Abstract
Transplantation and drug discovery programs for liver diseases are hampered by the shortage of donor tissue. While recent studies have shown that hepatic cells can be derived from human embryonic stem cells (hESCs), few cases have shown selective enrichment of hESC-derived hepatocytes and their integration into host liver tissues. Here we demonstrate that the dissociation and reaggregation procedure after an endodermal differentiation of hESC produces spheroids mainly consisted of cells showing hepatic phenotypes in vitro and in vivo. A combined treatment with Wnt3a and bone morphogenic protein 4 efficiently differentiated hESCs into definitive endoderm in an adherent culture. Dissociation followed by reaggregation of these cells in a nonadherent condition lead to the isolation of spheroid-forming cells that preferentially expressed early hepatic markers from the adherent cell population. Further differentiation of these spheroid cells in the presence of the hepatocyte growth factor, oncostatin M, and dexamethasone produced a highly enriched population of cells exhibiting characteristics of early hepatocytes, including glycogen storage, indocyanine green uptake, and synthesis of urea and albumin. Furthermore, we show that grafted spheroid cells express hepatic features and attenuate the serum aspartate aminotransferase level in a model of acute liver injury. These data suggest that hepatic progenitor cells can be enriched by the spheroid formation of differentiating hESCs and that these cells have engraftment potential to replace damaged liver tissues.
Introduction
The liver is a crucial and multifunctional organ that plays numerous roles in maintenance of homeostasis. Due to its pivotal roles, transplantation of the liver has been performed for the treatment of irreversible liver dysfunctions, including cirrhosis and fibrosis. More than 5,000 patients receive liver transplantation every year in the United States (www.unos.org/), but this therapeutic option is available to a limited number of people due to the scarcity of donor livers. Although the usage of a bioartificial liver and primary hepatocyte transplantation have been recognized as a temporal bridge to liver transplantation, the culturing of human hepatocytes is still a major obstacle to cell-based clinical applications.
One promising approach to overcome the shortage of donor livers and primary human hepatocytes is to use human embryonic stem cells (hESCs) capable of self-renewal and differentiation into a variety of somatic cell types [1,2]. We have previously shown that neurons and pancreatic cells derived from ESCs improved organ functions by grafting into animal models of Parkinson's disease and diabetes [3,4]. In early embryonic development, the Wnt signaling pathway is indispensable for the formation primitive streak that subsequently generates the mesoderm and definitive endoderm [5]. The Wnt signaling also induces intestinal commitment of the early definitive endoderm by activating the Cdx2 gene [6]. The liver rudiment arises from the definitive foregut endoderm by the convergent fibroblast growth factor and bone morphogenic protein signaling from the cardiac mesoderm and the septum transversum mesenchyme [7]. Many previous studies have tried to mimic a series of sequential cell-fate commitment during embryonic liver development in vitro and have demonstrated that hepatocytes can be derived from hESCs by exposing them to different growth factors, cytokines, extracellular matrices, and/or synthetic chemicals [8–12]. However, few studies have described the enrichment or purification of hepatocyte-like cells from the heterogeneous population of differentiating hESCs and their engraftment potential in vivo [9,13].
Spheroid formation has been used as a method of culture and enrichment for various types of stem cells [14–16]. For example, neural stem cells generate multipotential and self-renewing spherical clusters, named as neurospheres, by adhering to each other as they proliferate [17,18]. The basic principle of the spheroid culture is based on the fact that cells of the same embryonic lineage express common adhesion molecules, promoting aggregation [19]. The three-dimensional spheroid culture is also similar to the environment of normal embryonic organogenesis, which facilitates a cell-to-cell interaction. Previously, this technique has been used to maintain the viability and function of primary hepatocytes in vitro and to enrich hepatic progenitor cells from dissociated fetal liver tissues [20,21]. Here, we show that a highly enriched population of hepatoblast-like cells can be obtained by promoting hepatic endodermal differentiation of hESCs followed by multicellular spheroid formation. We also demonstrate that, following differentiation, the spheroid-forming cells are able to acquire multiple features of fetal hepatocytes in vitro and can efficiently engraft into the host liver and improve its function in a model of acute liver injury.
Materials and Methods
hESC differentiation and spheroid formation
hESCs (HSF6 and Miz-hES4) were maintained as described previously [4]. The Wnt3a-conditioned medium (Wnt3a-CM) was prepared from Wnt3a producing L cells (ATCC No. CRL-2647). To examine the effects of Wnt3a and the bone morphogenic protein 4 (BMP4) on endodermal differentiation, cells were differentiated as a monolayer in the presence or absence of Wnt3a [80% Wnt 3a-CM plus 20% fresh Dulbecco's modified Eagle's medium (DMEM)/F12 medium (1:1) supplemented with 20% Knock-Out serum replacement (Gibco), 1 mM nonessential amino acid (Gibco), and 0.1 mM beta-mercaptoethanol (Sigma)] and/or BMP4 (10 ng/mL) on 5 μg/mL collagen type IV (Sigma)-coated surfaces and cultured for 4 days. The medium was changed daily.
For spheroid formation, cells treated with Wnt3a and/or BMP4 were dissociated with 0.05% trypsin-ethylenediaminetetraaceticacid (EDTA; Gibco-BRL). To promote cell–cell interactions, the dissociated cells were plated on suspension culture dishes (SPL Life Sciences) at a high density of 2.5×105 cells/cm2 in the DMEM/F12 medium supplemented with different concentrations of B27 (Gibco) without rocking or rotating. After 1 day, spherical cell clusters and adherent cells were separated and cultured in different culture dishes under the same culture condition for additional 5 days. To investigate their differentiation potential in vitro, the resulting multicellular spheroids were then plated onto 5 μg/mL collagen type I (Sigma)-coated surfaces without dissociation. The attached spheroids were further differentiated for 10 days in the ITS medium (1:1 DMEM/F12 with the addition of 5 ug/ml insulin, 50 ug/ml transferrin, 30 nM sodium selenite) [22] supplemented with 20 ng/mL of the hepatocyte growth factor (R&D Systems), 10 ng/mL oncostatin M (OSM; Sigma), and 10−6 M dexamethasone (DEX; Sigma). All experimental procedures involving hESCs were approved by the Ministry of Health and Welfare and Korean Research Foundation (IRB No. 78).
Reverse transcriptase–polymerase chain reaction and real-time quantitative polymerase chain reaction analysis
Total RNA was isolated using Trizol (Invitrogen) and cDNA was synthesized from 1 μg of total RNA using the RevertAid™ H Minus First Strand cDNA Synthesis Kit (Fermentas) according to the manufacturer's instructions. Subsequent polymerase chain reaction (PCR) amplification was performed in a 20 μL reaction volume using AccuPower® PCR-Premix (Bioneer). PCR products were visualized by 1.2% agarose gel electrophoresis. To analyze the relative expression of mRNAs, the amount of cDNA was normalized based on the signal from glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. Data are expressed as means±standard deviation (SD). Quantitative real-time PCR (qPCR) was also performed using the CFX-96 real-time PCR detection system (Bio-Rad) with iQ SYBR Green Supermix (Bio-Rad). Relative gene expression was normalized by the amount of GAPDH for each sample. Primer sequences used in PCR and qPCR are listed in Table 1.
Table 1.
