Abstract
The mammary gland is an organ with a remarkable regenerative capacity that can undergo multiple cycles of proliferation, lactation, and involution. Growing evidence suggests that these changes are driven by the coordinated division and differentiation of mammary stem cell populations (MaSC). Whereas information regarding MaSC and their role in comparative mammary gland physiology is readily available in human and mice, such information remains scarce in most veterinary mammal species such as cows, horses, sheep, goats, pigs, and dogs. We believe that a better knowledge on the MaSC in these species will not only help to gain more insights into mammary gland (patho) physiology in veterinary medicine, but will also be of value for human medicine. Therefore, this review summarizes the current knowledge on stem cell isolation and characterization in different mammals of veterinary importance.
The Mammary Gland
The mammary gland, the structure that distinguishes mammals from all other animal species, has the unique task of providing nutrition for the young in the form of milk proteins and fat [1,2]. In a mammal's life time, the mammary gland probably undergoes more and greater changes in size, structure, composition, and activity than any other tissue or organ and it is considered as one of the few body organs that undergoes repeated cycles of structural development, functional differentiation, and regression. As a result, mammary growth is a complex process involving local and systemic factors, as well as external influences such as environment, climate, and diet [3,4]. Furthermore, normal development of the mammary gland is a multidimensional process that is controlled, in part, by its own microenvironment that encompasses the stromal tissue and the epithelium [5–7].
The stromal compartment is generally composed of (1) a number of different cell types and (2) the extracellular matrix consisting of, for example, laminin, fibronectin, collagen, and proteoglycans [5]. The best-studied stromal component is the fat tissue pad, composed of adipocytes, in which the network of ductal branches is embedded. There are, however, some striking differences with regard to the architecture of the fat pad in different animal species. For instance, the mammary fat pad in the postnatal mouse consists primarily of adipocytes irregularly interspersed in between the fine septa of fibroblasts and connective tissue [8,9]. In contrast, there is a much greater abundance of interlobular fibroblastic connective tissue and only a limited number of adipocytes in the bovine and human mammary fat pad [8,10–12].
The epithelial compartment, on the other hand, forms the ductal network of the mammary gland and there are basically two epithelial cell types in the mammary gland: luminal and basal. The luminal epithelium forms the ducts and the secretory alveoli, whereas the basal epithelium consists of myoepithelial cells [13–15]. It has been suggested that all these differentiated mammary cells, which possess different morphologies, characteristics, and potency, are derived from mammary gland stem cells [16–18].
Mammary Stem Cells and Their Progeny
Because there is no consensus yet on the requirements of a mammary cell to be typed as a true mammary stem cell, and specific definitions are lacking in literature, we propose in this review to abbreviate mammary stem cells into mammary stem cell population (MaSC), taken into account that this abbreviation accounts for mammary stem cells as well as mammary progenitor stem cells.
Over the last decades, evidence for the existence of MaSC has been accumulated mainly in mice and humans. The groups of DeOme and Daniel were the first to demonstrate the existence of self-renewing and multipotent adult stem cells in the mammary tissue of mice by limiting dilution transplantation experiments [19–21]. This was confirmed by Kordon and Smith, who showed that an entire murine mammary gland could be regenerated with the progeny of a single cell following transplantation of a tissue fragment into mammary glands cleared from their fat pad [4]. Furthermore, it was also found that single mouse MaSC, isolated according to their expression of the cell surface markers CD24 and CD49f (or CD29), were capable of reconstituting a functional mammary gland upon transplantation at limiting cell dilutions [4,22,23]. Intense research has been performed over the years to elucidate the murine mammary epithelial hierarchy. The generally accepted classical model describes a common stem cell-derived progenitor cell, which commits to either a luminal or a myoepithelial cell fate [24]. Recently, however, a revised model has been proposed, based on genetic lineage-tracing experiments and clonal analysis of the murine mammary gland during the different development stages [25]. This model describes the existence of different types of long-lived stem cells and suggests that, in contrast to other tissues where one stem cell pool gives rise to specific differentiated cells that will populate the organ, the mammary gland is maintained by two separate populations of unipotent progenitor cells.
In humans, MaSC have also been identified, although the knowledge on human MaSC and breast hierarchy lags behind the knowledge on the murine MaSC and mammary gland hierarchy, mainly because of the limited access to healthy mammary gland biopsy material [26]. To overcome this limitation, the presence of putative mammary stem cells in human breast milk has been proposed as a noninvasive, alternative source of MaSC. In human breast milk, a population of nestin-positive cells has been identified [27,28], and upon isolation and characterization, these cells were typed as multipotent mesenchymal stem cells [29].
In striking contrast, MaSC of veterinary animals have received little attention to date and no information is currently available on the presence of stem/progenitor cells in the milk of these animals.
Why Study MaSC in Veterinary Animals?
For understanding the functional role of MaSC in normal mammary gland development, the cleared fat pad mouse model is frequently used [24,30]. This in vivo model allows the transplantation and growth of MaSC into their normal anatomical site and under the influence of a normal physiological environment [24,30,31]. Also, this murine mammary gland-free fat pad transplantation system, together with transgenic mice overexpressing oncogenes, is the animal model of choice for human breast cancer research [32,33]. Indeed, mice have played an indispensable and pivotal role in the study of breast cancer and this animal species will keep on being a major research resource in comprehending this devastating disease [34]. However, by studying (patho) physiological mechanisms in such models, some key insights might be lost due to the absence of variation in the mouse model and due to important interspecies differences between murine and human mammary tumorigenesis. Moreover, most mammary gland tumors in mice are viral- or toxin-induced, which is in contrast to the natural occurrence of breast cancer in women [35].
Intriguingly, whereas both in humans and all other mammals, the mammary gland undergoes repeated cycles of development, function, and dedifferentiation, mammary cancer prevalence varies greatly among these species. Mammary cancer is common in humans and carnivores [35], whereas cows, horses, sheep, pigs, and even the old world primate macaques only very rarely develop mammary tumors [36]. Several influences, such as genetic, hormonal, dietary, and geographic factors, have been proposed to account for this difference in prevalence, but to date, no univocal proof for this has been found [35]. Another factor that might be involved in the susceptibility to develop mammary tumors could be differences in the functional behavior and regulation of MaSC, the driving force behind mammogenesis. Therefore, we believe it is of eminent importance to study the MaSC in different species.
