1MT and its inactive enantiomer D-1MT. Both ADSC-PL and BMMSC-FCS activity was completely inhibited by L-1MT, whereas D-1MT was inefficient (Fig. 5B). These data clearly confirmed that IDO was the central effector of the T-cell-suppressive function for all MSC and pMSC subsets.
FIG. 5.
IDO is involved in the inhibition of T-cell proliferation by MSCs unlike NOS and PGE2. (A) Resting MSCs (n=5) were cocultured at a 10 T:1 MSC ratio with CFSE-labeled purified T-cells stimulated with anti-CD3/anti-CD28 antibodies in the presence or not of l-N-monomethyl arginine (L-NMMA), l-1-methyltryptophan (L-1MT), or NS398 to inhibit iNOS, IDO-1, or Cox-2 activity, respectively, or in the presence of an IFN-γ-blocking antibody. T-cell proliferation was evaluated at day 6, and data are expressed relatively to T-cells alone (assigned to 100%). Results are expressed as mean±SD of the five experiments. NS: not significant; **p<0.01. (B) Representative example of IDO blockade in coculture of activated T-cells with one ADSC-PL and one BMMSC-FCS. The IDO-2 inhibitor D-1MT was used as a negative control. IDO, indoleamine-2,3-dioxygenase; iNOS, inducible nitric oxide synthase; PGE2, prostaglandin-E2.
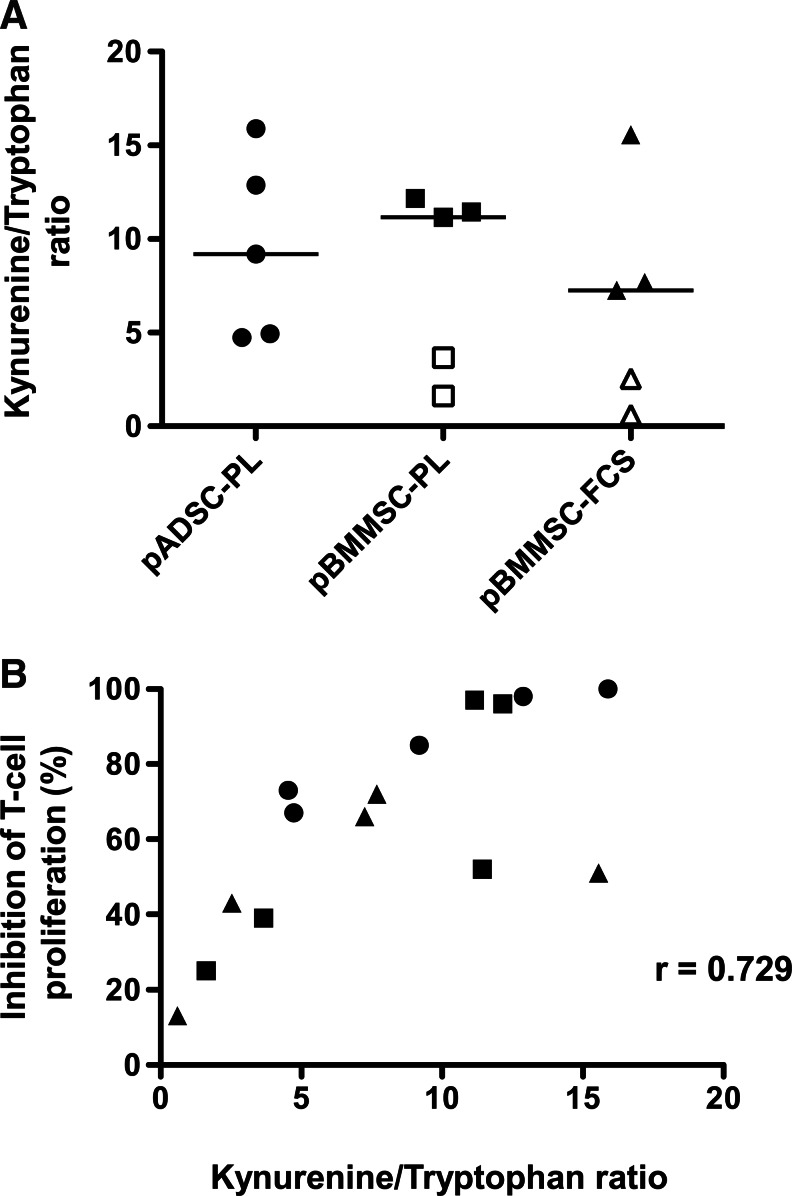
A recent study reported a correlation between the amount of IDO evaluated by western blot and the inhibition of T-cell proliferation by BMMSCs [42]. To extend these data to ADSCs and since IDO activity is regulated by transcriptional, traductional, and post-traductional mechanisms [43], we decided to determine the IDO activity through the quantification of tryptophan and kynurenine concentrations in pMSC supernatants (Fig. 6A). Strikingly, while IDO activity was not significantly different among pADSC-PL, pBMMSC-PL, and pBMMSC-FCS, the four MSC batches inhibiting T-cell proliferation by <50% (Fig. 4A, left) also displayed a low IDO activity upon stimulation with inflammatory cytokines (ratio tryptophan/kynurenine <4). Moreover, we demonstrated a significant correlation between IDO activity detected in the 15 pMSC batches and the capacity of corresponding resting MSCs to restrain T-cell proliferation (Fig. 6B). Conversely, neither TSG6 and PTGS2 mRNA nor the expression of CD200 or PD-L1 was positively correlated to the inhibition of T-cell proliferation, thus confirming the central role of IDO in this process (data not shown).
FIG. 6.
