Abstract
Sulf1A expression, which is a characteristic of embryonic muscle, is undetectable in mature muscle fibres and quiescent satellite cells, but is re-activated in vivo upon injury and in vitro following activation of satellite cells. Sulf1A is known to enhance canonical Wnt signalling, and its association with Wnt1-induced satellite cell proliferation in vitro in the present study further confirmed this. However, exogenous Wnt6 decreased satellite cell proliferation but promoted the adoption of a hyper-elongated cell morphology in myoblasts on isolated single fibres in culture. Such Wnt6-induced cellular hyper-elongation and inhibition of proliferation was found to be dependent upon Sulf1A, as treatment with Sulf1A neutralising antibodies abolished both these effects. This indicates that Sulf1A can regulate Wnt6 signalling and cellular differentiation in skeletal muscle.
Keywords: Wnt1, Wnt6, Sulf1, Satellite cells, Muscle growth
Highlights:
▸ Sulf1 regulates not only Wnt1 but also Wnt6 signalling. ▸ Wnt1 and Wnt6 play contrasting roles in skeletal muscle growth regulation. ▸ Reduction in Sulf1 activity level can reverse Wnt6-induced myogenic differentiation.
1. Introduction
The satellite cells generated during embryonic muscle development persist postnatally as quiescent muscle progenitors under the basal lamina surrounding the muscle fibres [1]. The quiescent muscle progenitors are activated during postnatal growth and in response to muscle injury to repair and regenerate the damaged muscle [1–3]. This resident satellite cell pool is usually sufficient for normal muscle growth and repair. However, in degenerative muscle diseases, satellite cell supply becomes limited due to repeated cell cycles leading to progressive loss of regenerative potential and an ability to maintain muscle mass and function. It is therefore important to understand factors regulating muscle mass since the contributions of different molecular signalling pathways that regulate satellite cell proliferation and differentiation remain poorly defined. The level of satellite cell dependent muscle regeneration relies on the activities of specific growth factors and signalling molecules. These activities are further regulated by the availability of primary and also secondary receptors since many growth factors in addition to their specific primary receptors also require heparan sulphate proteoglycans (HSPGs) as secondary receptors for ligand binding [4]. HSPGs interact with growth factors and receptors through their heparan sulphate (HS) chains, the sulfation status of which can regulate the activities of growth factors requiring HSPG interaction.
HSPG sulfation is not only regulated intracellularly by the activities of Sulfotransferases in the Golgi but is further modified extracellularly by Sulf1 and Sulf2 enzymes, members of the sulfatase gene family related to the lysosomal N-acetyl glucosamine sulfatases [5–7]. Sulf1, the subject of this present study, has been shown to modulate the activities of many growth factors and signalling molecules by regulating the sulfation status of specific HSPGs as it reduces the 6-O-sulfation of heparan sulphate, a component of HSPGs. For example, FGF2 and FGF4 function has been shown to be inhibited by Sulf1 (a full length variant), during chick angiogenesis and in ovarian cells by removing 6-O sulphates from glucosamine residues in heparan sulphate [8,9]. Our recent study [10] also identified a shorter alternatively spliced Sulf1 variant (Sulf1B) in some developing Quail embryonic tissues although full length Sulf1 (Sulf1A) was found to be the major isoform present in developing skeletal muscle. The present study therefore specifically investigated the role of Sulf1A in satellite cell growth that has been shown to enhance canonical Wnt signalling [9,10] although the precise mechanism by which Sulf1A regulates Wnt signalling is not clear but 6-O-desulfation of HSPGs by Sulf1A is believed to modulate the binding affinity of Wnts to heparan sulphate to promote the HSPG-mediated presentation of Wnt ligand to its Frizzled receptor [9]. Wnt signalling has been shown to regulate embryonic muscle development [11] as well as early muscle regeneration [12,13]. To understand the role of Sulf1A and Wnt signalling in muscle growth and regeneration, we used in vitro model of satellite cell activation and growth on dissociated whole skeletal muscle fibres. Dissociated single fibres were grown under normal culture conditions and in the presence of Wnt1 or Wnt6-secreting cells. The quiescent satellite cells in this study were identified from their expression of a widely used marker of these cells called Pax7, a paired-box transcription factor that is expressed in both quiescent and proliferating satellite cells or their progeny required for the maintenance of postnatal skeletal muscle [14,15]. Both Wnt1 and Wnt6 signalling re-activated Sulf1A expression in satellite cells in vitro. While Wnt1 induced a much earlier and increased satellite cell proliferation, Wnt6 inhibited satellite cell proliferation by promoting muscle cell differentiation indicated by hyper-elongation of progenitor cells. A reduction in Sulf1A expression by neutralising antibodies, however, reversed the Wnt6 induced inhibition of satellite cell proliferation as well as myoblast hyper-elongation.
