Abstract
Cold-inducible RNA-binding protein (CIRBP) induced by cold stress modulates the molecular circadian clock in vitro. The present study examines the effect of a ketogenic diet (KD) and fasting on Cirbp expression in the mouse liver. Chronic KD administration induced time-dependent Cirbp expression with hypothermia in mice. The circadian expression of clock genes such as Bmal1 and Clock was phase-advanced and augmented in the liver of mice fed with a KD. Transient food deprivation also induced time-dependent Cirbp expression with hypothermia in mice. These findings suggest that hypothermia is involved in the increased expression of Cirbp under ketogenic or fasting conditions.
Keywords: Circadian rhythm, Body temperature, Ketogenesis, Torpor, Peripheral clock
Highlights
-
•
A ketogenic diet and fasting induce time-of-day-dependent hypothermia in mice.
-
•
A ketogenic diet and fasting induce time-of-day-dependent Cirbp expression in mice.
-
•
A ketogenic diet affects the expression of circadian genes in the mouse liver.
1. Introduction
The central clock that regulates most physiological and behavioral rhythms in mammals is located in the suprachiasmatic nucleus (SCN) of the hypothalamus in the brain [1,2]. The mammalian circadian clock system consists of a master pacemaker in the SCN and peripheral oscillators in most tissues. Many studies at the molecular level have found that circadian oscillators in both the SCN and peripheral tissues are driven by negative feedback loops comprising the periodic expression of clock genes [1,2]. Peripheral oscillators are self-sustained and cell-autonomous because peripheral tissues maintain circadian clock gene expression even in vitro [1,2]. However, peripheral clocks are entrained to the central clock in the SCN by systemic time cues such as neural, humoral and other signals including body temperature [1–3].
Cold-inducible RNA-binding protein (CIRBP) has been identified as a protein that is induced by cold stress in cultured cells [4]. Levels of Cirbp expression rhythmically fluctuate in the mouse liver independently of the molecular clock, suggesting that changes in body temperature are involved in daily Cirbp expression [3,5]. Morf et al. [5] demonstrated direct interaction between CIRBP and transcripts encoding circadian clock proteins in vitro and revealed via loss-of-function experiments that CIRBP enhances the amplitude of circadian gene expression.
Ketogenic diets (KDs) comprise high-fat with low carbohydrate and protein contents, and they have been used as an approach to weight loss for both obese and non-obese individuals. Such diets mimic the metabolic conditions of fasting or caloric restriction and are based on theoretical concepts of the effects of dietary component ratios on energy expenditure [6]. We previously demonstrated that circadian clock gene expression is disrupted in peripheral tissues of hypothermic mice fed with a KD (KD mice) [7–9]. We examined the temporal expression profiles of Cirbp mRNA in mice under KD feeding and fasting to elucidate the regulation mechanism of CIRBP expression in vivo.
2. Materials and methods
2.1. Animals
Male ICR mice (Japan SLC Inc., Hamamatsu, Japan) aged 7–8 weeks were maintained under a 12:12 h light–dark cycle (lights on at 0:00 and lights off at 12:00). A white fluorescent lamp served as a daytime light source.
In the KD feeding experiment, mice were fed ad libitum for two weeks with a normal diet (ND) (CE-2; Clea Japan Inc., Tokyo, Japan) or with a KD (73.9% fat, 8.3% protein and 0.73% carbohydrates, w/w; modified AIN-93G; Oriental Yeast Co. Ltd., Tokyo, Japan). The proportions of calories derived from fat, carbohydrate and protein in ND and KD were 12.6%, 58.3% and 29.3%, and 94.8%, 0.1% and 4.8%, respectively. The mice were then sacrificed at 2:00, 6:00, 10:00, 14:00, 18:00, and 22:00, and tissues were dissected, quickly frozen and stored in liquid nitrogen.
Mice were sacrificed under food deprivation at 2:00, 8:00, 14:00, and 20:00 after an overnight fast.
All animal care, handling and experimentation proceeded under the approval of our institutional Animal Care and Use Committee (Permissions #2010–020 and #2012–020).
2.2. Monitoring core body temperature
Mice were surgically implanted intra-abdominally with data loggers (TempDisk TD-LAB, Labo Support Co. Ltd., Suita, Osaka, Japan) that were programmed to record Tb ± 0.1 °C every 10 min. The data obtained from each logger were analyzed using RhManager Ver.2.09 (KN Laboratories Inc., Ibaraki, Osaka, Japan). Hourly Tb data were averaged.
