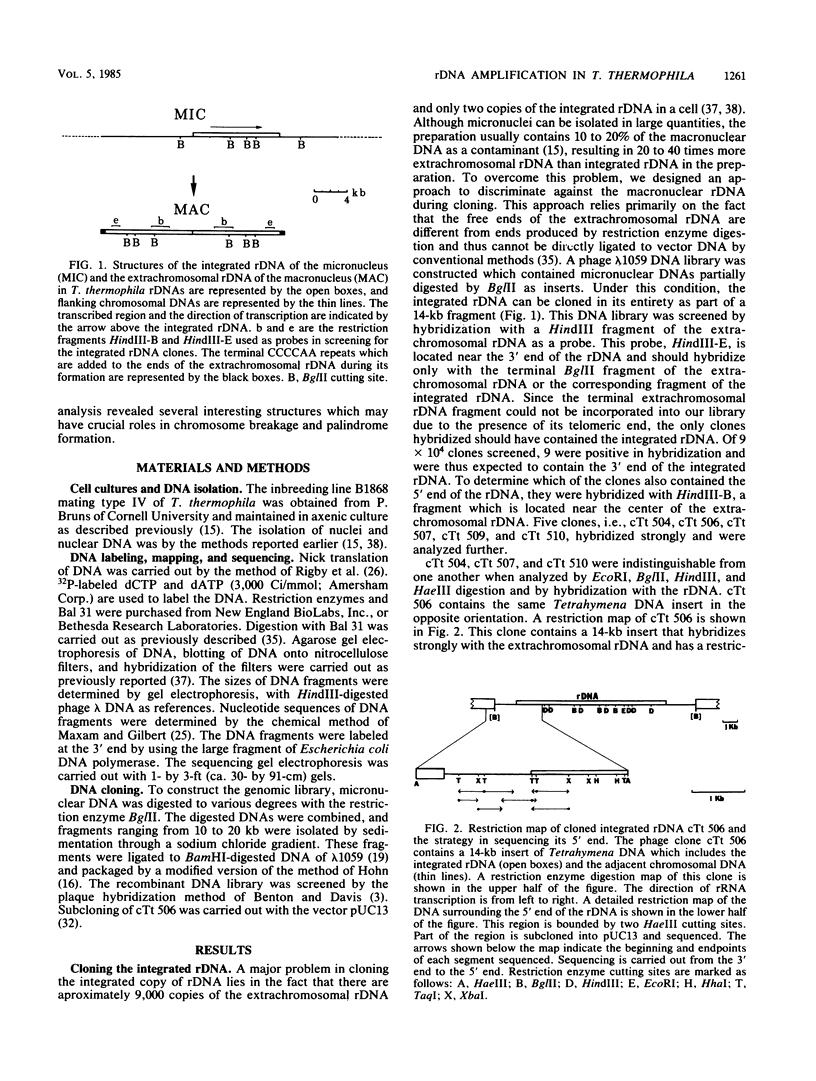
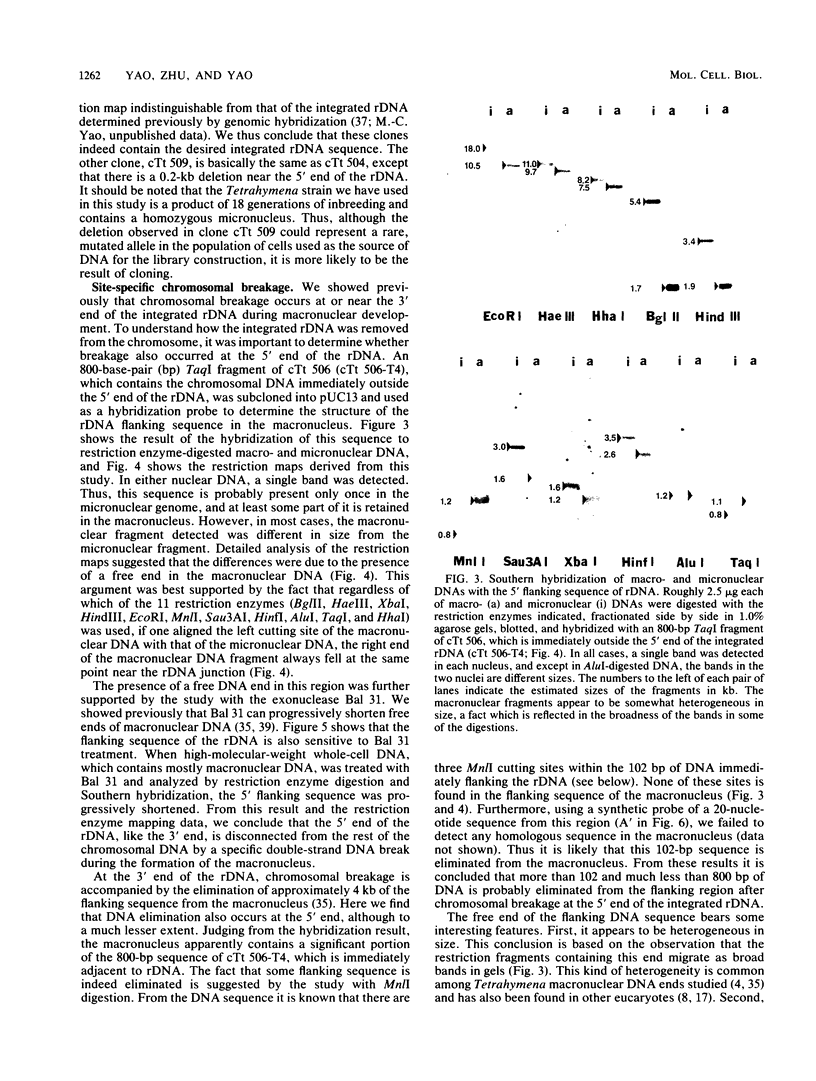
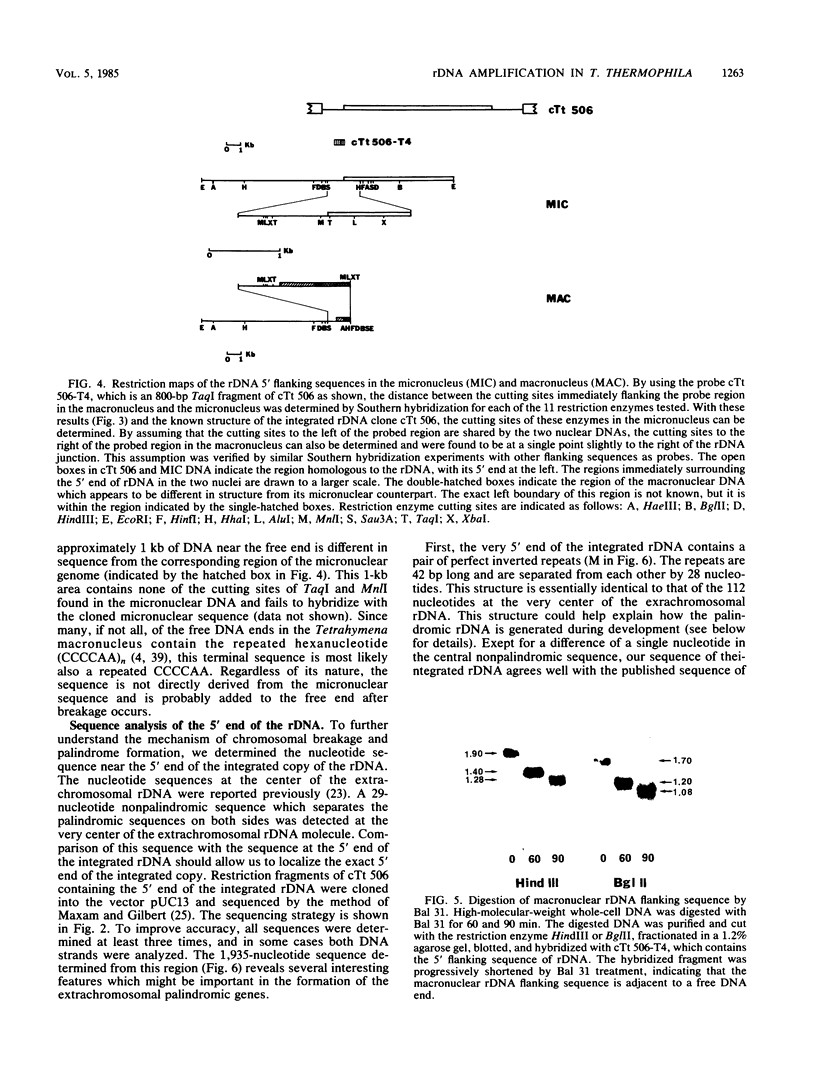
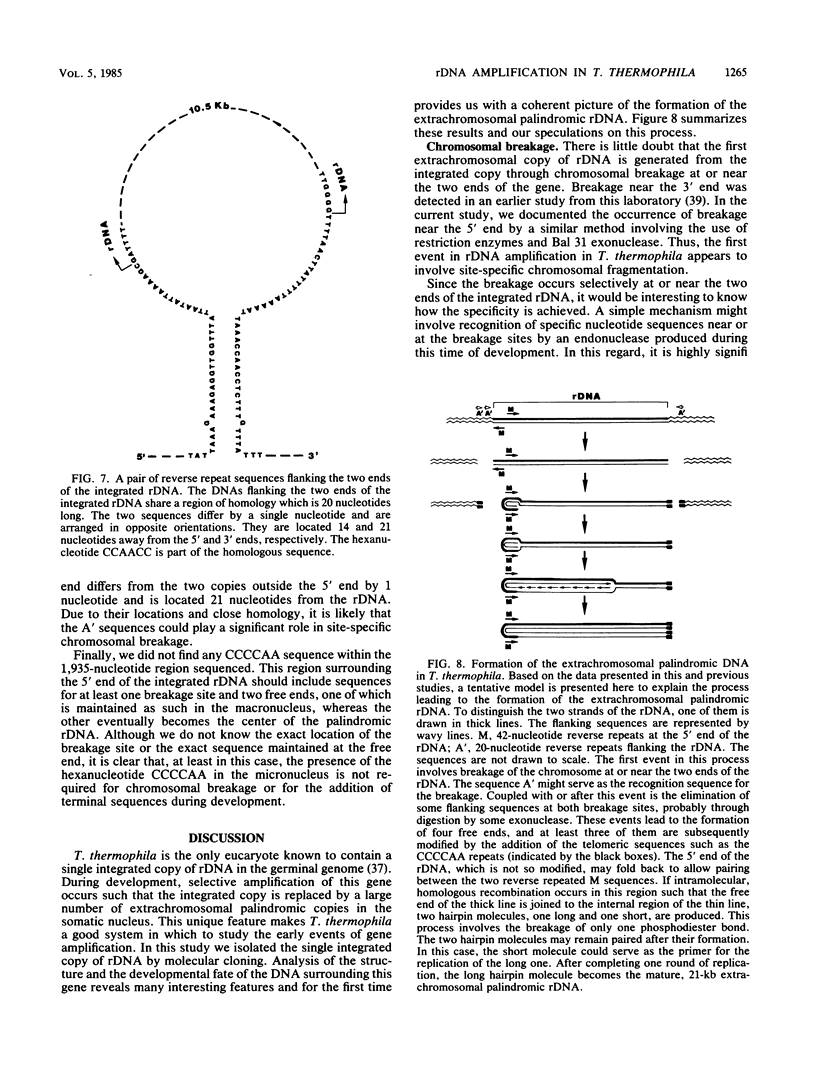
Abstract
Tetrahymena thermophila contains in the macronucleus multiple copies of extrachromosomal palindromic genes coding for rRNA (rDNA) which are generated from a single chromosomal copy during development. In this study we isolated the chromosomal copy of rDNA and determined the structure and developmental fate of the sequence surrounding its 5' junction. The result indicates that specific chromosomal breakage occurs at or near the 5' junction of rDNA during development. The breakage event is associated with DNA elimination and telomeric sequence addition. Similar results were also found previously for the 3' junction of this gene. These results could explain how the extrachromosomal rDNA is first generated. Near both junctions of the chromosomal rDNA, a pair of 20-nucleotide repeats was found. These sequences might serve as signals for site-specific breakage. In addition, we found a pair of perfect inverted repeats at the 5' junction of this gene. The repeats are 42 nucleotides long and are separated by 28 nucleotides. The existence of this structure provides a simple explanation for the formation of the palindromic rDNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Schwab M., Lin C. C., Varmus H. E., Bishop J. M. Homogeneously staining chromosomal regions contain amplified copies of an abundantly expressed cellular oncogene (c-myc) in malignant neuroendocrine cells from a human colon carcinoma. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1707–1711. doi: 10.1073/pnas.80.