2. Materials and methods
2.1. In situ hybridisation procedure
Extensor digitorum longus (EDL) muscles of control and mdx mice were fixed overnight in 4% paraformaldehyde (PFA) at 4 °C before Paraffin embedding and sectioning at 6 or 10 μM thickness. A digoxigenin-labelled 292 bp riboprobe to mouse Sulf1A (1686–1978 bp) was used to analyse mRNA expression using in situ hybridisation procedure described by Moorman et al. [16].
Ethical statement: Muscles for all experiments in this study were removed from animals sacrificed following the Home Office (UK) licence Schedule 1 procedure in accordance with the Animals (Scientific Procedures) Act 1986 and with approval from the Royal Veterinary College ethical review process.
2.2. Cell culture and muscle fibre isolation
The EDL muscles were carefully dissected from 8 to 10 week old CD mice (killed using Schedule 1 procedure) and muscle fibres dissociated in 0.12% Type 1 Collagenase (Sigma)/DMEM [13,17]. Single fibres were cultured in 24-well plates with 12–15 fibres/well in 0.5 ml/well culture medium (10% FCS/0.5% Chick embryo extract/DMEM). The single muscle fibres were incubated at 37 °C, 5% CO2 before fixation in 4% PFA for 15 min at different time intervals indicated in Section 3. Some muscle fibres were also fixed at time 0 to investigate quiescent satellite cell phenotype. For satellite cell growth in the presence of Wnt1 and Wnt6, mouse NIH-3T3 Wnt1 (provided by Dr. A. Kispert), Wnt6 (provided by Dr. S. Vainio) and Lac-Z-transfected control cells were grown in 24-well plates to 50–60% confluency before 12–15 dissociated muscle fibres were added and co-cultured for 24, 48 or 72 h before fixation in 4% PFA. In some experiments, 100 ng or 300 ng/ml neutralising antibody to Sulf1A [17] was added to the culture medium at time 0 and the satellite cells on single fibres cultured for 72 h before fixation and staining with different antibodies.
2.3. Immunocytochemical procedure
Different polyclonal and monoclonal antibodies were used to study the expression patterns of Sulf1A, Pax7 and MyoD. A Sulf1A specific rabbit polyclonal antibody (B) raised to an 18 amino acid peptide in the hydrophilic domain was used for most analyses [10] although another Sulf1 antibody (C) raised to a 20 amino acid peptide present in the C-terminal domain of Sulf1 was also used in some experiments [10]. Pax7 mouse monoclonal antibody was obtained from the Developmental Studies hybridoma bank while anti MyoD mouse monoclonal (clone 5.8A) was obtained from DakoCytomation. For immunochemical staining, fixed muscle fibres or tissue sections were washed with PBS and permeabilised for 15 min using a double immunofluorescence procedure described previously [10,13]. Sulf1 primary antibodies were diluted 1/200, Pax7 supernatant antibody 1/4, MyoD antibody 1/150, while all fluorochrome-labelled secondary antibodies were diluted 1/300 [10]. Muscle fibres were stained with antibody 83B6 that reacts with all skeletal muscle type myosin heavy chain isoforms [18,19]. Binding of 83B6, Pax7 and MyoD mouse monoclonals was detected using Alexa Fluor 488 fluorochrome-conjugated goat anti-mouse IgG (Molecular Probes). Sulf1 rabbit primary antibodies were visualised using goat anti-rabbit biotinylated IgG (DAKO E0353) followed by Streptavidin-conjugated Alexa Fluor 594 (Molecular Probes). Single fibres or tissue sections were mounted using fluorescent mounting medium (Sigma Aldrich) containing 2.5 μg/ml DAPI for nuclear visualisation and photographed using Leica DM4000B fluorescent microscope. For quantification, the total number of satellite cells/cluster for each category at different time points was counted from Sulf1 antibody staining. A minimum of 30 cell clusters from at least 15 different muscle fibres were counted for each group. The data from multiple clusters were pooled to get a mean (±SEM) for each category. Similarly, measurements for elongation index, were taken from a minimum of 25 cells to get a mean (±SEM) in each category. Data are presented as mean ± standard deviation. Statistical analysis was performed using student t-test, regarding p < 0.05 as statistically significant.