Different Techniques to Isolate and Characterize MaSC
For the isolation and characterization of MaSC, several methods have been described and are briefly outlined below. However, because of these different techniques, it is sometimes difficult to accurately compare the nature of the MaSC populations obtained by these different methods.
Light staining cells
This technique is often used as a method to identify mammary stem cells in situ, although functional data confirming the true identity of these cells are still missing, due to the lack of appropriate cellular markers [37,38]. When applying the key features of multipotent stem cells, namely, division competence, mitotic quiescence, (a)symmetric mitosis, and an undifferentiated cytology [39,40], two distinct forms have been identified using electron microscopy, i.e., small light staining cells (SLC) and undifferentiated large light staining cells (ULLC) [41]. In SLC, both the nucleoplasm and cytoplasm are characteristically pale staining, and the nuclear-to-cytoplasmic ratio is rather high. Organelles are small and show no structural evidence of specialized function. SLC are often present in pairs, which indicates that SLC engage in asymmetric mitosis. They are usually free of specialized membrane contacts with neighboring cells and present a basal location. ULLC on the other hand, are two to three times larger than SLC and show a more intermediate position. In contrast to SLC, ULLC sometimes contact the lumen. Although their membrane systems are more abundant, they are only slightly more developed than those of SLC. Even so, ULLC can contain small secretory granules in their Golgi and lipid droplets in their cytoplasm [41]. In addition to electron microscopy, histological analysis using light microscopy is another way to identify light staining cells. Different histological stains can be chosen, but a mixture of a basic dye like toluidine blue and azure II is the most frequently used option. On light microscopy, SLC typically show a very sparse distribution of mitochondria and almost no visible fibrillar mesh [38].
Functional markers
Flow cytometry analysis using the DNA-binding dye Hoechst 33342, and to a lesser extent Rhodamine-123, is a unique method to identify potential MaSC [42]. This method, which depends solely on dual-wavelength flow cytometric analysis of cells stained with the DNA-binding dye, was developed by Goodell and his coworkers originally as a method to identify murine hematopoetic stem cells and was later adapted to study stem cells in a variety of tissues, including the mammary gland [43]. This method is based on the fact that MaSC have the capacity to exclude this dye since stem cells express ATP-binding cassette transporters, leading to a Hoechst-negative population termed the side population (SP), which is highly enriched in MaSC.
Another functional characteristic to identify potential MaSC is their high aldehyde dehydrogenase-1 (ALDH-1) activity, which can be detected by flow cytometry as well as immunohistochemistry (IHC). IHC has the advantage that it can be used to identify MaSC in situ in slides of mammary gland tissues. Unfortunately, ALDH-1 expression is not MaSC-specific, since progenitor cells also express this enzyme, and therefore, ALDH-1 should always be combined with cell surface markers to increase the purity of the MaSC population [17].
A third approach to functionally identify MaSC is label-retention studies [42]. For these studies, bromodeoxyuridine (BrdU) is most commonly used and this synthetic analogue of thymidine is incorporated into newly synthesized DNA of replicating cells. MaSC are considered label-retaining cells, which keep their DNA template strand during self-renewal or symmetric division, while passing on the newly synthesized BrdU-labeled daughter strands to their progeny during asymmetric division.
Surface markers
A commonly used method for isolation and characterization of (cancer) stem cells is their selection based on the presence and/or absence of tissue-specific surface markers (Tables 1 and 2). However, a single marker selective for MaSC is still missing and several mammary stem cell markers [e.g., stem cell antigen-1 (Sca-1)] are controversial in literature. The latter may be due to different isolation protocols and culture media used in different laboratories and for different mammal species. Moreover, it still remains elusive whether all markers used for selection also have a functional role in MaSC biology.
Table 1.
Overview of the Most Commonly Used Cell Surface Markers to Immunophenotype Mammary Gland Stem/Progenitor Cells
| |
|
Mammalian species |
||||
|---|---|---|---|---|---|---|
| Marker | Function/expression | Humana | Mouse/rata | Cow | Dog | Horse |
| CD10/CALLA | Membrane-bound protein, highly expressed on myoepithelial and basal cells | + | − or + | |||
| CD24 | P-selectin ligand, expressed on luminal cells | + | Medium | + | ||
| CD29 | Beta1 integrin, highly expressed on basal cells | + | High | + | +b | + |
| CD31 | Endothelial cell marker | − | − | − | ||
| CD44 | Involved in cell–cell interactions, adhesion, and migration. Highly expressed on basal cells | High | + | +b | + | + |
| CD45 | Regulates cell growth, differentiation, and oncogenic transformation | − | − | − | ||
| CD49f | Alpha6 integrin with low expression on luminal cells and high expression on basal cells | High | High | + | +b | + |
| CD61 | Beta 3 integrin | + | + | |||
| EpCAM (ESA/CD326) | Epithelial-specific antigen with high expression on luminal cells and low expression on basal cells | Low or + | + | NC | ||
| MUC1 (EMA) | Epithelial membrane antigen with high expression on luminal cells | − or+ | − or+ | NC | ||
| Sca-1 | Stem cell antigen-1 | − or + | Low or + | − | ||
| Ter119 (Ly76) | Associates with murine glycophorin A | − | − | |||
| ER | Estrogen receptor | − | − | − or + | ||
| PR | Progesterone receptor | − | − | |||
Table 2.