Reverse Transcriptase–Polymerase Chain Reaction and Quantitative Real-Time Polymerase Chain Reaction for Differentiation Markers
| RT-PCR | 5′→3′ | 3′→5′ | Size |
|---|---|---|---|
| AFP | tgaaaaccctcttgaatgcc | tcttgcttcatcgtttgcag | 492 |
| Albumin | cttcctgggcatgtttttgt | ggttcaggaccacggataga | 401 |
| Brachyury | agccactgcttccctgagac | aggctggggtactgactgga | 500 |
| C/EBPβ | gccctcgcaggtcaagagca | ttgaacaagttccgcagggtg | 231 |
| CD34 | tgaagcctagcctgtcacct | cgcacagctggaggtcttat | 200 |
| Foxa2 | ctacgccaacatgaactcca | aaggggaagaggtccatgat | 207 |
| GAPDH | agccacatcgctcagacacc | gtactcagcgccagcatcg | 302 |
| GATA4 | ctcccctggcaaaacaagag | tgccgtgtcttagcagtcgt | 422 |
| Hex | gcccttttacatcgaggaca | ttcttctccagctcgatggt | 375 |
| HNF4α | tctcatgttgaagccactgc | ggtttgttttctcgggttga | 516 |
| Mixl1 | ggtaccccgacatccactt | tgagtccagctttgaaccaa | 335 |
| Nestin | cagctggcgcacctcaagatg | agggaagttgggctcaggactgg | 209 |
| Pax6 | ccgagagtagcgactccag | cttccggtctgcccgttc | 239 |
| Pdx1 | cccatggatgaagtctacc | gtcctcctcctttttccac | 262 |
| Prox1 | cagcccgaaaagaacagaag | gggtctagctcgcacatctc | 235 |
| Sox1 | caatgcggggaggagaagtc | ctctggaccaaactgtggcg | 464 |
| Sox17 | agcgcccttcacgtgtacta | cttgcacacgaagtgcagat | 246 |
| Tubulin βIII | catggacagtgtccgctcag | caggcagtcgcagttttcac | 175 |
| qPCR | 5′→3′ | 3′→5′ | Size |
|---|---|---|---|
| Oct-4 | actgcagcagatcagccacatcg | atcctctcgttgtgcatagtcgc | 123 |
| Brachyury | tgcttccctgagacccagtt | gatcacttctttcctttgcatcaag | 121 |
| Foxa2 | ttctccatcaacaacctcatgtcc | gtagtgcatcacctgttcgtagg | 108 |
| GAPDH | gtcagtggtggacctgacct | caccaccctgttgctgtagc | 256 |
| Mixl1 | ggtaccccgacatccactt | gcctgttctggaaccatacct | 87 |
| Sox1 | gcccaggagaaccccaag | cgtcttggtcttgcggc | 177 |
| Sox17 | acgccgagttgagcaaga | tctgcctcctccacgaag | 82 |
| Sox7 | gctgtctcccagtggaatgttc | caagtctgtccccccattagtt | 75 |
RT-PCR, reverse transcriptase–polymerase chain reaction; qPCR, quantitative real-time polymerase chain reaction.
Histochemistry and immunostaining
Cells, spheroids, and liver tissues were fixed in cold 4% paraformaldehyde in phosphate-buffered saline (PBS). The fixed spheroids were equilibrated in 20% sucrose overnight at 4°C, frozen in the O.C.T compound (Tissue Tek®), and sectioned at 10 μm. Immunostaining was carried out using standard protocols and the antibodies used in the present study are listed in Table 2. Appropriate fluorescence-tagged secondary antibodies (Molecular Probe) were used for visualization. The Apotome-Axiovert 200M (Carl Zeiss), was used for optical sectioning. To assess cell viability and cytotoxicity, spheroids were incubated with 50 μM of calcein acetoxymethyl (calcein AM) dye from Molecular Probes (Invitrogen) at 37°C for 30 min followed by 10 μg/mL propidium iodide (PI; Sigma) for 5 min and observed under fluorescence microscopy. For in vitro proliferation assays, spheroids were incubated for 4 h with 10 μg/mL 5-bromo-2′-deoxyuridine (BrdU; Becton Dickinson) in culture media and processed as described previously [22]. For paraffin-embedded sections, liver tissues were embedded in paraffin and sectioned at 5 μm. After deparaffinization and rehydration, the sections were boiled in a 10 mM sodium citrate buffer and stained with hematoxylin/eosin or immunolabeled with primary antibodies listed in Table 2. The sections were then labeled by the biotin-streptavidin-peroxidase method (Vectastatin ABC Elite Kit).
Table 2.
Antibodies and Dilution Factors for Immunostaining
| Antibody | Company | Origin | Dilution factor |
|---|---|---|---|
| AFP | Sigma | Mouse | 1:500 |
| Albumin | Sigma | Rabbit | 1:800 |
| Beta-catenin | Santa Cruz | Rabbit | 1:400 |
| Biotinylated anti-mouse lgG | Vector LAB | Goat | 1:100 |
| Cytokeratin-18 | DAKO | Mouse | 1:200 |
| Cytokeratin-19 | DAKO | Mouse | 1:200 |
| E-cadherin | Santa Cruz | Mouse | 1:500 |
| Foxa2 | Santa Cruz | Goat | 1:200 |
| GATA4 | Santa Cruz | Rabbit | 1:200 |
| Hep Par1 | DAKO | Mouse | 1:50 |
| Hex | Chemicon | Rabbit | 1:200 |
| Nestin | R&D Systems | Mouse | 1:100 |
| Pax6 | Chemicon | Rabbit | 1:2,000 |
| Sox17 | R&D Systems | Goat | 1:100 |
Fluorescence-activated cell sorting analysis
Differentiated cells were dissociated and resuspended with PBS with 1% fetal bovine serum. For intracellular markers staining, cells were fixed and permeabilized with the Cytofix/cytoperm solution (BD), and stained with appropriate primary antibodies, followed by fluorescence-labeled secondary antibodies (Molecular Probe). After washing, cells were analyzed by fluorescence-activated cell sorting (FACS)-Calibur with CellQuest software (BD Bioscience).
Hepatocyte functional assay
The γ-Glutamyl transpeptidase (GGT) activity was detected as previously reported [23]. Briefly, fixed cells were incubated for 15 min at room temperature with a substrate solution [γ-glutamyl-4-methoxy-2-naphthylamide, 2.5% dimethylsulfoxide, 1 N sodium hydroxide, glycylglycine, and fast blue BB salt (diazotized 4′-amino-2′,5′-diethoxybenzanilide)]. After rinsing with 0.85% saline, cells were incubated with 0.1 M cupric sulfate for 2 min at room temperature. The GGT activity was detected as red staining. For indocyanine green (ICG) uptake assay, cells were incubated at 37°C with the ICG solution (1 mg/mL in a culture medium) for 30 min, washed three times with PBS, and examined with an inverted microscope. Glycogen storage was detected by Periodic acid Schiff (PAS) and Diastase/PAS (D/PAS). For the PAS staining, cells were fixed with 10% formalin in 95% cold ethyl alcohol and exposed to a 1% periodic acid solution (Sigma) for 5 min. After rinsing, cells were stained with the Schiff's reagent (Sigma) for 15 min at room temperature. To remove excess staining, cells were washed with 0.55% potassium metabisulfite (Sigma), and then rinsed in water for 10 min. Glycogen granules were detected as a purple staining. For D/PAS staining, cells were incubated with the diastase solution (0.01 g/L in phosphate buffer, pH 6.0; Sigma) for 1 h at 37°C. After washing, cells were treated as described above for the PAS staining. The cytochrome P450 activity was measured after incubation of spheroid-derived cells, hESCs, HepG2, Hep3B, and Huh7 cells in the presence of 1 mM phenobarbital for 48 h using a P450-Glo CYP3A4 Assay Kit (Promega), according to the manufacturer's instructions. The luminescent signals were measured with a Multilabel Plate Reader (Perkin Elmer).