3. Results
3.1. Sulf1A, expressed in embryonic myotubes is undetectable in mature muscle fibres and quiescent satellite cells but is re-activated in vitro and in vivo regenerating myotubes
Sulf1A is expressed in embryonic myotubes (Fig. 1(A)) that stained positive for skeletal muscle type myosin heavy chain but is undetectable in adult chicken and postnatal mouse muscle fibres (Fig. 1(B) and (C)). Quiescent satellite cells identified from their Pax7 expression also showed no Sulf1A expression in either adult chicken (Fig. 1(B)) or 2-week old mouse EDL muscle fibres although Sulf1A was detectable in some other cell types e.g. endothelial cells (Fig. 1(B)). Virtually all Pax7 positive quiescent satellite cells were found to be located under the basement membrane at the periphery of the muscle fibres as expected [17] although an occasional Pax7-positive satellite-like cell was also observed inside a blood capillary (arrowed cell in Fig. 1(B) within a circle), with blood capillary identified from its Sulf1A staining of endothelial cells. In addition some Pax7-positive satellite-like cells were also observed amongst some interstitial cells (arrowed cell within a circle in Fig. 1(C)) as is apparent from the presence of a Pax7 positive cell amongst a cluster of cells identified from their blue DAPI staining. Unlike the normal postnatal skeletal muscle, Sulf1A expression at both mRNA and protein levels was clearly apparent in regenerating myogenic cells of spontaneously regenerating myotubes of postnatal mdx mouse muscles using in situ hybridisation (arrowed cells in Fig. 1(D.ii)) and immunocytochemical procedures (arrowed cells in Fig. 1(D.iii) and (D.iv)). In contrast, adjacent larger original mature muscle fibres remained unstained for Sulf1A mRNA as well as Sulf1A protein (as marked by asterisks in Fig. 1(D)) while smaller regenerating myotubes stained positive for both Sulf1A mRNA and protein (as marked by arrows in Fig. 1(D)). The immunocytochemical staining of freshly isolated muscle fibres from adult mouse EDL also showed little or no staining of satellite cells with antibodies to Sulf1A at time 0 (Fig. 1(E)) but Sulf1A protein expression was re-activated in satellite cells (as shown for 62 h) in vitro (Fig. 1(F)). The presence of satellite cells in both muscle tissue sections and isolated single muscle fibres that did not stain for Sulf1A was confirmed from their positive staining for Pax7, a ubiquitously expressed marker of satellite cells.
Fig. 1.