2.3. Quantitative reverse transcription (RT)-PCR
Total RNA was extracted using RNAiso (Takara Bio Inc., Otsu, Japan). Single-stranded cDNA was synthesized using PrimeScriptTM RT reagent kits with gDNA Eraser (Takara Bio Inc., Otsu, Japan). Real-time RT-PCR proceeded using SYBR® Premix Ex TaqTM II (Takara Bio Inc., Otsu, Japan) and a LightCyclerTM (Roche Diagnostics, Mannheim, Germany). The reaction conditions were 95 °C for 10 s followed by 45 cycles of 95 °C for 5 s, 57 °C for 10 s and 72 °C for 10 s. Supplemental Table 1 shows the sequences of the primer pairs. The amount of target mRNA was normalized relative to that of 18S rRNA.
2.4. Statistical analysis
All values are expressed as means ± SEM. Data were analyzed using a two-way ANOVA followed by Tukey's multiple comparison test. Circadian rhythms were analyzed using the modified cosinor method (nonlinear least-squares (NLLS) Marquardt–Levenberg algorithm) [10]. We defined the function as f(x) = M + A cos(2π/T(x − ø)) and set four variables (M, MESOR (mean statistics of rhythm); A, amplitude (one-half of the total peak-trough variation); T, period; ø, acrophase) as the fit parameters. The circadian period (T) was 24 h under LD 12:12. Acrophase is expressed in hours elapsed from 0:00. The significance of the circadian rhythm was tested by the zero-amplitude test; P < 0.05 was considered evidence of statistically significant rhythmicity for the given period of the cosine curve approximation. Data from cosinor analyses were compared between groups using Welch's or Student's t-test. P < 0.05 indicated a statistically significant difference.
3. Results
Core Tb fluctuated bimodally with a peak at early night (activity onset) and at the night-to-day transition under ND feeding (Fig. 1). Chronic KD administration significantly decreased Tb particularly at midday and late in the dark period, suggesting the induction of time-of-day-dependent torpor in these mice. Averaged daily Tb declined from 37.3 °C to 36.3 °C during two weeks of KD feeding.
Fig. 1.
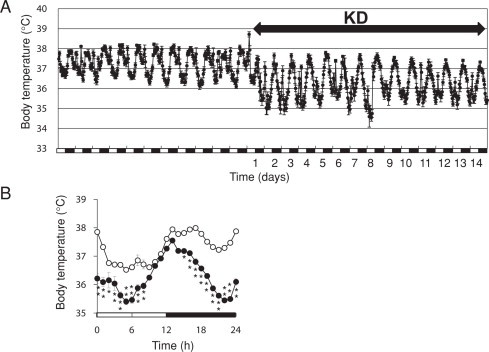
Circadian rhythms of core body temperature (Tb) in mice fed with a ketogenic diet (KD). Mice were fed with normal (ND; unfilled circles) or KD (filled circles) diets under 12 h light–12 h dark cycles (LD 12:12; lights on at 0 h). (A) Continuous Tb change during the experiment. (B) Averaged circadian rhythms of Tb under ND and KD feeding. Data are averaged Tb values before (ND; unfilled circles) and after (filled circles) two weeks of KD feeding. Values are shown as means ± SEM (n = 7). Significant differences between ND and KD are indicated as *P < 0.05, **P < 0.01. Horizontal open and solid bars, day and night, respectively.
Expression levels of Cirbp mRNA fluctuated in a circadian manner and peaked during late nighttime in the liver of ND mice (Fig. 2). The abundance of Cirbp mRNA was elevated during late nighttime, which corresponded to daily torpor (Fig. 1). On the other hand, levels of Cirbp mRNA expression were continuously elevated in the heart (Supplemental Fig. 1), although the relative abundance of basal Cirbp expression was 5.2-fold higher in the liver than in the heart (data not shown).
Fig. 2.
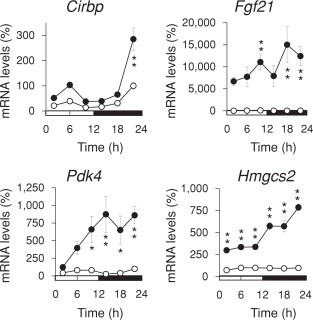
Temporal mRNA expression profiles of Cirbp and ketogenesis-related genes in the KD mouse liver. Mice were fed with normal (ND; unfilled circles) or ketogenic (KD filled circles) diets for 14 days under LD 12:12 (lights on at 0 h) and then total RNA was extracted from livers after sacrifice. Messenger RNA levels were measured by quantitative RT-PCR. Maximal value for ND mice is expressed as 100%. Values are shown as means ± SEM (n = 4). Significant differences between groups are indicated as *P < 0.05, **P < 0.01. Horizontal open and solid bars, day and night, respectively.