Correlation between IDO activity and T-cell inhibition. MSCs (n=15) were stimulated or not by IFN-γ+TNF-α for 40 h. Culture supernatants were collected for IDO activity quantification, and MSCs were cocultured with CFSE-labeled purified T-cells stimulated with anti-CD3/anti-CD28 antibodies for 6 days. (A) The IDO activity of pMSCs was evaluated as the ratio of kynurenine/tryptophan levels as determined by high-performance liquid chromatography. Open symbols correspond to MSC batches that inhibit T-cell proliferation by <50%. (B) The correlation between IDO activity produced by a given pMSCs and the capacity of corresponding resting MSCs to inhibit T cell proliferation was determined by a two-tailed Spearman's test. Each symbol corresponds to a different MSC batch, with circles representing ADSC-PL (n=5); squares, BMMSC-PL (n=5); and triangles, BMMSC-FCS (n=5).
Discussion
In this study, we have developed a panel of robust assays, validated in two independent immune-monitoring laboratories, allowing the quantitative comparison of immunological properties of clinical-grade MSCs. We identified the most critical variables to look for to ensure result consistency as (i) the references of antibodies; (ii) the concentration of IFN-γ and TNF-α used to preactivate MSCs with a careful control of the specific activity of IFN-γ that is highly variable depending on the distributor and batch; (iii) the quality of responding cells, that is, viability and proliferation rate in response to activation, especially for B cells (viability >80%, proliferation >50%) and NK cells (viability >50%, proliferation >70%), to avoid interference with the antiapoptotic activity of MSCs; (iv) for the same reason, the use of the best stimulatory system for each purified cell subset (stimulatory cocktail composition, including culture medium and batch-to-batch validation of nonclinical-grade CD40 ligand and CpG, and duration of the stimulation); and (v) the use of RRI instead of cpm for measurement of proliferation by thymidine incorporation and the normalization of carboxyfluorescein succinimidyl ester (CFSE) dilution with MSCs to the CFSE dilution without MSCs.
We applied these read-out systems to the evaluation of ADSC-PL, BMMSC-PL, and BMMSC-FCS produced by three cell therapy units according to the GMP rules. We clearly demonstrated that MSCs derived from various tissues and expanded through different culture procedures display some significant differences in their capacity to inhibit the immune response, a property that is of utmost interest for their clinical application.