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beermann S. The diminution of Heterochromatic chromosomal segments in Cyclops (Crustacea, Copepoda). Chromosoma. 1977 Apr 20;60(4):297–344. doi: 10.1007/BF00292858. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H., Gall J. G. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol. 1978 Mar 25;120(1):33–53. doi: 10.1016/0022-2836(78)90294-2. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Dawid I. B. Specific gene amplification in oocytes. Oocyte nuclei contain extrachromosomal replicas of the genes for ribosomal RNA. Science. 1968 Apr 19;160(3825):272–280. doi: 10.1126/science.160.3825.272. [DOI] [PubMed] [Google Scholar]
- Cockburn A. F., Taylor W. C., Firtel R. A. Dictyostelium rDNA consists of non-chromosomal palindromic dimers containing 5S and 36S coding regions. Chromosoma. 1978 Dec 21;70(1):19–29. doi: 10.1007/BF00292212. [DOI] [PubMed] [Google Scholar]
- Cowell J. K. Double minutes and homogeneously staining regions: gene amplification in mammalian cells. Annu Rev Genet. 1982;16:21–59. doi: 10.1146/annurev.ge.16.120182.000321. [DOI] [PubMed] [Google Scholar]
- Emery H. S., Weiner A. M. An irregular satellite sequence is found at the termini of the linear extrachromosomal rDNA in Dictyostelium discoideum. Cell. 1981 Nov;26(3 Pt 1):411–419. doi: 10.1016/0092-8674(81)90210-5. [DOI] [PubMed] [Google Scholar]
- Engberg J., Andersson P., Leick V., Collins J. Free ribosomal DNA molecules from Tetrahymena pyriformis GL are giant palindromes. J Mol Biol. 1976 Jun 25;104(2):455–470. doi: 10.1016/0022-2836(76)90281-3. [DOI] [PubMed] [Google Scholar]