Overview of the Most Commonly Used Cell Surface Markers Used to Immunophenotype Mammary Gland Cancer Stem Cells
| |
|
Mammalian species |
|||
|---|---|---|---|---|---|
| Marker | Function/expression | Humana | Mousea | Dog | Cat |
| CD10/CALLA | Membrane-bound protein, highly expressed on myoepithelial and basal cells | − | − | − | |
| CD24 | P-selectin ligand, expressed on luminal cells | − or low | − or + | − | |
| CD29 | Beta1 integrin, highly expressed on basal cells | High | High | − or + | |
| CD34 | Cell–cell adhesion factor | High | + | ||
| CD44 | Involved in cell–cell interactions, adhesion, and migration. Highly expressed on basal cells | + | + | − or + | + |
| CD49f | Alpha6 integrin with low expression on luminal cells and high expression on basal cells | + or − | + | ||
| CD61 | Beta 3 integrin | + | + | ||
| CD133 (prominin 1) | Unknown function | + | + | + | |
| EpCAM (ESA/CD326) | Epithelial-specific antigen with high expression on luminal cells and low expression on basal cells | + | |||
| MUC1 (ESA) | Tumor antigen | + | |||
| PROCR | Basal breast epithelial marker | + | + | ||
| ER-alpha | Estrogen receptor alpha | + | + | ||
| EGFR | Epidermal growth factor receptor | + | + | + | |
| Lineage markers | CD2, CD3, CD10, CD16, CD18, CD31, CD64, CD140b | − | − | ||
Mammospheres, self-renewal and differentiation tests
Mammospheres are nonadherent spheres, which are enriched in MaSC [44]. This is based on the notice that normal epithelial cells are anchorage dependent and undergo apoptosis, named anoikis, when they are cultured under circumstances they cannot attach to a substratum. This culture technique is frequently applied to enrich for MaSC from freshly isolated mammary gland tissues or cell lines in different mammal species (Fig. 1). Testing the two main properties of MaSC, namely, self-renewal and differentiation, is a frequently used technique to characterize these cells [44]. The sphere formation efficiency of serially cultivated cells can be used as a self-renewal test. To this end, a limited number of cells is seeded at a clonal density and cultivated for 7 days, after which the number of colony-forming units (CFU) is evaluated [45]. For the differentiation test, cells are cultured under differentiation conditions and immunostained with lineage-specific markers. The most common markers to identify luminal epithelial cells are CK8, CK18, CK19, ESA, and MUC-1; whereas CK5, CK14, smooth muscle actin, and vimentin are the most frequently used markers to detect basal myoepithelial cells [18,46–48].
FIG. 1.
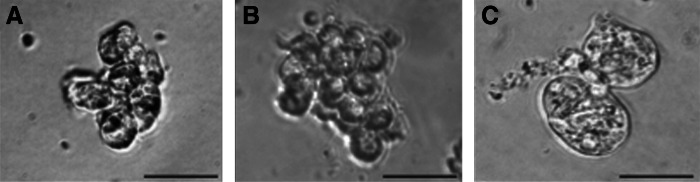
Representative pictures of mammospheres from bovine (A), canine (B), and equine (C) mammary gland tissues.
Bovine MaSC
Several research laboratories are focused on the study of bovine MaSC because these cells are considered interesting targets for manipulation to manage cell growth and tissue regeneration, thereby improving dairy cow productivity and udder health [49]. Moreover, the bovine mammary gland may also provide an alternative model for biomedical research aimed at evaluating the role of mammary stem cells in mammary gland function and carcinogenesis [50,51]. For the latter, it is intriguing to note that cows do not suffer from mammary cancer [3] and as such, studying the regulation and functioning of bovine MaSC could identify protective factors against mammary tumor development.
The first report of a highly proliferative cell population in the bovine mammary gland came from Ellis and Capuco [38]. They identified, using histological analysis of mammary gland samples from cows, which were injected with BrdU before euthanasia, a lightly stained parenchymal cell population, which was proposed to function as putative MaSC. This was based on the observation that these light cells comprised only 10% of the parenchymal cell population, but accounted for nearly 50% of the proliferation [38]. One year later, the group of Holland et al. isolated a cell population with stem cell characteristics from monolayers of bovine mammary gland cells [52]. In line with what Ellis and Capuco found, Holland et al. observed ultrastructurally a population of small light undifferentiated cells that was deficient in functional gap junctions, a characteristic of stem cells. Moreover, these cells immunohistochemically expressed casein, but not connexin 43, a gap junction protein [52]. This group named these cells bovine mammary gland progenitor cells or BMGPC [14,53]. A couple of years later, Capuco identified putative bovine MaSC by their retention of labeled DNA strands and characterized these cells more thoroughly based on the expression of the nuclear proliferation antigen Ki67 and estrogen receptor (ER)/progesterone receptor (PR) expression [51]. Because the label retaining Ki67-positive cell population contained ER-positive as well as ER-negative cells, he suggested that the ER-negative cells represent the stem cells and the ER-positive cells their progenitors [51]. An in vivo study by the same group, in which xanthosine was injected into the mammary gland of female calves, was initiated to evaluate whether this product could enhance the MaSC population. This was based on the fact that xanthosine suppresses the function of p53, a protein that promotes asymmetric cell division (resulting in a stem cell and a progenitor cell of committed cell lineage) [49]. Their salient findings were that xanthosine treatment resulted in a temporary increase of label retaining mammary epithelial cells from 0.4% in the control gland to 0.8% in xanthosine-treated glands [49]. This finding was more recently also confirmed in vitro, using bovine mammary epithelial cell lines [54].
Aside from the (immuno)histological and ultrastructural studies described above, a couple of reports also describe the actual isolation and characterization of bovine MaSC from mammary gland tissue. Li et al. isolated, cultured, and characterized bovine mammary epithelial cells with stem cell characteristics based on (1) their growth in suspension (mammosphere cultures, Fig. 1A), (2) differentiation characteristics into epithelial-like, myoepithelial-like, and secretory cells, and (3) their expression of beta 1-integrin and alpha 6-integrin [16]. Martignani et al. used CFU assays to study the clonogenic expansion capacities of bovine MaSC and they found three distinct colonies, according to the size and morphology [55,56]. One type of colony had a myoepithelial phenotype based on a CK18-negative and CK14-positive expression profile, whereas the two other colonies had a luminal phenotype characterized by a CK18-positive and CK14-negative phenotype [55]. Moreover, analysis of the ALDH activity showed that ALDHhigh cells had luminal features, whereas ALDHlow cells produced colonies with myoepithelial features [56]. The group of Motyl identified putative MaSC in a suspension of bovine mammary epithelial cells using the fluorescent dye Hoechst 33342 [57]. This bovine SP was estimated to account for ∼0.5% of the total cell number present in the bovine mammary gland [57]. In addition, they used the marker Sca-1 to phenotype bovine MaSC using IHC and scanning cytometry of the mammary tissue section. Hereby, it was found that Sca-1-positive cells were located predominantly in the basal layer of the epithelium and comprised around 2% of the total cell number [57]. However, transcriptional profiling, using bovine gene expression microarrays, revealed that these Sca-1-positive cells had a high expression of genes linked with cells of hematopoietic origin, suggesting that these cells represent a cell population of nonepithelial lineage [57]. More recently, Rauner and Barash used a murine mammary stem cell enrichment kit to obtain a pure population of bovine epithelial cells [19]. Subsequently, the cells were divided in 4 subpopulations based on their expression of CD24 and CD49f: CD24medCD49fpos (named putative stem cells), CD24highCD49fneg (named putative progenitor cells), CD24negCD49fpos (named basal cells), and CD24medCD49fneg (named luminal cells). Those 4 populations were then further characterized for expression of epithelial lineage markers, stem cell markers, CFU capacity, and differences in the ALDH activity. Based on the results, a cell hierarchy and lineage commitment model was designed (Fig. 2).The putative stem cells were put on top of the hierarchy, based on their basal origin, bipotent character, and their capacity to form organized colonies at a high growth rate. The putative progenitor cells were unipotent since they could only form CK18-positive colonies, but had a high sphere formation capacity and were therefore typed as luminal-restricted progenitor cells. The basal cells were bipotent, but in contrast to the putative stem cells, showed a low sphere formation capacity and a medium growth rate and were therefore typed as a mix of bipotent progenitor cells and more differentiated myoepithelial cells. The last subpopulation, the luminal cells formed unorganized, strong CK18-positive colonies and had a low sphere formation capacity along with a very slow growth rate. Based on this, these cells were proposed as the milk producing cells with hardly any proliferation and were placed at the bottom of the hierarchy model [18].
