Abstract
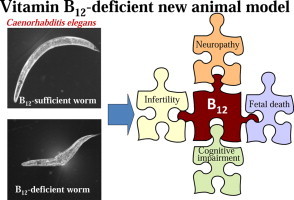
Vitamin B12 (B12) deficiency has been linked to developmental disorders, metabolic abnormalities, and neuropathy; however, the mechanisms involved remain poorly understood. Caenorhabditis elegans grown under B12-deficient conditions for five generations develop severe B12 deficiency associated with various phenotypes that include decreased egg-laying capacity (infertility), prolonged life cycle (growth retardation), and reduced lifespan. These phenotypes resemble the consequences of B12 deficiency in mammals, and can be induced in C. elegans in only 15 days. Thus, C. elegans is a suitable animal model for studying the biological processes induced by vitamin deficiency.
Keywords: Caenorhabditis elegans, Cobalamin, Methionine synthase, Methylmalonic acid, Methylmalonyl-CoA mutase, Vitamin B12
Abbreviations: Ado-B12, 5′-deoxyadenosylcobalamin; B12, vitamin B12; C. elegans, Caenorhabditis elegans; CH3-B12, methylcobalamin; Hcy, homocysteine; MCM, methylmalonyl-CoA mutase; MMA, methylmalonic acid; MS, methionine synthase.
Graphical abstract
Highlights
▸ Worms grown under B12-deficient conditions have significantly reduced B12 content. ▸ B12-deficient C. elegans exhibit loss of fertility and a prolonged life cycle. ▸ B12-deficient C. elegans have a significantly shorter lifespan. ▸ B12 is essential for C. elegans and functions as a cofactor for B12-enzymes. ▸ B12-deficient C. elegans could be rapidly generated in only 15 days.
1. Introduction
Vitamin B12 (B12), the largest and most complex vitamin molecule, is exclusively synthesized by certain bacteria, and is most abundant in higher order predators in the natural food chain. After B12 is taken up by living cells, it is converted into two coenzyme forms, 5′-deoxyadenosylcobalamin (Ado-B12) and methylcobalamin (CH3-B12), which function as the coenzymes for methylmalonyl-CoA mutase (MCM; EC 5.4.99.2) [1] and methionine synthase (MS; EC 2.1.1.13) [2], respectively.
Food derived from animal products is the major dietary source of B12; therefore, strict vegetarians and/or the elderly are at a high risk of developing B12 deficiency [3]. The major symptoms of B12 deficiency are developmental disorders, megaloblastic anemia, metabolic abnormalities, and neuropathy [4], although the underlying disease mechanisms are poorly understood [5,6]. Developing animal models of B12-deficiency is essential for investigating the molecular mechanisms that are defective in this metabolic disorder. However, such animal models have proven difficult to generate because animals must be fed with a B12-deficient diet for long periods to achieve B12 deficiency [7]. The lack of robust B12-deficient animal models has limited investigations to the biochemical mechanisms induced by B12-deficiency.
Caenorhabditis elegans offers several advantages for genetic and biochemical studies, including a short lifespan, a 3-day life cycle, a completely sequenced genome, <1000 somatic cells, and the ability to change reproductive rates, life cycle, and locomotive behavior [8]. In addition, many molecular and cellular processes are conserved between nematodes and mammals. Most human disease genes and pathways are present in the worm [9]. Thus, this animal has been widely used as a model organism for studying a variety of biological processes including apoptosis, cell signaling, cell cycle regulation, gene regulation, metabolism, and aging [8].
The enzymes responsible for human methylmalonic aciduria, a disease caused by B12 deficiency, have been partially characterized in C. elegans using RNA interference techniques [10,11]. However, whether B12 is an absolute requirement for normal growth and physiological function in C. elegans is unknown. If a method for creating viable B12-deficient worms can be found, C. elegans could serve as a suitable model organism for studying the effects of B12 deficiency.
In this study, we report a novel method for inducing B12 deficiency in C. elegans, describe the effects of B12 deficiency on various biomarkers, and characterize the physiological roles of B12 in this model organism.
