Abstract
We report changes of the content of anionic phospholipids in Bacillus subtilis in response to hypoxic conditions and inhibition of terminal respiration. Cardiolipin accumulates rapidly when bacteria are suspended in non-growth medium under reduced aeration or exposed to the inhibitor cyanide; the increase of cardiolipin occurs at the expense of its precursor phosphatidylglycerol and is temperature-dependent. Depending on the extent of hypoxic stress, membranes containing different levels of cardiolipin can be isolated from B. subtilis cells. The NADH oxidase activity in cardiolipin-enriched membranes is cyanide-resistant; furthermore O2 consumption measurements indicated that cardiolipin-enriched cells are resistant to cyanide. Results point out a possible interdependence between the effect of cyanide on cardiolipin metabolism and the effect of cardiolipin on the effectiveness of cyanide inhibition.
Keywords: Cardiolipin, Phosphatidylglycerol, Hypoxia, Cyanide
Abbreviations: CL, cardiolipin; L-PG, lysyl-phosphatidylglycerol; NAO, 10-N-nonyl-acridine orange; NGM, non-growth medium; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PGL, phosphoglycolipid.
Highlights
▸ Bacillus subtilis lipids were analyzed by TLC and MALDI-TOF/MS. ▸ Hypoxic stress stimulates the conversion of PG in CL. ▸ Membrane CL levels correlate with the extent of hypoxic stress. ▸ The respiratory poison cyanide induces CL membrane enrichment. ▸ CL-rich cells or membranes are resistant to cyanide.
1. Introduction
The soil bacterium Bacillus subtilis, typically exposed to stress and starvation in natural ecosystems, forms tough protective endospores allowing the organism to tolerate extreme environmental conditions. Furthermore B. subtilis, like other desiccation exposed bacteria, has the ability to avoid loss of internal water by adjusting the chemical composition of cytoplasm. The first defence to restore and maintain cell turgor under hyperosmotic conditions is the uptake of K+ ions [1]; then the bacterium starts to actively take up or synthesize so-called ‘compatible solutes’ or osmolytes [2].
As cell survival depends on membrane lipid homeostasis, bacteria have also evolved the ability to modify their membrane lipid composition to acclimatize to different environmental changes, such as in temperature, osmolarity, salinity and pH [3]. The stability and permeability of the cellular membranes play a fundamental role on the adaptation to different kinds of stresses and they might be closely related to the fatty acid composition and phospholipid content.
In this study we focus on changes in cardiolipin (CL) content of B. subtilis induced by hypoxia. CL is a dimeric phospholipid which may accumulate in specific situations depending on the physiological state of bacteria; its amount is highly variable, ranging from 1% to 80% in membranes of different Gram-positive and Gram-negative bacteria. Although it is a minor component during the exponential bacterial growth phase, CL level increases in the stationary growth phase [4]. An increased ratio of anionic to zwitterionic membrane phospholipids has been observed when Gram-positive, as well as Gram-negative, halophilic and halotolerant bacteria are grown in high salt containing media [5]. Changes in the CL content have also been described in Staphylococcus aureus and B. subtilis protoplasts [6,7] and in Rhodobacter sphaeroides exposed to hyperosmotic shock [8]. In particular previous results documented that in B. subtilis CL level increased when cells were cultivated in hyperosmotic medium [9]. Osmotic induction of the genes encoding CL synthase in both B. subtilis and Escherichia coli has been reported; thus phosphatidylglycerol (PG) synthesis is also osmotically induced and/or phosphatidylethanolamine (PE) synthesis is osmotically repressed [5,9].
Apart from lipids, several proteins are also up or down regulated in order to cope with particular stress, determining complex cell responses. Among these responses, numerous proteins involved in maintaining redox balance of the cell are up-regulated in some stress conditions [10].
Here we report results of experiments showing anti-parallel changes in CL and PG levels occurring when B. subtilis cells are stressed by oxygen-limiting conditions, such as hypoxia and cyanide exposure, and examine whether the high CL-content in the stressed cells and membranes correlates with changes in respiratory chain activity.
2. Experimental procedures
2.1. Materials
Organic solvents, staining reagents and 10-N-nonyl acridine orange (NAO) were obtained from Sigma–Aldrich; Plates for TLC (Silica gel 60A) from Merck.