Urea synthesis and albumin secretion assay
hESCs, hepG2, human hepatocytes, and differentiated cells on day 20 of differentiation were rinsed with PBS and incubated in culture media for 48 h. Media samples were assayed for urea contents using a commercially available QuantiChrom Urea Assay Kit (DIUR-500; BioAssay Systems) according to the manufacturer's protocol. Absorbance readings were obtained at 520 nm using a spectrophotometer (Libra S22). Data were normalized by the standard urea solution provided by the manufacturer. Albumin secretion was assayed by using the Human Albumin ELISA quantitation kit (Bethyl Laboratories, Inc.) according to the manufacturer's protocol.
Transplantation of hepatoblast-like cells into acute liver disease model
Five- to seven-week-old male Balb/c nude mice (n=4) were subjected to an intraperitoneal injection of 10% carbon tetrachloride (CCl4, 100 μL per 20 g body weight) dissolved in olive oil 1 day before transplantation. Spheroid-forming cells and attached cells on suspension culture dishes were dissociated with 0.05% trypsin-EDTA. After 5 days of spheroid culture, in the presence of B27, the spheroids were incubated in a 0.05% trypsin-EDTA solution in microcentrifuge tubes for 15 min, while rotating the tubes on an inverter (40 rpm) at 37°C. After dissociation, the cell viability was determined by the trypan blue exclusion test and transplantation experiments were conducted only when the viability was >80%. About 1.0×106 viable cells were transplanted into the mouse via the spleen and sham animals received only the tissue culture medium used to support the cell suspension. After 7 days of transplantation, graft survival and liver function were assessed by enzyme linked immunosorbent assay (ELISA) for human albumin (hALB) levels in serum of host animals and immunohistochemistry for hepatic markers. Quantification of the hALB-positive area in the liver section was carried out using NIH ImageJ software (National Institutes of Health; http://rsb.info.nih.gov/nih-image/).
To assay aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels, blood serum was collected at 1, 2, 5, and 7 days after transplantation. AST and ALT levels were measured using Asan Set (Asan Pharmaceutical) by the manufacturer's protocol.
Statistical analysis
Numerical values were expressed as the mean±SD of three independent experiments performed in triplicates. Statistical significance was determined using a one-way analysis of variance followed by the Bonferroni's test procedure for multiple comparisons with the appropriate control. P values less than 0.05 were judged to be statistically significant.
Results
Wnt3a and BMP4 efficiently differentiate hESCs into definitive endoderm
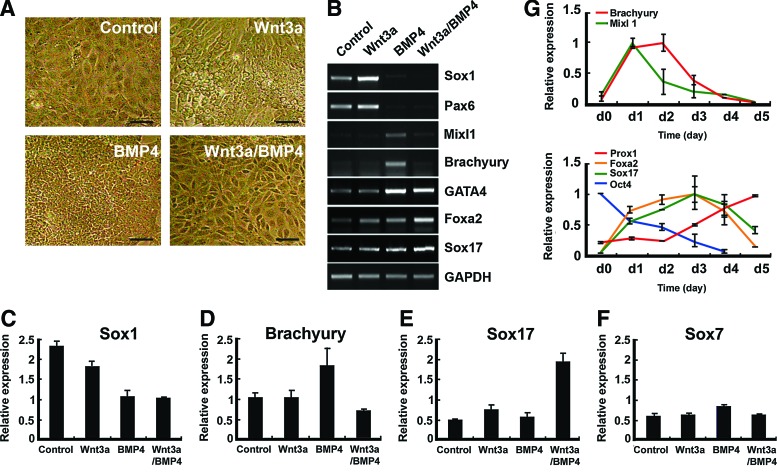
Because both Wnt3a and BMP4 are involved not only in definitive endoderm specification, but also in early liver development [10,24,25], we tested the effects of these two cytokines on hepatic endodermal differentiation of hESCs. hESCs were differentiated in the presence or absence of Wnt3a-CM (obtained from L cells overexpressing Wnt3a) or BMP4 on the collagen-IV-coated culture dishes for 4 days. Compared to other treatments, a combined treatment with Wnt3a and BMP4 generated a relatively homogeneous population of epithelial-like cells with a clear cell–cell boundary (Fig. 1A). These cells were morphologically similar to mesendodermal cells that were previously reported to express multiple endodermal markers, including GATA4 and E-cadherin [26]. Reverse transcriptase–polymerase chain reaction (RT-PCR) analysis showed that BMP4 alone or together with Wnt3a profoundly downregulated the expressions of neuroectodermal genes, Sox1 and Pax6 (Fig. 1B). Expressions of mesoendodermal markers, Mixl1 and Brachyury, were detected in BMP4-treated cells, but decreased in the presence of both Wnt3a and BMP4 (Wnt3a/BMP4). Endoderm-associated genes, GATA4, Foxa2, and Sox17 were expressed in all culture conditions, but at different levels (Fig. 1B). We next performed qPCR analysis to quantitatively measure the transcript levels of germ-layer-specific markers. Consistent with RT-PCR results, Sox1 was profoundly downregulated both in BMP4 and Wnt3a/BMP4-treated cells, but Brachyury expression was increased after BMP4 treatment (Fig. 1C, D). The highest level of Sox17 was obtained when cells were exposed to Wnt3a/BMP4 (Fig. 1E). qPCR analysis revealed that the primitive endodermal marker Sox7 was slightly increased upon BMP4 treatment (Fig. 1F). However, no significant increase in Sox7 expression was found in Wnt3a/BMP4-treated cells compared to other treatments. We also analyzed the time-course profile of gene expression during the 5 days of Wnt3a/BMP4 treatment. The result showed that early mesendodermal markers Brachyury and MixL1 were transiently upregulated after Wnt3a/BMP4 treatment, and decreased in the last 2–3 days of treatment (Fig. 1G). Reduction of these two gene expressions occurred concomitantly with the increased expression of endodermal genes Sox17 and Foxa2 (Fig. 1G). Although the upregulated Sox17 and Foxa2 expressions decreased in the later phase of endodermal differentiation, the expression of Prox1, one of the earliest marker genes of the developing liver gradually increased (Fig. 1G).
FIG. 1.