- Engberg J., Christiansen G., Leick V. Autonomous rDNA molecules containing single copies of the ribosomal RNA genes in the macronucleus of Tetrahymena pyriformis. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1356–1365. doi: 10.1016/0006-291x(74)90463-x. [DOI] [PubMed] [Google Scholar]
- Engberg J. Strong sequence conservation of a 38 bp region near the center of the extrachromosomal rDNA palindrome in different Tetrahymena species. Nucleic Acids Res. 1983 Jul 25;11(14):4939–4946. doi: 10.1093/nar/11.14.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall J. G. Differential synthesis of the genes for ribosomal RNA during amphibian oögenesis. Proc Natl Acad Sci U S A. 1968 Jun;60(2):553–560. doi: 10.1073/pnas.60.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall J. G. Free ribosomal RNA genes in the macronucleus of Tetrahymena. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3078–3081. doi: 10.1073/pnas.71.8.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover D. M., Zaha A., Stocker A. J., Santelli R. V., Pueyo M. T., De Toledo S. M., Lara F. J. Gene amplification in Rhynchosciara salivary gland chromosomes. Proc Natl Acad Sci U S A. 1982 May;79(9):2947–2951. doi: 10.1073/pnas.79.9.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovsky M. A., Yao M. C., Keevert J. B., Pleger G. L. Isolation of micro- and macronuclei of Tetrahymena pyriformis. Methods Cell Biol. 1975;9(0):311–327. doi: 10.1016/s0091-679x(08)60080-1. [DOI] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Johnson E. M. A family of inverted repeat sequences and specific single-strand gaps at the termini of the Physarum rDNA palindrome. Cell. 1980 Dec;22(3):875–886. doi: 10.1016/0092-8674(80)90564-4. [DOI] [PubMed] [Google Scholar]