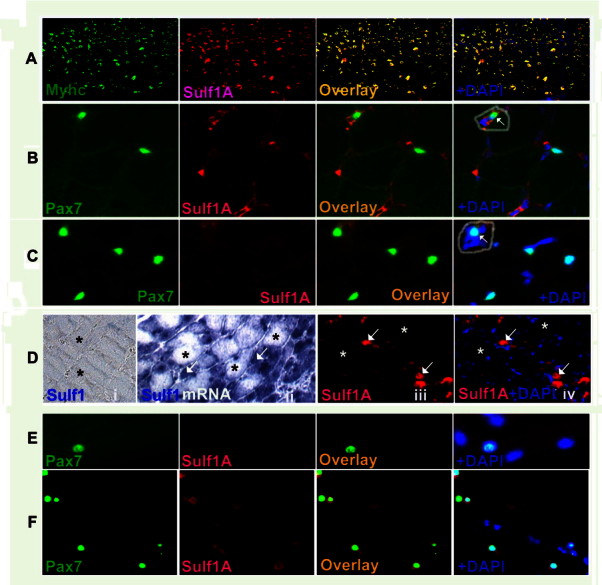
Sulf1A expressed in embryonic developing muscle becomes undetectable in adult muscle fibres and quiescent satellite cells but is re-activated in vitro and in vivo regenerating myogenic cells. For example, embryonic myotubes of a 7 day chick limb muscle express both skeletal muscle type myosin heavy chain and Sulf1A as is apparent from the individual and superimposed images (A). Unlike the embryonic muscle, neither adult chicken skeletal muscle fibres (B) or EDL muscle (C) from a 2-week old mouse or their Pax7-positive quiescent satellite cells show any Sulf1A staining by double immunofluorescence procedure. Unlike the skeletal muscle fibres and satellite cells, Sulf1A expression in the same sections is clearly detectable in endothelial cells of blood capillaries (B). Pax7-positive satellite-like cell present in a blood capillary is highlighted by a circle in (B). Pax7-positive satellite-like cell amongst interstitial cells is highlighted by a circle in (C). Myogenic Sulf1 expression, is re-activated in small sized myotubes of spontaneously regenerating myotubes indicated by white arrows while larger unaffected mature muscle fibres in EDL muscle of 4-week old mdx mouse show no Sulf1 expression at either mRNA (D.i, D.ii) or protein (D.iii, D.iv) levels (indicated by asterisks) using in situ hybridisation (D.i, D.ii) or immunofluorescence (D.iii, D.iv) procedures. Similarly, Sulf1A expression is undetectable in Pax7 positive quiescent satellite cells in isolated muscle fibres (E) at time 0 but detectable in most activated Pax7-positive satellite cells at 62 h (F) using double immunofluorescence procedure.
3.2. Sulf1A expression is also re-activated in Wnt1 and Wnt6 treated satellite cells
Sulf1A and MyoD, characteristic markers of early embryonic muscle, are undetectable in Pax7-positive quiescent satellite cells but are both re-expressed in activated satellite cells (Figs. 1 and 2). Satellite cell development therefore was further investigated in the presence of Lac-Z transfected control and Wnt1 and Wnt6 secreting cells using Sulf1A and MyoD as markers of satellite cell activation and growth. MyoD and Sulf1A expression was apparent in both control and Wnt1 or Wnt6 treated satellite cells. Compared with the control cultures, the level of satellite cell proliferation (measured as an increase in the number of cells/cluster) in the presence of Wnt1 secreting cells, however, was markedly increased (Figs. 2 and 4) as also demonstrated by Otto et al. [13]. The onset of satellite cell division in the presence of Wnt1 was found to be much earlier when compared with control growth conditions. For example, while satellite cells in normal growth medium or in the presence of Lac-Z transfected cells are rarely observed undergoing cell division before 24 h, satellite cell proliferation in the presence of Wnt1 was frequently apparent at 12–13 h in vitro (Fig. 2).
Fig. 2.
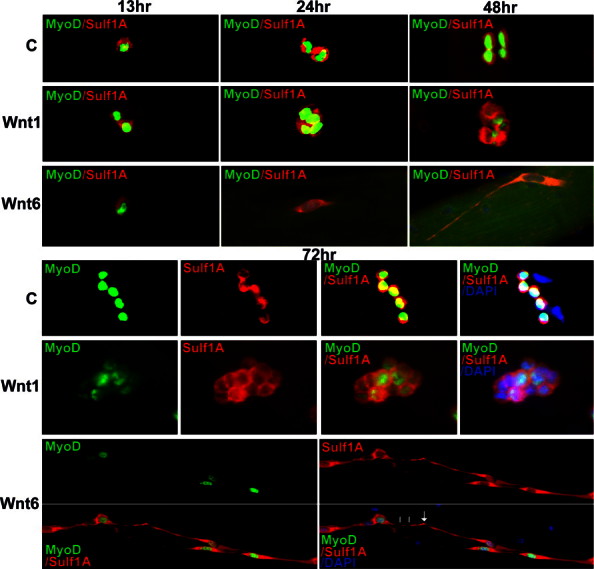
Not only Sulf1A but also MyoD is undetectable in quiescent satellite cells (0 h) but reactivated in these cells in vitro, in normal culture medium or in the presence of Lac-Z transfected (control), and Wnt1 and Wnt6 secreting cells. Satellite cells on single fibres were cultured in the presence of Lac-Z-transfected control cells (C), and Wnt1 and Wnt6 secreting cells for 13, 24, 48 and 72 h before staining for MyoD and Sulf1A using double immunofluorescence procedure. Satellite cells at 13, 24 and 48 h are shown as a single image with superimposed image of both Sulf1 and MyoD while individual images for Sulf1A and MyoD are shown for 0 and 72 h time points; h: hour. Individually spaced single satellite cells on freshly isolated muscle fibres gradually develop into small clusters in normal culture medium while the cluster size is increased in the presence of Wnt1 exposure. Wnt6 in contrast shows little or no proliferation but a marked change in cell morphology indicating its inhibitory action on growth.
