Abstract
LDH-C4 is a lactate dehydrogenase that catalyzes the interconversion of pyruvate with lactate. In mammals the, Ldh-c gene was originally thought to be expressed only in testis and spermatozoa. Plateau pika (Ochotona curzoniae), belonging to the genus Ochotona of the Ochotonidea family, is a hypoxia tolerant mammal living at 3000–5000 m above sea levelon the Qinghai-Tibet Plateau. We found that the expression pattern of six LDH isoenzymes in the somatic tissues of female and male plateau pikas to be the same as those in testis and sperm, suggesting that LDH-C4 was expressed in somatic tissues of plateau pika. Here we report the detection of LDHC in the somatic tissues of plateau pika using RT-PCR, Western blotting and immunohistochemistry. Our results indicate that Ldh-c mRNA is transcribed in the heart, liver, lung, kidney, brain, skeletal muscle and testis. In somatic tissues LDHC was translated in the cytoplasm, while in testis it was expressed in both cytoplasm and mitochondria. The third band from cathode to anode in LDH isoenzymes was identified as LDH-C4. The finding that Ldh-c is expressed in both somatic tissues and testis of plateau pika provides important implications for more in-depth research into the Ldh-c function in mammals.
Keywords: Testis-specific lactate dehydrogenase C, Plateau pika (Ochotona curzoniae), Hypoxia, Somatic tissues, Qinghai-Tibet Plateau
Abbreviations: LDH-C4, testis-specific lactate dehydrogenase; NBT, nitrobenzene thiocyanate chloride; PMS, phenazine methosulfate; cDNA, complementary DNA; EST, expressed sequence tag; CRE, cAMP-response element; IPTG, isopropyl-β-d-thiogalactopyranoside.
Highlights
▸ Plateau pika (Ochotona curzoniae) express 6 isoforms of lactate dehydrogenase (LDH). ▸ LDH-C4 was thought to be expressed only in the testis and sperm of mammals. ▸ We detected LDH-C4 in somatic tissues as well as the testis and sperm of plateau pika.
1. Introduction
The lactate dehydrogenase (LDH) family enzymes catalyze the interconversion of pyruvate to lactate with the concomitant oxidation/reduction of NADH to NAD+ [1]. Different forms of LDH are the product of three different genes: Ldh-a, Ldh-b, and Ldh-c which encode A, B and C subunits, respectively [2,3]. LDH consists of A and B subunits that assemble into homo- or heterotetramers that are distributed in the body in combinations reflecting the metabolic requirements of different tissues and consistent with the catalytic properties of the isozymes. However, the homotetramer LDH-C4 was previously only detected in testis and spermatozoa and not in other tissues or cells [4,5]. More recent studies have detected the LDH-C4 protein by immunohistochemistry in germinal-vesicle stage oocytes and fertilized eggs, which persists into the pre-implantation blastocyst stage, but the enzymatic activity of LDH-C4 was not detected in egg extracts [6]. In addition computational methods were used to predict the amino acid sequences and gene locations for mammalian lactate dehydrogenase (LDH) genes and proteins using genome sequence databanks. Their results indicated that LDHA, LDHB and LDH6B genes are present in all mammalian genomnes examined, including a monotreme species (platypus), whereas the LDHC gene may have arise more recently in marsupial mammals [7].
Plateau pika (Ochotona curzoniae), belonging to the genus Ochotona of the Ochotonidea family, is a small, non-hibernating rodent that lives in remote mountain areas at high altitudes. The plateau pika evolved as a hypoxia and low temperature tolerant mammal with a markedly high resting metabolic rate, non-shivering thermogenesis [8] and a high ratio of oxygen utilization to cope with the cold and hypoxic plateau environment [9–11]. While other studies have indicated that only five LDH isoenzymes are present in the somatic tissues of mammals, we observed six bands corresponding to LDH isoenzymes using native polyacrylamide gel electrophoresis (PAGE) in the tissues of female and male plateau pikas, and these patterns in the tissues were the same as those in testis and sperm. A comparison of the electrophoretic mobility of LDH isoenzymes in testes of Sprague-Dawley rats and plateau pika (data not shown) suggested that the third band from cathode to anode may be LDH-C4 in somatic tissues of plateau pika. To verify this observation, we detected Ldh-c mRNA in somatic tissues of plateau pika by RT-PCR. Production and purification of polyclonal antibodies against the LDHC was performed for distribution of LDHC in both cytoplasm and mitochondria. Furthermore, the mobility of His-tagged LDH-C4 protein was compared with that of LDH isoenzymes from somatic tissues of plateau pika in order to confirm the LDH-C4 band within the LDH isoenzyme electrophoretic spectrum.
2. Materials and methods
2.1. Animal procedures
Plateau pikas were live-trapped from the Haibei Alpine Meadow Ecosystem Research Station in Qinghai Province in China. Pathogen-free Sprague-Dawley rats were purchased from the Animal Center of Lanzhou Medicine College (1500 m altitude). All animals were first anesthetized with sodium pentobarbital (5%) and then sacrificed by cervical dislocation immediately before dissection. Heart, liver, lung, kidney, brain, skeletal muscle and testis were rapidly removed and frozen in liquid nitrogen for storage. All procedures involved in the handling and care of animals were in accordance with the China Practice for the Care and Use of Laboratory Animals and were approved by the China Zoological Society (permit number: GB 14923-2010).