2. Materials and methods
2.1. Organisms and growth conditions
The N2 Bristol wild-type C. elegans strain was maintained at 20 °C on nematode growth medium (NGM) plates using the Escherichia coli OP50 strain as the food source [12]. To induce B12 deficiency, worms were grown on 1.7% (w/v) agar plates containing M9 medium (3 g/L KH2PO4, 6 g/L Na2HPO4, 0.5 g/L NaCl, 1 g/L NH4Cl, 1 mmol/L MgSO4, 50 μmol/L CaCl2, 2 g/L glucose, 4 mg/L thiamine hydrochloride, and 5 mg/L cholesterol) in 1 L H2O. Plates containing B12-supplemented (100 μg/L cyanocobalamin) M9-medium each received one egg obtained from worms grown on NGM plates with B12-deficient OP50 E. coli (described below). Eggs were allowed to hatch and develop into egg-laying adult worms. The adult worms were then removed from each plate, eggs were collected, and each egg was transferred onto a new control plate. After this procedure was repeated at least 10 times, the resultant worms were used as experimental controls.
2.2. Preparation of B12-deficient E. coli cells
E. coli OP50 was grown in M9 medium (3 g/L KH2PO4, 6 g/L Na2HPO4, 0.5 g/L NaCl, 1 g/L NH4Cl, 1 mmol/L MgSO4, 50 μmol/L CaCl2, 2 g/L glucose, and 4 mg/L thiamine hydrochloride) at 37 °C for 3 days. Cells were inoculated every 3 days into fresh M9 medium and used as a food source for C. elegans. The B12 content (0.2 μg/g wet weight) of E. coli cells grown in the M9 medium was significantly reduced compared with cells grown in the standard Luria–Bertani medium (11.1 μg/g wet weight).
2.3. Preparation of B12-deficient C. elegans
C. elegans were grown at 20 °C on B12 (100 μg/L)-supplemented medium using B12-deficient E. coli OP50 as a food source. An individual worm egg was transferred onto each plate, which contained fresh B12-deficient medium seeded with the B12-deficient E. coli OP50. After the eggs hatched, worms were allowed to grow until they became adults and had laid eggs (yielding the F1 generation). Individual eggs were removed from the plate and each was transferred onto a fresh plate containing B12-deficient medium and grown to maturity under the same conditions (yielding the F2 generation). This process was repeated for five generations. After 3 days, adult worms of each generation (F1–F5) were used for experiments. This protocol is shown in Fig. 1.
Fig. 1.
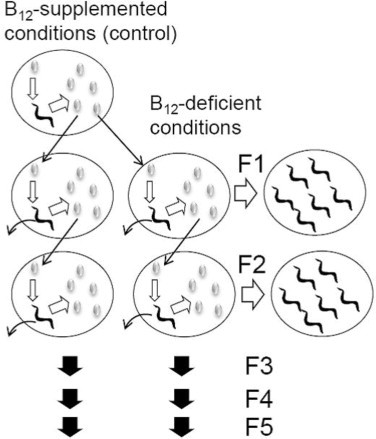
Preparation of B12-deficient C. elegans. Single eggs from C. elegans adults grown on NGM plate were transferred onto individual plates containing B12-supplemented M9 seeded with B12-deficient E. coli OP50 (control) and grown for 3 days. The eggs hatched and developed into adult worms, which then laid eggs. The adult worm was removed from each plate and individual eggs were transferred individually onto fresh identical plates. This procedure was repeated at least 10 times. These worms were used as experimental control worms. To prepare B12-deficient worms, single eggs from the control worm were transferred onto individual plates containing B12-deficient M9 medium seeded with B12-deficient E. coli and allowed to reach maturity and lay eggs (F1 generation). F1 worms obtained from these eggs were used to generate F2 worms following the same procedures. This process was repeated to prepare F5 B12-deficient worms.
2.4. Vitamin B12 assay
F1–F5 worms grown under B12-supplemented or B12-deficient conditions were harvested and incubated for 1 h at 20 °C in fresh M9 medium to remove any residual E. coli cells. Worms (2 g wet weight) were then disrupted using a hand homogenizer (AS ONE Corp., Osaka, Japan) and sonicated (6 kHz for 60 s) three times. The worm homogenate was resuspended in 100 mL of 57 mmol/L sodium acetate buffer (pH 4.8) containing 0.05% (w/v) KCN and boiled for 30 min. The extract was centrifuged at 15,000 g for 15 min at 4 °C and the supernatant was used for assaying B12 concentrations by standard microbiological methods utilizing Lactobacillus delbrueckii subsp. lactis ATCC 7830, as described previously [13].
2.5. B12-related biomarker assays
F1–F5 worms grown under B12-supplemented or B12-deficient conditions were collected and washed in M9 medium as described above. The harvested worms were resuspended in 0.5 mL of 100 mmol/L potassium phosphate buffer (pH 7.0) at 2 °C and homogenized using a hand homogenizer. The cell homogenate was centrifuged at 15,000 g for 15 min at 4 °C and the supernatant was used as a crude homogenate for subsequent biomarker assays.