- Kan N. C., Gall J. G. Sequence homology near the center of the extrachromosomal rDNA palindrome in Tetrahymena. J Mol Biol. 1981 Dec 25;153(4):1151–1155. doi: 10.1016/0022-2836(81)90472-1. [DOI] [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L., Cesareni G. Novel bacteriophage lambda cloning vector. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5172–5176. doi: 10.1073/pnas.77.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer K. M., Gall J. G. The macronuclear ribosomal DNA of Tetrahymena pyriformis is a palindrome. J Mol Biol. 1976 Jun 25;104(2):421–453. doi: 10.1016/0022-2836(76)90280-1. [DOI] [PubMed] [Google Scholar]
- Katzen A. L., Cann G. M., Blackburn E. H. Sequence-specific fragmentation of macronuclear DNA in a holotrichous ciliate. Cell. 1981 May;24(2):313–320. doi: 10.1016/0092-8674(81)90321-4. [DOI] [PubMed] [Google Scholar]
- King B. O., Yao M. C. Tandemly repeated hexanucleotide at Tetrahymena rDNA free end is generated from a single copy during development. Cell. 1982 Nov;31(1):177–182. doi: 10.1016/0092-8674(82)90417-2. [DOI] [PubMed] [Google Scholar]
- Kiss G. B., Pearlman R. E. Extrachromosomal rDNA of Tetrahymena thermophila is not a perfect palindrome. Gene. 1981 Apr;13(3):281–287. doi: 10.1016/0378-1119(81)90032-9. [DOI] [PubMed] [Google Scholar]
- Klobutcher L. A., Jahn C. L., Prescott D. M. Internal sequences are eliminated from genes during macronuclear development in the ciliated protozoan Oxytricha nova. Cell. 1984 Apr;36(4):1045–1055. doi: 10.1016/0092-8674(84)90054-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Varmus H. E., Bishop J. M., George D. A cellular oncogene (c-Ki-ras) is amplified, overexpressed, and located within karyotypic abnormalities in mouse adrenocortical tumour cells. Nature. 1983 Jun 9;303(5917):497–501. doi: 10.1038/303497a0. [DOI] [PubMed] [Google Scholar]