2.2. LDH isoenzymes
All tissues were washed with cold 0.9% physiological saline, dried on filter paper and weighed. The tissues were homogenized on ice as a 1:4 (w/v) dilution in 0.9% physiological saline. The homogenate was centrifuged at 15,000 revs/min at 4 °C for 10 min, and the supernatant was collected. Cauda epididymides collected in 1× PBS (Ca2+/Mg2+-free) were carefully dissected to remove blood vessels and fat, and several small cuts were made with iridectomy scissors to allow the sperm to swim out in 1 ml PBS for 10 min at room temperature. To remove residual substrates present in the epididymal fluid, sperm were washed by dilution in 3 ml PBS. After freezing and thawing twice, the suspension was centrifuged at 4 °C for 20 min at 15,000g, and the supernatant was collected. Native PAGE was performed with a DY-200 steady current and voltage electrophoresis apparatus (Beijing Liuyi Instrument Factory). The electrode buffer was Tris–glycine (pH 8.3), and 6 μl samples were loaded. The current was 10 mA in the stacking gel and 25 mA in the separating gel. The LDH bands were stained at 37 °C in a mixture of 4 ml of 5 mg/ml NAD+, 2.5 ml of 0.1 M NaCl, 10 ml of 1 mg/ml nitrobenzene thiocyanate chloride (NBT), 1 ml of 1 mg/ml phenazine methosulfate (PMS), 2.5 ml of 1 M sodium lactate and 0.5 M phosphate buffer (pH 7.5) for 30 min in the dark. The gels were rinsed with distilled water and stored in 10% glycerol, 7% acetic acid.
2.3. Cloning and sequencing of Ldh-a, Ldh-b and Ldh-c in testis
The pika Ldh-a specific primers for EST amplification were designed from the Ldh-a gene of Oryctolagus cuniculus (NM_001082277.1). The pika Ldh-b specific primers for EST amplification were designed from the alignment of highly conserved coding sequence regions of the Ldh-b gene of Mus musculus (NM_008492.2), Homo sapiens (NM_002300.5), Bos taurus (NM_174100.1) and Rattus norvegicus (NM_012595.1). For Ldh-c, the specific primer for 5′RACE (not for EST) was designed from the alignment of highly conserved coding sequence regions of the Ldh-c gene of M. musculus (NM_013580.4), H. sapiens (NM_002301.4), B. taurus (NM_001113249.1) and R. norvegicus (NM_017266.2).
Total cellular RNA was extracted from frozen testis tissue using the EZ Spin Column Total RNA Isolation Kit (Sangon, Shanghai, China). Two micrograms of total RNA was subjected to reverse transcription and PCR using the AMV one-step RT-PCR Kit (Sangon, Shanghai, China). The Ldh-a and Ldh-b PCR conditions were 25 min at 45 °C, 5 min at 94 °C, 40 cycles of PCR with 40 s at 94 °C, 30 s at 59 °C annealing temperature, 75 s at 72 °C, and a final elongation step at 72 °C for 10 min. The PCR products were subsequently cloned into the PMD19-T vector (Takara, Dalian, China) and sequenced.
The antisense primer for 5′RACE was designed according to the sequence of an EST fragment previously cloned. 5′RACE first-strand cDNA was primed with SMARTer RACE cDNA Amplification Kit (Clontech, Mountain View, CA, USA). For PCR amplification of Ldh-a, Ldh-b and Ldh-c, 2.5 μl of 5′RACE first-strand cDNA were amplified using the Advantage 2 PCR Kit (Clontech, Mountain View, CA, USA) in a 50 μl reaction. The PCR conditions were 5 min activation at 95 °C, 40 cycles of PCR with 1 min at 94 °C, 30 s at 60 °C annealing temperature, 2 min at 72 °C, and a final elongation step at 72 °C for 10 min. The PCR products were subsequently cloned into the PMD19-T vector and sequenced.
The sense primer for 3′RACE was designed according to the sequence of a 5′RACE fragment previously cloned. 3′RACE first-strand cDNA was primed with SMARTer RACE cDNA Amplification Kit. For PCR amplification, 2.5 μl of 3′RACE first-strand cDNA was amplified using Advantage 2 PCR Kit in a 50 μl reaction. The PCR conditions were 5 min activation at 95 °C, 40 cycles of PCR with 30 s at 94 °C, 30 s at annealing temperature (60 °C, 59 °C and 62 °C for Ldh-a, Ldh-b and Ldh-c, respectively), 2 min at 72 °C, and a final elongation step at 72 °C for 10 min. The PCR products were subsequently cloned into the PMD19-T vector and sequenced. The nucleotide sequences of oligonucleotides used for cloning of Ldh-a, Ldh-b and Ldh-c in plateau pika testis are shown in Table 1.
Table 1.
Sequences of oligonucleotides used for cloning of Ldh-a, ldh-b, Ldh-c in plateau pika testis.
| Gene | Primer | Sequence |
|---|---|---|
| Ldh-a | EST primers | F:5′-GGCACGGCAGCAAGAGGGAGAAA-3′ |
| R:5′-TGA AGGGACCAGAAGTTCCGTAGCAC-3′ | ||
| 5′REAC PCR primer | 5′-GTGCCAAGATGCGAAGAACACTCAGGAA-3′ | |
| 3′REAC PCR primer | 5′- TCC TGGGCCATT GGATTGTCTGTAGCA-3′ | |
| Ldh-b | EST primers | F:5′-ACAGCCAATTCTAAGATTGTGGT-3′ |
| R:5′-TCCCTTCACCATTGTAGACACT-3′ | ||
| 5′REAC PCR primer | 5′-GGTGCTTGGGCAGTCCACTTAGTTTCC-3′ | |
| 3′REAC PCR primer | 5′-AGCGATGGGAACAGACAATGACAGTGAA-3′ | |
| Ldh-c | 5′REAC PCR primer | 5′-ACACCACTCCATATGGGCACACTGGA-3′ |
| 3′REAC PCR primer | 5′-AAGGCAGCAGGAGGGAGAAGGTCG-3′ |
The full-length Ldh-a and Ldh-b cDNA of plateau pika were spliced and the ORF determined according to the overlapping regions formed by the three fragments, and Ldh-c was spliced into two fragments. Translation of the cDNA nucleotide sequence was performed using the EditSeq program of DNASTAR. The nucleotide and deduced amino sequences were compared with the sequences in the GenBank database by using BLAST at NCBI. Multiple alignments of nucleotide sequences were performed using the DNAMAN program.