Generation of definitive endoderm by treatment of Wnt3a and the bone morphogenic protein 4 (BMP4). (A) Phase-contrast images of cells cultured in control, Wnt3a, BMP4, or Wnt3a/BMP4 conditions. Scale bars: 20 μm. (B) Reverse transcriptase–polymerase chain reaction (RT-PCR) analysis of cells cultured in the four different conditions for marker genes of embryonic germ layers. (C–F) Quantitative real-time polymerase chain reaction analysis of differentiated cells for indicated genes in each culture condition. (G) Dynamics of gene expressions over a 5-day period of differentiation in Wnt3a/BMP4 conditions. Color images available online at www.liebertpub.com/scd
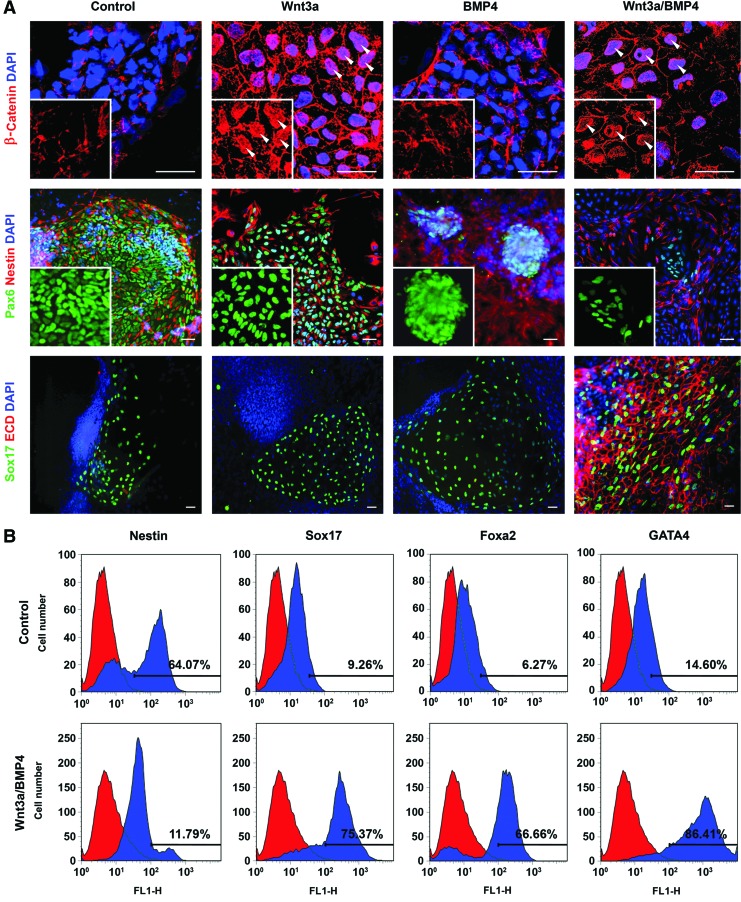
The gene expression data were further confirmed at protein levels by immunostaining and flow cytometric analyses after 4 days of differentiation. It is well known that the Wnt/β-catenin signaling pathway plays a crucial role in the development of different tissues or organs, including the gut endoderm and liver [27,28]. Activation of Wnt/β-catenin signaling leads to the inhibition of glycogen synthase kinase-3β, resulting in nuclear translocation of β-catenin. Immunohistochemistry using anti-β-catenin antibodies showed the increased nuclear β-catenin in cells treated with Wnt3a-CM (Wnt3a and Wnt3a/BMP4) (Fig. 2A, upper panels, insets). This data verified that the Wnt3a-CM obtained from Wnt3a-trasfected L cells was able to activate the β-catenin signaling pathway in our culture system. Control and Wnt3a-treated cultures contained a number of large colonies consisting mainly of cells positive for both Pax6 and Nestin, markers of neurectoderm (Fig. 2A, middle panels, insets show Pax6 images separately). In contrast, small colonies expressing both markers were occasionally observed in BMP4-treated culture. Particularly, these Pax6/Nestin-double-positive colonies were hardly detectable in cultures treated with Wnt3a/BMP4, although a few Pax6+ cells, which did not coexpress Nestin were found (Fig. 2A, middle panels). Instead, a large number of Wnt3a/BMP4-treated cells expressed Sox17 and E-cadherin, both of which are frequently used to identify definitive endoderm (Fig. 2A, lower panels) [26,29,30]. Interestingly, most of the cells expressing Sox17 in other conditions were relatively more flat and did not coexpress E-cadherin (Fig. 2A, lower panels). Flow cytometric quantitation showed that Wnt3a/BMP4 decreased the number of Nestin+ cells, but increased the cells expressing endodermal markers, Sox17, Foxa2, and GATA4 up to about 10-fold (Fig. 2B).
FIG. 2.
Expression of endodermal marker proteins in Wnt3a/BMP4-treated cells. (A) Immunostaining of differentiated cells for β-catenin (top panels, insets show β-catenin only, arrowheads indicate nuclear localization of β-catenin), Pax6/Nestin (middle panels, inset show Pax6 only) and Sox17/E-cadherin (bottom panels) in different culture conditions. Scale bars: 20 μm. (B) Flow cytometric analysis of Nestin, Sox17, Foxa2, and GATA4-expressing cells in control and Wnt3a/BMP4 conditions. Data shown are representative histograms from three independent experiments. The percentage of positive cells was indicated. Red, isotype control. Color images available online at www.liebertpub.com/scd
Enrichment of hepatic endoderm by spheroid formation
Formation of multicelluar spheroids has been observed in culture of different tissue-specific stem cells [14,17,18,31]. In addition, the spheroid culture can maintain the viability and function of primary hepatocytes and enrich hepatic progenitor cells from dissociated fetal liver tissues [20,21]. After 4 days of Wnt3a/BMP4 treatment, cells were dissociated and replated on suspension culture dishes in the presence of B27. This free-floating culture system generated two types of cells, spherical cluster-forming cells and adherent cells over 24 h after plating (Fig. 3A). Calcein AM/PI staining and BrdU incorporation assay showed that most of the cells in spherical clusters were viable and mitotic (Fig. 3B, C). B27 was necessary for high levels of spheroid formation as the number of spheroids was decreased at low concentrations of B27 (Fig. 3D). After an additional 5 days of culture, we analyzed the expression of genes specific for various tissues, including the liver. Interestingly, an enhanced expression of endodermal and hepatic marker genes (Foxa2, Hex, AFP, HNF4a, C/EBPβ, and ALB) was observed in spherical clusters. In contrast, adherent cells expressed only some of these markers, while many different nonhepatic markers were detected at high levels compared to spheroids (Fig. 3E).
FIG. 3.
Enrichment of spheroid-producing cells by dissociation and reaggregation of Wnt3a/BMP4-treated cells. (A) Spheroid formation from dissociated Wnt3a/BMP4-treated cells in the presence of B27. The dissociation and reaggregation procedure generates two types of cells, spherical cluster-forming cells and adherent cells over 24 h after plating. (B) Phase-contrast image (left) and calcein acetoxymethyl ester (calcein AM, green)/PS (red) staining (right) of spheroid-forming cells. (C) 5-Bromo-2′-deoxyuridine (BrdU, green) staining of spheroid-forming cells. 4′,6-Diamidino-2-phenylindole, blue. (D) Efficacy of spheroid formation in different B27 concentrations (% in the culture medium, v/v) *<0.05 and **<0.01. (E) RT-PCR analysis of genes expressed in spheroid-forming and adherent cells. Color images available online at www.liebertpub.com/scd
Neural stem cells generate neurospheres, self-renewing spherical clusters, by adhering to each other as they proliferate [17,18]. We next attempted to generate neurospheres and compare those with spherical clusters derived from the cells treated with Wnt3a/BMP4. The neurospheres were obtained from our control cells that preferentially differentiated into neurectodermal cells (Figs. 1B, C, and 2A). Immunostaining of serial frozen spheroid sections showed that a large number of control spheroids consisted of cell expressing Nestin (Fig. 4A, upper left, B). Only very few control spheroids were positive for cytokeratin 18 (CK18), which are detected in fetal hepatocytes, but not in extraembryonic tissues such as the primitive endoderm (Fig. 4A, upper right, B) [32]. In contrast, Wnt3a/BMP4-treated cells produced many spheroids expressing CK18, but only a few spheroids expressing Nestin (Fig. 4A, lower panels, B). Interestingly, individual spheroids were composed exclusively of either Nestin or CK18 (Fig. 4A), suggesting that dissociation and reaggregation in suspension provides an efficient tool to enrich a similar lineage during differentiation. Immunostaining of serial sections of CK18+ clusters showed that many cells coexpressed CK19, CK18, and AFP, all of which are expressed in hepatoblasts and some of these cells also produced albumin (Fig. 4C) [33,34]. Flow cytometric analysis revealed that compared to the adherent cell population, spheroids are highly enriched for cells that express AFP and Hex, which are expressed in hepatoblasts and essential for the onset of embryonic liver development (Fig. 4D) [35,36].