Methylmalonic acid (MMA) and homocysteine (Hcy), two indices of B12-deficiency, were assayed using the high performance liquid chromatography (HPLC) methods of Al-Dirbashi et al. [14] and Febriani et al. [15], respectively. MCM (methylmalonyl-CoA mutase)- [16] and MS (methionine synthase)- [17,18] activities were assayed at 37 °C as previously described. Total- and holo-enzyme activities were determined in the presence or absence of each B12 coenzyme (Ado-B12 for MCM and CH3-B12 for MS).
2.6. Analysis of egg-laying capacity, life cycle, and lifespan
Measurement of egg-laying capacity was based on the method of Byerly et al. [19]. Individual L4-stage worms grown under B12-supplemented or B12-deficient conditions were selected, transferred onto the fresh plates containing the same culture medium, and incubated for 1 day at 20 °C. After laying eggs, each worm was removed from the plate, and the eggs were counted. Egg counting was performed in triplicate.
The life cycle and lifespan of B12-deficient F5 worms were determined at 20 °C using the synchronization method of Johnson and Wood [20]. Worms were scored as dead when they no longer responded to prodding with a pick. In each survival experiment, 100 worms were used.
2.7. Protein quantitation
Proteins were assayed by the method of Bradford [21] using ovalbumin as a standard.
2.8. Statistical analysis
The effects of B12-deficiency on various C. elegans biomarkers were evaluated by one-way ANOVA, and a post-hoc analysis was performed using Tukey's multiple comparison tests. Analyses were performed with GraphPad Prism 3 for Windows version 2.01 (GraphPad software Inc., La Jolla, CA, USA). All data are presented as the mean ± SD. Differences were considered statistically significant when p < 0.01.
3. Results and discussion
3.1. Effects of B12-deficient growth conditions on various B12-related biomarkers in C. elegans
Nematodes grown on B12-supplemented M9 medium with B12-deficient E. coli OP50 as a food source (control) showed identical growth rates that were identical to that of worms grown under normal conditions (data not shown), indicating that the experimental control conditions were adequate for the normal growth of C. elegans. The control worms were able to ingest both B12-enriched agar medium and B12-deficient E. coli cells. Therefore, they would mainly absorb sufficient amount of free B12 from the agar medium because of low B12 content in E. coli.
Under B12-deficient conditions, the B12 content of the worms decreased gradually over four generations (Fig. 2). The B12 concentration in F5 generation worms was only 4% compared with that in the control worms. These results indicate that dietary B12 deprivation over five generations leads to a significantly decreased B12 status in C. elegans. The MMA and Hcy indices of B12 deficiency were assayed in C. elegans grown under both control and B12-deficient conditions. There was a significant increase in the levels of both compounds between F3 and F5 generation worms (Fig. 3A and B). Hcy and MMA levels were approximately four and five times greater, respectively, in F5 worms grown under B12-deficient conditions than in the control worms. Although holo-MCM activity significantly decreased in F4 and F5 worms grown under B12-deficient conditions (Fig. 3E), the total MCM activity (holo- and apo-enzymes) increased (Fig. 3C), which indicates that apo-MCM activity is significantly increased by B12 deficiency. Both total- and holo-MS activities significantly decreased with each generation until the F4 generation and was maintained at a constant level thereafter (Fig. 3D and F). These results indicate that F5 generation worms grown under B12-deficient conditions develop severe B12 deficiency. However, one day after transfer of B12-deficient worms onto the B12-supplemented medium, the level of these B12-related biomarkers recovered considerably (data not shown). These results indicate that B12 functions as a cofactor for both MCM and MS in C. elegans and that B12-dependent changes in both MCM and MS enzyme activities occur in C. elegans and mammals [22,23]. However, using the nematode model, the time needed to produce a severely B12-deficient animal is only 15 days (i.e. five generations).
Fig. 2.
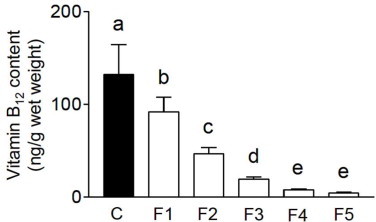
B12 content is reduced in worms grown under B12-supplemented and -deficient conditions. The B12 content of F1–F5 worms grown under control (black bar) and B12-deficient (white bars) conditions was assayed using microbiological methods. Data represent the mean ± SD of 10 independent experiments. Different letters (a–e) indicate values that are significantly different (p < 0.01); identical letters indicate values that are not significantly different.
Fig. 3.