2.4. Cloning and sequencing of Ldh-c in somatic cells
The specific primers for the ORF of Ldh-c for amplification from somatic tissues amplification were designed according to the sequence of testis Ldh-c previously cloned, and specificity of the primers was analyzed using the BLAST (NCBI) program. Total cellular RNA was extracted from frozen tissues using TRIzol reagent (Invitrogen). Four micrograms of this RNA was primed with a dT18 oligonucleotide and reverse-transcribed with the First Strand cDNA Synthesis Kit (Sangon) according to the manufacturer's instructions. For somatic amplification of Ldh-c from tissues, 2 μl first-strand cDNA was used in 25 μl RT-PCR reactions using the Premix Ex Taq Version Kit (Takara); PCR conditions were 3 min at 94 °C, 40 cycles of 30 s at 94 °C, 30 s at 60 °C, 2 min at 72 °C, and a final elongation step at 72 °C for 10 min. The amplified PCR products were subsequently cloned into the PMD19-T vector and sequenced. Primers used for Ldh-c to yield a 999-bp sequence: 5′-ATGTCGACAGTCAAGGAGCAACT-3′ (sense) and 5′-TTAAAACACCAGGTCCTTCTGGA-3′ (antisense). Primers used for Ldh-c to yield a 130-bp sequence: 5′-TATCGAGAATCTGATCGCAGAAGAC-3′ (sense) and5′-GGGCAAGTTCATCAGCCAAATCC-3′ (antisense).
2.5. Plasmid construction and preparation of recombinant proteins
A 996 bp BamHI/XhoI fragment representing the entire Ldh-c coding sequence was amplified by PCR from cDNA of plateau pika testis using the Premix Ex Taq Version Kit. The PCR primers were 5′-CGGGATCCATGTCGACAGTCAAGGAGC-3′ (sense) and 5′-CCGCTCGAGAAACACCAGGTCCTTCTGGAC-3′ (antisense). PCR conditions were 5 min at 95 °C, 30 cycles of 45 s at 95 °C, 45 s at 65 °C, 1 min at 72 °C, and a final elongation step at 72 °C for 10 min. The BamHI/XhoI fragment was subsequently cloned into the pET-30a(+) expression vector (Novagen). The recombinant expression shuttle (pET-30a-Ldh-c) was transformed into Escherichia coli BL21 cells and grown in LB media. The recombinant protein was expressed in E. coli BL21 cells by induction with 0.8 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 37 °C for 2 h. Purification of the recombinant protein was performed using a His-trapR chelating column (Pharmacia Biotech Inc.). SDS–PAGE was performed in Tris/glycine buffer, pH 8.3, on a 12% (w/v) separating gel with a 4% (w/v) stacking gel, and then electroblotted onto a nitrocellulose membrane at a constant current of 80 mA at 4 °C for 1 h. After blocking in TBS (150 mM NaCl, 20 Mm Tris-base, pH 7.4) with 5% (w/v) skimmed milk, the membrane was incubated with an anti-His polyclonal antibody (1:2000 diluted in TBS) at 37 °C for 2 h. After washing three times, goat anti-rabbit IgG conjugated with HRP was added and incubated for 1 h at room temperature. Antibody binding was visualized with the ECL kit from Pierce Biotechnology.
2.6. Production and purification of polyclonal antibodies against the LDHC
Antibodies against recombinant LDHC were raised in a male New Zealand white rabbit. The rabbit was injected subcutaneously with 0.3 mg of highly purified recombinant LDHC protein dissolved in 0.2 M NaCl and emulsified in 0.5 ml of Freund's complete adjuvant to enhance the response to the immunogen. Two booster injections were given with 0.3 mg recombinant protein each in incomplete Freund's adjuvant at 2-week interval to obtain a prolonged persistence of the immunogen in tissues and a continuous stimulation to the immune system. Ten days after the final injection, 60 ml of blood was collected and kept overnight at room temperature to allow clotting of blood. The crude antiserum was collected by centrifugation (4200g for 5 min), and purification of the anti-LDHC was performed using immunoaffinity chromatography.
2.7. Western blotting
Tissues of plateau pika was homogenized in extraction buffer (1% Triton X-100; 150 mM sodium chloride; 10 mM TrisCl (pH 7.4); 1 mM EDTA; 0.2 mM Na3VO4; 0.2 mM phenylmethanesulfonyl fluoride; 0.5% NP-40; 50 mM NaF) and centrifuged for 10 min at 4 °C and 15,000g. The supernatant was recovered, and protein concentration was measured with the Pierce protein assay kit. Proteins were separated on a 12% SDS–PAGE gel, transferred to PVDF membrane, blocked by 5% milk, and incubated with anti-LDHC antibody at 4 °C overnight. The blots were incubated with secondary antibody at room temperature for 2 h and washed by TBST. Antibody binding was visualized with the ECL kit from Pierce Biotechnology.
2.8. Mitochondrion isolation
The mitochondrion isolation medium consisted of 10 mM potassium phosphate, 0.25 M sucrose, and 0.5 mM EDTA, at pH 7.4. All isolation procedures were carried out at 0–4 °C. Mitochondrion were prepared as described previously [12]. Sample of heart, liver, lung, kidney, brain, skeletal muscle and testis of plateau pika were homogenized in 10 volumes of buffer followed by differential centrifugation: cells and cell debris were spun down by centrifugation at 600g for 10 min. Mitochondrion were spun down from the supernatant by centrifugation at 9000g for 10 min. The mitochondrial pellet was then washed 3 times before use. The mitochondrion were lysed by using 2× SDS–PAGE loading buffer (Sangon, Shanghai, China) at 95 °C for 5 min, centrifuged at 16,000 rpm for 2 min, and the supernatant was collected for the Western blotting, primary antibody was anti-LDHC.