FIG. 2.
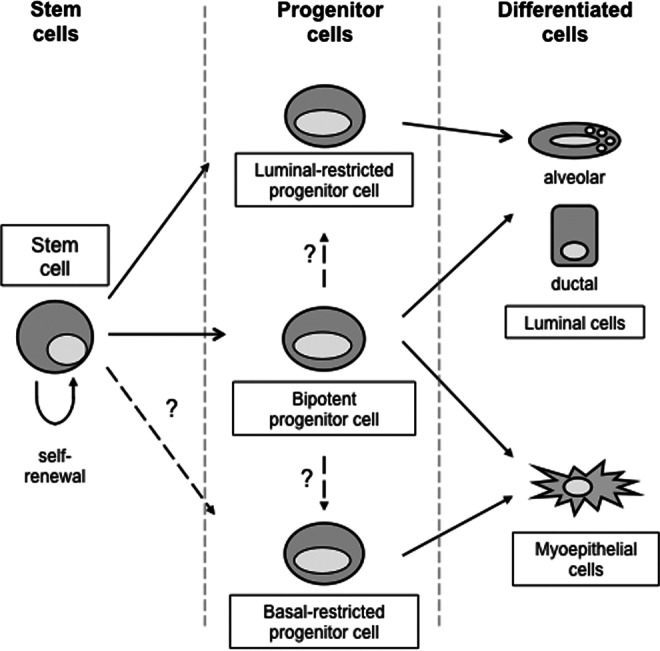
Schematic illustration of the proposed hierarchy of mammary epithelial cells in the bovine mammary gland, based on Rauner and Barash [18] and others.
Finally, one group attempted to confirm in vivo the existence of stem cells in the bovine mammary gland through xenotransplantation of bovine MaSC into the cleared mouse mammary fat pad, but this was found to be extremely challenging [58]. This is most likely due to the inherent differences between the fat pad in mice, which mainly consist of adipocytes, and the much more fibrous bovine fat pad. A possible solution to this technical problem might be the simultaneous addition of bovine stromal fibroblasts, in line with what has been described for a human-in-mouse model. Hereby, functionally normal human breast tissue could be reproducibly recreated in the cleared fat pad of mice when human stromal fibroblasts were simultaneously incorporated [59].
Canine/Feline MaSC
The interest of researchers active in the field of canine and feline stem cell research is predominantly focused on the cancer stem cell (CSC) theory. This theory states that in essence, cancer is a true stem cell disease, based on the fact that stem cells are the only cell type in an adult organ that persist long enough to acquire enough mutations to transform into malignant cancer cells [60]. CSC have been identified in several tumoral tissues as colonies of cells (spheres) that exhibit stem cell properties, defined by their capacity for self-renewal, gene expression profiles, and their ability to recapitulate tumors in model systems [60]. However, whether CSC are normal tissue stem cells, which have undergone malignant transformation or whether they are more differentiated cells, which have acquired more primitive, stem cell-like characteristics due to mutations or dedifferentiation, is not fully elucidated yet [61].
In women, breast cancer has a high incidence and the existence of CSC or tumor-initiating cells in breast cancer tissues has been well documented over the years [62]. In the female dog, mammary cancer is the most common cancer and in female cats the third most prevalent cancer [63,64]. Also, mammary gland tumors in these companion animals share many features with human breast cancer, including histological appearance, biological behavior, and genetics and therefore, these species are proposed as very valuable animal models to study breast cancer [65,66]. However, information regarding MaSC and CSC in dogs and cats remains scarce.
Cocola et al. were the first to report the isolation of normal MaSC and CSC from dog mammary gland tissues [67]. These cells were propagated as mammospheres and tumor spheres for three to five passages in nonadherent cultures. Moreover, these cells displayed self-renewing properties, multilineage differentiation potential, could form tubular structures in vitro and tumors in vivo in NOD/SCID mice. Marker expression analysis using western blot showed that canine CSC were clearly positive for CD49f, CD133, p21, and CK14 and moderately positive for CD29 and CD44 [67] (Table 2). Unfortunately, no data on the expression of these markers in normal MaSC was given, so it remains to be determined what the expression pattern is on normal MaSC and whether there is a difference between normal MaSC and CSC. Preliminary data from our research group, however, showed that canine MaSC isolated from healthy mammary gland tissue and grown in mammospheres (Fig. 1B) were positive for CD29, CD44, CD49f, and the proliferation marker Ki67 (unpublished data, Table 1). CD44 is a highly conserved transmembrane glycoprotein, which is used as a marker to identify human CSC in primary tumors, including breast cancer and cancer cell lines of diverse origin [68–70]. Recently, it was investigated whether CD44 could also prove a valuable marker to identify canine CSC [71]. Hereby, it was found that, using the canine adenocarcinoma cell line REM134, CD44high cells proliferated more rapidly and had a much higher colony forming and tumor sphere formation capacity in comparison to CD44low cells. Although this observation was indicative for a CD44high CSC population, the fluctuation in expression patterns when culturing these CD44high and CD44low populations led the authors to conclude that the use of CD44 as a CSC marker is limited in canine cancer research [71]. Another research group characterized spheres derived from canine mammary gland adenocarcinoma cell lines using flow cytometry and RT-PCR analyses. Their salient findings were that the sphere cell population was predominantly CD44+CD24− and had a higher expression of stem cells markers, including CD133, CD34, Notch 3, and the multidrug resistance protein, when compared to the adherent cell population [56]. Moreover, these CD44+CD24− populations showed a high ALDH activity and were capable of initiating tumors in NOD/SCD mice [72]. CSC spheres, isolated from REM134, were shown to express the embryonic stem cell markers Oct4 and Nanog as assessed by nonquantitative RT-PCR analysis [73]. The group of Ferletta et al. [74] isolated spheres from the mammary tumor cell line CMT-U229 (an atypical benign mixed mammary tumor), characterized these spheres by IHC and found a similar expression pattern to what had been described by other research groups [72,73].