FIG. 4.
Characterization of spheroid-forming cells. (A) Immunostaining of serial sections of spheroids for cytokeratin 18 (CK18) and Nestin. Dashed white circles indicate a CK18+ spheroid and a Nestin+ spheroid produced under the control and Wnt3a/BMP4 condition, respectively. Scale bars: 20 μm. (B) Quantitation of CK18+ or Nestin+ spheroids produced under control and Wnt3a/BMP4 conditions [one-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test **P<0.01]. (C) Immunostaining of Wnt3a/BMP4 spheroids for early hepatic markers. Immunostaining was performed on serial sections of the same spheroids. Scale bars: 50 μm. (D) Hex (upper panels) and AFP (lower panels) expressions in adherent (left) and spheroid (right) cells. Data shown are representative histograms from three independent experiments. The percentage of positive cells was indicated. Blue, isotype control. Color images available online at www.liebertpub.com/scd
Maturation of spheroid cells into hepatocyte-like cells
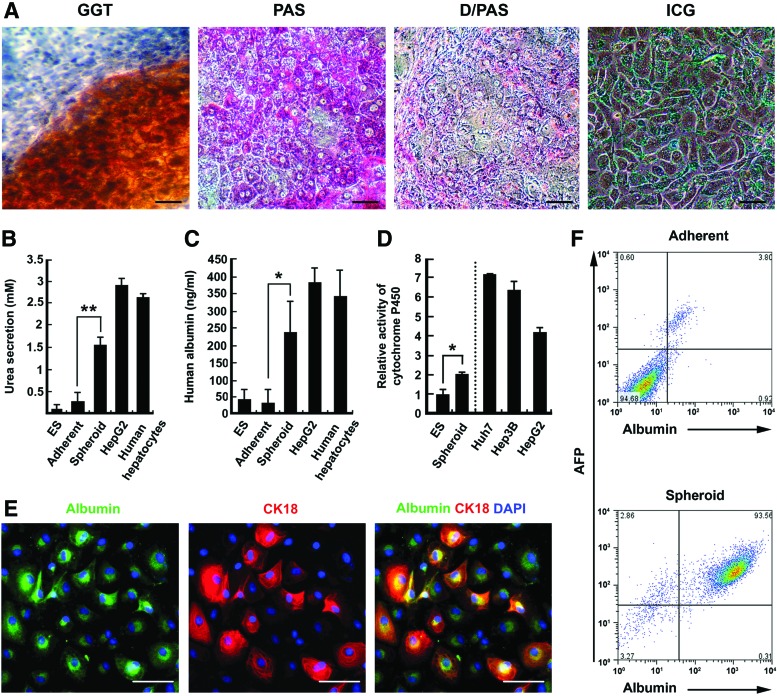
To investigate their differentiation potential into hepatocytes, Wnt3a/BMP4-treated spheroids were plated, without dissociation, onto tissue culture dishes coated with collagen I in the ITS medium supplemented with the hepatocyte growth factor (HGF), OSM, and DEX, which are known to play important roles in the commitment and maturation of hepatic cell types [37–40]. GGT is a metabolic enzyme expressed in fetal hepatocytes and bile duct cells, but not in adult hepatocytes [23,41]. Over the following 10 days, many polygonal cells showing a high GGT activity migrated from spheroids attached on culture dishes (Fig. 5A). Glycogen accumulation is a characteristic of liver cells and a central component of metabolic regulation [42]. Many polygonal cells were positive for PAS staining and the PAS-positive signals were profoundly reduced upon diastase digestion, suggesting the glycogen accumulation in differentiated cells (Fig. 5A: PAS and D/PAS). These cells exhibited a specific uptake of ICG, a nontoxic organic dye that is clinically used to test liver function (Fig. 5A). The end point of nitrogen metabolism is the cytotoxic compound ammonia. The urea cycle in hepatocytes converts ammonia to urea [43]. Low levels of urea and albumin secretion were detected in undifferentiated hESCs and differentiated adherent cells (Fig. 5B, C). However, the levels were significantly increased after hepatic differentiation of hESC-derived spheroid cells although the levels were lower than HepG2 cells and primary hepatocytes (Fig. 5B, C). The spheroid cells also exhibited the cytochrome P450 3A4 activity after phenobarbital sodium induction (Fig. 5D). The CYP activity increased significantly after hepatic differentiation of spheroid cells compared to undifferentiated hESCs, although the level is lower than those of hepatic cell lines (Huh7, Hep3B, and HepG2). Immunostaining and quantitative analysis using flow cytometry demonstrated that some albumin-positive cells expressed CK18 (Fig. 5E) and 93.5% of the spheroid-derived cells were double-positive for albumin and AFP, suggesting these cells have characteristics of early hepatocytes (Fig. 5F).
FIG. 5.
Differentiation of spheroid-forming cells into hepatocyte-like cells. (A) Differentiated cells from Wnt3a/BMP4 spheroids show a high γ-glutamyl transpeptidase (GGT) activity, accumulate cytoplasmic glycogen [Periodic acid Schiff (PAS) and Diastase/PAS (D/PAS)], and uptake indocyanine green (ICG). Scale bars: 20 μm. (B, C) Urea and albumin secretion measured by enzyme-linked immunosorbent assay show that these cells secrete comparable levels to HepG2 cells and human hepatocytes (one-way ANOVA followed by the Bonferroni post hoc test **P<0.01, *P<0.05). (D) The cytochrome P450 activity was measured after pentobarbital sodium induction. Hepatic cell lines (Huh7, Hep3B, and HepG2) were used as positive controls. (E) Immunostaining of spheroid-derived cells for albumin and CK18. Scale bars: 20 μm. (F) Flow cytometric analysis of albumin/AFP-double-positive cells in cells differentiated from adherent cells and spheroid-forming cells. Color images available online at www.liebertpub.com/scd
Engraftment of spheroid cells in injured liver tissues
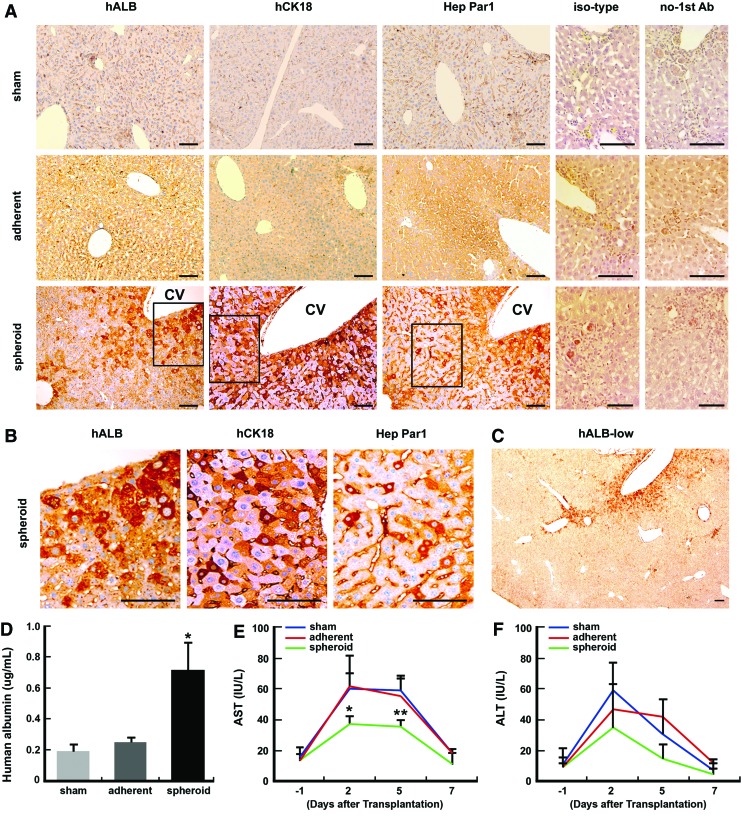
We next explored the ability of spheroid-forming cells to integrate, differentiate, and function in vivo. Immunodeficient BALB/c mice preintoxicated with CCl4 were injected intrasplenically with 1×106 spheroid-derived cells or differentiated adherent cells. Sham-operated mice received a medium used to support the cell suspension. Immunohistochemical analyses showed that human-specific signals of albumin, CK18, and Hep Par1 (human hepatocellular antigen) were distributed mostly around central veins 7 days after transplantation with spheroid-derived cells (Fig. 6A, B). The distribution of grafted cells was confined to multiple, but not all central veins (Fig. 6C), and the overall repopulation rate estimated from the hALB-positive area was approximately below 2%. In contrast, no signals were detected in the sham-operated and adherent cell-grafted liver. The presence of circulating hALB in the mouse serum was confirmed by ELISA assay showing that the hALB level significantly increased upon transplantation of spheroid-derived cells compared to sham-operated and adherent cell-grafted animals (Fig. 6D). CCl4 induces centrilobular necrosis, which is known to resolve by 7–10 days, providing an acute model of liver injury. As expected, CCl4 injection caused a dramatic elevation in the serum levels of liver enzymes, AST, and ALT, and the levels returned to basal levels after 7 days (Fig. 6E, F, sham). Transplantation of spheroid-forming cells at 1 day after CCl4 administration significantly decreased the level of AST at day 2 and 5, compared to sham-operated and adherent cell-grafted animals (Fig. 6E). Serum ALT levels were not significantly different between different treatments, but showed a similar trend of attenuation after grafting spheroid-forming cells (Fig. 6F). In our conditions, no teratoma formation was observed in a series of grafts, although further studies using different animal models of liver diseases are needed to address the long-term safety and efficacy of the spheroid cells.