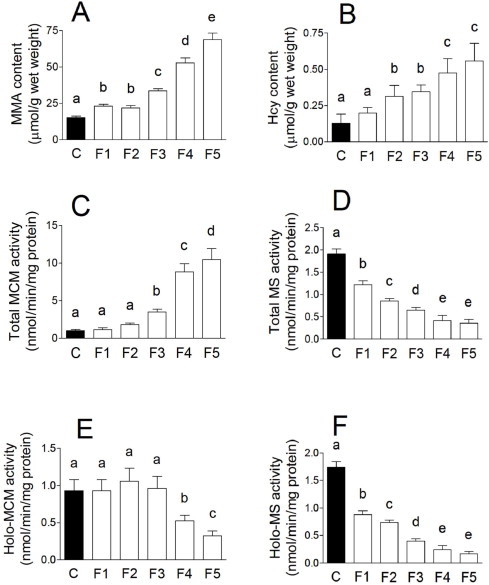
Vitamin B12 deficiency changes the concentration of various B12-related biomarkers. (A) Methylmalonic acid (MMA) and (B) homocysteine (Hcy) content, (C) total- and (E) holo-methylmalonyl-CoA mutase (MCM) activity, and (D) total- and (F) holo-methionine synthase (MS) activity were measured in extracts of worms grown under B12-supplemented (black bar) and B12-deficient conditions (white bars) for up to five generations. For (E) holo-MCM and (F) holo-MS activities, measurements were made in the absence of specific coenzymes. Data represent mean ± SD of 10 independent experiments. Different letters (a–e) indicate values that are significantly different (p < 0.01); identical letters indicate values that are not significantly different.
Holo-MS activity was rapidly decreased by F1 generation under B12-deficient conditions (Fig. 3F), but holo-MCM activity was not changed until the F4 generation (Fig. 3E), which indicated that MS is more sensitive to cellular B12 concentrations than MCM. Yamada et al. have demonstrated that most MS activity is derived from holo-enzyme in B12-sufficient or -deficient mammals because the apo-enzyme is very unstable [22]. In contrast, Nakao et al. have indicated that holo-MCM activity was less than 5% of the total enzyme activity in B12-sufficient rats and that a marked increase in the apo-enzyme activity occurred under B12-deficient conditions [23]. In this C. elegans study, holo-MCM activity was 97% of the total enzyme activity in the control worms and holo-enzyme activity gradually decreased in the F1 (92%), F2 (50%), and F3 (24%) generations, under B12-deficient conditions. However, the specific activity of holo-MCM did not change until the F4 generation even if the B12 content of the worms significantly decreased along with increased MMA content. The details of the occurrence of this MCM-independent increase in MMA concentration soon after the onset of B12-deficiency remain to be elucidated.
3.2. B12 deficiency affects C. elegans egg-laying capacity, life cycle and lifespan
Egg-laying rates significantly decreased in B12-deficient worms (Fig. 4A), which also showed a significantly prolonged life cycle compared with the control worms (Fig. 4B). Similarly, B12-deficient rats have been reported to show severe growth retardation [24] and infertility [25].
Fig. 4.
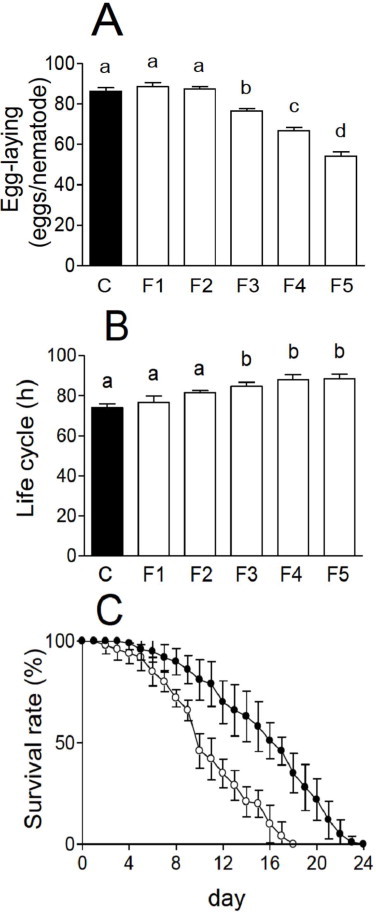
Vitamin B12 deficiency reduces egg-laying capacity and lifespan, and increases the length of the life cycle in C. elegans. (A) Total number of eggs per worm, (B) the length of the life cycle (h), and (C) the lifespan were determined in the control (black bar) and B12-deficient (white bars) F5 worms. Data represent mean ± SD of 10 independent experiments. Different letters (a–d) indicate values that are significantly different (p < 0.01); identical letters indicate values that are not significantly different.