The first description on the isolation of feline CSC came from the research group of Argyle, where they derived putative CSC from the feline mammary carcinoma (FMC) cell line CAT-MT [75]. Later on, another group also characterized CSC from FMC. These FMC-derived CSC showed in vitro self-renewal, long-lasting proliferation and in vivo tumorigenicity [76]. Immunofluorescence staining demonstrated that these feline CSC were positive for CD44, ER-alpha, and EGFR [76].
Several research groups have used the sphere assay, using feline and canine tumors or cell lines, to evaluate the effects of anticancer drugs. For example, the effect of recombinant feline interferon-omega, alone or in combination with anthracycline chemotherapeutic drugs, was evaluated using CSC derived from the carcinoma cell lines REM134 and CAT-MT. Hereby, it was found that the CSC were more resistant to the actions of interferon-omega than the REM134 and CAT-MT cell lines [75]. A similar result was found with the chemotherapeutic drug doxorubicin, where spheres derived from the canine adenocarcinoma cell line CHMp and REM134 had chemoresistant characteristics [72,73]. Taken together, it can be stated that such sphere assays are suitable models to test therapeutic strategies aimed at eradicating CSC.
Equine MaSC
To date, there is only one report on MaSC in horses [45]. In this study, MaSC were isolated from healthy mammary gland tissue and grown as mammospheres (Fig. 1C). These cells were immunophenotyped as CD29, CD44, CD49f, and casein kinase 2beta-positive (Table 1) and they were able to differentiate into luminal epithelial and myoepithelial cells during culture in a selective differentiation medium. Moreover, the authors assessed their clonogenic expansion capacities using CFU assays and studied equine MaSC in more detail during lactation. Hereby, it was observed that equine lactating mammary glands contained significantly more mammosphere-initiating cells than the inactive, nonlactating gland (a reflection of MaSC self-renewal) and moreover, that these spheres were significantly larger in size upon initial cultivation (a reflection of progenitor cell proliferation) [45].
MaSC in Other Veterinary Animals
No information is available to date on MaSC in other animal species such as pigs, rabbits, or marine mammals, with the exception of a few articles on small ruminants. One group described the expression of the putative stem cell marker Musaschi 1 (Msi 1) in the mammary glands of ewes [77,78]. Msi 1 is an RNA binding protein associated with self-renewal of neural, intestinal, and mammary progenitor cells [79]. They found that Msi 1 was expressed in epithelial cells, using IHC, and showed a strong correlation with the proliferation marker Ki-67 [77]. Moreover, they found that the expression of Msi 1 was upregulated during mammary gland differentiation and downregulated during lactation [78]. The actual isolation and characterization of MaSC in sheep, however, has not been described so far. In a very recent article, in vitro and in vivo functional assays were used to identify the presence of MaSC in the mammary gland of caprines [80]. Single-cell suspensions were made from lactating mammary tissue of goats and in vitro CFU indicated the appearance of three morphologically distinct colony types, which arose with frequencies of 1%–8% of the total cells seeded. In addition, transplantation of these cells under the kidney capsule of NOD/SCID mice resulted in the formation of organized, bilayered epithelial structures that were positive for CK14 and CK18 [80].
Hurdles in (Veterinary) MaSC Research and Future Prospects
Despite extensive research, markers that allow for a clear and consistent identification of MaSC have not been identified yet. Although several research groups, working on human and murine MaSC, have reached an agreement on the cell surface expression profiles of mammary stem and progenitor populations, only a handful of potential stem cell markers are currently being used to search for MaSC populations in the mammary gland of mammals of veterinary importance (Tables 1 and 2). This can partially be explained by the limited availability of species-specific antibodies and the lack of cross-reacting antibodies. Hereby, detection of gene expression on the mRNA level could be a valuable alternative. Unfortunately, this technique cannot be used to isolate defined cell populations through sorting, so evaluating several antibody clones in search for cross reactivity will remain required. Moreover, markers used in mice and humans to identify specific stem cell populations are not always valid in other mammal species. For example, CD44 expression has been linked to mammary gland CSC in humans, but this marker was recently shown to be inadequate as a canine mammary gland CSC marker, due to a transient and fluctuating expression [71]. Also, it needs to be kept in mind that flow cytometry data on marker expression can be influenced by the different fluorochromes conjugated to the antibodies, antibody titrations, and the use of proper negative and positive controls. All this makes it sometimes difficult to interpret and compare data on the immunophenotypic profiles of MaSC between different research groups, as well as between different mammal species. Lastly, whereas in murine, and to a lesser extent human, MaSC research, the hierarchy of stem and progenitor cells in relation to marker expression has been well defined, the actual hierarchy remains to be determined in the mammary gland of other mammals.
Another major hurdle in MaSC research is the lack of consistency in nomenclature. Mammary epithelial stem cells, mammary gland progenitor cells, mammary stem/progenitor cells, potential stem cells, stem-like cells, mammary repopulating units, and others, are being used to define cells in the mammary gland with stem cell properties. Moreover, a plethora of isolation and identification assays are employed to obtain these cells, which makes it very difficult to compare the obtained cellular populations. All this emphasizes the need for more universal studies as well as more standardized assays. Hereby, it is crucial to functionally validate each marker and method used. So in conclusion, there is a huge need for a consensus on the minimal requirements to isolate and characterize MaSC in veterinary and human medicine.
Finally, in regard to breast cancer research and the potential role of cancer MaSC, the spontaneous canine/feline cancer models are still an under-used resource. However, several research groups have isolated and characterized CSC successfully from these species, using similar protocols designed for the human/rodent model systems, indicating the promising potential of these cells in basic as well as translational oncology research.
