Abstract
This study evaluated the effect of gamma irradiation on the reduction of the toxicity of mistletoe lectin using both in vitro and in vivo models. To extract the lectin from mistletoe, an (NH4)2SO4 precipitation method was employed and the precipitant purified using a Sepharose 4B column to obtain the pure lectin fraction. Purified lectin was then gamma-irradiated at doses of 0, 5, 10, 15, and 20 kGy, or heated at 100 °C for 30 min. Toxic effects of non-irradiated, irradiated, and heat-treated lectins were tested using hemagglutination assays, cytotoxicity assays, hepatotoxicity, and a mouse survival test and immunological response was tested using cytokine production activity. Hemagglutination of lectin was remarkably decreased (P < 0.05) by irradiation at doses exceeding 10 kGy and with heat treatment. However, lectin irradiated with 5 kGy maintained its hemagglutination activity. The cytotoxicity of lectin was decreased by irradiation at doses over 5 kGy and with heat treatment. In experiments using mouse model, glutamate oxaloacetate transaminase (GOT) and glutamic pyruvic transaminase (GPT) levels were decreased in the group treated with the 5 kGy irradiated and heat-treated lectins as compared to the intact lectin, and it was also shown that 5 kGy irradiated and heat-treated lectins did not cause damage in liver tissue or mortality. In the result of immunological response, tumor necrosis factor (TNF-α) and interleukin (IL-6) levels were significantly (P < 0.05) increased in the 5 kGy gamma-irradiated lectin treated group. These results indicate that 5 kGy irradiated lectin still maintained the immunological response with reduction of toxicity. Therefore, gamma-irradiation may be an effective method for reducing the toxicity of lectin maintaining the immune response.
Keywords: Mistletoe lectin, Toxicity, Gamma irradiation, Immune response, Cytotoxicity, Cytokine
Highlights
▸ Cytotoxicity of lectin against immune cells was decreased by gamma-irradiation at doses above 5 kGy. ▸ The liver toxicity of gamma-irradiated lectin (5 kGy) was decreased, as shown by levels of GOT and GPT. ▸ Gamma-irradiated lectin (5 kGy) was not lethal for mice. ▸ Gamma-irradiated lectin (5 kGy) still maintained immunological responses.
1. Introduction
Cancer is one of the leading causes of death worldwide. Recently, many anticancer reagents have been developed to solve this severe problem. However, most of chemicals have side effects such as immune system damage, normal cell toxicity, hepatotoxicity, induction of heart disease. Therefore, it is very important to investigate novel antitumor drugs that offer improved immune potential without harming the host [1].
Mistletoe (Viscum album), a common evergreen semi-parasite in trees, has been used for various medicinal purposes from ancient times [2,3]. Various mistletoe preparations such as capsules and tea have been used as subsidiary treatments for cancer, cardiovascular diseases, arthritis, hemorrhage, pleurisy, gout, and heart disease [4,5]. In Europe especially, one commercial product using mistletoe is a form of complementary medicine used as an adjuvant therapy in cancer patients to stimulate the innate immune system via activation of macrophage/monocytes with stimulation of cytokines and nitric oxide for the purpose of improving quality of life [6,7]. Mistletoe contains various bioactive components such as lectin [8], alkaloids [9], viscotoxins [10], and polysaccharides [11,12], and among them the most active anticancer compound is identified as a lectin [13,14]. However, the use of mistletoe in humans has been limited due to its toxicity [15]. Thus, the development of technology to reduce this toxicity is necessary for the increased application of lectin for health purposes.
Recently several studies have reported that heat treatment effectively reduced the toxicity of mistletoe lectin. However, heat treatment also diminished the bioactivity of mistletoe lectin due to denaturation of the protein [16]. Therefore a novel method is required to reduce the toxicity of mistletoe lectin without compromising its bioactivity.
Gamma irradiation induces a change in both structural and physiological properties of some molecules, which can result in improved quality or bioactivity of the component [17]. In previous studies, gamma irradiation was used to reduce the toxicity of lipopolysaccharide [18–20], and to diminish the allergens in eggs, milk, and shrimp [21–24]. In addition, it has been demonstrated that gamma-irradiated doxorubicin used as an anti-cancer drug significantly reduced immunotoxicity without changes in the anti-cancer activity in both in vitro and in vivo models [25].
Therefore, the objective of this study was to evaluate the effect of gamma irradiation on reducing the toxicity of mistletoe lectin and immunomodulatory activities.
2. Materials and methods
2.1. Preparation of mistletoe and isolation of the lectin
Fresh mistletoe grown on oak trees was collected in January 2007 from Naejang mountain in Jeollabuk-Do, Republic of Korea, and stored at −70 °C until used. To prepare the mistletoe extract, mistletoe (100 g) was homogenized in 1 L of saline solution (0.85% NaCl) followed by centrifugation (20,000 g, 4 °C, 20 min). The supernatant was filtered through a 0.45 μm filter (Millipore Corp., Billerica, MA, USA). (NH4)2SO4 was then added to the extract up to 70% saturation, and this mixture was centrifuged at 20,000 g for 20 min. The precipitate from the centrifugation was resuspended in 20 mL of phosphate buffered saline (PBS, InvitrogenTM, Carlsbad, CA, USA), and dialyzed in PBS for 5 days.