The lifespan of B12-deficient F5 worms were significantly decreased by B12 deficiency (Fig. 4C). The maximal lifespan of B12-deficient worms was reduced to 18 days, compared with a lifespan of 24 days in the control worms (Fig. 4C). These data demonstrate for the first time that B12 deficiency significantly reduces the lifespan of animals.
B12 deficiency causes severe growth retardation and various metabolic disorders in mammals [24]. The B12 coenzyme Ado-B12 functions as a coenzyme of MCM, catalyzing the isomerization of R-methylmalonyl-CoA to succinyl-CoA in the mitochondria. Odd-numbered fatty acids, branched chain amino acids, and cholesterols are metabolized by methylmalonyl-CoA to the tricarboxylic acid (TCA) cycle intermediate succinyl-CoA by MCM [1]. When MCM activity was significantly decreased by B12 deficiency, MMA abnormally accumulated in the cells [24]. The elevated concentration of MMA mainly inhibits mitochondrial respiration because of competitive inhibition of succinate dehydrogenase (EC 1.3.99.1) by MMA [25]. TCA cycle inhibition by MMA accumulation contributes to various metabolic disorders associated with B12 deficiency [24], including severe growth retardation (prolonged life cycle) in the B12-deficient worms.
Many studies have demonstrated that there is a relationship between B12 deficiency and infertility in males and females [26,27]. However, the mechanism whereby B12 deficiency causes infertility is poorly elucidated. CH3-B12 functions as a coenzyme of MS, which catalyzes the methyl transfer from methyltetrahydrofolate to homocysteine, resulting in the donation of a methyl group to homocysteine, forming methionine [2]. MS is important to re-synthesize methionine and to metabolize methyltetrahydrofolate. Tetrahydrofolate is the precursor for the methylene derivative of folate, which is essential for thymidine supply and normal DNA replication in cells [28]. Furthermore, methionine is one of the amino acid building blocks of protein and acts as the universal methyl group donor (S-adenosylmethionine) for a large number of methylation reactions. Yamada et al. [29] reported that reduced testicular MS activity is the primary cause of pathological impairment of spermatogenesis owing to B12 deficiency and that methionine supplementation to the diet can reduce this impairment. Bennet [30] has reported that B12 deficiency may lead to recurrent fetal death owing to the elevated Hcy levels. The epigenetic regulation of gene expression involves remodeling of chromation by either the addition of methyl group to DNA and/or the post-translational modification of histone amino acid residues. S-adenosylmethionine is a critical substrate for histone methyltransferases, whereas S-adenosylhomocysteine is a potent inhibitor of the enzymatic reaction [31]. It has been observed that the concentration of S-adenosylmethionine is reduced, with a concomitant increase in S-adenosylhomocysteine concentration, in B12-deficient animal models or humans [32]. Our preliminary experiments indicated that S-adenosylmethionine/S-adenosylhomocysteine ratios significantly decrease in B12-deficient C. elegans relative to control worms (data not shown). The decreased S-adenosylmethionine/S-adenosylhomocysteine ratios may lead to abnormal epigenetic regulation of gene expression, including gene relevant to fertility.
These observations suggest that decreased egg-laying and prolonged life cycle found in B12-deficient worms are because of various B12-associated metabolic disorders, which results in abnormal epigenetic regulation of the expression of certain genes.
Approximately, 1% of B12-deficient worms showed a specific morphological abnormality (Fig. 5A), similar to the short and plump “dumpy” mutant phenotype that is formed because of disordered cuticle collagen biosynthesis [33,34]. However, there is no information available on the relationship between B12 deficiency and collagen biosynthesis. Hcy, which is significantly increased by B12 deficiency, has been shown to interfere with post-translational modifications of collagen directly by inhibiting lysyl oxidase (EC 1.4.3.13), which is involved in collagen cross-linking [35]. This observation and our data indicate a possible link between B12 deficient and collagen biosynthesis including post-translational modifications of collagen cross-linking.
Fig. 5.
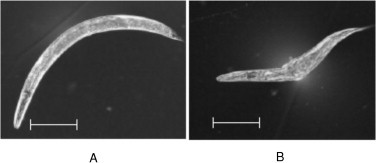
B12-deficient C. elegans show morphological changes. Differential interference microscopy images of the (1) control and (2) B12-deficient worms were obtained using an IX71 microscope (OLYMPUS Corp., Tokyo, Japan). The length of individual worms was measured using Image J software, bar = 200 μm.