Acknowledgments
B.B. is supported by the Netherlands University Foundation for International Cooperation (NUFFIC), G. VdW. is supported by the Morris Animal Foundation (grant no. D12MS-002).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hurley WL. Growth, Development and Involution. 2nd. Academic Press; USA: 2002. Encyclopedia of Dairy Sciences: Mammary gland anatomy; pp. 1689–1697. [Google Scholar]
- 2.Peaker M. The mammary gland in mammalian evolution: a brief commentary on some of the concepts. J Mammary Gland Biol Neoplasia. 2002;3:347–353. doi: 10.1023/a:1022860902083. [DOI] [PubMed] [Google Scholar]
- 3.Knight CH. Peaker M. Development of the mammary gland. J Reprod Fert. 1982;65:521–536. doi: 10.1530/jrf.0.0650521. [DOI] [PubMed] [Google Scholar]
- 4.Kordon EC. Smith GH. An entire functional mammary gland may comprise the progeny from a single cell. Development. 1998;125:1921–1930. doi: 10.1242/dev.125.10.1921. [DOI] [PubMed] [Google Scholar]
- 5.Li L. Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 6.Kass L. Erler JT. Dembo M. Weaver VM. Mammary epithelial cell: influence of extracellular matrix composition and organization during development and tumorigenesis. Int J Biochem Cell Biol. 2007;39:1987–1994. doi: 10.1016/j.biocel.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarzynska J. Motyl T. Apoptosis and autophagy in involuting bovine mammary gland. J Physiol Pharmacol. 2008;59:275–288. [PubMed] [Google Scholar]
- 8.Hovey RC. McFadden TB. Akers RM. Regulation of mammary gland growth and morphogenesis by the mammary fat pad: a species comparison. J Mammary Gland Biol Neoplasia. 1999;4:53–68. doi: 10.1023/a:1018704603426. [DOI] [PubMed] [Google Scholar]
- 9.Parmar H. Cunha GR. Epithelial-stromal interactions in the mouse and human mammary gland in vivo. Endocr Relat Cancer. 2004;11:437–458. doi: 10.1677/erc.1.00659. [DOI] [PubMed] [Google Scholar]
- 10.Sheffield LG. Organization and growth of mammary epithelia in the mammary gland fat pad. J Dairy Sci. 1988;71:2855–2874. doi: 10.3168/jds.S0022-0302(88)79881-1. [DOI] [PubMed] [Google Scholar]
- 11.Akers RM. Lactational physiology: a ruminant animal perspective. Protoplasma. 1990;159:96–111. [Google Scholar]
- 12.Hovey RC. Auldist DE. Mackenzie DD. McFadden TB. Preparation of an epithelium-free mammary fat pad and subsequent mammogenesis in ewes. J Anim Sci. 2000;78:2177–2185. doi: 10.2527/2000.7882177x. [DOI] [PubMed] [Google Scholar]
- 13.Jones PH. Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 14.Holland MS. Holland RE. The cellular perspective on mammary gland development: stem/progenitor cells and beyond. J Dairy Sci. 2005;88:E1–E8. doi: 10.3168/jds.S0022-0302(05)73132-5. [DOI] [PubMed] [Google Scholar]
- 15.Watson CJ. Khaled WT. Mammary development in the embryo and adult: a journey of morphogenesis and commitment. Development. 2008;135:995–1003. doi: 10.1242/dev.005439. [DOI] [PubMed] [Google Scholar]
- 16.Li JX. Zhang Y. Ma LB. Sun JH. Yin BY. Isolation and culture of bovine mammary epithelial stem cells. J Vet Med Sci. 2009;71:15–19. doi: 10.1292/jvms.71.15. [DOI] [PubMed] [Google Scholar]
- 17.Stingl J. Detection and analysis of mammary gland stem cells. J Pathol. 2009;217:229–241. doi: 10.1002/path.2457. [DOI] [PubMed] [Google Scholar]
- 18.Rauner G. Barash I. Cell hierarchy and lineage commitment in the bovine mammary gland. PLoS One. 2012;7:e30113. doi: 10.1371/journal.pone.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeOme KB. Faulkin LJ., Jr. Bern HA. Blair PB. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959;19:515–520. [PubMed] [Google Scholar]
- 20.Daniel CW. De Ome KB. Young JT. Blair PB. Faulkin LJ., Jr The in vivo life span of normal and preneoplastic mouse mammary glands: a serial transplantation study. Proc Natl Acad Sci U S A. 1968;61:53–60. doi: 10.1073/pnas.61.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniel CW. Young LJ. Medina D. DeOme KB. The influence of mammogenic hormones on serially transplanted mouse mammary gland. Exp Gerontol. 1971;6:95–101. doi: 10.1016/0531-5565(71)90053-2. [DOI] [PubMed] [Google Scholar]
- 22.Shackleton M. Vaillant F. Simpson KJ. Stingl J. Smyth GK. Asselin-Labat ML. Wu L. Lindeman GJ. Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 23.Stingl J. Eirew P. Ricketson I. Shackleton M. Vaillant F. Choi D. Li HI. Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 24.Bruno RD. Smith GH. Functional characterization of stem cell activity in the mouse mammary gland. Stem Cell Rev. 2011;7:238–247. doi: 10.1007/s12015-010-9191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Keymeulen A. Rocha AS. Ousset M. Beck B. Bouvencourt G. Rock J. Sharma N. Dekoninck S. Blanpain C. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479:189–193. doi: 10.1038/nature10573. [DOI] [PubMed] [Google Scholar]
- 26.Fridriksdottir AJ. Peterson R. Ole W. Jessen LR. Mammary gland stem cells: current status and future challenges. Int J Dev Biol. 2011;55:719–729. doi: 10.1387/ijdb.113373af. [DOI] [PubMed] [Google Scholar]
- 27.Cregan MD. Fan Y. Appelbe A. Brown ML. Klopcic B. Koppen J. Mitoulas LR. Piper KM. Choolani MA. Chong YS. Hartmann PE. Identification of nestin-positive putative mammary stem cells in human breastmilk. Cell Tissue Res. 2007;329:129–136. doi: 10.1007/s00441-007-0390-x. [DOI] [PubMed] [Google Scholar]
- 28.Fan Y. Chong YS. Choolani MA. Cregan MD. Chan JK. Unravelling the mystery of stem/progenitor cells in human breast milk. PLoS One. 2010;5:e14421. doi: 10.1371/journal.pone.0014421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patki S. Kadam S. Chandra V. Bhonde R. Human breast milk is a rich source of multipotentmesenchymal stem cells. Hum Cell. 2010;23:35–40. doi: 10.1111/j.1749-0774.2010.00083.x. [DOI] [PubMed] [Google Scholar]