A Sepharose 4B column (Sigma, St. Louis, MO, USA) was hydrolyzed with 0.2 N HCl for 2.5 h in a 50 °C water bath and then washed with PBS, and the dialyzed sample was passed through the hydrolyzed Sepharose column, followed by elution using a lactose-containing buffer (0.1 M lactose and 10 mM sodium phosphate in 0.14 M NaCl, pH 7.3) to obtain potential lectin fractions. The fractions were examined using a blood agglutination test to determine lectin-containing fractions, and the lectin-containing fractions were dialyzed using a membrane (molecular weight cut-off of 10,000 Da; Spectrum Laboratories Inc., Rancho Dominguez, CA, USA) in distilled water. The dialyzed fractions were then freeze-dried for use in this study.
2.2. Gamma-irradiation and heat-treatment
Lectin samples were dissolved in PBS to obtain a concentration of 1 mg/mL (w/v), and the samples were irradiated at doses of 5, 10, 15, and 20 kGy. The heat treatment was carried out by heating the samples in boiling water (100 °C) for 30 min [15]. Irradiation was conducted using a cobalt-60 irradiator (point source AECL, IR-221, MDS Nordion International Co. Ltd., Ottawa, ON, Canada) with 11.1 peta-becquerel (PBq) source strength. Dosimetry was performed using an alanine dosimeter with a 5-mm diameter (Bruker Instruments, Rheinstetten, Germany). The dosimeter was calibrated using an International Atomic Energy Agency (Vienna, Austria) standard.
2.3. Experimental animals
BALB/c mice (7 weeks old, 18–20 g body weight (BW)) were purchased from Orient Bio (Seongnam-si, Gyeonggi-Do, Republic of Korea). The mice were placed in a polycarbonate cage, and a standard animal diet and water ad libitum were provided to mice under controlled conditions (22 ± 2 °C, 60% humidity) with 12 h light and dark cycles. All animals received proper care according to methods approved under institutional guidelines and experiments were conducted according to principles enunciated in the Animal Care Act prepared by the Ministry of Agriculture and Forestry, Republic of Korea.
2.4. Hemagglutination assay
Hemagglutination assays were performed as previously described by Kang et al. [26]. Briefly, erythrocyte was separated from the BALB/c mouse and then diluted in a 10 mM sodium–phosphate buffer (pH 7.4) to a concentration of 2% mouse erythrocytes. Various concentrations (0, 3.125, 6.25, 12.5, 25, 50, 100, and 200 μg/mL) of intact, gamma-irradiated, and heat-treated lectin samples were then mixed with 2% mouse erythrocytes at a 1:1 ratio to obtain 0, 1.562, 3.125, 6.25, 12.5, 25, 50, and 100 μg/mL of final lectin concentrations. After gentle mixing, the mixtures were placed at room temperature for 1 h, and agglutination of samples was observed.
2.5. Cytotoxicity and cytokine assay
RAW 264.7 macrophage cells (Korean Cell Line Bank No. 40071) grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 100 units/mL penicillin (InvitrogenTM, CA, USA), 100 μg/mL streptomycin (InvitrogenTM, Carlsbad, CA, USA), and 10% fetal bovine serum (FBS) were incubated in a 5% CO2 incubator at 37 °C for 48 h. Cells (5 × 104 cells/well) were inoculated into 96-well plates containing 90 μL of DMEM per well and incubated at 37 °C in a 5% CO2 incubator for 4 h. Various levels (0, 0.25, 1, and 2 μg/mL) of intact, gamma-irradiated, and heat-treated lectins were inoculated into each well, followed by further incubation at 37 °C in a 5% CO2 incubator for 24 h, with PBS and lipopolysaccharide (LPS) added to wells as negative and positive controls, respectively. Cell viability was then measured according to the XTT method as described by Goodwin et al. [27], and the upper layer of the cell cultures was used to evaluate cytokine levels (IL-6 and TNF-α), using a BD OptEIATM cytokine detection kit (BD Biosciences, San Jose, CA, USA) according to the manufacturer's instructions.
2.6. Measurement of liver function index enzymes
Animals (5 per treatment) were intravenously injected with (i) PBS alone, various concentrations (25, 50, 100, and 250 μg/g BW) of (ii) intact lectin (0 kGy), (iii) 5 kGy gamma-irradiated lectin, or (iv) heat-treated lectin. After 1 h, animals were anesthetized under ether and sacrificed by cervical decapitation, and blood samples taken from the experimental animals and centrifuged at 1500g for 10 min to obtain clear serum. Serum was then analyzed using a Hitachi 7180 (Hitachi Medico, Tokyo, Japan) equipped with user interface operability features to determine GOT and GTP levels. Wako control serum (Wako, Richmond, VA, USA) was used as a marker. Liver tissue samples were also removed for histopathological analysis.