- Sor F., Fukuhara H. Unequal excision of complementary strands is involved in the generation of palindromic repetitions of rho- mitochondrial DNA in yeast. Cell. 1983 Feb;32(2):391–396. doi: 10.1016/0092-8674(83)90458-0. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Mahowald A. P. Amplification of genes for chorion proteins during oogenesis in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1096–1100. doi: 10.1073/pnas.77.2.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Braun R. Structure of ribosomal DNA in Physarum polycephalum. J Mol Biol. 1976 Sep 25;106(3):567–587. doi: 10.1016/0022-2836(76)90252-7. [DOI] [PubMed] [Google Scholar]
- Yao M. C., Gall J. G. A single integrated gene for ribosomal RNA in a eucaryote, Tetrahymena pyriformis. Cell. 1977 Sep;12(1):121–132. doi: 10.1016/0092-8674(77)90190-8. [DOI] [PubMed] [Google Scholar]
- Yao M. C., Kimmel A. R., Gorovsky M. A. A small number of cistrons for ribosomal RNA in the germinal nucleus of a eukaryote, Tetrahymena pyriformis. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3082–3086. doi: 10.1073/pnas.71.8.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C. Ribosomal RNA gene amplification in Tetrahymena may be associated with chromosome breakage and DNA elimination. Cell. 1981 Jun;24(3):765–774. doi: 10.1016/0092-8674(81)90102-1. [DOI] [PubMed] [Google Scholar]
- Yao M. C., Yao C. H. Repeated hexanucleotide C-C-C-C-A-A is present near free ends of macronuclear DNA of Tetrahymena. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7436–7439. doi: 10.1073/pnas.78.12.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]