- 30.Visvader JE. Smith GH. Murine mammary epithelial stem cells: discovery, function, and current status. Cold Spring Harb Perspect Biol. 2011;3:a004879. doi: 10.1101/cshperspect.a004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molofsky AV. Pardal R. Morrison SJ. Diverse mechanisms regulate stem cell self-renewal. Curr Opin Cell Biol. 2004;16:700–707. doi: 10.1016/j.ceb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Deng CX. Brodie SG. Knockout mouse models and mammary tumorigenesis. Semin Cancer Biol. 2001;11:387–394. doi: 10.1006/scbi.2001.0394. [DOI] [PubMed] [Google Scholar]
- 33.Cardiff RD. Bern HA. Faulkin LJ. Daniel CW. Smith GH. Young LJ. Medina D. Gardner MB. Wellings SR, et al. Contributions of mouse biology to breast cancer research. Comp Med. 2002;52:12–31. [PubMed] [Google Scholar]
- 34.Young LJ. Mus tales: a hands-on view. J Mammary Gland Biol Neoplasia. 2008;13:343–349. doi: 10.1007/s10911-008-9088-2. [DOI] [PubMed] [Google Scholar]
- 35.Munson L. Moresco A. Comparative pathology of mammary gland cancers in domestic and wild animals. Breast Dis. 2007;28:7–21. doi: 10.3233/bd-2007-28102. [DOI] [PubMed] [Google Scholar]
- 36.Knight CH. Sorensen A. Windows in early mammary development: critical or not? Reproduction. 2001;122:337–345. doi: 10.1530/rep.0.1220337. [DOI] [PubMed] [Google Scholar]
- 37.Alvi AJ. Clayton H. Joshi C. Enver T. Ashworth A. Vivanco MM. Dale TC. Smalley MJ. Functional and molecular characterization of mammary side population cells. Breast Cancer Res. 2003;5:R1–R8. doi: 10.1186/bcr547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellis S. Capuco AV. Cell proliferation in bovine mammary epithelium: identification of the primary proliferative cell population. Tissue Cell. 2002;34:21–28. doi: 10.1016/s0040-8166(02)00025-3. [DOI] [PubMed] [Google Scholar]
- 39.Horvitz HR. Herskowits I. Mechanisms of asymmetric cell division: to Bs or not two Bs, that is the question. Cell. 1992;68:237–255. doi: 10.1016/0092-8674(92)90468-r. [DOI] [PubMed] [Google Scholar]
- 40.Seaberg RM. van der Kooy D. Stem and progenitor cells: the premature desertion of rigorous definitions. Trends Neurosci. 2003;26:125–131. doi: 10.1016/S0166-2236(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 41.Chepko G. Smith GH. Mammary epithelial stem cells: our current understanding. J Mammary Gland Biol Neoplasia. 1999;4:35–52. doi: 10.1023/a:1018752519356. [DOI] [PubMed] [Google Scholar]
- 42.Woodward WA. Chen MS. Behbod F. Rosen JM. On mammary stem cells. J Cell Sci. 2005;118:3585–3594. doi: 10.1242/jcs.02532. [DOI] [PubMed] [Google Scholar]
- 43.Goodell MA. Rosenzweig M. Kim H. Marks DF. DeMaria M. Paradis G. Grupp SA. Sieff CA. Mulligan RC. Johnson RP. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 44.Dontu G. Al-Hajj M. Abdallah WM. Clarke MF. Wicha MS. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36:59–72. doi: 10.1046/j.1365-2184.36.s.1.6.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spaas JH. Chiers K. Bussche L. Burvenich C. Van de Walle GR. Stem/progenitor cells in non-lactating versus lactating equine mammary gland. Stem Cells Dev. 2012;31:3055–3067. doi: 10.1089/scd.2012.0042. [DOI] [PubMed] [Google Scholar]
- 46.Stingl J. Eaves CJ. Zandich I. Emerman T. Characterization of bipotent mammary epithelial progenitor cells in normal adult breast tissue. Breast Cancer Res Treat. 2001;67:93–109. doi: 10.1023/a:1010615124301. [DOI] [PubMed] [Google Scholar]
- 47.Gudjonsson T. Villadsen R. Lind Nielsen H. Rønnov-Jessen L. Bissel MJ. Wiliam Petersen O. Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev. 2002;16:693–706. doi: 10.1101/gad.952602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clayton H. Titley I. Vivanco MdM. Growth and differentiation of progenitor/stem cells derived from the human mammary gland. Exp Cell Res. 2004;297:444–460. doi: 10.1016/j.yexcr.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 49.Capuco AV. Vock-Clover CME. Minuti A. Wood DL. In vivo expansion of the mammary stem/progenitor cell population by xanthosine infusion. Exp Biol Med. 2009;234:475–482. doi: 10.3181/0811-RM-320. [DOI] [PubMed] [Google Scholar]
- 50.Bierla JB. Osinska E. Motyl T. Bovine mammary stem cells studies—current status—a review. Anim Sci Pap Rep. 2012;30:195–204. [Google Scholar]
- 51.Capuco AV. Identification of putative bovine mammary epithelial stem cells by their retention of labeled DNA strands. Exp Biol Med. 2007;232:1381–1390. doi: 10.3181/0703-RM-58. [DOI] [PubMed] [Google Scholar]
- 52.Holland MS. Tai MH. Trosko JE. Griffin LD. Stasko JA. Cheville NC. Holland RE. Isolation and differentiation of bovine mammary gland progenitor cell populations. Am J Vet Res. 2003;64:396–403. doi: 10.2460/ajvr.2003.64.396. [DOI] [PubMed] [Google Scholar]
- 53.Holland MS. Stasko JA. Holland RE. Influence of extracellular matrix on bovine mammary gland progenitor cell growth and differentiation. Am J Vet Res. 2007;68:476–482. doi: 10.2460/ajvr.68.5.476. [DOI] [PubMed] [Google Scholar]
- 54.Choudhary RK. Capuco AV. In vitro expansion of the mammary stem/progenitor cell population by xanthosine treatment. BMC Cell Biol. 2012;14:13–14. doi: 10.1186/1471-2121-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martignani E. Eirew P. Eaves C. Baratta M. Functional identification of bovine mammary epithelial stem/progenitor cells. Vet Res Commun. 2009;33:S101–S103. doi: 10.1007/s11259-009-9254-z. [DOI] [PubMed] [Google Scholar]