FIG. 6.
Transplantation of spheroid-forming cells into carbon tetrachloride (CCl4)-intoxicated mice. (A) Immunohistochemistry of human-specific hepatocyte markers (hALB, human albumin; hCK18, human cytokeratin 18; Hep Par1, human hepatocyte paraffin 1) in liver sections 7 days after sham operation and transplantation of spheroid-forming cells and adherent cells. CV, central vein. Sham and adherent, scale bars: 50 μm. Spheroid, scale bars: 20 μm. (B) High magnifications of boxed areas in (A). Scale bars: 20 μm. (C) A representative low-magnification image of hALB staining after transplantation of spheroid-forming cells. Note that grafted spheroid cells are confined to only some of central veins. Scale bars: 50 μm. (D) Human albumin levels in the blood of mice 7 days after transplantation (one-way ANOVA followed by the Bonferroni post hoc test *P<0.05). (E, F) Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels in the blood of mice at different time points over the 7 days of transplantation (one-way ANOVA followed by the Bonferroni post hoc test **P<0.01, *P<0.05). Color images available online at www.liebertpub.com/scd
Discussion
Developing enrichment or purification methods is essential to address before clinical application of hESC-derived hepatocytes. In this study, we demonstrate that directed differentiation followed by spheroid formation of hESCs promotes the enrichment for the hepatic endoderm. The spheroid cells were not only able to differentiate into cells displaying multiple features of hepatocytes in vitro, but also engrafted and survived in injured liver tissues.
The initial differentiation of hESCs into the endoderm was enhanced by Wnt3a and BMP4 in adherent culture. Our gene and protein expression data showed that endodermal markers, GATA4, Foxa2, and Sox17 were all upregulated upon the combined treatment of Wnt3a and BMP4. The endodermal gene expressions were increased after transient upregulations of mesendodermal genes, Brachyury and MixL1, suggesting that hESCs were differentiated into a bipotent mesendoderm and subsequently into endodermal cells. Because GATA4, Foxa2, and Sox17 are expressed in the primitive endoderm as well as the definitive endoderm, we also analyzed the expression of Sox7, which expresses in the primitive endoderm, but not in the definitive endoderm. We showed that the enhanced expression of endodermal genes induced by Wnt3a/BMP4 was not accompanied by the induction of a primitive endodermal marker gene Sox7. Therefore, these data suggest that the Wnt3a/BMP4 treatment promoted differentiation of the definitive endoderm, the founder tissue of hepatocytes, rather than the primitive endoderm.
Since pluripotent stem cells exhibit heterogeneous differentiation, directed differentiation of hESCs should be followed by enrichment or purification of hepatic cells before transplantation. Previous studies have raised the possibility that hESC-derived hepatic cells could be enriched by genetically transforming hESCs or by immunopanning with hepatocyte-specific antibodies [13,44]. These strategies, however, require genetic modification or binding of antibodies to cell surface antigens that may cause unexpected alterations in signaling pathways. In the present study, we successfully enriched for the hepatic endoderm by a simple dissociation and reaggregation of the differentiated cells. This method produced spheroids consisting primarily of cells actively dividing and expressing a series of markers of the hepatic endoderm (AFP, ALB, CK18, CK19, C/EBPβ, Hex, and HNF4α) at the gene or protein levels.
Adhesion between cells is crucial to cellular function, survival, and differentiation and mediated by a different set of cell adhesion molecules spatiotemporally produced in early organogenesis [45]. In vitro formation of floating multicellular aggregates has been used to enrich and expand somatic stem or progenitor cells of various cell types, including neural cells, cardiac cells, and mammary gland cells [18,46,47]. A conventional study of the early embryonic development demonstrated that the same lineage of cells recognize and adhere to one another when ectodermal and mesodermal cells are mixed together [48,49]. In addition, recent studies showed that a highly enriched population of hepatic progenitor cells can be obtained by spheroid formation without the use of FACS [14]. In line with these findings, our data show that dissociation and reaggregation of differentiating hESCs produced spherical multicellular aggregates of 50–250 μm in diameter in the presence of B27. They were composed exclusively either of nestin or CK18 and the number of CK18+ spheroids was profoundly increased by a combined treatment of Wnt3a and BMP4. Although they were different in size, many resulting spheroids were composed entirely of cells expressing CK18/19, AFP, and albumin, markers of hepatocyte and this phenotype was not depending on the size of spheroids. Thus, we speculate that efficacy of hepatic differentiation may not depend on the size of spheroids at least under our differentiation condition. Although the detailed mechanism remains to be elucidated, it is speculated that a set of cell adhesion molecules specifically produced in the hepatic endoderm contributed to the selective aggregation of hepatoblast-like cells derived from hESCs.