Expression levels of Fgf21 (a transcription target of peroxisome proliferator-activated receptor α (PPARα) that plays an important role in adaptation to fasting and starvation [11]), Pdk4 (a transcription target of PPARα that suppresses glucose oxidation to increase fatty acid utilization), and Hmgcs2 (that encodes the rate-limiting enzyme in ketogenesis) were obviously induced in the liver of KD mice (Fig. 2). Notably, the KD-induced expression of these genes and Cirbp was considerably enhanced during the nighttime.
The MESOR of Bmal1, Clock, Per3, and Per2 expression and of the amplitude of Bmal1 and Per3 expression were significantly increased in the liver of KD mice (Fig. 3 and Table 1). The KD advanced the acrophase of circadian gene expression by 3.6, 3.8, 3.8, 7.3 and 3.9 h for Bmal1, Clock, Per3, Per2, Dbp, respectively, in the liver.
Fig. 3.
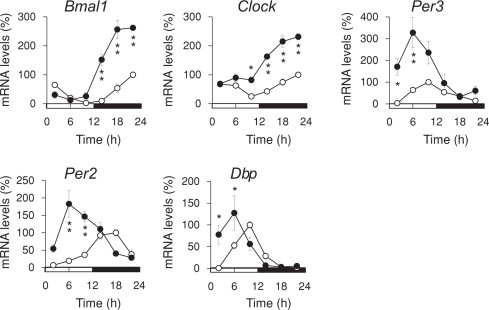
Temporal mRNA expression profiles of circadian clock-related genes in the liver of mice fed with a ketogenic diet (KD). Mice were fed with normal (ND; unfilled circles) or KD (filled circles) diets for 14 days under LD 12:12 (lights on at 0 h) and then total RNA was extracted from livers after sacrifice. Messenger RNA levels were measured by quantitative RT-PCR. Maximal value for ND mice is expressed as 100%. Values are shown as means ± SEM (n = 4). Significant differences between groups are indicated as *P < 0.05, **P < 0.01. Horizontal open and solid bars, day and night, respectively.
Table 1.
Cosinor analysis of Bmal1, Clock, Per3, Per2, and Dbp mRNA expression in the liver of mice fed with a ketogenic diet.
| MESOR | Amplitude | Acrophase (h) | ||
|---|---|---|---|---|
| Bmal1 | ND | 41.8 ± 3.9 | 47.8 ± 5.6 | 22.5 ± 0.44 |
| KD | 123.4 ± 17.2** | 144.7 ± 24.3** | 18.9 ± 0.64** | |
| Clock | ND | 62.1 ± 4.9 | 31.2 ± 6.9 | 22.5 ± 0.85 |
| KD | 141.7 ± 15.5** | 83.9 ± 21.9 | 18.7 ± 1.0* | |
| Per3 | ND | 45.2 ± 7.1 | 42.2 ± 10.0 | 10.6 ± 0.90 |
| KD | 153.9 ± 12.0** | 143.2 ± 17.0** | 6.8 ± 0.45** | |
| Per2 | ND | 48.8 ± 5.3 | 48.1 ± 7.6 | 16.0 ± 0.60 |
| KD | 93.8 ± 10.7** | 76.8 ± 15.2 | 8.7 ± 0.75** | |
| Dbp | ND | 31.1 ± 8.9 | 46.1 ± 12.5 | 9.5 ± 1.0 |
| KD | 46.1 ± 8.3 | 62.0 ± 11.8 | 5.6 ± 0.73* | |
KD, ketogenic diet; ND, normal diet. MESOR, mean statistics of rhythm; amplitude, one-half the total peak-trough variation; acrophase, delay from 0:00 (lights on). Data are shown as means ± SEM (n = 4). Significant differences compared with ND value are indicated as *P < 0.05, **P < 0.01.
The MESOR of circadian mRNA expression in the heart was essentially unaffected by KD except for the Per2 gene (Supplemental Fig. 1 and Table 2). The amplitude of the circadian mRNA expression of Bmal1, Clock, Per3, and Per2 was unaffected, whereas that of Dbp was significantly decreased in the hearts of KD mice. On the other hand, the phase-advancing effect in the heart was relatively larger (by 6.2, 7.3, 4.8, 5.6, 6.0 h for Bmal1, Clock, Per3, Per2, Dbp, respectively) than that in the liver.
Food deprivation notably and time-dependently reduced Core Tb (Fig. 4(B)). Fasting decreased Tb particularly at midday and late in the dark period as well as chronic KD administration (Fig. 1). Average daily Tb declined from 37.0 °C to 35.6 °C on the sampling day. Furthermore, hepatic expression levels of Cirbp were significantly increased in accordance with the timing of the Tb decrease (Fig. 4(C)).