2.9. Immunohistochemistry
Tissues of plateau pika were placed in 4% paraformaldehyde (Sigma) fixative at 4 °C overnight. The tissue was dehydrated and paraffin embedded. Four-micrometer microtome sections were obtained and mounted on slides. For immunohistochemistry, the slides were deparaffinized in xylenes and then rehydrated for 3 min each in 100% ethyl alcohol, 95% ethyl alcohol, 70% ethyl alcohol, 50% ethyl alcohol, and ddH2O. Antigen retrieval was accomplished by incubating slides in 10 mM sodium citrate and heating in a microwave oven on high for 2 min and on low for 7 min. The slides were cooled in sodium citrate solution for 20 min, washed in TBS-T (Tween) to permeabilize, and then incubated in 3% hydrogen peroxide in TBS for 15 min. The sections were blocked for 1 h in 10% serum (from host of secondary antibody) in 3% BSA-TBS at room temperature and incubated overnight in primary antibody (anti-LDHC) diluted 1:500 in the blocking solution. A ChemMateTM EnvisionTM Detection kit was used for immunohistochemistry staining according to the manufacturer's instructions. Immunohistochemical images were acquired on an OLYMPUS DP71 microscope and DP CONTROLLER software.
3. Results
3.1. Characteristics of LDH isoenzyme spectrum in tissues of plateau pika
Earlier studies indicated that the LDH isoenzyme spectrum includes five bands, LDH-B4, LDH-B3A1, LDH-B2A2, LDH-B3A1 and LDH-A4, in the somatic tissues of mammals except the testis. However, we have found six electrophoretic bands of LDH isoenzymes in the somatic tissues of female and male plateau pika, and the patterns in the somatic tissues were the same as those in testis and sperm (Fig. 1). LDH-C4 is known to have high thermostability [13,14], so that thermostability of LDH isoenzymes in plateau pika liver was analyzed by electrophoresis on a native polyacrylamide gel, which was stained for LDH activity with lactate as the substrate. After incubation of the plateau pika liver homogenate at 50 °C for 40 min and at 55 °C for 55 min, the third band from cathode to anode (indicated by an arrow in Fig. 1) still retained partial activity as compared to the virtually complete inactivation of the fourth band (Fig. 1(D)), suggesting that the band may be LDH-C4 in somatic tissues of plateau pika.
Fig. 1.
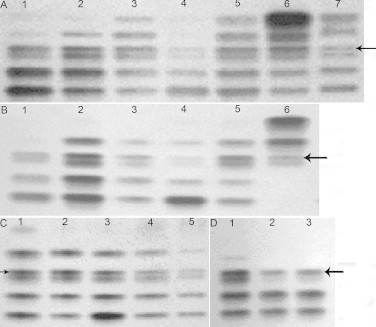
Characteristics of LDH isoenzyme spectrum in tissues of plateau pika. There were six LDH isoenzyme bands in all tissues (Panels A and B), and the patterns in the somatic tissues were same as that in testis and sperm (Panel C). The third band from cathode to anode (indicated by an arrow) in LDH isoenzymes was heat-stable (Panel D). Panels A and B, LDH isoenzyme spectrum of male and felmale plateau pika, lanes 1–7 are heart, liver, lung, kidney, brain, skeletal muscle and testis of plateau pika, respectively. Panel C, LDH isoenzyme spectrum in plateau pika's sperm. Lanes 1–5 are brain, lung, kidney, testis and sperm of plateau pika, respectively. Panel D, thermal stability of LDH isoenzymes in plateau pika's liver. Lanes 1–3 are the homogenate of plateau pika liver frozen in liquid nitrogen, incubated at 50 °C for 40 min, and incubated at 55 °C for 55 min, respectively. From the anode (bottom of gel) to cathode is follows: LDH-B4, LDH-B3A1, LDH-B2A2, LDH-C4 (indicated by an arrow), LDH-B1A3 and LDH-A4.
3.2. Cloning and sequencing of Ldh-c in somatic tissues
In order to confirm expression of Ldh-c mRNA in plateau pika's somatic tissues, Ldh-a, Ldh-b and Ldh-c firstly were cloned in plateau pika testis, the primers amplified Lhd-c in plateau pika's somatic tissues was designed by according to the sequence of the Ldh-c in testis, and then, we used PCR to amplify the Ldh-c mRNA in plateau pika's somatic tissues. Ldh-a, Ldh-b and Ldh-c of plateau pika testis were cloned and deposited in GenBank with the accession numbers HQ704676, HQ704677 and HQ704678, respectively. The cDNA sequences of Ldh-a, Ldh-b and Ldh-c were, respectively, 1693 bp, 1299 bp and 1624 bp, with open reading frames (ORF) of 999 bp, 1005 bp and 999 bp, encoding 332, 334 and 332 amino acids, with predicted molecular weights of 36,557.5 Da, 36,464.3 Da and 36,052.9 Da and pI values of 8.16, 6.19 and 8.08. Plateau pika Ldh-c ORF shares 74% and 68% nucleotide sequence homology with that of Ldh-a and Ldh-b of plateau pika, respectively. According to the sequence of testis Ldh-c (HQ704678), the primers were designed to amplify an ORF transcript. A single band of ∼1000 bp was amplified from the heart, liver, lung, kidney, brain, skeletal muscle and testis of plateau pika (Fig. 2(A)). By sequencing and alignment, the PCR products amplified from somatic tissues were determined to be 999 bp in length and identical to that of Ldh-c (HQ704678). The primers that would ensure specificity of the reaction were designed to amplify a 130-bp fragment of the transcript according to the sequence of somatic tissues Ldh-c. RT-PCR showed a single band as the amplification product from every tissue (Fig. 2(B)). The sequence of the130-bp transcripts was identical to that in the GenBank for Ldh-c (HQ704678), suggesting that Ldh-c mRNA was transcribed in the heart, liver, lung, kidney, brain, skeletal muscle and testis.
Fig. 2.
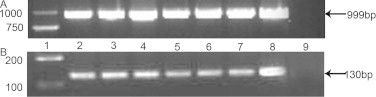
RT-PCR screening of Ldh-c expression in somatic tissues of plateau pika. RNA was isolated from heart, liver, lung, kidney, brain, skeletal muscle and testis of plateau pika. Primers were designed to amplify both full-length and 130-bp sequences specific to Ldh-c mRNA (Panels A and B). The sequence of the130-bp and 999-bp transcripts was identical to that in the GenBank for testis Ldh-c, suggesting that Ldh-c mRNA was transcribed in the heart, liver, lung, kidney, brain and skeletal muscle. Lane 1, size markers; lanes 2–8, PCR products are in heart, liver, lung, kidney, brain skeletal muscle and testis of plateau pika, respectively; lane 9, water blank.