Our data also demonstrate that the spheroid cells are able to differentiate into cells displaying characteristics of liver cells in vitro and after transplantation. After further differentiation in the presence of HGF/OSM/DEX, the spheroid cells showed the GGT activity, exhibited specific uptake of ICG, accumulated glycogen, and released urea and albumin. After intrasplenic injection, the spheroid-forming cells, but not adherent cells, integrated into the pericentral area of the injured liver, expressed human-specific signals of albumin, CK18, and Hep Par1, and attenuated the AST and ALT levels after transplantation. The hALB level detected in mouse serum (700 ng/mL) is comparable to other reports [9,13], but these values are much lower than the levels previously observed after transplantation of primary human hepatocytes [8]. It has been previously shown that hepatocyte-like cells derived from hESCs and induced pluripotent stem cells had properties of early hepatocytes, showing immature phenotypes and exhibiting low levels of CYP expression or activities [50–52]. In our study, a large number of spheroid cells still produced AFP and exhibited a relatively low CYP activity even after hepatic differentiation in vitro. Thus, it is likely that these spheroid cells need further maturation processes in the host liver to acquire more functional phenotypes after transplantation. In line with this speculation, a recent long-term in vivo study demonstrated that the ability of hESC-derived hepatocytes to produce albumin began to increase sharply after one month of grafting and reached a plateau level within an additional month [9].
In summary, our data show that the dissociation and reaggregation method efficiently enriched for hepatic endoderm in spheroids during differentiation of hESCs. These spheroid-forming cells were able to differentiate into early hepatic cells and attenuate AST and ALT levels after grafting into injured liver tissues. Although further studies are necessary to characterize the spheroid cells in vitro and in vivo, these results suggest the potential application of hESC-derived spheroid cells for therapy of liver injuries or diseases.
Acknowledgment
This research was supported by the Bio and Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MEST) (2012M3A9B4028636 and 2012M3A9C7050139)
Author Disclosure Statement
All authors declare they have no actual or potential competing financial interests.
References
- 1.Thomson JA. Itskovitz-Eldor J. Shapiro SS. Waknitz MA. Swiergiel JJ. Marshall VS. Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Biswas A. Hutchins R. Embryonic stem cells. Stem Cells Dev. 2007;16:213–222. doi: 10.1089/scd.2006.0081. [DOI] [PubMed] [Google Scholar]
- 3.Kim JH. Auerbach JM. Rodriguez-Gomez JA. Velasco I. Gavin D. Lumelsky N. Lee SH. Nguyen J. Sanchez-Pernaute R. Bankiewicz K. McKay R. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- 4.Shim JH. Kim SE. Woo DH. Kim SK. Oh CH. McKay R. Kim JH. Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia. 2007;50:1228–1238. doi: 10.1007/s00125-007-0634-z. [DOI] [PubMed] [Google Scholar]
- 5.Kelly OG. Pinson KI. Skarnes WC. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development. 2004;131:2803–2815. doi: 10.1242/dev.01137. [DOI] [PubMed] [Google Scholar]
- 6.Sherwood RI. Maehr R. Mazzoni EO. Melton DA. Wnt signaling specifies and patterns intestinal endoderm. Mech Dev. 2011;128:387–400. doi: 10.1016/j.mod.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaret KS. Regulatory phases of early liver development: paradigms of organogenesis. Nat Rev Genet. 2002;3:499–512. doi: 10.1038/nrg837. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal S. Holton KL. Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells. 2008;26:1117–1127. doi: 10.1634/stemcells.2007-1102. [DOI] [PubMed] [Google Scholar]
- 9.Basma H. Soto-Gutierrez A. Yannam GR. Liu L. Ito R. Yamamoto T. Ellis E. Carson SD. Sato S, et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136:990–999. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hay DC. Zhao D. Fletcher J. Hewitt ZA. McLean D. Urruticoechea-Uriguen A. Black JR. Elcombe C. Ross JA. Wolf R. Cui W. Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells. 2008;26:894–902. doi: 10.1634/stemcells.2007-0718. [DOI] [PubMed] [Google Scholar]
- 11.Touboul T. Hannan NR. Corbineau S. Martinez A. Martinet C. Branchereau S. Mainot S. Strick-Marchand H. Pedersen R, et al. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology. 2010;51:1754–1765. doi: 10.1002/hep.23506. [DOI] [PubMed] [Google Scholar]
- 12.Bukong TN. Lo T. Szabo G. Dolganiuc A. Novel developmental biology-based protocol of embryonic stem cell differentiation to morphologically sound and functional yet immature hepatocytes. Liver Int. 2012;32:732–741. doi: 10.1111/j.1478-3231.2011.02743.x. [DOI] [PubMed] [Google Scholar]
- 13.Duan Y. Catana A. Meng Y. Yamamoto N. He S. Gupta S. Gambhir SS. Zern MA. Differentiation and enrichment of hepatocyte-like cells from human embryonic stem cells in vitro and in vivo. Stem Cells. 2007;25:3058–3068. doi: 10.1634/stemcells.2007-0291. [DOI] [PubMed] [Google Scholar]
- 14.Tsuchiya A. Heike T. Fujino H. Shiota M. Umeda K. Yoshimoto M. Matsuda Y. Ichida T. Aoyagi Y. Nakahata T. Long-term extensive expansion of mouse hepatic stem/progenitor cells in a novel serum-free culture system. Gastroenterology. 2005;128:2089–2104. doi: 10.1053/j.gastro.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 15.Moliner A. Enfors P. Ibanez CF. Andang M. Mouse embryonic stem cell-derived spheres with distinct neurogenic potentials. Stem Cells Dev. 2008;17:233–243. doi: 10.1089/scd.2007.0211. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Q. Nguyen AL. Shi S. Hill C. Wilder-Smith P. Krasieva TB. Le AD. Three-dimensional spheroid culture of human gingiva-derived mesenchymal stem cells enhances mitigation of chemotherapy-induced oral mucositis. Stem Cells Dev. 2012;21:937–947. doi: 10.1089/scd.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer TD. Markakis EA. Willhoite AR. Safar F. Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds BA. Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 19.Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988;102:639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- 20.Matsushita T. Nakano K. Nishikura Y. Higuchi K. Kiyota A. Ueoka R. Spheroid formation and functional restoration of human fetal hepatocytes on poly-amino acid-coated dishes after serial proliferation. Cytotechnology. 2003;42:57–66. doi: 10.1023/B:CYTO.0000009819.28689.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong JZ. Sarrazin S. Cassio D. Gauthier F. Alvarez F. Application of spheroid culture to human hepatocytes and maintenance of their differentiation. Biol Cell. 1994;81:77–81. doi: 10.1016/0248-4900(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 22.Gil JE. Woo DH. Shim JH. Kim SE. You HJ. Park SH. Paek SH. Kim SK. Kim JH. Vitronectin promotes oligodendrocyte differentiation during neurogenesis of human embryonic stem cells. FEBS Lett. 2009;583:561–567. doi: 10.1016/j.febslet.2008.12.061. [DOI] [PubMed] [Google Scholar]