Fig. 4.
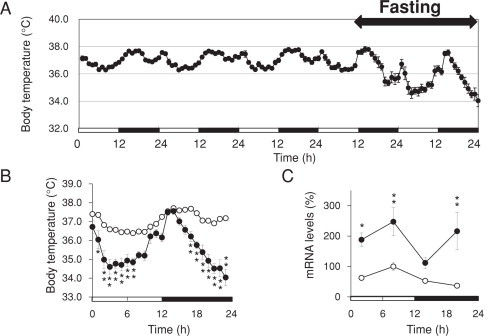
Circadian rhythms of core body temperature (Tb) and hepatic Cirbp expression of fasted mice. Mice were fed (unfilled circles) or fasted (filled circles) under 12 h light–12 h dark cycles (LD 12:12; lights on at 0 h). (A) Continuous Tb change during the experiment. (B) Averaged circadian rhythms of Tb under fed and fasted conditions. Data are averaged Tb values during fed (unfilled circles) and fasted (filled circles). Values are shown as means ± SEM (n = 7). (C) Temporal mRNA expression profiles of Cirbp in the liver of fasted mice. Messenger RNA levels were measured by quantitative RT-PCR. Maximal value for fed mice is expressed as 100%. Values are means ± SEM (n = 4). Significant differences between fed and fasted groups are indicated as *P < 0.05, **P < 0.01. Horizontal unfilled and solid bars, day and night, respectively.
4. Discussion
Mammalian peripheral circadian clocks are self-sustained and cell autonomous, although they are synchronized by the central clock in the SCN. Several circadian genes are expressed in peripheral tissues. Peripheral circadian rhythms are controlled by both local oscillators and systemic cues such as hormones, neural signals, and body temperature [1–3]. Originally identified as a cold stress-induced protein in cultured cells [4], CIRBP was later found to directly interact with circadian RNAs such as Clock, Per3 and Dbp, and to maintain robust (high amplitude) circadian gene expression in vitro [5].
The present study showed that chronic KD administration and fasting induce time-dependent hepatic Cirbp expression with hypothermia in mice. Expression levels of Cirbp that were obviously increased late in the dark period corresponded to daily torpor, suggesting that the KD- and fasting-induced mRNA expression of Cirbp was a direct effect of hypothermia. To the best our knowledge, this is the first study to demonstrate the induction of Cirbp expression in vivo.
The magnitude and amplitude of circadian gene expression were obviously increased in the KD mouse liver. Daily body temperature fluctuations are presently considered as possible systemic cues for resetting peripheral clocks [12]. Hypothermia-induced CIRBP expression might be responsible for the KD-induced robustness of peripheral oscillators in the liver, because the depletion of CIRBP by siRNA results in a reduction in the amplitude of circadian gene expression in fibroblasts [5]. The expression of CLOCK is extremely reduced in cells depleted of CIRBP, indicating that CIRBP positively regulates CLOCK [5]. Temperature-dependent peripheral clock regulation might be mediated by CIRBP through the induction of Clock gene expression, because CLOCK completely rescues disrupted circadian gene expression in CIRBP-depleted fibroblasts [5].
On the other hand, the magnitude and amplitude of circadian gene expression remained essentially unaffected in the hearts of KD mice, although Cirbp expression levels were significantly increased in both tissues. Systemic signals tissue-dependently regulate peripheral oscillators. Non-neuronal signals are sufficient to generate peripheral rhythms in the liver, whereas neural signals are critical for generating circadian rhythms in the heart [13]. Oscillators in the liver might be more sensitive to changes in Tb than those in the heart.
The phase of circadian gene expression was obviously advanced both in the KD mouse liver and heart as described [8,9]. We demonstrated the phase-advancing effects of a KD on the biological clock that governs rhythmic behavioral activity as well as the rhythmic expression of circadian genes in mouse peripheral tissues [8,9]. Therefore, chronic KD feeding seems to affect the circadian phase of the central nervous systems. Several studies have indicated that caloric restriction or fasting induces a phase-advance and enlarges the amplitude of various circadian rhythms such as locomotor activity, Tb and circadian gene expression [14,15]. Fasting or caloric restriction induces time-dependent hypothermia in mammals [16,17]. Hypothermia-induced CIRBP expression might be involved in the phase-advancing effect of KD or fasting on circadian clocks.