Fig. 4.
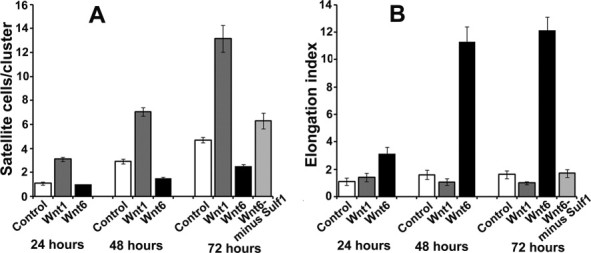
The quantification of the proliferation and hyper-elongation of satellite cells grown in the presence of normal, Wnt1 and Wnt6 secreting cells, and following Sulf1A inhibition by 100 ng/ml Sulf1A neutralising antibodies. Satellite cell elongation index represents length divided by maximum diameter at the level of nuclear centre. Data are presented as mean ± standard deviation. Statistical analysis was performed using student t-test, regarding p < 0.05 as statistically significant.
The in vitro culture of isolated single muscle fibres in the presence of Wnt6 secreting cells also led to a marked change in satellite cell growth. However, unlike Wnt1 cells showing earlier and increased level of satellite cell proliferation, Wnt6 exposure inhibited satellite cell proliferation measured as number of cells/cluster (Figs. 2–4). Reduced satellite cell proliferation in the presence of Wnt6 cells was observed throughout the 72 h culture time period (Figs. 2–4). The other marked feature of Wnt6 on satellite cell growth was its very distinctive effect on myogenic cell morphology (Figs. 2 and 3). Unlike satellite cells grown in the presence of Wnt1 secreting cells and normal culture medium, satellite cell generated myogenic cells in the presence of Wnt6 were usually hyper-elongated (Figs. 2–4). The onset of such morphological change often became apparent within 24 h in vitro, initially adopting a spindle shape resembling myoblast morphology, although the degree of elongation increased with time and appeared to be related to the distance between neighbouring myogenic cells. For example, the elongation index (obtained by the maximal myogenic cell length divided by the maximum cell width at the centre of the nucleus) for Wnt6 exposed satellite cells at 24, 48 and 72 h had significantly increased when compared with satellite cells grown in the presence of Wnt1 and Lac-Z control or normal growth medium (Fig. 4). Unlike Wnt6, the elongation index for the satellite cells grown in the presence of Wnt1 and control cultures over 24–72 h time period showed only a minor change in morphology (Fig. 4). Sulf1A expression in Wnt6-induced satellite cell extensions was not uniform but regularly punctuated (indicated by white lines in Fig. 2) until it reached or contacted the extension on its nearest neighbouring myogenic cell with increased expression level (an arrow in Fig. 2).
Fig. 3.