2.2. Strain and growth conditions
B. subtilis strain 168 (trpC2 mutant) was grown at 37 °C with vigorous agitation in the standard Luria–Bertani (LB) medium, containing 0.5% NaCl, 1% tryptone, 0.5% yeast extract. Overnight cultures were diluted (1:500) into fresh broth and incubated in the same above conditions. Cells at exponential growth phase (OD600 about 1) were harvested by centrifugation (3000g for 5 min) and washed with half volume non-growth medium (NGM), containing 0.5% NaCl, 50 mM Tris/Cl, pH 7.4. The cellular pellets were suspended in appropriate solutions and immediately processed for further uses.
2.3. Cell incubation in hypoxic conditions
Cells from 250 ml-culture at exponential growth phase (OD600 about 1) were harvested as above described and incubated in 40 ml NGM without shaking for 1 or 2 h at room temperature. By using a Clark electrode, we found that oxygen levels rapidly fall to zero in less than 1 min in these experimental conditions.
2.4. Time-course of CL increase during hypoxic incubation
Four aliquots of 250 ml-cell culture were washed and resuspended in about 2 ml NGM each. Hypoxic incubation was terminated at 0, 15, 30 and 60 min at 20 or 37 °C. Lipids were then extracted from equivalent cell suspension aliquots (i.e. aliquots having the same protein content).
2.5. Isolation of membranes containing different CL-levels
Membranes (M1 and M2) were isolated from cells under different hypoxic conditions. Cells from 250 ml-culture were harvested and incubated in 40 ml NGM for either 1 or 2 h. Then the cells were supplemented with 1 mM phenylmethanesulfonyl fluoride (PMSF), 0.1 mM benzamidine, 0.5 mg/ml lysozyme and some milligrams of DNase I. After incubation at 35 °C for 30 min, cells were sonicated and immediately centrifuged at 40,000g for 30 min. The resulting pellet was resuspended in 10 ml Buffer A, containing 20 mM Tris–HCl/1 mM EDTA, pH 8.0, 0.2 mM PMSF, 0.1 mM benzamidine and then homogenized with Potter. Protein concentration of membranes was determined by the biuret method.
2.6. Oxidase activity and spectral analysis
Isolated membranes in Buffer A were solubilized by adding laurylmaltoside (2 mg/mg proteins). After an overnight incubation at 4 °C, the mixture was centrifuged at 100,000g for 2 h and the resulting pellet discarded. NADH-oxidase activity of solubilized membranes was determined spectrophotometrically by adding increasing amounts of KCN (0–5 μM) and following the absorbance variations at 340 nm after the addition of 0.1 mM NADH as substrate, using ɛ340 = 6.22 × 103 M−1 cm−1. The oxidized form of solubilized membranes was taken as reference and then few grains of sodium dithionite were added to obtain the reduced form. For the heme a content ɛ605–630 = 13.5 mM−1 cm−1 was used.
2.7. Cell exposure to KCN
Three equivalent aliquots of cells (250-ml culture, OD600 = 1) were incubated in the absence, in the presence of 0.5 mM KCN and in the presence of 10.5 mM KCN, pH 8. After 30 min in an orbital shaker at 20 °C, the cells were harvested and suspended in a small NGM volume and quickly processed for lipid extraction. Lipids were extracted from equivalent cell aliquots. Endogenous respiration measurements were also performed on control and on cells incubated with 10.5 mM KCN.
2.8. Lipid extraction and thin-layer chromatography (TLC)
Lipids were extracted from whole cells or membranes according to the Bligh and Dyer method. Lipid extracts were analyzed by TLC on silica gel plates in solvent chloroform/methanol/acetic acid/water (85:15:10:3.5, v/v). Phospholipids were detected with Molybdenum Blue Reagent. The lipid quantitative analysis was performed by video densitometry, using the ImageJ software.
2.9. MALDI-TOF mass spectrometry
Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectra were acquired on a Bruker Microflex RLF mass spectrometer (Bruker Daltonics, Bremen, Germany). Lipid analysis was performed by using 9-aminoacridine as matrix, as previously described [11].