Several immune markers were proposed as differentially expressed on ADSCs versus BMMSCs and/or on MSC expanded in PL versus FCS. Among them, we found that CD274 and CD200, two immunosuppressive molecules that cooperate in blocking T-cell activation, are poorly expressed by ADSC-PL unlike BMMSC-FCS. As of yet, a direct role of the PD-1/PD-L1 pathway in T-cell inhibition has been documented only for mouse BMMSCs [23]. Further, blocking of the CD200/CD200R interaction does not prevent the decrease of T-cell proliferation induced by MSCs, even when using CD200hi Wharton's jelly-derived MSCs [44]. In agreement, our work confirms that CD274loCD200lo ADSC-PL strongly inhibit the proliferation of purified T-cells, thus suggesting the implication of distinct dominant factors. CD54 and CD106 adhesion molecules were recently highlighted as crucial for MSC-driven immunoregulation, at least in a mouse model [45]. Our data confirmed an opposite pattern of expression of CD54 versus CD106 on the various MSC subsets. Nevertheless, all resting MSC batches expressed at least one of these two molecules, whereas pMSCs displayed high levels of both. Interestingly, although CD54 has already been described as strongly expressed by ADSCs [9], we further demonstrated that BMMSC-PL overexpress CD54 as compared to BMMSC-FCS. This could be related to the presence of very high levels of all three isoforms of platelet-derived growth factors (PDGFs) in PL [32]. PDGF-BB upregulates CD54 expression on rat BMMSCs through the activation of p38MAPK [46], indicating that PL could be involved in the induction of CD54 on BMMSCs and in its increase on ADSCs.
The composition of PL could influence many other MSC characteristics. Importantly, whole-blood-derived PL used in Germany and apheresis-derived PL used in France have been previously compared and displayed a very similar composition and capacity to sustain MSC growth [32]. Soluble VCAM-1 and soluble CD40L are two important components of PL [32] that could activate MSCs, in particular ADSCs. In fact, ADSCs, unlike BMMSCs, strongly expressed the VCAM-1 ligand VLA-4 that has been previously involved in murine MSC homing through the interaction with an inflamed endothelium [47]. Moreover, we demonstrated that pADSCs strongly express CD40, a marker that was also detected on native human BMMSCs [48]. The role of CD40 signaling in MSCs has never been explored, but CD40 is also inducible by IFN-γ on human fibroblasts where its ligation by CD40L enhances the secretion of inflammatory cytokines and chemokines and proangiogenic factors [49,50]. Adipocytes also express functional CD40 molecules [51]. Given the major role of inflammatory chemokines in the immunosuppressive function of MSCs [22], a detailed analysis of the CD40 function on cultured MSCs would be of great interest. Finally, we never detected HLA-DR expression by BMMSC-PL despite the use of unprocessed BM as a starting material and culture with 8% PL containing about 500 pg/mL of FGF-2 [32], suggesting that high amounts of FGF-2 are required to drive HLA-DR expression.
It should be noted that this study was designed to compare the validated clinical-grade processes currently used in several countries for therapeutic purposes, making it not possible to directly conclude on the specific role of tissue origin in MSC properties. As an example, the total PD was similar for BMMSCs obtained using both PL and FCS (see Supplementary Data), but was higher for BMMSCs than for ADSCs, a discrepancy that could have some impact on MSC features. A parallel evaluation of autologous BMMSCs and ADSCs obtained using identical procedures would be mandatory to answer this important question.
Our functional assays first confirmed that IDO is the pivotal mechanism of T-cell inhibition in all human MSC subtypes. Given the multiple levels of regulation of IDO, we propose to use the measurement of its enzymatic activity after stimulation by inflammatory cytokines to validate clinical-grade MSCs. In fact, a good correlation exists between the expression by MSCs of functional IDO after priming with IFN-γ and TNF-α and their capability of suppressing T-cells. Conversely, we found no correlation between the IDO mRNA level and T-cell inhibition, whereas the quantification of IDO protein expression by western blot seems hardly realizable in terms of standardization for multicentric studies. In addition, IDO activity also contributes to the induction of IL-10-producing M2 macrophages by MSCs [42], thus reinforcing the interest for its accurate evaluation. Blood or urinary IDO activity has been recently correlated to severity and outcome in several immune disorders [36,52]. In addition to its interest as validation criteria of clinical-grade MSCs, IDO activity should thus also be finely evaluated in vivo as a biomarker for the immunosuppressive activity of MSCs in forthcoming MSC-based clinical trials. Of note, we previously demonstrated that IDO is also involved in the inhibition of B-cell proliferation by MSCs [37]. Accordingly, as activated human B-cells do not produce IFN-γ, MSC priming by inflammatory stimuli was required to reveal their IDO-dependent B-cell inhibitory potential [20]. We used an optimal cocktail to trigger IDO activity, so that all MSCs that have been previously activated by IFN-γ+TNF-α inhibited both T-cell and B-cell proliferation in a secondary culture. In line with this, injected MSCs seem to be highly effective in some mouse models of acute GVHD only when the concentration of IFN-γ is sufficient to allow their in vivo licensing, or after in vitro priming by IFN-γ [53]. Surprisingly, while we could extend to ADSC-PL our previous data showing that resting BMMSC-FCS favor B-cell growth, resting BMMSC-PL displayed no B-cell supportive activity. Further studies targeting potential stroma-derived B-cell growth factors would be helpful to understand this phenomenon.