2.7. Histopathological analysis
Liver tissues were separated from the PBS, intact lectin (0 kGy), 5 kGy gamma-irradiated lectin, and heat-treated lectin treated mice after 1 h of intravenous injection (50 μg/kg BW) and washed with a cold saline solution (0.85% NaCl), diced into small pieces (5 × 5 × 5 mm), and fixed immediately in a solution of 10% formalin for 48 h. Small pieces were sectioned at 5 μm thickness after dehydration and paraffin embedding and stained with haematoxylin and eosin (H&E). The severity of liver tissue damage was observed under a Nikon Eclipse e400 microscope (Nikon Corporation, Kanagawa, Japan) and imaged using Focus Pro software for the Windows XP platform.
2.8. Survival test
To measure mouse survival, animals were divided into 5 groups (n = 10) and intravenously injected with (i) PBS alone, various concentrations (25, 50, 100, and 250 μg/kg BW) of (ii) intact lectin (0 kGy), (iii) 5 kGy gamma-irradiated lectin, or (iv) heat-treated lectin; 10 animals were subjected to each treatment. Mouse survival was then counted for 7 days.
2.9. Statistical analysis
Data were analyzed with a two-tailed Student's t-test using the Statistical Package for the Social Science software (SPSS Inc., 10.0, 2000). Means among treatments were compared using a Duncan multiple comparison test at P < 0.05.
3. Results
3.1. Isolation of lectin from mistletoe
Fig. 1(A) shows the elution profile of mistletoe lectin from the Sepharose 4B column in the presence of 100 mM lactose. In total, we obtained 30 fractions with the possibility of containing the lectin component. Among these fractions, the optical density at 280 nm was increased in fraction numbers 10–11 and the highest optical density was found in fraction number 10. Next, we used the hemagglutination assay to confirm the presence of lectin in the separated fractions (Fig. 1(B)). As shown in Fig. 1(B), the 10th and 11th fractions show the strongest hemagglutination activity. Thus, in subsequent examinations these fractions (fractions 10th and 11th) were collected and were used for further experiments.
Fig. 1.
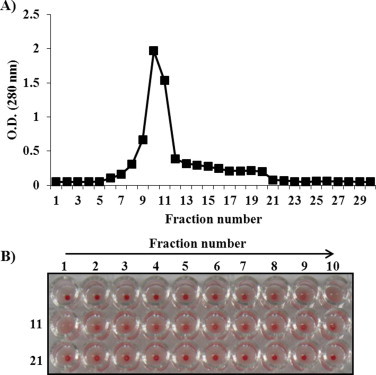
Identification of mistletoe lectin by using Sephrose 4B column (A) and hemagglutination assay (B). Erythrocytes were separated from the BALB/c mice and then diluted in a 10 mM sodium–phosphate buffer (pH 7.4) to a concentration of 2% mouse erythrocytes. Separated fractions were mixed with 2% mouse erythrocytes at a 1:1 ratio. Agglutination of lectin samples was observed after 1 h.
3.2. Hemagglutination of gamma-irradiated and heat-treated lectin
Fig. 2 shows that intact and 5 kGy gamma-irradiated lectin samples at a concentration more than 6.2 μg/mL did cause agglutination of erythrocytes, but gamma-irradiated lectin at a dose of more than 10 kGy or heat-treated lectin did not cause agglutination of erythrocytes. This result indicates that 5 kGy irradiated lectin may sustain its hemagglutination activity due to the presence of the B-chain, which can bind to the erythrocyte membrane. Thus, in this study, our next investigation looked at cytokine production of gamma-irradiated (5 kGy) and non-irradiated lectin to determine whether the immunological response was still present.
Fig. 2.
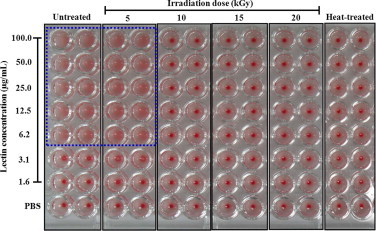
Hemagglutination activity of gamma-irradiated (5, 10, 15, and 20 kGy), and non-irradiated (intact; 0 kGy) and heat-treated lectin to erythrocytes. Erythrocytes were separated from the BALB/c mice and then diluted in a 10 mM sodium–phosphate buffer (pH 7.4) to a concentration of 2% mouse erythrocytes. Various concentrations (0, 3.125, 6.25, 12.5, 25, 50, 100, and 200 μg/mL) of intact, gamma-irradiated, and heat-treated lectin samples were then mixed with 2% mouse erythrocytes at a 1:1 ratio to obtain 0, 1.562, 3.125, 6.25, 12.5, 25, 50, and 100 μg/mL final lectin concentrations. Agglutination of lectin samples was observed after 1 h.