2.10. Oxygen consumption measurements
Cells were harvested by centrifugation and resuspended in LB-tricine medium to a final OD600 of about 10. The endogenous respiratory activity was determined in an oxygraphic chamber containing 1 ml of LB-tricine medium at 25 °C, using 50 μl of the bacterial suspension. When a constant slope of the oxygen trace was reached, KCN was added in order to determine the cyanide sensitivity of the endogenous respiratory activity in the range 0–10 mM. Each experimental data point has been repeated at least three times starting from at least three independent cultures.
2.11. Fluorescence microscopy
10-N-nonyl acridine orange (NAO) was added to a final concentration of 100 nM to B. subtilis cultures (OD600 = 0.5). The same NAO concentration was added to cells after incubation in NGM (see before). After 1 h at room temperature, the cells were supplemented with 0.5 μg/ml 4′,6-diamidino-2-phenylindole (DAPI), fixed on object slides coated with a layer of 1% agarose gel in water and viewed with a Leica epifluorescence microscope equipped with 100× fluorite oil immersion objectives. NAO fluorescence was detected by using a standard fluorescein isothiocyanate (FITC) filter set. Images were captured with a cooled charge-coupled camera device and manipulated in Iplab Adobe Photoshop 7.0.
3. Results
3.1. Lipid composition of hypoxic cells
Fig. 1A compares the phospholipid pattern of the hypoxic cells (lane H) with that of control ones (lane C). In the case of hypoxic cells lipid extraction was performed after prolonged incubation of B. subtilis in a small volume of non-growth medium (NGM). The individual lipid components were identified by their Rf values relative to those of authentic standard markers, by staining behaviour with specific reagents and by MALDI-TOF/MS analysis. As previously reported in the literature [9], phospholipids present in the membranes of B. subtilis are (in Rf order) a phosphoglycolipid (PGL), lysyl-phosphatidylglycerol (L-PG), PG, PE, plus small amount of CL. By comparing the two TLC profiles (Fig. 1A), it is evident that the cells stressed by low O2 availability have a CL content higher than control ones. Furthermore it can be seen that the level of PG, the precursor phospholipid of CL synthesis, decreases in hypoxic cells.
Fig. 1.
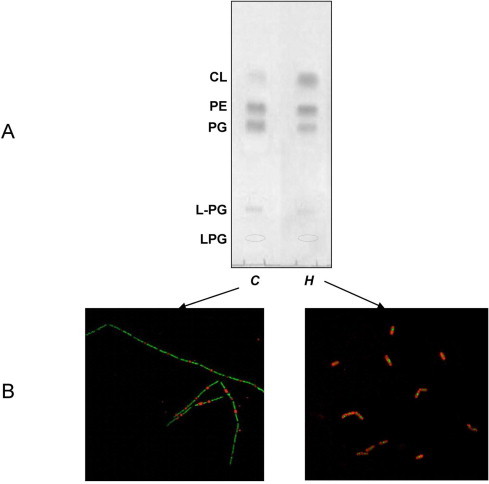
Hypoxia increases CL level in B. subtilis cells. (A) TLC of the lipid extracts of control cells and cells under hypoxic conditions. Two aliquots of fresh cultures (OD600 1, 250 ml each) were harvested; control cells (C) were suspended in a small volume and quickly processed for lipid extraction, while hypoxic cells (H) were harvested and incubated in 40 ml NGM without shaking for 120 min at 20 °C before lipid extraction. Forty micrograms of lipids were loaded in each lane; phospholipids were detected with Molybdenum Blue Reagent. (B) CL domains stained with NAO in living cells. NAO-stained CL domains have been coloured red and DAPI-stained DNA domains coloured green by Adobe Photoshop software. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Further evidences of the ability of B. subtilis to modulate CL levels under hypoxia can be observed by using the dye 10-N-nonyl-acridine orange (NAO), considered an indicator of CL-rich domains in the membranes of various living cells [12]. Fig. 1B shows microscopy fluorescence images of cells incubated with NAO together with the dye DAPI, a well known staining of DNA; in the merged fluorescence images shown, the cells are shown stained green by DAPI, while CL-rich membrane domains are shown stained red by NAO. It can be clearly observed that red CL-rich membrane domains were localized at the cell poles and at the division septum in control cells, as previously reported [13].