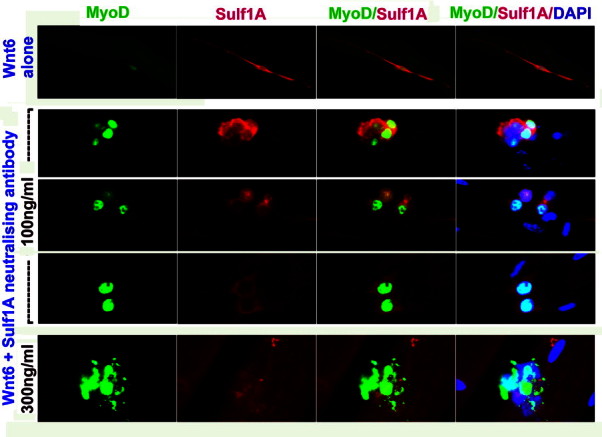
Pattern of MyoD and Sulf1A expression in satellite cells on dissociated single muscle fibres grown in the presence of only Wnt6 secreting cells as a control (lane 1) and in the presence of 100 ng/ml (lanes 2–4 representing three different experiments to demonstrate reproducibility) or 300 ng/ml (lane 5) Sulf1A peptide antibodies for 72 h investigated by double immunofluorescence procedure. Sulf1A inhibition by neutralising antibodies clearly apparent from reduced Sulf1A expression in a large majority of the satellite cells, reverses Wnt6-induced satellite cell differentiation and proliferation inhibition. A further increase in the Sulf1 antibody concentration triggering initial proliferation appeared to lead to some apoptosis.
3.3. Sulf1A inhibition by neutralising antibodies reverses Wnt6-induced inhibition of satellite cell proliferation and myoblast hyper-elongation
Since exogenous Wnt6 inhibited satellite cell proliferation and induced marked changes in cellular morphology despite endogenous Sulf1A being present in these cells, we further investigated the function of Sulf1A by reducing Sulf1A levels in satellite cells cultured in the presence of Wnt6 secreting cells. Sulf1A reduction by 100 ng/ml antibody concentration did not only restore cell proliferation but also reversed the cell shape change leading to near normal cell morphology after 72 h in vitro growth (Fig. 3) as indicated by the increased satellite cell number/cluster and reduction in myogenic hyper-elongation index (Fig. 4). A further increase in neutralising Sulf1A antibody concentration to 300 ng/ml initially enhanced proliferation but was followed by apparent apoptosis and was therefore avoided in later experiments by using only 100 ng/ml in the present study.
4. Discussion
Our previous study demonstrated the importance of Sulf1A in embryonic muscle development since Sulf1A inhibition by antisense oligos led to down-regulation of MyoD transcription factor, an early marker of myogenesis [20]. Unlike the embryonic muscle, Sulf1A was undetectable in postnatal mature muscle fibres as well as Pax7 positive quiescent satellite cells associated with post-natal skeletal muscle fibres. Sulf1A expression, however, was re-activated in satellite cells on isolated muscle fibres cultured in vitro and in vivo regenerating myotubes of mouse muscles, thus re-capitulating early muscle development where it may regulate the activities of specific growth factors or signalling molecules. Once activated, Sulf1A expression in vitro continued in most satellite cells up to the 72 h time period investigated in this study. During this early phase of myogenesis, Sulf1A could regulate multiple growth factor signalling pathways including Wnt signalling since canonical Wnt signalling has been shown to be enhanced by Sulf1A [9,10,20,21].
In addition to increased satellite cell proliferation, in vitro exposure to Wnt1 induced earlier onset of satellite cell division. We did not, however, experimentally alter the Sulf1A levels in the presence of Wnt1 but Sulf1A was expressed in all satellite cells during Wnt1 exposure. The co-expression of Sulf1A with Wnt1 could enhance canonical Wnt signalling as shown for other cells in earlier studies [9,10,20,21]. Unlike Wnt1, Wnt6 did not only show much reduced satellite cell proliferation but also a marked change in cell morphology, indicating possible onset of differentiation or its role in cell fusion. For example, while satellite cells grown in the presence of Wnt1 maintained the regular cell size and shape observed in satellite cells grown under normal culture conditions, satellite cells grown in the presence of Wnt6 showed hyper-elongation of myoblasts demonstrating that different Wnt signalling pathways do not only selectively regulate satellite cell proliferation but also myogenic differentiation and possibly cell fusion distinctly. Sulf1A inhibition achieved by the addition of Sulf1A neutralising antibodies [17], however, overcame Wnt6-induced inhibition of satellite cell proliferation as well as morphological change indicating onset of differentiation. The level of satellite cell proliferation following Sulf1A inhibition had increased to that seen in control culture levels. Sulf1A is known to enhance canonical Wnt signalling whereas the present study demonstrates that Sulf1A also plays a role in Wnt6 signalling as indicated by restoring near normal satellite cell proliferation and morphology following Sulf1A inhibition. This may be due to Sulf1A's distinct role in Wnt6 signalling since Wnt6 has the capacity to act via both canonical and non-canonical pathways. Further studies, however, would be required to confirm the role of Sulf1A in Wnt6 regulation by not only Sulf1A inhibition but also using purified Sulf1A protein. While the expression and role of canonical Wnt signalling in muscle development is well established, little is known about either the expression or role of Wnt6 in muscle growth. Otto et al. did not observe Wnt6 expression in activated satellite cells at 24 h in vitro [13] but considering its inhibitory role in satellite cell proliferation, Wnt6 expression may need to be investigated during later stages of satellite cell growth to understand its role in myoblast cell fusion and differentiation.