There is accumulating evidence that BMMSCs inhibit NK cell proliferation and cytotoxicity, but could be killed by IL-2-activated NK [27,41], a process that could contribute to their lack of long-term engraftment [54]. While the general consensus describes high levels of CD112, CD155, and MICA/B on MSCs, expression of ULBP remains still controversial [41,55,56]. As recently described for adult BMMSC-FCS [57], we found in our study a strong expression of ULBP-2, but not ULBP-1 and ULBP-3, on both ADSCs and BMMSCs. In addition, the use of PL instead of FCS did not alter the expression of NK-activating ligands in contrast to previous reports [45]. Nevertheless, HLA-ABC was expressed on BMMSC-FCS at a lower level than on BMMSC-PL, in line with the capacity of FGF-2 to downregulate HLA class I expression [58]. This could contribute to a general decrease of NK cell degranulation and cytotoxicity induced by BMMSC-PL compared to BMMSC-FCS [45]. Priming of MSCs rescues them from killing by NK cells, the findings corroborated by others and associated with the higher expression of HLA class I [41]. Similar to Spaggiari et al. [41], we detected in pBMMSCs an increase in the expression of the DNAM-1 ligands, that is, CD112 and CD155, but not NKG2D ligands. However, CD155 was not induced on ADSCs. Differential regulation of DNAM-1 ligand expression has been already reported in activated T-cells or dendritic cells [59,60]. Further investigations are required to definitively conclude on the origin and the functional consequences of this lower expression of CD155 by pADSCs. Interestingly, we could not reverse the MSC-dependent inhibition of NK cell proliferation using an IDO inhibitor (data not shown). In agreement with the reported implication of both IDO and Cox-2 in NK cell inhibition, resting BMMSC-FCS expressed higher levels of Cox-2 and strongly decreased NK cell proliferation [27].
Among the other proposed mechanisms of MSC-related immunosuppression, TSG-6 makes a major contribution to the decrease in neutrophil recruitment observed in response to MSC infusion in several mouse models of acute inflammation [29,61], at least in part by reducing the release of inflammatory cytokines by macrophages [62]. We confirmed here that exposure to IFN-γ+TNF-α induces TSG-6 at the transcriptional level in MSCs. Moreover, we identified ADSC-PL as strong producers of TSG-6, making them interesting candidates as anti-inflammatory MSCs.
In conclusion, our study paves the way for the definition of quantitative and reproducible validation techniques to select the best GMP procedures and to establish the release criteria for clinical-grade MSC production. Besides the well-known interindividual variability, differences among MSCs produced from different tissues could be highlighted in a different expansion medium. In particular, ADSC-PL clearly emerge as a very interesting alternative to the classical BMMSC-FCS. In fact, we demonstrated that ADSC-PL produce high levels of IDO and TSG-6, thus targeting both innate and adaptative immunity, and inhibit reproducibly T-cell proliferation. The next step will be to evaluate how these tools will be helpful to predict the clinical response to MSC infusion in controlled clinical trials.
Supplementary Material
Acknowledgments
The authors would like to thank Olivier Tribut for his help in the determination of IDO activity and Nadège Bescher for her technical assistance.