- 56.Martignani E. Eirew P. Accornero P. Eaves CJ. Baratta M. Human milk protein production in xenografts of genetically engineered bovine mammary epithelial stem cells. PLoS One. 2010;5:e13372. doi: 10.1371/journal.pone.0013372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Motyl T. Bierła JB. Kozłowski M. Gajewska M. Gajkowska B. Koronkiewicz K. Identification, quantification and transcriptional profile of potential stem cells in bovine mammary gland. Livest Sci. 2010;136:136–149. [Google Scholar]
- 58.Ellis S. Akers RM. Xenotransplantation of immortalized bovine mammary epithelial cells into the cleared fat pads of immunocompetent mice. J Anim Sci. 1995;73:77–78. [Google Scholar]
- 59.Proia DA. Kuperwasser C. Reconstruction of human mammary tissues in a mouse model. Nat Protoc. 2006;1:206–214. doi: 10.1038/nprot.2006.31. [DOI] [PubMed] [Google Scholar]
- 60.Reya T. Morrison SJ. Clarke MF. Weissman IL. Stem cells, cancer and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 61.Polyak K. Hahn WC. Roots and stems: stem cells in cancer. Nat Med. 2006;12:296–300. doi: 10.1038/nm1379. [DOI] [PubMed] [Google Scholar]
- 62.Ercan C. van Diest PJ. Vooijs M. Mammary development and breast cancer: the role of stem cells. Curr Mol Med. 2011;11:270–285. doi: 10.2174/156652411795678007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brodey RS. Goldschmidt MH. Roszel JF. Canine mammary gland neoplasms. J Am Anim Hosp Assoc. 1983;19:61–90. [Google Scholar]
- 64.Schmidt RE. Langham RF. A survey of feline neoplasms. J Am Vet Med Assoc. 1967;151:1325–1328. [Google Scholar]
- 65.Kumaraguruparan R. Prathiba D. Nagini S. Of humans and canines: immunohistochemical analysis of PCNA, Bcl-2, p53, cytokeratin and ER in mammary tumours. Res Vet Sci. 2006;81:218–224. doi: 10.1016/j.rvsc.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Uva P. Aurisicchio L. Watters J. Loboda A. Kulkarni A. Castle J. Palombo F. Viti V. Mesiti G, et al. Comparative expression pathway analysis of human and canine mammary tumors. BMC Genomics. 2009;10:135. doi: 10.1186/1471-2164-10-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cocola C. Anastasi P. Astigiano S. Piscitelli E. Pelucchi P. Vilardo L. Bertoli G. Beccaglia M. Veronesi MC, et al. Isolation of canine mammary cells with stem cell properties and tumour-initiating potential. Reprod Dom Anim. 2009;44:214–217. doi: 10.1111/j.1439-0531.2009.01413.x. [DOI] [PubMed] [Google Scholar]
- 68.Al-Hajj M. Wicha MS. Benito-Hernandez A. Morrison SJ. Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prince JM. Klinowska TC. Marshman E. Lowe ET. Mayer U. Miner J. Aberdam D. Vestweber D. Gusterson B. Streuli CH. Cell–matrix interactions during development and apoptosis of the mouse mammary gland in vivo. Dev Dyn. 2002;223:497–516. doi: 10.1002/dvdy.10070. [DOI] [PubMed] [Google Scholar]
- 70.Takaishi S. Okumura T. Tu S. Wang SS. Shibata W. Vigneshwaran R. Gordon SA. Shimada Y. Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006–1020. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blacking TM. Waterfall M. Argyle DJ. CD44 is associated with proliferation, rather than a specific cancer stem cell population, in cultured canine cancer cells. Vet Immunol Immunopathol. 2011;141:46–57. doi: 10.1016/j.vetimm.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 72.Michishita M. Akiyoshi R. Yoshimura H. Katsumoto T. Ichikawa H. Ohkusu-Tsukada K. Nakagawa T. Sasaki N. Takahashi K. Characterization of spheres derived from canine mammary gland adenocarcinoma cell lines. Res Vet Sci. 2011;91:254–260. doi: 10.1016/j.rvsc.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 73.Pang LY. Alejandro CA. Rod WE. David JA. Canine mammary cancer stem cells are radio- and chemo- resistant and exhibit an epithelial-mesenchymal transition phenotype. Cancers. 2011;3:1744–1762. doi: 10.3390/cancers3021744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferletta M. Grawé J. Hellmén E. Canine mammary tumors contain cancer stem-like cells and form spheroids with an embryonic stem cell signature. Int J Dev Biol. 2011;55:791–799. doi: 10.1387/ijdb.113363mf. [DOI] [PubMed] [Google Scholar]
- 75.Penzo C. Ross M. Muirhead R. Else R. Argyle DJ. Effect of recombinant feline interferon-omega alone and in combination with chemotherapeutic agents on putative tumour-initiating cells and daughter cells derived from canine and feline mammary tumours. Vet Comp Oncol. 2009;7:222–229. doi: 10.1111/j.1476-5829.2009.00192.x. [DOI] [PubMed] [Google Scholar]
- 76.Barbieri F. Wurth R. Ratto A. Campanella C. Vito G. Thellung S. Daga A. Cilli M. Ferrari A. Florio T. Isolation of stem-like cells from spontaneous feline mammary carcinomas: phenotypic characterization and tumorigenic potential. Exp Cell Res. 2012;318:847–860. doi: 10.1016/j.yexcr.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 77.Colitti M. Farinacci M. Expression of a putative stem cell marker, Musashi 1, in mammary glands of ewes. J Mol Histol. 2009;40:139–149. doi: 10.1007/s10735-009-9224-3. [DOI] [PubMed] [Google Scholar]
- 78.Colitti M. Expression of putative stem cell markers related to developmental stage of sheep mammary glands. Anat Histol Embryol. 2010;39:555–562. doi: 10.1111/j.1439-0264.2010.01028.x. [DOI] [PubMed] [Google Scholar]
- 79.Glazer RI. Wang XY. Yuan H. Yin Y. Musashi1: a stem cell marker no longer in search of a function. Cell Cycle. 2008;7:2635–2639. doi: 10.4161/cc.7.17.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prpar S. Martignani E. Dovc P. Baratta M. Identification of goat mammary stem/progenitor cells. Biol Reprod. 2012;86:117. doi: 10.1095/biolreprod.111.095489. [DOI] [PubMed] [Google Scholar]
- 81.Molyneux G. Regan J. Smalley MJ. Mammary stem cells and breast cancer. Cell Mol Life Sci. 2007;64:3248–3260. doi: 10.1007/s00018-007-7391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