3.3. LDH-C4 in somatic tissues of plateau pika
A recombinant LDH-C protein was produced for a subsequent experiment to produce polyclonal antibodies against the LDHC and to identify the electrophoretic band corresponding to an active LDH-C4 enzyme present in somatic tissues of plateau pika. SDS–PAGE (Fig. 3(A)) and Western blot (Fig. 3(B)) using an anti-His polyclonal antibody indicated the purified recombinant protein migrated with an apparent molecular mass within 33–45 KD, native PAGE stained for LDH activity demonstrated that the purified recombinant protein was an active LDH enzyme (Fig. 3(C)), suggesting that the purified recombinant protein was LDH-C4 of plateau pika. Antibodies against recombinant LDHC were produced and immunohistochemistry was performed. The result shows that LDHC has distribution in the heart, liver, lung, kidney, brain, skeletal muscle and testis of plateau pika (Fig. 4). Extracts of cytoplasm and mitochondria of heart, liver, lung, kidney, brain and testis were resolved by SDS–PAGE, blotted to PVDF for Western blots, and probed with the antibody. A positive signal was obtained for extracts of cytoplasm of heart, liver, lung, kidney, brain, skeletal muscle and testis (Fig. 5(A)) as well as extracts of testis mitochondria (Fig. 5(B)). No positive signal was observed for extracts of mitochondria of somatic tissues (Fig. 5(B)). This finding suggested that LDHC in somatic tissues was translated only in the cytoplasm while in testis it was expressed in both cytoplasm and mitochondria. To directly confirm that the third band in LDH isoenzymes was LDH-C4 of plateau pika, mobility of the recombinant C-terminal His-tagged LDH-C4 was compared with that of LDH isoenzymes in the somatic tissues and testis by native PAGE stained for LDH activity. The mobility of the third band (indicated by an arrow) in the LDH isoenzyme spectrum of somatic tissues and testes of plateau pika was identical with that of the purified LDH-C4 (Fig. 6). The result is consistent with analyses of the thermal stability, suggesting that the third band (indicated by an arrow) was LDH-C4 of plateau pika and the active LDH-C4 enzyme is presented in somatic tissues of plateau pika.
Fig. 3.
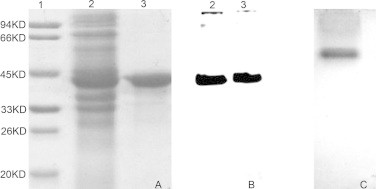
SDS–PAGE, Western blot and enzymic activity analysis of purified recombinant LDH-C. Panel A, lanes 1–3 are molecular mass standards, cell-free extract of E. coli expressing the pET-30a-Ldh-c plasmid, and purified recombinant LDH-C respectively, and stained with Coomassie Brilliant Blue R250. Panel B, lanes 2 and 3 are the same as panel A and probed with horse radish peroxidase conjugate, directed specifically against the 6× histidine tag on the recombinant LDH-C protein. Panel C, native PAGE stained for LDH activity demonstrated that the purified recombinant protein was an active LDH enzyme.
Fig. 4.

Histochemical analyses of LDH-C in somatic tissues of plateau pika. Histochemical analyses show that LDHC has distribution in the plateau pika's somatic tissues. Yellow-brown positive signal and the royal purple nucleus were observed. (Panels A1–A7) Sections incubated with antibody to LDHC. (Panels B1–B7) Sections incubated with PBS as control. Numbers 1–7 are heart, liver, lung, kidney, brain, skeletal muscle and testis of plateau pika. Scale bars = 100 × magnification with a 50-μm calibration bar. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
Subcellular distribution of LDH-C4 in somatic tissues of plateau pika. A positive signal in cytoplasm in heart, liver, lung, kidney, brain, skeletal muscle and testis (Panel A) was obtained using Western blotting as well as in testicular mitochondria (Panel B). No positive signal (Panel B) for extracts of mitochondria of somatic tissues was observed, suggesting that LDHC in somatic tissues was translated only in the cytoplasm while in testis it was expressed in both cytoplasm and mitochondria. Panels A and B, Western blotting for extracts of cytoplasm and mitochondria respectively. Numbers 1–7 are heart, liver, lung, kidney, brain, skeletal muscle and testis of plateau pika.
Fig. 6.
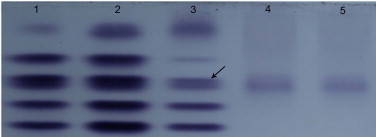
Location of LDH-C4 in LDH isoenzymes of plateau pika. Mobility of the recombinant C-terminal His-tagged LDH-C4 was compared with that of LDH isoenzymes in the somatic tissues and testis by native PAGE stained for LDH activity. The mobility of the third band (indicated by an arrow) in the LDH isoenzyme spectrum of somatic tissues and testes of plateau pika was identical with that of the purified LDH-C4. The finding suggested that the third band (indicated by an arrow) was LDH-C4 of plateau pika. Lanes 1–3: LDH isoenzyme spectrum in lung, brain and testis of plateau pika; lanes 4, 5: purified LDH-C4 protein.
4. Discussion
Ldh-c was previously identified to be exclusively expressed in testis and spermatozoa. In the present study, RT-PCR, Western blots and immunohistochemistry provide solid evidence that the Ldh-c gene is transcribed and translated in somatic tissues of plateau pika. LDHC in somatic tissues was translated only in the cytoplasm while in testis it was expressed in both cytoplasm and mitochondria.