- 23.Rutenburg AM. Kim H. Fischbein JW. Hanker JS. Wasserkrug HL. Seligman AM. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969;17:517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- 24.Gouon-Evans V. Boussemart L. Gadue P. Nierhoff D. Koehler CI. Kubo A. Shafritz DA. Keller G. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat Biotechnol. 2006;24:1402–1411. doi: 10.1038/nbt1258. [DOI] [PubMed] [Google Scholar]
- 25.Rossi JM. Dunn NR. Hogan BL. Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tada S. Era T. Furusawa C. Sakurai H. Nishikawa S. Kinoshita M. Nakao K. Chiba T. Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development. 2005;132:4363–4374. doi: 10.1242/dev.02005. [DOI] [PubMed] [Google Scholar]
- 27.Lickert H. Domon C. Huls G. Wehrle C. Duluc I. Clevers H. Meyer BI. Freund JN. Kemler R. Wnt/(beta)-catenin signaling regulates the expression of the homeobox gene Cdx1 in embryonic intestine. Development. 2000;127:3805–3813. doi: 10.1242/dev.127.17.3805. [DOI] [PubMed] [Google Scholar]
- 28.Hussain SZ. Sneddon T. Tan X. Micsenyi A. Michalopoulos GK. Monga SP. Wnt impacts growth and differentiation in ex vivo liver development. Exp Cell Res. 2004;292:157–169. doi: 10.1016/j.yexcr.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 29.Izumi N. Era T. Akimaru H. Yasunaga M. Nishikawa S. Dissecting the molecular hierarchy for mesendoderm differentiation through a combination of embryonic stem cell culture and RNA interference. Stem Cells. 2007;25:1664–1674. doi: 10.1634/stemcells.2006-0681. [DOI] [PubMed] [Google Scholar]
- 30.Nakanishi M. Kurisaki A. Hayashi Y. Warashina M. Ishiura S. Kusuda-Furue M. Asashima M. Directed induction of anterior and posterior primitive streak by Wnt from embryonic stem cells cultured in a chemically defined serum-free medium. FASEB J. 2009;23:114–122. doi: 10.1096/fj.08-111203. [DOI] [PubMed] [Google Scholar]
- 31.Li L. Li F. Qi H. Feng G. Yuan K. Deng H. Zhou H. Coexpression of Pdx1 and betacellulin in mesenchymal stem cells could promote the differentiation of nestin-positive epithelium-like progenitors and pancreatic islet-like spheroids. Stem Cells Dev. 2008;17:815–823. doi: 10.1089/scd.2008.0060. [DOI] [PubMed] [Google Scholar]
- 32.Moll R. Franke WW. Schiller DL. Geiger B. Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 33.Shiojiri N. Lemire JM. Fausto N. Cell lineages and oval cell progenitors in rat liver development. Cancer Res. 1991;51:2611–2620. [PubMed] [Google Scholar]
- 34.Germain L. Blouin MJ. Marceau N. Biliary epithelial and hepatocytic cell lineage relationships in embryonic rat liver as determined by the differential expression of cytokeratins, alpha-fetoprotein, albumin, and cell surface-exposed components. Cancer Res. 1988;48:4909–4918. [PubMed] [Google Scholar]
- 35.Shiojiri N. Enzymo- and immunocytochemical analyses of the differentiation of liver cells in the prenatal mouse. J Embryol Exp Morphol. 1981;62:139–152. [PubMed] [Google Scholar]
- 36.Hunter MP. Wilson CM. Jiang X. Cong R. Vasavada H. Kaestner KH. Bogue CW. The homeobox gene Hhex is essential for proper hepatoblast differentiation and bile duct morphogenesis. Dev Biol. 2007;308:355–367. doi: 10.1016/j.ydbio.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belanger L. Frain M. Baril P. Gingras MC. Bartkowiak J. Sala-Trepat JM. Glucocorticosteroid suppression of alpha1-fetoprotein synthesis in developing rat liver. Evidence for selective gene repression at the transcriptional level. Biochemistry. 1981;20:6665–6672. doi: 10.1021/bi00526a022. [DOI] [PubMed] [Google Scholar]
- 38.Kamiya A. Kinoshita T. Miyajima A. Oncostatin M and hepatocyte growth factor induce hepatic maturation via distinct signaling pathways. FEBS Lett. 2001;492:90–94. doi: 10.1016/s0014-5793(01)02140-8. [DOI] [PubMed] [Google Scholar]
- 39.Michalopoulos GK. Bowen WC. Mule K. Luo J. HGF-, EGF-, and dexamethasone-induced gene expression patterns during formation of tissue in hepatic organoid cultures. Gene Expr. 2003;11:55–75. doi: 10.3727/000000003108748964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt C. Bladt F. Goedecke S. Brinkmann V. Zschiesche W. Sharpe M. Gherardi E. Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 41.Houssaint E. Differentiation of the mouse hepatic primordium. I. An analysis of tissue interactions in hepatocyte differentiation. Cell Differ. 1980;9:269–279. doi: 10.1016/0045-6039(80)90026-3. [DOI] [PubMed] [Google Scholar]
- 42.Shelley HJ. Carbohydrate reserves in the newborn infant. Br Med J. 1964;1:273–275. doi: 10.1136/bmj.1.5378.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farrar WE., Jr. Corwin LM. The essential role of the liver in detoxification of endotoxin. Ann N Y Acad Sci. 1966;133:668–684. doi: 10.1111/j.1749-6632.1966.tb52397.x. [DOI] [PubMed] [Google Scholar]
- 44.Chiao E. Elazar M. Xing Y. Xiong A. Kmet M. Millan MT. Glenn JS. Wong WH. Baker J. Isolation and transcriptional profiling of purified hepatic cells derived from human embryonic stem cells. Stem Cells. 2008;26:2032–2041. doi: 10.1634/stemcells.2007-0964. [DOI] [PubMed] [Google Scholar]
- 45.Hynes RO. Lander AD. Contact and adhesive specificities in the associations, migrations, and targeting of cells and axons. Cell. 1992;68:303–322. doi: 10.1016/0092-8674(92)90472-o. [DOI] [PubMed] [Google Scholar]
- 46.De Bruijne J. Jongsma HJ. van Ginneken AC. The passive electrical properties of spheroidal aggregates cultured from neonatal rat heart cells. J Physiol. 1984;355:281–293. doi: 10.1113/jphysiol.1984.sp015419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sansone P. Storci G. Tavolari S. Guarnieri T. Giovannini C. Taffurelli M. Ceccarelli C. Santini D, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117:3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moscona A. Rotation-mediated histogenetic aggregation of dissociated cells. A quantifiable approach to cell interactions in vitro. Exp Cell Res. 1961;22:455–475. doi: 10.1016/0014-4827(61)90122-7. [DOI] [PubMed] [Google Scholar]
- 49.Steinberg MS. Gilbert SF. Townes and Holtfreter (1955): directed movements and selective adhesion of embryonic amphibian cells. J Exp Zool A Comp Exp Biol. 2004;301:701–706. doi: 10.1002/jez.a.114. [DOI] [PubMed] [Google Scholar]
- 50.Sharma AD. Cantz T. Vogel A. Schambach A. Haridass D. Iken M. Bleidissel M. Manns MP. Scholer HR. Ott M. Murine embryonic stem cell-derived hepatic progenitor cells engraft in recipient livers with limited capacity of liver tissue formation. Cell Transplant. 2008;17:313–323. doi: 10.3727/096368908784153896. [DOI] [PubMed] [Google Scholar]
- 51.Song Z. Cai J. Liu Y. Zhao D. Yong J. Duo S. Song X. Guo Y. Zhao Y, et al. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19:1233–1242. doi: 10.1038/cr.2009.107. [DOI] [PubMed] [Google Scholar]
- 52.Takayama K. Inamura M. Kawabata K. Sugawara M. Kikuchi K. Higuchi M. Nagamoto Y. Watanabe H. Tashiro K, et al. Generation of metabolically functioning hepatocytes from human pluripotent stem cells by FOXA2 and HNF1alpha transduction. J Hepatol. 2012;57:628–636. doi: 10.1016/j.jhep.2012.04.038. [DOI] [PubMed] [Google Scholar]