This work was supported by grants from the 7th Framework Program of the European Commission: CASCADE (FP7-HEALTH-233236) and REBORNE (FP7-HEALTH-241879) and by grants from the European Center for Transplantation Sciences and Immunotherapy (IHU CESTI, ANR-10-IBHU-0005).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Uccelli A. Moretta L. Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 2.Tan J. Wu W. Xu X. Liao L. Zheng F. Messinger S. Sun X. Chen J. Yang S, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. 2012;307:1169–1177. doi: 10.1001/jama.2012.316. [DOI] [PubMed] [Google Scholar]
- 3.Connick P. Kolappan M. Crawley C. Webber DJ. Patani R. Michell AW. Du MQ. Luan SL. Altmann DR, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11:150–156. doi: 10.1016/S1474-4422(11)70305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciccocioppo R. Bernardo ME. Sgarella A. Maccario R. Avanzini MA. Ubezio C. Minelli A. Alvisi C. Vanoli A, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. 2011;60:788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- 5.Le Blanc K. Frassoni F. Ball L. Locatelli F. Roelofs H. Lewis I. Lanino E. Sundberg B. Bernardo ME, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 6.Dominici M. Le Blanc K. Mueller I. Slaper-Cortenbach I. Marini F. Krause D. Deans R. Keating A. Prockop D. Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 7.Sensebe L. Bourin P. Tarte K. Good manufacturing practices production of mesenchymal stem/stromal cells. Hum Gene Ther. 2011;22:19–26. doi: 10.1089/hum.2010.197. [DOI] [PubMed] [Google Scholar]
- 8.De Ugarte DA. Alfonso Z. Zuk PA. Elbarbary A. Zhu M. Ashjian P. Benhaim P. Hedrick MH. Fraser JK. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol Lett. 2003;89:267–270. doi: 10.1016/s0165-2478(03)00108-1. [DOI] [PubMed] [Google Scholar]
- 9.Pachon-Pena G. Yu G. Tucker A. Wu X. Vendrell J. Bunnell BA. Gimble JM. Stromal stem cells from adipose tissue and bone marrow of age-matched female donors display distinct immunophenotypic profiles. J Cell Physiol. 2011;226:843–851. doi: 10.1002/jcp.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strioga M. Viswanathan S. Darinskas A. Slaby O. Michalek J. Same or not the same? comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 11.Ivanova-Todorova E. Bochev I. Mourdjeva M. Dimitrov R. Bukarev D. Kyurkchiev S. Tivchev P. Altunkova I. Kyurkchiev DS. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol Lett. 2009;126:37–42. doi: 10.1016/j.imlet.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Bernardo ME. Avanzini MA. Perotti C. Cometa AM. Moretta A. Lenta E. Del Fante C. Novara F. de Silvestri A, et al. Optimization of in vitro expansion of human multipotent mesenchymal stromal cells for cell-therapy approaches: further insights in the search for a fetal calf serum substitute. J Cell Physiol. 2007;211:121–130. doi: 10.1002/jcp.20911. [DOI] [PubMed] [Google Scholar]
- 13.Noel D. Caton D. Roche S. Bony C. Lehmann S. Casteilla L. Jorgensen C. Cousin B. Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Exp Cell Res. 2008;314:1575–1584. doi: 10.1016/j.yexcr.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Tarte K. Gaillard J. Lataillade JJ. Fouillard L. Becker M. Mossafa H. Tchirkov A. Rouard H. Henry C, et al. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood. 2010;115:1549–1553. doi: 10.1182/blood-2009-05-219907. [DOI] [PubMed] [Google Scholar]
- 15.Bocelli-Tyndall C. Zajac P. Di Maggio N. Trella E. Benvenuto F. Iezzi G. Scherberich A. Barbero A. Schaeren S, et al. Fibroblast growth factor 2 and platelet-derived growth factor, but not platelet lysate, induce proliferation-dependent, functional class II major histocompatibility complex antigen in human mesenchymal stem cells. Arthritis Rheum. 2010;62:3815–3825. doi: 10.1002/art.27736. [DOI] [PubMed] [Google Scholar]