3.3. Changes in cytotoxicity and cytokine production
The survival ratio of RAW 264.7 macrophage cells with added intact lectin (0.25, 0.5, 1, and 2 μg/mL) decreased as the lectin concentration increased (Fig. 3). The highest cytotoxic effect appeared at a concentration of 2 μg/mL (38 ± 7.97), but 5 kGy gamma-irradiated (104 ± 11.5) and heat-treated (97 ± 8.9) lectin did not cause cell death (Fig. 3). This result indicated that lectin-induced cytotoxicity in macrophages was decreased by gamma-irradiation and heat treatment. The cytokine production of macrophage cell treated with gamma-irradiated and non-irradiated and heated lectin at the same dose of 2 μg/mL was also carried out and the results are shown in Fig. 4. As the levels of TNF-α (938 ± 186.4) and IL-6 (1830 ± 48.3) treated with LPS were high, the immunological activity of RAW 264.7 cells was shown to be normal. In Fig. 4, cytokine production was decreased in the intact (non-irradiated) lectin-treated group (TNF-α; 134 ± 98.7, IL-6; 98 ± 11.5) due to cell death. The levels of TNF-α and IL-6 were higher (P < 0.05) in the 5 kGy gamma-irradiated lectin-treated group (TNF-α; 632 ± 13.2, IL-6; 1193 ± 25.3) than in the heated lectin-treated group (TNF-α; 275 ± 57.3, IL-6; 179 ± 24.8) (Fig. 4). This result suggests that the immunological activity can be sustained at the 5 kGy gamma-irradiated lectin, even though the heated lectin did not maintain immunological activity.
Fig. 3.
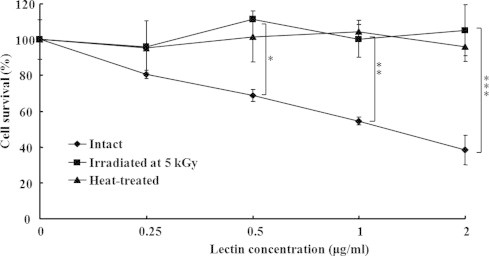
Cell survival of intact (0 kGy), irradiated (5 kGy), and heated lectin treated macrophage cells line (RAW 264.7). Each sample was treated at the concentrations of 0.25, 0.5, 1, and 2 μg/mL, respectively. Cell survival percentage was measured by MTT assay. Results are expressed as the mean ± S.D. Statistical analysis was performed using Student's two tails t-test with significant level of *P < 0.05, **P < 0.01, ***P < 0.001 compared to intact (0 kGy).
Fig. 4.
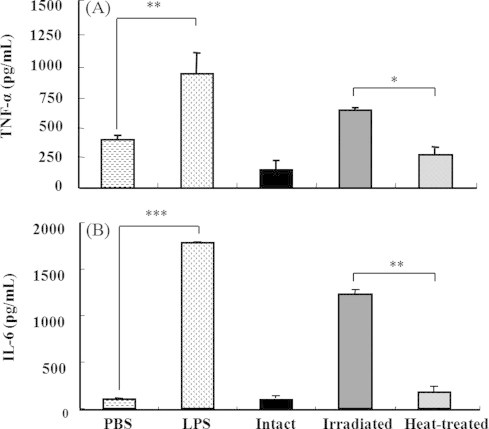
Cytokine productions (TNF-α; (A) and IL-6; (B)) of intact (0 kGy), irradiated (5 kGy), and heated lectin treated macrophage cells line (RAW 264.7). Each sample was treated at the concentration of 2 μg/mL. Results are expressed as the mean ± S.D. Statistical analysis was performed using Student's two tails t-test with significant level of *P < 0.05, **P < 0.01, ***P < 0.01 compared to intact (0 kGy). PBS: phosphate buffered saline, LPS: lipopolysaccharide.
3.4. Effect of gamma-irradiated lectin on liver tissue damage
GOT and GTP levels in the sera of mice treated with intact lectin (50 μg/kg BW) were higher (P < 0.05) than those of mice treated with PBS alone (Fig. 5). However, GOT and GTP levels could not be measured in the liver of mice treated with intact lectin at a high dose of 100 and 250 μg/kg BW because the administration of these higher concentrations of lectin was lethal (Fig. 5). However, the levels of GOT and GTP were not increased in mice treated with 5 kGy gamma-irradiated and heat-treated lectin, compared to the intact lectin treated group. Fig. 6 shows the morphology of liver tissue in mice treated with intact, 5 kGy gamma-irradiated, and heat-treated lectin after 1 h of intravenous injection (50 μg/kg BW). In this research, intact lectin induced hepatocyte damage and cytoplasmic vacuolization, and caused severe damage to the hepatic artery and bile duct (Fig. 6(B)). However, 5 kGy gamma-irradiated lectin attenuated the liver tissue damages (Fig. 6(C)). A similar effect was also shown in the group of heat-treated lectin (Fig. 6(D)). Therefore, our finding indicated that 5 kGy gamma-irradiated lectin highly reduced the acute liver toxicity.
Fig. 5.