Our results indicate that B12 is essential for C. elegans growth and that prolonged B12 deficiency induces a number of phenotypes, including decreased egg-laying capacity (infertility), prolonged life cycle (growth retardation), and a reduced lifespan. Therefore, we propose that C. elegans is an ideal model organism for investigating the mechanisms driving such B12-deficient phenotypes, as B12 deficiency can be induced in this animal in only 15 days. However, there are some limitations to this animal model; for example, C. elegans does not have any blood corpuscle systems. Moreover, bioinformatic analyses indicate that C. elegans also does not have any orthologs of three B12-transport proteins (haptocorrin, intrinsic factor, and transcobalamin II) involved in human gastrointestinal absorption and subsequent blood circulation of B12. Thus, C. elegans is not suitable for use as a model organism to study the mechanisms of some human B12-deficient disease phenotypes, such as megaloblastic anemia and dysfunctions of intestinal absorption and transport of B12. However, this animal is widely used as a model organism for studying the mechanisms of fertilization [36] and embryonic cell division [37]. Moreover, C. elegans is often used for understanding human brain and neuronal disorders [38] in addition to the effects of certain molecules on learning and memory [39]. C. elegans may become a suitable organism and a powerful new tool for the study of B12-deficient human diseases such as infertility [26,27,29], fetal death [30,40], neuropathy [41], and cognitive impairment [42].
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Fenton W., Hack A., Willard H., Gertler A., Rosenberg L. Purification and properties of methylmalonyl coenzyme A mutase from human liver. Arch. Biochem. 1982;214:815–823. doi: 10.1016/0003-9861(82)90088-1. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z., Crippen K., Gulati S., Banerjee R. Purification and kinetic mechanism of a mammalian methionine synthase from pig liver. J. Biol. Chem. 1994;269:27193–27197. [PubMed] [Google Scholar]
- 3.Watanabe F. Vitamin B12 sources and bioavailability. Exp. Biol. Med. 2007;232:1266–1274. doi: 10.3181/0703-MR-67. [DOI] [PubMed] [Google Scholar]
- 4.Institute of Medicine . Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Institute of Medicine, National Academy Press; Washington, DC: 1998. Vitamin B12; pp. 306–356. [PubMed] [Google Scholar]
- 5.Pepper M.R., Black M.M. B12 in fetal development. Semin. Cell Dev. Biol. 2011;22:619–623. doi: 10.1016/j.semcdb.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Ebara S., Toyoshima S., Matsumura T., Adachi S., Takenaka S., Yamaji R., Watanabe F., Miyatake K., Inui H., Nakano Y. Cobalamin deficiency results in severe metabolic disorder of serine and threonine in rats. Biochim. Biophys. Acta. 2001;1568:111–117. doi: 10.1016/s0304-4165(01)00207-0. [DOI] [PubMed] [Google Scholar]
- 7.Ebara S., Nakao M., Tomoda M., Yamaji R., Watanabe F., Inui H., Nakano Y. Vitamin B12 deficiency results in the abnormal regulation of serine dehydratase and tyrosine aminotransferase activities correlated with impairment of the adenylyl cyclase system in rat liver. Br. J. Nutr. 2008;99:503–510. doi: 10.1017/S0007114507812025. [DOI] [PubMed] [Google Scholar]
- 8.Susana G.M., Ana M., Laura D., Simone P., Felipe S.L., Montserrat D., Celestino S.B. Oxidative status of stressed Caenorhabditis elegans treated with Epicatechin. J. Agric. Food Chem. 2012;60:8911–8916. doi: 10.1021/jf3004256. [DOI] [PubMed] [Google Scholar]
- 9.Culetto E., Sattelle D.B. A role for Caenorhabditis elegans in understanding the function and interactions of human disease genes. Hum. Mol. Genet. 2000;9:869–877. doi: 10.1093/hmg/9.6.869. [DOI] [PubMed] [Google Scholar]