- 16.Sotiropoulou PA. Perez SA. Salagianni M. Baxevanis CN. Papamichail M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006;24:462–471. doi: 10.1634/stemcells.2004-0331. [DOI] [PubMed] [Google Scholar]
- 17.Tsutsumi S. Shimazu A. Miyazaki K. Pan H. Koike C. Yoshida E. Takagishi K. Kato Y. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun. 2001;288:413–419. doi: 10.1006/bbrc.2001.5777. [DOI] [PubMed] [Google Scholar]
- 18.Ng F. Boucher S. Koh S. Sastry KS. Chase L. Lakshmipathy U. Choong C. Yang Z. Vemuri MC. Rao MS. Tanavde V. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112:295–307. doi: 10.1182/blood-2007-07-103697. [DOI] [PubMed] [Google Scholar]
- 19.Le Blanc K. Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 20.Krampera M. Cosmi L. Angeli R. Pasini A. Liotta F. Andreini A. Santarlasci V. Mazzinghi B. Pizzolo G, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 21.Krampera M. Mesenchymal stromal cell “licensing”.: a multistep process. Leukemia. 2011;25:1408–1414. doi: 10.1038/leu.2011.108. [DOI] [PubMed] [Google Scholar]
- 22.Ren G. Zhang L. Zhao X. Xu G. Zhang Y. Roberts AI. Zhao RC. Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Augello A. Tasso R. Negrini SM. Amateis A. Indiveri F. Cancedda R. Pennesi G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–1490. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- 24.Liotta F. Angeli R. Cosmi L. Fili L. Manuelli C. Frosali F. Mazzinghi B. Maggi L. Pasini A, et al. Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells. 2008;26:279–289. doi: 10.1634/stemcells.2007-0454. [DOI] [PubMed] [Google Scholar]
- 25.Gieseke F. Bohringer J. Bussolari R. Dominici M. Handgretinger R. Muller I. Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood. 2010;116:3770–3779. doi: 10.1182/blood-2010-02-270777. [DOI] [PubMed] [Google Scholar]
- 26.Selmani Z. Naji A. Zidi I. Favier B. Gaiffe E. Obert L. Borg C. Saas P. Tiberghien P, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 27.Spaggiari GM. Capobianco A. Abdelrazik H. Becchetti F. Mingari MC. Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 28.Chabannes D. Hill M. Merieau E. Rossignol J. Brion R. Soulillou JP. Anegon I. Cuturi MC. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110:3691–3694. doi: 10.1182/blood-2007-02-075481. [DOI] [PubMed] [Google Scholar]
- 29.Lee RH. Pulin AA. Seo MJ. Kota DJ. Ylostalo J. Larson BL. Semprun-Prieto L. Delafontaine P. Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren G. Su J. Zhang L. Zhao X. Ling W. L'Huillie A. Zhang J. Lu Y. Roberts AI, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 31.Bourin P. Peyrafitte JA. Fleury-Cappellesso S. A first approach for the production of human adipose tissue-derived stromal cells for therapeutic use. Methods Mol Biol. 2011;702:331–343. doi: 10.1007/978-1-61737-960-4_24. [DOI] [PubMed] [Google Scholar]
- 32.Fekete N. Gadelorge M. Furst D. Maurer C. Dausend J. Fleury-Cappellesso S. Mailander V. Lotfi R. Ignatius A, et al. Platelet lysate from whole blood-derived pooled platelet concentrates and apheresis-derived platelet concentrates for the isolation and expansion of human bone marrow mesenchymal stromal cells: production process, content and identification of active components. Cytotherapy. 2012;14:540–554. doi: 10.3109/14653249.2012.655420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fekete N. Rojewski MT. Fürst D. Kreja L. Ignatius A. Dausend J. Schrezenmeier H. GMP-compliant isolation and large-scale expansion of bone marrow-derived MSC. Plos One. 2012;7:e43255. doi: 10.1371/journal.pone.0043255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Francois M. Copland IB. Yuan S. Romieu-Mourez R. Waller EK. Galipeau J. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-gamma licensing. Cytotherapy. 2012;14:147–152. doi: 10.3109/14653249.2011.623691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y. Wang L. Kikuiri T. Akiyama K. Chen C. Xu X. Yang R. Chen W. Wang S. Shi S. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-gamma and TNF-alpha. Nat Med. 2011;17:1594–1601. doi: 10.1038/nm.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tattevin P. Monnier D. Tribut O. Dulong J. Bescher N. Mourcin F. Uhel F. Le Tulzo Y. Tarte K. Enhanced indoleamine 2,3-dioxygenase activity in patients with severe sepsis and septic shock. J Infect Dis. 2010;201:956–966. doi: 10.1086/650996. [DOI] [PubMed] [Google Scholar]