The plateau pika, a hypoxic-tolerant rodent, inhabits meadows only at altitudes 3000–5000 m above sea level on the Qinghai-Tibet Plateau. In the present study, our results indicated that Ldh-c is expressed not only in testis and sperm but also in somatic tissues of plateau pika. LDH-C4 catalyzes the interconversion of pyruvate to lactate with the concomitant oxidation of NADH to NAD+, which is essential for the continued production of ATP by glycolysis. The enzymatic kinetics characteristics of LDH-C4 have been studied in detail in other species. The biochemical properties separating LDH-C4 from the other LDH isoforms may contribute to the high glycolytic flux. Compared with LDH-A4, LDH-C4 has a low Km for pyruvate (∼0.030 mM) and a high Km for lactate (∼2.0 mM) [14–18]. This finding implies that LDH-C4 has an affinity for pyruvate that is 60-fold higher than that for lactate and suggests that pyruvate turnover to lactate may be high even at high concentrations of endogenous or extracellular lactate. This notion is supported by experiments in which addition of excess lactate (50-fold excess in relation to pyruvate) did not influence ATP production in capacitating spermatozoa [19]. A high rate of ATP production in sperm is known to be essential for maintaining a high level of motility for a prolonged period of time, and to induce sperm capacitation and hyperactivity [20–23]. Odet et al. [24] found that targeted disruption of the Ldh-c results in male infertility due to sperm with decreased progressive motility, a failure to develop the hyperactivated motility pattern essential for fertilization and a rapid decline in ATP levels. The authors confirmed that lactate production in Ldh-c null male mice sperm is extremely low (around 30 times lower than in wild-type male mice sperm) and that glucose utilization occurs at a very low level, indicating that the loss of LDH-C4 directly perturbs the process of glycolysis. LDH-C4 accounts for 80–100% of the LDH activity in mammalian spermatozoa [15,25]. Hereng et al. [19] found that exogenous pyruvate increases intracellular ATP levels. When human spermatozoa mitochondrial respiration is blocked, a combination of pyruvate and glucose maintains ATP production, the exogenous pyruvate increases the glycolysis flux, and all of the exogenous pyruvate is then completely converted into lactate. Therefore, LDH-C4 increases ATP levels and decreases oxygen consumption significantly by enhancing the process of glycolysis in spermatozoa. The findings of the studies above suggest that, by regulating the expression of Ldh-c in somatic tissues, plateau pika obtains most of its cellular ATP through enhancement of anaerobic glycolysis, thereby reducing its dependence on oxygen and increasing the capacity to adapt to the hypoxic environment. Previous studies have indicated that fecundity of human and mammals decrease in hypoxic conditions at high elevations [26,27]. Furthermore, Ldh-c is expressed in tumors [28,29]. Many different histopathologic classifications of human solid tumors are now known to contain significant numbers of cells that exist at less than normal physiological oxygen levels. Hypoxia is a significant component of the microenvironment in human tumors [30,31]. Determining how the expression of Ldh-c is regulated by hypoxia will require further investigation.
In the last decade, scientists have made progress in understanding the regulation of Ldh-c gene expression [32–34]. It was concluded that simultaneous occupancy of the GC box and CRE sites in the core promoter is necessary for full expression of Ldh-c in the testis [29,35]. Tang and Goldberg found that MYB (MYBL1) stimulates murine testis-specific Ldh-c expression via the cAMP-responsive element (CRE) site [36]. Genes frequently are hypomethylated in the testis [37]. In earlier studies, no differences were found in the methylation patterns of Ldh-c between somatic and germ cells [38]. Other studies demonstrated that the human promoter contains a mini-CpG island [39], and that its methylation in non-expressing cells serves as the likely mechanism to suppress Ldh-c activation [29]. However, the regulatory mechanism of Ldh-c expression in spermatozoa and non-expression in somatic tissues is still unclear. Therefore, we utilized the plateau pika as a model for studying the expression of Ldh-c in somatic tissues and testis, which would provide important insights into the regulatory mechanisms of Ldh-c in mammals.
The level of Ldh-c transcripts is higher in whole testis than those of Ldh-a and Ldh-b transcripts [24,38]. Earlier experiments suggested that LDH-C4 is the only LDH isozyme present in spermatozoa [40]. Although recent studies have shown that LDHA is also present in spermatozoa [24,41,42], LDH-C4 remains the major LDH in germ cells, being responsible for more than 80% of the total LDH activity in mouse spermatozoa [24]. Odet et al. found that Ldh-c null male mice sperm are able to convert pyruvate into lactate at the same rate as wild-type male mice sperm, showing that sperm lacking LDH-C4 still contain appreciable levels of LDH activity [24], probably due to the presence of LDHA. It is worth mentioning that sperm from mice heterozygous for the Ldh-c mutation are fertile, even though Ldh-c transcript levels were found to be reduced by 40% in the testes of these mice, and global LDH activity reduced by 19.1% in testis and 24.7% in sperm. These observations indicate that sperm contain substantially more LDH-C4 than is required to maintain normal fertility. Odet et al. [43] identified 27 proteins associated with LDH-C4 by co-immunoprecipitation coupled with mass spectrometry. A majority of these proteins are implicated in ATP synthesis, utilization, transport and/or sequestration. These results suggest that, in addition to its role in glycolysis, LDH-C4 is part of a complex involved in ATP homeostasis, and has non-catalytic functions essential for regulation of glycolysis in sperm that other LDH isozymes are unable to provide. The plateau pika would be a suitable model animal for further research on non-catalytic functions of the LDH-C4 protein.
In conclusion, previous findings supported that Ldh-c was expressed in both male and female germ cells. Our finding that Ldh-c is expressed in both somatic tissues and testis of plateau pika provides important implications for more in-depth research into the Ldh-c function in mammals.