The present study showed that KD and fasting induce time-dependent hepatic Cirbp expression accompanied by hypothermia in mice. Further study is required to elucidate direct interactions between hypothermia-induced CIRBP expression and circadian clocks in vivo.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplementary material
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.fob.2013.03.005.
Appendix. Supplementary materials
Supplementary materials for Ketogenic diet and fasting induce the expression of cold-inducible RNA-binding protein with time-dependent hypothermia in the mouse liver.
References
- 1.Reppert S.M., Weaver D.R. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi J.S., Hong H.K., Ko C.H, McDearmon E.L. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kornmann B., Schaad O., Bujard H., Takahashi J.S., Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 2007;5:e34. doi: 10.1371/journal.pbio.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishiyama H., Itoh K., Kaneko Y., Kishishita M., Yoshida O., Fujita J. A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth. J. Cell Biol. 1997;137:899–908. doi: 10.1083/jcb.137.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morf J., Rey G., Schneider K., Stratmann M., Fujita J., Naef F., Schibler U. Cold-inducible RNA-binding protein modulates circadian gene expression posttranscriptionally. Science. 2012;338:379–383. doi: 10.1126/science.1217726. [DOI] [PubMed] [Google Scholar]
- 6.Astrup A., Meinert Larsen T., Harper A. Atkins and other low-carbohydrate diets: hoax or an effective tool for weight loss? Lancet. 2004;364:897–899. doi: 10.1016/S0140-6736(04)16986-9. [DOI] [PubMed] [Google Scholar]
- 7.Oishi K., Sakamoto K., Konishi M., Murata Y., Itoh N., Sei H. FGF21 is dispensable for hypothermia induced by fasting in mice. Neuro Endocrinol. Lett. 2010;31:198–202. [PubMed] [Google Scholar]
- 8.Oishi K., Uchida D., Ohkura N., Doi R., Ishida N., Kadota K., Horie S. Ketogenic diet disrupts the circadian clock and increases hypofibrinolytic risk by inducing expression of plasminogen activator inhibitor-1. Arterioscler. Thromb. Vasc. Biol. 2009;29:1571–1577. doi: 10.1161/ATVBAHA.109.190140. [DOI] [PubMed] [Google Scholar]
- 9.Oishi K., Uchida D., Ohkura N., Horie S. PPARalpha deficiency augments a ketogenic diet-induced circadian PAI-1 expression possibly through PPARgamma activation in the liver. Biochem. Biophys. Res. Commun. 2010;401:313–318. doi: 10.1016/j.bbrc.2010.09.060. [DOI] [PubMed] [Google Scholar]
- 10.Ohkura N. Circadian variations in coagulation and fibrinolytic factors among four different strains of mice. Chronobiol. Int. 2007;24:651–669. doi: 10.1080/07420520701534673. [DOI] [PubMed] [Google Scholar]
- 11.Cuevas-Ramos D., Aguilar-Salinas C.A., Gomez-Perez F.J. Metabolic actions of fibroblast growth factor 21. Curr. Opin. Pediatr. 2012;24:523–529. doi: 10.1097/MOP.0b013e3283557d22. [DOI] [PubMed] [Google Scholar]
- 12.Saini C., Morf J., Stratmann M., Gos P., Schibler U. Simulated body temperature rhythms reveal the phase-shifting behavior and plasticity of mammalian circadian oscillators. Genes Dev. 2012;26:567–580. doi: 10.1101/gad.183251.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo H., Brewer J.M., Champhekar A., Harris R.B, Bittman E.L. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc. Natl. Acad. Sci. USA. 2005;102:3111–3116. doi: 10.1073/pnas.0409734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Challet E. Interactions between light, mealtime and calorie restriction to control daily timing in mammals. J. Comp. Physiol. B. 2010;180:631–644. doi: 10.1007/s00360-010-0451-4. [DOI] [PubMed] [Google Scholar]
- 15.Froy O., Chapnik N., Miskin R. Effect of intermittent fasting on circadian rhythms in mice depends on feeding time. Mech. Ageing Dev. 2009;130:154–160. doi: 10.1016/j.mad.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Overton J.M., Williams T.D. Behavioral and physiologic responses to caloric restriction in mice. Physiol. Behav. 2004;81:749–754. doi: 10.1016/j.physbeh.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Tokizawa K., Uchida Y., Nagashima K. Thermoregulation in the cold changes depending on the time of day and feeding condition: physiological and anatomical analyses of involved circadian mechanisms. Neuroscience. 2009;164:1377–1386. doi: 10.1016/j.neuroscience.2009.08.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials for Ketogenic diet and fasting induce the expression of cold-inducible RNA-binding protein with time-dependent hypothermia in the mouse liver.