- 10.Chandler R.J., Venditti C.P. Genetic and genomic systems to study methylmalonic acidemia. Mol. Genet. Metab. 2005;86:34–43. doi: 10.1016/j.ymgme.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandler R.J., Aswani V., Tsai M.S., Falk M., Wehrli N., Stabler S., Allen R., Sedensky M., Kazazian H.H., Venditti C.P. Propionyl-CoA and adenosylcobalamin metabolism in C.elegans: Evidence for a role of methylmalonyl-CoA epimerase in intermediary metabolism. Mol. Genet. Metab. 2006;89:64–73. doi: 10.1016/j.ymgme.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe F., Tanioka Y., Miyamoto E., Fujita T., Takenaka H., Nakano Y. Purification and characterization of corrinoid-compounds from the dried powder of edible cyanobacterium, Nostoc commune (Ishikurage) J. Nutr. Sci. Vitaminol. 2007;53:183–186. doi: 10.3177/jnsv.53.183. [DOI] [PubMed] [Google Scholar]
- 14.Al-Dirbashi O.Y., Jacob M., Al-Hassnan Z., Chabayta R.W., El-Badaoui F., Rashed M.S. Determination of methylmalonic acid in urine by HPLC with intramolecular excimer-forming fluorescence derivatization. Biomed. Chromatogr. 2006;20:54–60. doi: 10.1002/bmc.527. [DOI] [PubMed] [Google Scholar]
- 15.Febriani A.D., Sakamoto A., Ono H., Sakura N., Ueda K., Yoshii C., Kubota M., Yanagawa J. Determination of total homocysteine in dried blood spots using high performance liquid chromatography for homocystinuria newborn screening. Pediatr. Int. 2004;46:5–9. doi: 10.1111/j.1442-200X.2004.01825.x. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto E., Tanioka Y., Nishizawa-Yokoi A., Yabuta Y., Ohnishi K., Misono H., Shigeoka S., Nakano Y., Watanabe F. Characterization of methylmalonyl-CoA mutase involved in the propionate photoassimilation of Euglena gracilis Z. Arch. Microbiol. 2010;192:437–446. doi: 10.1007/s00203-010-0572-x. [DOI] [PubMed] [Google Scholar]
- 17.Tanioka Y., Yabuta Y., Yamaji R., Shigeoka S., Nakano Y., Watanabe F., Inui H. Occurrence of pseudovitamin B12 and its possible function as the cofactor of cobalamin-dependent methionine synthase in a cyanobacterium synechocystis sp. PCC6803. J. Nutr. Sci. Vitaminol. 2009;55:518–521. doi: 10.3177/jnsv.55.518. [DOI] [PubMed] [Google Scholar]
- 18.Huang L., Zhang J., Hayakawa T., Tsuge H. Assays of methylenetetrahydrofolate reductase and methionine synthase activities by monitoring 5-methyltetrahydrofolate and tetrahydrofolate using high-performance liquid chromatography with fluorescence detection. Anal. Biochem. 2001;299:253–259. doi: 10.1006/abio.2001.5421. [DOI] [PubMed] [Google Scholar]
- 19.Byerly L., Cassada R.C., Russell R.L. The life cycle of the nematode Caenorhabditis elegans. I. Wild-type growth and reproduction. Dev. Biol. 1976;51:23–33. doi: 10.1016/0012-1606(76)90119-6. [DOI] [PubMed] [Google Scholar]
- 20.Johnson T., Wood W. Genetic analysis of life-span in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 1982;79:6603–6607. doi: 10.1073/pnas.79.21.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 22.Yamada K., Kawata T., Wada M., Isshiki T., Onoda J., Kawanishi T., Kunou A., Tadokoro T., Tobimatsu T., Maekawa A., Toraya T. Extremely low activity of methionine synthase in vitamin B12-deficient rats may be related to effects on coenzyme stabilization rather than to changes in coenzyme induction. J. Nutr. 2000;130:1894–1900. doi: 10.1093/jn/130.8.1894. [DOI] [PubMed] [Google Scholar]
- 23.Nakao M., Hironaka S., Harada N., Adachi T., Bito T., Yabuta Y., Watanabe F., Miura T., Yamaji R., Inui H., Nakano Y. Cobalamin deficiency results in an abnormal increase in l-methylmalonyl-co-enzyme-A mutase expression in rat liver and COS-7 cells. Br. J. Nutr. 2009;101:492–498. doi: 10.1017/S0007114508023398. [DOI] [PubMed] [Google Scholar]
- 24.Toyoshima S., Watanabe F., Saido H., Pezacka E.H., Jacobsen D.W., Miyatake K., Nakano Y. Accumulation of methylmalonic acid caused by vitamin B12-deficiency disrupts normal cellular metabolism in rat liver. Br. J. Nutr. 1996;75:929–938. doi: 10.1079/bjn19960198. [DOI] [PubMed] [Google Scholar]