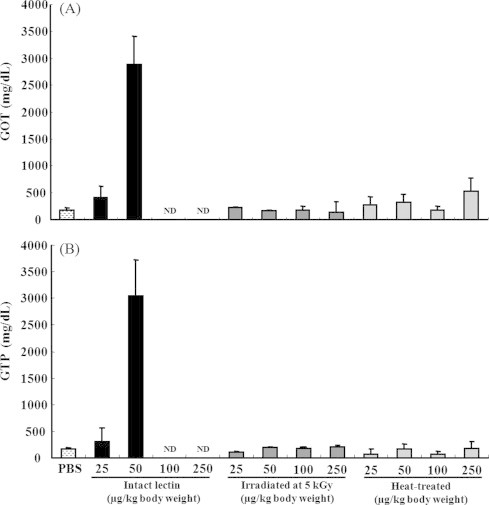
Levels of GOT (glutamic-oxaloacetic transaminase) (A) and GPT (glutamic-pyruvate transaminase) (B) in blood sera. Mice were intravenously injected with PBS (alone), various concentrations (25, 50, 100, and 250 μg/kg BW) of intact lectin (0 kGy), 5 kGy gamma-irradiated lectin, or heat-treated lectin. After 1 h, blood samples were taken from the experimental animals and centrifuged at 1500 g for 10 min to obtain clear serum. Serum was then analyzed using a Hitachi 7180 equipped with user interface operability features to determine GOT and GTP levels. Wako control serum was used as a marker. Results are expressed as the mean ± S.D. ND: not detected.
Fig. 6.
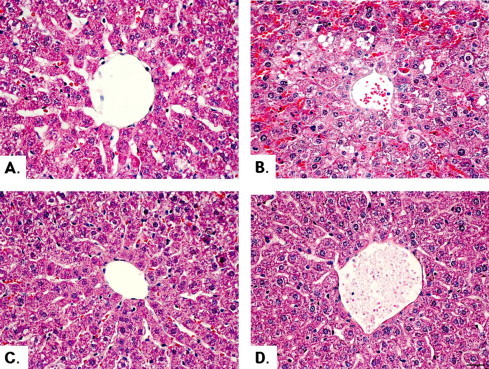
Microscopic picture of liver tissue from mice subjected to phosphate buffered saline (PBS) (A), intact lectin (B), 5 kGy irradiated lectin (C), and heat-treated lectin (D). Liver tissues were separated from the PBS, intact lectin (0 kGy), 5 kGy gamma-irradiated lectin, and heat-treated lectin treated mice after 1 h of intravenous injection (50 μg/kg BW) and washed with a cold saline solution (0.85% NaCl), and fixed immediately in a solution of 10% formalin for 48 h. Small pieces were sectioned at 5 μm thickness after dehydration and paraffin embedding and stained with haematoxylin and eosin (H&E). The severity of liver tissue damage was observed under a Nikon Eclipse e400 microscope.
3.5. Mortality of mice treated with lectin
Based on the above study of reduced toxicity (Figs. 1–6), the mortality of mice treated with intact, 5 kGy gamma-irradiated lectin, and heat-treated lectin was investigated. All mice treated with intact lectin at a dose of 25 μg/kg BW survived. However, 6 of 10 mice injected with 50 μg/kg BW of intact lectin died and no survival was observed in mice treated with intact lectin at 100 and 250 μg/kg BW (Table 1). Meanwhile, mice treated with 5 kGy irradiated and heat-treated lectins all survived at the tested doses (20, 50, 100 and 250 μg/kg BW, Table 1). Accordingly, the LD50 of intact lectin is between 50 and 100 μg/kg BW and this LD50 is in agreement with previous results [28]. These results indicate that a 5 kGy irradiation effectively reduced mouse mortality by lectin.
Table 1.
Survivals of mice administrated with phosphate buffered saline (PBS), intact (non-irradiated, 0 kGy), 5 kGy gamma-irradiated, and heat-treated mistletoe lectin.
| Treatment | Dose (μg/kg body weight) | Days after treatment |
Total mortalityb (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| PBS | 0/10a | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0 | |
| Intact lectin | 25 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0 |
| 50 | 0/10 | 4/10 | 6/10 | 6/10 | 6/10 | 6/10 | 6/10 | 40 | |
| 100 | 5/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 100 | |
| 250 | 8/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 100 | |
| 5 kGy irradiated lectin | 25 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0 |
| 50 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0 | |
| 100 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0 | |
| 250 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0 | |
| Heat-treated lectin | 25 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0 |
| 50 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0 | |
| 100 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0 | |
| 250 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0 | |
Data represent the number of dead mice to total number of tested mice (n = 10).
The count of survival mice in the seven days after lectin injection was calculated to represent the total mortality (%).