Enzymes
Lactate dehydrogenase commission numbers is EC 1.1.1.27.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 30960054 and 31040011), scientific research key project fund of Ministry of Education of China (209132) and Natural Science Foundation of Qinghai Province (No. 2012-Z-905).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Everse J., Kaplan N.O. Lactate dehydrogenases: structure and function. Adv. Enzymol. Relat. Areas Mol. Biol. 1973;37:61–133. doi: 10.1002/9780470122822.ch2. [DOI] [PubMed] [Google Scholar]
- 2.Li S.S. Lactate dehydrogenase isoenzymes A (muscle), B (heart) and C (testis) of mammals and the genes coding for these enzymes. Biochem. Soc. Trans. 1989;17:304–307. doi: 10.1042/bst0170304. [DOI] [PubMed] [Google Scholar]
- 3.Li S.S., O’Brien D.A., Hou E.W., Versola J., Rockett D.L., Eddy E.M. Differential activity and synthesis of lactate dehydrogenase isozymes A (muscle), B (heart), and C (testis) in mouse spermatogenic cells. Biol. Reprod. 1989;40:173–180. doi: 10.1095/biolreprod40.1.173. [DOI] [PubMed] [Google Scholar]
- 4.Blanco A., Zinkham W.H. Lactate dehydrogenases in human testes. Science. 1963;139:601–602. doi: 10.1126/science.139.3555.601. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg E. Lactate Dehydrogenases and malate dehydrogenases in sperm: studied by polyacrylamide gel electrophoresis. Ann. N.Y. Acad. Sci. 1964;121:560–570. doi: 10.1111/j.1749-6632.1964.tb14226.x. [DOI] [PubMed] [Google Scholar]
- 6.Coonrod S., Vitale A., Duan C., Bristol-Gould S., Herr J., Goldberg E. Testis-specific lactate dehydrogenase (LDH-C4; Ldh3) in murine oocytes and preimplantation embryos. J. Androl. 2006;27:502–509. doi: 10.2164/jandrol.05185. [DOI] [PubMed] [Google Scholar]
- 7.Holmes R.S., Goldberg E. Computational analyses of mammalian lactate dehydrogenases: human, mouse, opossum and platypus LDHs. Comput. Biol. Chem. 2009;33:379–385. doi: 10.1016/j.compbiolchem.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q., Sun R., Huang C., Wang Z., Liu X., Hou J., Liu J., Cai L., Li N., Zhang S., Wang Y. Cold adaptive thermogenesis in small mammals from different geographical zones of China. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001;129:949–961. doi: 10.1016/s1095-6433(01)00357-9. [DOI] [PubMed] [Google Scholar]
- 9.Ge R.L., Kubo K., Kobayashi T., Sekiguchi M., Honda T. Blunted hypoxic pulmonary vasoconstrictive response in the rodent Ochotona curzoniae (pika) at high altitude. Am. J. Physiol. 1998;274 doi: 10.1152/ajpheart.1998.274.5.H1792. H1792—H1799. [DOI] [PubMed] [Google Scholar]
- 10.Qi X.Z., Wang X.J., Zhu S.H., Rao X.F., Wei L., Wei D.B. Hypoxic adaptation of the hearts of plateau zokor (Myospalax baileyi) and plateau pika (Ochotona curzoniae) Sheng Li Xue Bao. 2008;60:348–354. [PubMed] [Google Scholar]
- 11.Zhu S.H., Qi X.Z., Wang X.J., Rao X.F., Wei L., Wei D.B. Difference in oxygen uptake in skeletal muscles between plateau zokor (Myospalax rufescens baileyi) and plateau pika (Ochotona curzoniac) Sheng Li Xue Bao. 2009;61:373–378. [PubMed] [Google Scholar]
- 12.Suarez R.K., Hochachka P.W. Preparation and properties of rainbow trout liver mitochondria. J. Comp. Physiol. B: Biochem. Syst. Environ. Physiol. 1981;143:269–273. [Google Scholar]
- 13.Goldberg E. Amino acid composition and properties of crystalline lactate dehydrogenase X from mouse testes. J. Biol. Chem. 1972;247:2044–2048. [PubMed] [Google Scholar]
- 14.LeVan K.M., Goldberg E. Properties of human testis-specific lactate dehydrogenase expressed from Escherichia coli. Biochem. J. 1991;273(Pt 3):587–592. doi: 10.1042/bj2730587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clausen J., Ovlisen B. Lactate dehydrogenase isoenzymes of human semen. Biochem. J. 1965;97:513–517. doi: 10.1042/bj0970513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coronel C.E., Burgos C., Gerez de Burgos N.M., Rovai L.E., Blanco A. Catalytic properties of the sperm-specific lactate dehydrogenase (LDH X or C4) from different species. J. Exp. Zool. 1983;225:379–385. doi: 10.1002/jez.1402250305. [DOI] [PubMed] [Google Scholar]
- 17.Wong C., Rodriguez-Paez L., Nogueda B., Perez A., Baeza I. Selective inhibition of the sperm-specific lactate dehydrogenase isozyme-C4 by N-isopropyl oxamate. Biochim. Biophys. Acta. 1997;1343:16–22. doi: 10.1016/s0167-4838(97)00090-3. [DOI] [PubMed] [Google Scholar]
- 18.Battellino L.J., Jaime F.R., Blanco A. Kinetic properties of rabbit testicular lactate dehydrogenase isozyme. J. Biol. Chem. 1968;243:5185–5192. [PubMed] [Google Scholar]
- 19.Hereng T.H., Elgstoen K.B., Cederkvist F.H., Eide L., Jahnsen T., Skalhegg B.S., Rosendal K.R. Exogenous pyruvate accelerates glycolysis and promotes capacitation in human spermatozoa. Hum. Reprod. 2011;26:3249–3263. doi: 10.1093/humrep/der317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho H.C., Granish K.A., Suarez S.S. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMP. Dev. Biol. 2002;250:208–217. doi: 10.1006/dbio.2002.0797. [DOI] [PubMed] [Google Scholar]
- 21.Mukai C., Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol. Reprod. 2004;71:540–547. doi: 10.1095/biolreprod.103.026054. [DOI] [PubMed] [Google Scholar]