- 25.Toyoshima S., Watanabe F., Saido H., Miyatake K., Nakano Y. Methylmalonic acid inhibits respiration in rat liver mitochondria. J. Nutr. 1995;125:2846–2850. doi: 10.1093/jn/125.11.2846. [DOI] [PubMed] [Google Scholar]
- 26.Blair J.H., Stearns H.E., Simpson G.M. Vitamin B12 and fertility. Lancet. 1968;1:49–50. doi: 10.1016/s0140-6736(68)90044-5. [DOI] [PubMed] [Google Scholar]
- 27.Jackson I.M.D., Doig W.B., McDonald G. Pernicious anemia as a cause of infertility. Lancet. 1967;2:1159–1160. doi: 10.1016/s0140-6736(67)91887-9. [DOI] [PubMed] [Google Scholar]
- 28.Shane B, Stokstad E.L.R. Vitamin B12-folate interrelationships. Ann. Rev. Nutr. 1985;5:115–141. doi: 10.1146/annurev.nu.05.070185.000555. [DOI] [PubMed] [Google Scholar]
- 29.Yamada K., Kawata T., Wada M., Mori K., Tamai H., Tanaka N., Tadokoro T., Tobimatu T., Toraya T., Maekawa A. Testicular injury to rats fed on soybean protein-based vitamin B12-deficient diet can be reduced by methionine supplementation. J. Nutr. Sci. Vitaminol. 2007;53:95–101. doi: 10.3177/jnsv.53.95. [DOI] [PubMed] [Google Scholar]
- 30.Bennett M. Vitamin B12 deficiency, infertility and recurrent fetal loss. J. Reprod. Med. 2001;46:209–212. [PubMed] [Google Scholar]
- 31.Halsted C.H., Medici V. Vitamin-dependent methionine metabolism and alcoholic liver sisease. Adv. Nutr. 2011;2:421–427. doi: 10.3945/an.111.000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stabler S.P. B12 and nutrition. In: Banerjee R., editor. Chemistry and Biochemistry of B12. John Wiley & Sons, Inc.; New York, USA: 2000. pp. 343–365. [Google Scholar]
- 33.Meredith K., Edgar R.S. Genetic studies of unusual loci that affect body shape of the nematode Caenorhabditis elegans and may code for cuticle structural proteins. Genetics. 1986;113:621–639. doi: 10.1093/genetics/113.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iain L.J. The cuticle of the nematode Caenorhabditis elegans: a complex collagen structure. BioEssays. 1994;16:171–177. doi: 10.1002/bies.950160307. [DOI] [PubMed] [Google Scholar]
- 35.Thaler R., Agsten M., Spitzer S., Paschalis E.P., Karlic H., Klaushofer K. Homocysteine suppresses the expression of the collagen cross-linker lysyl oxidase involving IL-6, Fli 1, and epigenetic DNA methylation. J. Biol. Chem. 2011;286:5578–5588. doi: 10.1074/jbc.M110.166181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geldziler B.D., Marcello M.R., Shakes D.C., Singson A. The genetics and cell biology of fertilization. Methods Cell Biol. 2011;106:343–375. doi: 10.1016/B978-0-12-544172-8.00013-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Platzer U., Meinzer H.P. Genetic networks in the early development of Caenorhabditis elegans. Int. Rev. Cytol. 2004;234:47–100. doi: 10.1016/S0074-7696(04)34002-7. [DOI] [PubMed] [Google Scholar]
- 38.Calahoro F., Ruiz-Rubio M. Caenorhabditis elegans as an experimental tool for the study of complex neurological diseases: Parkinson's disease, Alzheimer's disease and autism spectrum disorder. Invert. Neurosci. 2011;11:73–83. doi: 10.1007/s10158-011-0126-1. [DOI] [PubMed] [Google Scholar]
- 39.Ardiel E.L., Rankin C.H. An elegant mind: learning and memory in Caenorhabditis elegans. Learn. Mem. 2012;17:191–201. doi: 10.1101/lm.960510. [DOI] [PubMed] [Google Scholar]
- 40.Candio M., Magnaldo S., Bayle J., Dor J.F., Gillet Y., Bongain A., Van Obberghen E. Clinical B12 deficiency in one case of recurrent spontaneous pregnancy loss. Clin. Chem. Lab. Med. 2003;41:1026–1027. doi: 10.1515/CCLM.2003.157. [DOI] [PubMed] [Google Scholar]
- 41.Metz J. Cobalamin deficiency and the pathogenesis of nervous system disease. Annu. Rev. Nutr. 1992;12:59–79. doi: 10.1146/annurev.nu.12.070192.000423. [DOI] [PubMed] [Google Scholar]
- 42.Lindballe D.L., Fedosov S., Sherliker P., Hin H., Clarke R., Nexo E. Association of cognitive impairment with combinations of vitamin B12-related parameters. Clin. Chem. 2011;57:1436–1443. doi: 10.1373/clinchem.2011.165944. [DOI] [PubMed] [Google Scholar]