4. Discussion
The objective of this study was to evaluate the effect of gamma irradiation on reduction of mistletoe lectin toxicity using both in vitro and in vivo models. Mistletoe lectin was extracted and purified by Sepharose 4B column, which has the affinity to lectin (Fig. 1). Mistletoe lectin was gamma-irradiated at various doses (0, 5, 10, 15, and 20 kGy), or heated for reduction of toxicity. First, we evaluated the binding activity of the lectin to erythrocytes using a hemagglutination assay [29]. This assay is based on the binding activity between the B-chain in lectin and cell surface carbohydrates in erythrocytes [30]. Intact and 5 kGy gamma-irradiated lectin (≥6.2 μg/mL) did agglutinate to erythrocytes, but lectin irradiated over 10 kGy and heat-treated lectin did not cause agglutination of erythrocytes (Fig. 2), which suggests that irradiation at 5 kGy may preserve the B-chain of mistletoe lectin.
Mistletoe lectin is a heterodimeric glycoprotein consisting of two subunits (A-chain and B-chain) linked by a disulfide bond. In the lectin, the toxic A-chain possesses a highly specific N-glycosidase activity and modifies the 28S rRNA of the eukaryotic ribosome 60S subunit [31], thus arresting protein synthesis in the cell. The B-chain is a lectin which binds to cell surfaces causing cell agglutination [32]. The B-chain of lectin plays an important immunomodulatory role by stimulation of immune cells through the activation of the iNOS gene [26]. Thus, breakdown of the B-chain in lectin decreases both the immunological activity and the cell surface binding activity. Proflammatory cytokines such as IL-6 and TNF-α secreted from macrophages played a role in activating T cells and rejecting tumor cells [33,34]. Especially, TNF-α, a multifunctional cytokine, is critical for eliciting tumor immunity [35,36]. Thus, IL-6, and TNF-α play a potent role in immune regulation and anti-tumor mechanisms, and have been extensively tested in vitro and in vivo, as well as in clinical trials for the immunotherapy of malignant diseases. In our investigation, cytokine productions (TNF-α and IL-6) for immunomodulatory activity were highly sustained in 5 kGy gamma-irradiated lectin unlike heated-lectin (Fig. 4). Cytokine production suggests that the heat-treated lectin easily destroyed the B-chain as well as the A-chain [12,13,31].
To investigate the effect of gamma irradiation on reduction of the toxicity of lectin, further studies were carried out including cytotoxicity and measurement of liver function index enzymes (GOT, GPT), histopathology of liver tissue (H&E), and a mouse survival test using lectin irradiated at 5 kGy. Heat-treated lectin was used for the control which is already known for reduction of lectin-mediated toxicity. Mistletoe lectin has a strong cytotoxic activity against cancer cells but also has cytotoxicity against immune cells such as macrophages [8]. In this study, the survival ratio of RAW 264.7 macrophage cells treated with the intact (non-irradiated; 0 kGy) lectin decreased as the lectin concentration increased (from 0.25 to 2 μg/mL), but 5 kGy gamma-irradiated and heat-treated lectin did not cause RAW 264.7 cell death in the same concentration range (from 0.25 to 2 μg/mL; Fig. 3).
In the assessment of liver damage by chemicals, the determination of enzyme levels such as GOT and GPT is widely used as a toxic index of liver. Necrosis or membrane damage releases the enzyme into circulation; therefore, it can be measured in serum. High levels of GOT indicate liver damage, such as that due to viral hepatitis as well as cardiac infarction and muscle injury. GPT is more specific to the liver, and is thus a better parameter for detecting liver injury [37]. Elevated levels of serum enzymes are indicative of cellular leakage and loss of functional integrity of cell membrane in liver [38]. In our results, GOT and GPT levels were decreased in the group treated with 5 kGy irradiated and heat-treated lectins as compared to the intact lectin (Fig. 5), and it was also shown that 5 kGy irradiated and heat-treated lectins did not cause damage to the liver tissue (Fig. 6) or mortality (Table 1). Therefore, gamma irradiation and heat treatment could be an effective method for detoxification of mistletoe lectin.
Based on the above results, our finding indicates that both gamma irradiation at a dose of 5 kGy and heat-treatment clearly decreases the toxicity of lectin (immune cell cytotoxicity, liver toxicity, and mice mortality), but the 5 kGy-irradiated lectin still sustains immunological activity by stimulating the immune cells as compared to heat-treated lectin.
5. Conclusion
In conclusion, the present study was conducted to evaluate the effect of gamma irradiation on reducing the toxicity of mistletoe (V. album) lectin. Lectin was irradiated at doses of 5, 10, 15, and 20 kGy, and the toxicity of irradiated and heat-treated lectins was compared to intact lectin (0 kGy) using a hemagglutination assay, cytotoxicity and cytokine assay, measurement of liver function index enzymes, and histopathological analysis. In all toxicity tests, gamma-irradiated and heated lectin decreased the lectin-induced toxicity. However, the 5 kGy gamma-irradiated lectin surprisingly sustained immunological activity. Therefore, gamma irradiation could be considered as a useful technology for the reduction of the toxicity of lectin without compromising its bioactivity.