- 37.Maby-El Hajjami H. Ame-Thomas P. Pangault C. Tribut O. DeVos J. Jean R. Bescher N. Monvoisin C. Dulong J, et al. Functional alteration of the lymphoma stromal cell niche by the cytokine context: role of indoleamine-2,3 dioxygenase. Cancer Res. 2009;69:3228–3237. doi: 10.1158/0008-5472.CAN-08-3000. [DOI] [PubMed] [Google Scholar]
- 38.Corcione A. Benvenuto F. Ferretti E. Giunti D. Cappiello V. Cazzanti F. Risso M. Gualandi F. Mancardi GL. Pistoia V. Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 39.Traggiai E. Volpi S. Schena F. Gattorno M. Ferlito F. Moretta L. Martini A. Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells. 2008;26:562–569. doi: 10.1634/stemcells.2007-0528. [DOI] [PubMed] [Google Scholar]
- 40.Francois M. Romieu-Mourez R. Stock-Martineau S. Boivin MN. Bramson JL. Galipeau J. Mesenchymal stromal cells cross-present soluble exogenous antigens as part of their antigen-presenting cell properties. Blood. 2009;114:2632–2638. doi: 10.1182/blood-2009-02-207795. [DOI] [PubMed] [Google Scholar]
- 41.Spaggiari GM. Capobianco A. Becchetti S. Mingari MC. Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 42.Francois M. Romieu-Mourez R. Li M. Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20:187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 43.Thomas SR. Terentis AC. Cai H. Takikawa O. Levina A. Lay PA. Freewan M. Stocker R. Post-translational regulation of human indoleamine 2,3-dioxygenase activity by nitric oxide. J Biol Chem. 2007;282:23778–23787. doi: 10.1074/jbc.M700669200. [DOI] [PubMed] [Google Scholar]
- 44.Najar M. Raicevic G. Jebbawi F. De Bruyn C. Meuleman N. Bron D. Toungouz M. Lagneaux L. Characterization and functionality of the CD200-CD200R system during mesenchymal stromal cell interactions with T-lymphocytes. Immunol Lett. 2012;146:50–56. doi: 10.1016/j.imlet.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 45.Abdelrazik H. Spaggiari GM. Chiossone L. Moretta L. Mesenchymal stem cells expanded in human platelet lysate display a decreased inhibitory capacity on T- and NK-cell proliferation and function. Eur J Immunol. 2011;41:3281–3290. doi: 10.1002/eji.201141542. [DOI] [PubMed] [Google Scholar]
- 46.Cheng P. Gao ZQ. Liu YH. Xue YX. Platelet-derived growth factor BB promotes the migration of bone marrow-derived mesenchymal stem cells towards C6 glioma and up-regulates the expression of intracellular adhesion molecule-1. Neurosci Lett. 2009;451:52–56. doi: 10.1016/j.neulet.2008.12.044. [DOI] [PubMed] [Google Scholar]
- 47.Constantin G. Marconi S. Rossi B. Angiari S. Calderan L. Anghileri E. Gini B. Bach SD. Martinello M, et al. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells. 2009;27:2624–2635. doi: 10.1002/stem.194. [DOI] [PubMed] [Google Scholar]
- 48.Delorme B. Ringe J. Gallay N. Le Vern Y. Kerboeuf D. Jorgensen C. Rosset P. Sensebe L. Layrolle P. Haupl T. Charbord P. Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood. 2008;111:2631–2635. doi: 10.1182/blood-2007-07-099622. [DOI] [PubMed] [Google Scholar]