The opposing roles of Wnt1 and Wnt6 on satellite cell proliferation and cell morphology demonstrate distinct functions of different Wnt signalling pathways in the regulation of muscle mass. Muscle growth therefore could be regulated by competing Wnt signalling pathways, with canonical signals promoting growth through increased proliferation while non-canonical signals favouring differentiation. This study demonstrated that Sulf1A is present in activated satellite cells and its inhibition can reverse Wnt6-induced satellite cell behaviour, presumably by blocking non-canonical actions of Wnt6. During muscle regeneration, the function of Sulf1A therefore may be to regulate the levels of different Wnt signalling pathways so that satellite cell proliferation and differentiation occur in a co-ordinated manner to regulate muscle mass. While Wnt6 signalling has been reported to inhibit myocardial growth in some other tissues such as developing heart [22], this is a first study to highlight the role of Wnt6 signalling pathway in skeletal muscle mass regulation by not only preventing satellite cell proliferation but also by preventing myogenic cell size growth.
Acknowledgements
We thank Tony Otto and Raymond Macharia for their help and instruction in muscle dissection and single fibre dissociation protocol. Laura Hitchins carried out this work during her final year BSc project and a summer studentship. Fenella Fletcher was supported by a Wellcome Trust summer studentship. We thank Elaine Shervill for skilled technical assistance with histology.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References:
- 1.Bischoff R. Proliferation of muscle satellite cells on intact myofibers in culture. Dev. Biol. 1986;115:129–139. doi: 10.1016/0012-1606(86)90234-4. [DOI] [PubMed] [Google Scholar]
- 2.Zammit P.S., Heslop L., Hudon V., Rosenblatt J.D., Tajbakhsh S., Buckingham M.E., Beauchamp J.R., Partridge T.A. Kinetics of myoblast proliferation show that resident satellite cells are competent to fully regenerate skeletal muscle fibers. Exp. Cell Res. 2002;281:39–49. doi: 10.1006/excr.2002.5653. [DOI] [PubMed] [Google Scholar]
- 3.Collins C.A., Olsen I., Zammit P.S., Heslop L., Petrie A., Partridge T.A., Morgan J.E. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Caser J.C., Cabello-Verrugio C., Olguin H., Aldunate R., Inestrosa N.C., Brandan E. Heparan sulfate proteoglycans are increased during skeletal muscle regeneration: requirement of syndecan-3 for successful fiber formation. J. Cell Sci. 2004;117:73–84. doi: 10.1242/jcs.00828. [DOI] [PubMed] [Google Scholar]
- 5.Lukatela G., Krauss N., Theis K., Selmer T., Gieselmann V., von Figura K., Saenger W. Crystal structure of human arylsulfatase A: the aldehyde function and the metal ion at the active site suggest a novel mechanism for sulfate ester hydrolysis. Biochemistry. 1998;37:3654–3664. doi: 10.1021/bi9714924. [DOI] [PubMed] [Google Scholar]
- 6.Knaust B., Schmidt T., Dierks R., von Bulow K., von Figura K. Residues critical for formylglycine formation and/or catalytic activity of arylsulfatase A. Biochemistry. 1998;37:13941–13946. doi: 10.1021/bi9810205. [DOI] [PubMed] [Google Scholar]