Acknowledgement
This study was supported by the Basic Research Support Program of the Korea Atomic Energy Research Institute and the Nuclear Research & Development Program of the Korea Science and Engineering Foundation grant funded by the Government of the Republic of Korea.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Yang X., Guo D., Zhang J., Wu M. Characterization and anti-tumor activity of pollen polysaccharide. Int. Immunopharmacol. 2007;7:427–434. doi: 10.1016/j.intimp.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Becker H. Botany of European mistletoe. Oncology. 1986;43:2–7. doi: 10.1159/000226413. [DOI] [PubMed] [Google Scholar]
- 3.Paine L.K., Harrison H.C. Mistletoe: its role in horticulture and human life. Hort-Technology. 1992;2:324–330. [Google Scholar]
- 4.Kanner L. Mistletoe, magic and medicine. Bull. Hist. Med. 1939;7:875–936. [Google Scholar]
- 5.Wagner H., Jordan E., Feil B. Studies on the standardization of mistletoe. Oncology. 1986;43:16–22. doi: 10.1159/000226416. [DOI] [PubMed] [Google Scholar]
- 6.Huber R., Klein R., Berg P.A., Lűdtke R., Werner M. Effect of a lectin- and viscotoxin-rich mistletoe preparation on clinical and hematologic parameters: a placebo-controlled evalution in healthy subjects. J. Altern. Complem. Med. 2002;8:857–866. doi: 10.1089/10755530260511847. [DOI] [PubMed] [Google Scholar]
- 7.Stein G.M., Henn W., V.Laue H.B., Berg P.A. Modulation of the cellular and humoral immune responses of tumor patients by mistletoe therapy. Eur. J. Med. Res. 1998;3:194–202. [PubMed] [Google Scholar]
- 8.Lee R.T., Gabius H.J., Lee Y.C. Ligand binding characteristics of the major mistletoe lectin. J. Biol. Chem. 1992;267:23722–23727. [PubMed] [Google Scholar]
- 9.Khwaja T.A., Manjikian S.P. Characterization of biologically active components of mistletoe. Cancer Res. 1990;31:412–416. [Google Scholar]
- 10.Konopa J., Woynarowski J.M., Lewandowska M. Isolation of viscotoxin, cytotoxic basic polypeptides from mistletoe Viscum album L. Hoppe Seyler's. Z. Physiol. Chem. 1980;361:1525–1533. doi: 10.1515/bchm2.1980.361.2.1525. [DOI] [PubMed] [Google Scholar]
- 11.Jordan E., Wagner H. Structure and properties of polysaccharides from Viscum album (L.) Oncology. 1986;43:16–22. doi: 10.1159/000226414. [DOI] [PubMed] [Google Scholar]
- 12.Mueller E.A., Anderer F.A. Synergistic action of a plant rhamno-galacturonan enhancing antitumor cytotoxicity of humar natural killer and lymphoking-acitivated killer cells: chemical specificity of target cell recognition. Cancer Res. 1990;50:3646–3651. [PubMed] [Google Scholar]
- 13.Hajto T., Hostanska K., Gabius H.J. Modulatory potency of the β-galactoside-specific lectin from mistletoe extract (Iscador) on the host defense system in vivo in rabbits and patients. Cancer Res. 1990;49:4803–4808. [PubMed] [Google Scholar]
- 14.Wu A.M., Clun L.K., Franz H., Pfuller U., Herp A. Carbohydrate specificity of the receptor sites of mistletoe toxic lectin I. Biochim. Biophys. Acta. 1992;1117:232–234. doi: 10.1016/0304-4165(92)90084-8. [DOI] [PubMed] [Google Scholar]
- 15.Van Wely M., Stoss M., Gorter R.W. Toxicity of a standardized mistletoe extract in immunocompromised and healthy individuals. Am. J. Therapeut. 1999;6:37–43. doi: 10.1097/00045391-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Park J.H., Hyun C.K., Shin H.K., Yeo I.H. Effects of heat-treatment, sugar addition and fermentation on cytotoxicity of Korean mistletoe. Kor. J. Food Sci. Technol. 1997;29:362–368. [Google Scholar]
- 17.Lee S.L., Lee M.S., Song K.B. Effect of gamma-irradiation on the physicochemical properties of gluten films. Food Chem. 2005;92:621–625. [Google Scholar]
- 18.Bertok L. Radio-detoxified endotoxin activates natural immunity: a review. Pathophysiology. 2005;12:85–95. doi: 10.1016/j.pathophys.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Nerkar D.P., Bandekar J.R. Stimulation of macrophages and antitumor activity of radiodetoxified endotoxin. Microbiol. Immunol. 1986;30:893–897. doi: 10.1111/j.1348-0421.1986.tb03016.x. [DOI] [PubMed] [Google Scholar]