- 49.Gelbmann CM. Leeb SN. Vogl D. Maendel M. Herfarth H. Scholmerich J. Falk W. Rogler G. Inducible CD40 expression mediates NFkappaB activation and cytokine secretion in human colonic fibroblasts. Gut. 2003;52:1448–1456. doi: 10.1136/gut.52.10.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Danese S. Scaldaferri F. Vetrano S. Stefanelli T. Graziani C. Repici A. Ricci R. Straface G. Sgambato A, et al. Critical role of the CD40 CD40-ligand pathway in regulating mucosal inflammation-driven angiogenesis in inflammatory bowel disease. Gut. 2007;56:1248–1256. doi: 10.1136/gut.2006.111989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Missiou A. Wolf D. Platzer I. Ernst S. Walter C. Rudolf P. Zirlik K. Kostlin N. Willecke FK, et al. CD40L induces inflammation and adipogenesis in adipose cells—a potential link between metabolic and cardiovascular disease. Thromb Haemost. 2010;103:788–796. doi: 10.1160/TH09-07-0463. [DOI] [PubMed] [Google Scholar]
- 52.Landfried K. Zhu W. Waldhier MC. Schulz U. Ammer J. Holler B. Wolff D. Edinger M. Peter K, et al. Tryptophan catabolism is associated with acute GVHD after human allogeneic stem cell transplantation and indicates activation of indoleamine 2,3-dioxygenase. Blood. 2011;118:6971–6974. doi: 10.1182/blood-2011-06-357814. [DOI] [PubMed] [Google Scholar]
- 53.Polchert D. Sobinsky J. Douglas G. Kidd M. Moadsiri A. Reina E. Genrich K. Mehrotra S. Setty S. Smith B. Bartholomew A. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38:1745–1755. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.von Bahr L. Batsis I. Moll G. Hagg M. Szakos A. Sundberg B. Uzunel M. Ringden O. Le Blanc K. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30:1575–1578. doi: 10.1002/stem.1118. [DOI] [PubMed] [Google Scholar]
- 55.Poggi A. Prevosto C. Massaro AM. Negrini S. Urbani S. Pierri I. Saccardi R. Gobbi M. Zocchi MR. Interaction between human NK cells and bone marrow stromal cells induces NK cell triggering: role of NKp30 and NKG2D receptors. J Immunol. 2005;175:6352–6360. doi: 10.4049/jimmunol.175.10.6352. [DOI] [PubMed] [Google Scholar]
- 56.DelaRosa O. Sanchez-Correa B. Morgado S. Ramirez C. del Rio B. Menta R. Lombardo E. Tarazona R. Casado JG. Human adipose-derived stem cells impair natural killer cell function and exhibit low susceptibility to natural killer-mediated lysis. Stem Cells Dev. 2012;21:1333–1343. doi: 10.1089/scd.2011.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gotherstrom C. Lundqvist A. Duprez IR. Childs R. Berg L. le Blanc K. Fetal and adult multipotent mesenchymal stromal cells are killed by different pathways. Cytotherapy. 2011;13:269–278. doi: 10.3109/14653249.2010.523077. [DOI] [PubMed] [Google Scholar]
- 58.Tsunematsu H. Tatsumi T. Kohga K. Yamamoto M. Aketa H. Miyagi T. Hosui A. Hiramatsu N. Kanto T. Hayashi N. Takehara T. Fibroblast growth factor-2 enhances NK sensitivity of hepatocellular carcinoma cells. Int J Cancer. 2012;130:356–364. doi: 10.1002/ijc.26003. [DOI] [PubMed] [Google Scholar]
- 59.Ardolino M. Zingoni A. Cerboni C. Cecere F. Soriani A. Iannitto ML. Santoni A. DNAM-1 ligand expression on Ag-stimulated T lymphocytes is mediated by ROS-dependent activation of DNA-damage response: relevance for NK-T cell interaction. Blood. 2011;117:4778–4786. doi: 10.1182/blood-2010-08-300954. [DOI] [PubMed] [Google Scholar]
- 60.Pende D. Castriconi R. Romagnani P. Spaggiari GM. Marcenaro S. Dondero A. Lazzeri E. Lasagni L. Martini S, et al. Expression of the DNAM-1 ligands, Nectin-2 (CD112) and poliovirus receptor (CD155), on dendritic cells: relevance for natural killer-dendritic cell interaction. Blood. 2006;107:2030–2036. doi: 10.1182/blood-2005-07-2696. [DOI] [PubMed] [Google Scholar]
- 61.Bieback K. Hecker A. Kocaomer A. Lannert H. Schallmoser K. Strunk D. Kluter H. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009;27:2331–2341. doi: 10.1002/stem.139. [DOI] [PubMed] [Google Scholar]
- 62.Oh JY. Roddy GW. Choi H. Lee RH. Ylostalo JH. Rosa RH., Jr. Prockop DJ. Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc Natl Acad Sci U S A. 2010;107:16875–16880. doi: 10.1073/pnas.1012451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.