- 7.Robertson D.A., Freeman C., Morris C.P., Hopwood J.J. A cDNA clone for human glucosamine-6-sulphatase reveals differences between arylsulphatases and non-arylsulphatases. Biochem. J. 1992;288:539–544. doi: 10.1042/bj2880539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morimoto-Tomita M., Uchimura K., Werb Z., Hemmerich S., Rosen S.D. Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. J. Biol. Chem. 2002;277:49175–49185. doi: 10.1074/jbc.M205131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ai X., Do A.T., Lozynska O., Kusche-Gullberg M., Lindahl U., Emerson Jr C.P. QSulf1 remodels the 6-O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. J. Cell Biol. 2003;162:341–351. doi: 10.1083/jcb.200212083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahota A.P., Dhoot G.K. A novel SULF1 splice variant inhibits Wnt signalling but enhances angiogenesis by opposing SULF1 activity. Exp. Cell Res. 2009;315:2752–2764. doi: 10.1016/j.yexcr.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 11.Munsterberg A.E., Kitajewski J., Bumcrot D.A., McMahon A.P., Lassar A.B. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes Dev. 1995;9:2911–2922. doi: 10.1101/gad.9.23.2911. [DOI] [PubMed] [Google Scholar]
- 12.Polesskaya A., Seale P., Rudnicki M.A. Wnt signalling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell. 2006;113:841–852. doi: 10.1016/s0092-8674(03)00437-9. [DOI] [PubMed] [Google Scholar]
- 13.Otto A., Schmidt C., Luke G., Allen S., Valasek P., Muntoni F., Lawrence-Watt D., Patel K. Canonical Wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration. J. Cell Sci. 2008;121:2939–2950. doi: 10.1242/jcs.026534. [DOI] [PubMed] [Google Scholar]
- 14.Seale P., Ishibashi J., Scime A., Rudnicki M.A. Pax7 is necessary and sufficient for the myogenic specification of CD45+:Sca1+ stem cells from injured muscle. PLoS Biol. 2004;2:E130. doi: 10.1371/journal.pbio.0020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zammit P.S., Relaix F., Nagata Y., Ruiz A.P., Collins C.A., Partridge T.A., Beauchamp J.R. Pax7 and myogenic progression in skeletal muscle satellite cells. J. Cell Sci. 2006;119:1824–1832. doi: 10.1242/jcs.02908. [DOI] [PubMed] [Google Scholar]
- 16.Moorman A.F., Houweling A.C., de Boer P.A., Christoffels V.M. Sensitive nonradioactive detection of mRNA in tissue sections: novel application of the whole-mount in situ hybridization protocol. J. Histochem. Cytochem. 2001;49:1–8. doi: 10.1177/002215540104900101. [DOI] [PubMed] [Google Scholar]
- 17.Gill R., Hitchins L., Fletcher F., Dhoot G.K. Sulf1A and HGF regulate satellite-cell growth. J. Cell Sci. 2010;123:1873–1883. doi: 10.1242/jcs.061242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ralphs J.R., Dhoot G.K., Tickle C. Differentiation of myogenic cells in micromass cultures of cells from chick facial primordia. Dev. Biol. 1989;131:189–196. doi: 10.1016/s0012-1606(89)80050-8. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J., Dhoot G.K. Localised and limited changes in the expression of myosin heavy chains in injured skeletal muscle fibres being repaired. Muscle Nerve. 1997;21:469–481. doi: 10.1002/(sici)1097-4598(199804)21:4<469::aid-mus5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Dhoot G.K., Gustafsson M.K., Ai X., Sun W., Standiford D.M., Emerson C.P. Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase. Science. 2001;293:1663–1666. doi: 10.1126/science.293.5535.1663. [DOI] [PubMed] [Google Scholar]
- 21.Nawroth R., van Zante A., Cervantes S., McManus M., Hebrok M., Rosen S.D. Extracellular sulfatases, elements of the Wnt signaling pathway, positively regulate growth and tumorigenicity of human pancreatic cancer cells. PLoS One. 2007;2:e392. doi: 10.1371/journal.pone.0000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavery D.L., Martin J., Turnbull Y.D., Hoppler S. Wnt6 signalling regulates heart muscle development during organogenesis. Dev. Biol. 2008;323:177–188. doi: 10.1016/j.ydbio.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]