- 20.Previte J.J., Chang Y., El-Bisi H.M. Detoxification of Salmonella typhimurium lipopolysaccharide by ionizing radiation. J. Bacteriol. 1967;93:1607–1612. doi: 10.1128/jb.93.5.1607-1614.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Byun M.W., Lee J.W., Yook H.S., Jo C., Kim H.J. Application of gamma irradiation for inhibition of food allergy. Radiat. Phys. Chem. 2002;63:369–370. [Google Scholar]
- 22.Jeon G.R., Lee J.W., Byun M.W., Lee S.Y. Reduced allergenicities of irradiated egg white ovalbumin determined by skin prick test and ELSA inhibition test. Kor. J. Asthma Allergy Clin. Immunol. 2002;22:711–719. [Google Scholar]
- 23.Lee J.W., Kim J.W., Yook H.S., Kang K.O., Lee S.Y., Hwang H.J., Byun M.W. Effects of gamma radiation on the allergenicity and antigenicity properties of milk proteins. J. Food Protect. 2001;64:272–276. doi: 10.4315/0362-028x-64.2.272. [DOI] [PubMed] [Google Scholar]
- 24.Seo J.H., Lee J.W., Kim J.H. Reduction of allergenicity of irradiated ovalbumin in ovalbumin-allergic mice. Radiat. Phys. Chem. 2007;76:1855–1857. [Google Scholar]
- 25.Kim J.H., Sung N.Y., Raghavendran H.B., Yoon Y.H., Song B.S., Choi J.I., Yoo Y.C., Byun M.W., Hwang Y.J., Lee J.W. Gamma irradiation reduces the immunological toxicity of doxorubicin, anticancer drug. Radiat. Phys. Chem. 2009;78:425–428. [Google Scholar]
- 26.Kang T.B., Yoo Y.C., Lee K.H., Yoon H.S., Her E., Kim J.B., Song S.K. Korean mistletoe lectin (KML-IIU) and its subchains induce nitric oxide (NO) production in murine macrophage cells. J. Biomed. Sci. 2008;15:197–204. doi: 10.1007/s11373-007-9210-2. [DOI] [PubMed] [Google Scholar]
- 27.Goodwin C.J., Holt S.J., Downes S., Marshall N.J. Microculture tetrazolium assays: a comparison between two new tetrazolium salts, XTT and MTS. J. Immunol. Meth. 1995;179:95–103. doi: 10.1016/0022-1759(94)00277-4. [DOI] [PubMed] [Google Scholar]
- 28.Kang T.B., Yoo Y.C., Kim J.B., Song S.K, Lee K.H., Kwak J.H. Preliminary toxicity and general pharmacology of KML-IIU, a purified lectin from Korean mistletoe (Viscum album coloratum) Pharmaceut. Soc. Kor. 2001;45:251–257. [Google Scholar]
- 29.Etzler M.E. Distribution and function of plant lectin. In: Liener I.E., Sharon N., editors. In the Lectin: Properties, Functions, and Application in Biology and Medicine. Academic Press; NY: 1986. pp. 371–435. [Google Scholar]
- 30.Lis H., Sharon N. Lectin. In: Sela M., editor. Their Chemistry and Application to Immunology, in the Antigens. Academic Press; NY: 1997. pp. 429–529. [Google Scholar]
- 31.Hussein F., Daniels R. Improvent of an enzyme linked lectin assay to determine recombinant mistletoe lectin I. J. Pharmaceut. Biomed. Anal. 2007;43:758–762. doi: 10.1016/j.jpba.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 32.Endo Y., Tsurugi K., Franz H. The site of action of the A-chain of mistletoe lectin I on eukaryotic ribosomes. The RNA N-glycosidase activity of the protein. FEBS Lett. 1988;2:378–380. doi: 10.1016/0014-5793(88)80853-6. [DOI] [PubMed] [Google Scholar]
- 33.Baxevanis C.N., Voutsas I.F., Tsitsilonis O.E., Tsiatas M.L., Gritzapis A.D., Amichail M. Compromised anti-tumor responses in tumor necrosis factor-alpha knockout mice. Eur. J. Immunol. 2000;30:1957–1966. doi: 10.1002/1521-4141(200007)30:7<1957::AID-IMMU1957>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 34.Tanigawa K., Craig R.A., Stoolman L.M., Chang A.E. Effects of tumor necrosis factor-alpha on the in vitro maturation of tumorreactive effector T cells. J. Immunother. 2000;23:528–535. doi: 10.1097/00002371-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Hunter C.A., Chizzonite R., Remington J.S. IL-1 beta is required for IL-12 to induce production of IFN-gamma by NK cells. A role for IL-1 beta in the T cell-independent mechanism of resistance against intracellular pathogens. J. Immunol. 1995;155:4347–4354. [PubMed] [Google Scholar]
- 36.Lee S., Lee S., Song K.B. Effect of gamma-irradiation on the physicochemical properties of porcine and bovine blood plasma proteins. Food Chem. 2003;82:521–526. [Google Scholar]
- 37.Moss D.W., Butterworth P.J. Pitman Medical; London: 1974. Enzymology and Medicine. pp. 139. [Google Scholar]
- 38.Drotman R.B., Lawhorn G.T. Serum enzymes are indicators of chemical induced liver damage. Drug Chem. Toxicol. 1978;1:163–171. doi: 10.3109/01480547809034433. [DOI] [PubMed] [Google Scholar]