- 22.Travis A.J., Tutuncu L., Jorgez C.J., Ord T.S., Jones B.H., Kopf G.S., Williams C.J. Requirements for glucose beyond sperm capacitation during in vitro fertilization in the mouse. Biol. Reprod. 2004;71:139–145. doi: 10.1095/biolreprod.103.025809. [DOI] [PubMed] [Google Scholar]
- 23.Miki K. Energy metabolism and sperm function. Soc. Reprod. Fertil. Suppl. 2007;65:309–325. [PubMed] [Google Scholar]
- 24.Odet F., Duan C., Willis W.D., Goulding E.H., Kung A., Eddy E.M., Goldberg E. Expression of the gene for mouse lactate dehydrogenase C (Ldhc) is required for male fertility. Biol. Reprod. 2008;79:26–34. doi: 10.1095/biolreprod.108.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zinkham W.H., Blanco A., Clowry Jr. L.J. An unusual isozyme of lactate dehydrogenase in mature testes: localization, ontogeny, and kinetic properties. Ann. N.Y. Acad. Sci. 1964;121:571–588. doi: 10.1111/j.1749-6632.1964.tb14227.x. [DOI] [PubMed] [Google Scholar]
- 26.Eckes L. Altitude adaptation. IV. Fertility and reproduction at high altitudes. Gegenbaurs Morphol. Jahrb. 1976;122:761–770. [PubMed] [Google Scholar]
- 27.Bangham C.R., Sacherer J.M. Fertility of Nepalese Sherpas at moderate altitudes: comparison with high-altitude data. Ann. Hum. Biol. 1980;7:323–330. doi: 10.1080/03014468000004391. [DOI] [PubMed] [Google Scholar]
- 28.Koslowski M., Tureci O., Bell C., Krause P., Lehr H.A., Brunner J., Seitz G., Nestle F.O., Huber C., Sahin U. Multiple splice variants of lactate dehydrogenase C selectively expressed in human cancer. Cancer Res. 2002;62:6750–6755. [PubMed] [Google Scholar]
- 29.Tang H., Goldberg E. Homo sapiens lactate dehydrogenase c (Ldhc) gene expression in cancer cells is regulated by transcription factor Sp1, CREB, and CpG island methylation. J. Androl. 2009;30:157–167. doi: 10.2164/jandrol.108.005785. [DOI] [PubMed] [Google Scholar]
- 30.Sutherland R.M. Tumor hypoxia and gene expression–implications for malignant progression and therapy. Acta Oncol. 1998;37:567–574. doi: 10.1080/028418698430278. [DOI] [PubMed] [Google Scholar]
- 31.Okuyama H., Inoue M. Hypoxic microenvironment and cancer dormancy. Gan To Kagaku Ryoho. 2011;38:1559–1564. [PubMed] [Google Scholar]
- 32.Li S., Zhou W., Doglio L., Goldberg E. Transgenic mice demonstrate a testis-specific promoter for lactate dehydrogenase, LDHC. J. Biol. Chem. 1998;273:31191–31194. doi: 10.1074/jbc.273.47.31191. [DOI] [PubMed] [Google Scholar]
- 33.Markert C.L., Amet T.M., Goldberg E. Human testis-specific lactate dehydrogenase-C promoter drives overexpression of mouse lactate dehydrogenase-1 cDNA in testes of transgenic mice. J. Exp. Zool. 1998;282:171–178. [PubMed] [Google Scholar]
- 34.Kroft T.L., Li S., Doglio L., Goldberg E. A transgenic analysis of mouse lactate dehydrogenase C promoter activity in the testis. J. Androl. 2003;24:843–852. doi: 10.1002/j.1939-4640.2003.tb03135.x. [DOI] [PubMed] [Google Scholar]
- 35.Tang H., Kung A., Goldberg E. Regulation of murine lactate dehydrogenase C (Ldhc) gene expression. Biol. Reprod. 2008;78:455–461. doi: 10.1095/biolreprod.107.064964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang H., Goldberg E. A-MYB (MYBL1) stimulates murine testis-specific Ldhc expression via the cAMP-responsive element (CRE) site. Biol. Reprod. 2012;86:30. doi: 10.1095/biolreprod.111.095661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oakes C.C., La Salle S., Smiraglia D.J., Robaire B., Trasler J.M. A unique configuration of genome-wide DNA methylation patterns in the testis. Proc. Natl. Acad. Sci. USA. 2007;104:228–233. doi: 10.1073/pnas.0607521104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alcivar A.A., Trasler J.M., Hake L.E., Salehi-Ashtiani K., Goldberg E., Hecht N.B. DNA methylation and expression of the genes coding for lactate dehydrogenases A and C during rodent spermatogenesis. Biol. Reprod. 1991;44:527–535. doi: 10.1095/biolreprod44.3.527. [DOI] [PubMed] [Google Scholar]
- 39.Bonny C., Goldberg E. The CpG-rich promoter of human LDH-C is differentially methylated in expressing and nonexpressing tissues. Dev. Genet. 1995;16:210–217. doi: 10.1002/dvg.1020160213. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg E. Lactate dehydrogenases in spermatozoa: subunit interactions in vitro. Arch. Biochem. Biophys. 1965;109:134–141. doi: 10.1016/0003-9861(65)90298-5. [DOI] [PubMed] [Google Scholar]
- 41.Sleight S.B., Miranda P.V., Plaskett N.W., Maier B., Lysiak J., Scrable H., Herr J.C., Visconti P.E. Isolation and proteomic analysis of mouse sperm detergent-resistant membrane fractions: evidence for dissociation of lipid rafts during capacitation. Biol. Reprod. 2005;73:721–729. doi: 10.1095/biolreprod.105.041533. [DOI] [PubMed] [Google Scholar]
- 42.Krisfalusi M., Miki K., Magyar P.L., O’Brien D.A. Multiple glycolytic enzymes are tightly bound to the fibrous sheath of mouse spermatozoa. Biol. Reprod. 2006;75:270–278. doi: 10.1095/biolreprod.105.049684. [DOI] [PubMed] [Google Scholar]
- 43.Odet F., Gabel S.A., Williams J., London R.E., Goldberg E., Eddy E.M. Lactate dehydrogenase C and energy metabolism in mouse sperm. Biol. Reprod. 2011;85:556–564. doi: 10.1095/biolreprod.111.091546. [DOI] [PMC free article] [PubMed] [Google Scholar]