According to TLC data (Fig. 1A), fluorescence data clearly show a marked increase in red NAO staining (i.e. CL-rich domains) in B. subtilis cells under hypoxic conditions (Fig. 1B). Microscopic observations also indicate that cell morphology changed in the two different experimental conditions: the filamentous phenotype of control cells was lost and cells appeared as shorter chains or clumps of cells when stressed by low O2 availability.
In order to investigate on the CL increase observed in stressed cells, we looked at the time course of CL and PG content during hypoxic conditions. BS168 cells were incubated in a small volume of NGM medium without shaking at 20 °C and total lipids were extracted from equivalent aliquots of cell suspensions (i.e. having the same protein content), at different incubation times (0, 15, 30 and 60 min). The TLC results in Fig. 2A demonstrate an increase in CL content paralleled by a decrease in PG content in stressed cells, indicating that CL synthesis occurs at the expense of its precursor PG. A similar behaviour was found in BS168 cells incubated in the same condition, but at higher temperature (37 °C). Fig. 2B compares the time-courses of membrane CL and PG contents during incubation at 20 or 37 °C; it is evident that CL increase at the expense of PG is dependent on temperature.
Fig. 2.
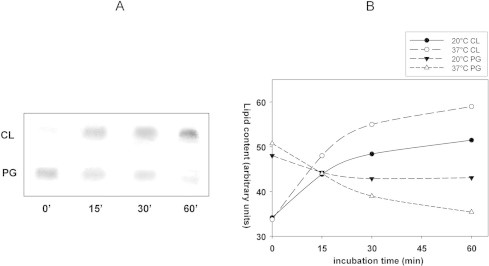
Time course of CL and PG content during cell incubation under hypoxic conditions. (A) Total lipids were extracted from equivalent cell aliquots incubated in a small NGM volume without shaking for different incubation times at 20 °C. Forty micrograms of lipids were loaded in each lane. (B) Phospholipid changes occurring in hypoxic cells at 20 and 37 °C. Values in the y axis, estimated by TLC video-densitometry, are representative of a typical experiment. Equivalent cell aliquots had the same protein content.
3.2. Isolation of CL-enriched membranes
Previous studies reported that the cultivation of the facultative anaerobe bacterium Paracoccus denitrificans at different oxygen tensions modulated the ratio of cardiolipin to heme a in the bacterial membranes, ranging from about 300 for anaerobic growth to 30 for aerobic growth [14]. In this study different levels of CL were detected in membranes isolated from B. subtilis depending on the extent of hypoxic stress. The availability of CL-enriched membranes prompted us to investigate on the effect of high CL level on the respiratory chain activity in the B. subtilis cells. We examined the heme a content and the respiratory chain activity, as NADH-oxidase with oxygen as terminal electron acceptor, in membranes having different CL-levels. Spectroscopic investigations indicated that the heme a contents of membranes isolated after 1 or 2 h of hypoxic incubations (M1 and M2) were 0.245 ± 0.006 and 0.198 ± 0.003 nmol mg−1 protein, respectively (not shown). Quantitative analyses of cardiolipin in M1 and M2 membranes, performed by densitometric analysis of the TLC plate, indicated that 20.50 ± 0.50 and 23.45 ± 0.80 μg CL mg−1 protein were present in M1 and M2, respectively. Then the CL to heme a molar ratios of M1 and M2 were about 6 and 9, respectively (Fig. 3A).
Fig. 3.
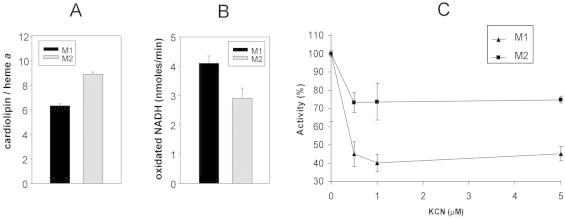
NADH-oxidase activity of membranes with different CL content. Membranes were isolated from cells incubated in hypoxic conditions for either 1 or 2 h (M1 and M2, respectively) and then solubilized, as described in Section 2. Lipid quantitative analysis and spectral assay were performed on M1 and M2. (A) Molar ratio of CL to heme a of M1 and M2 membranes. Heme a content was estimated spectrophotometrically. (B) NADH-oxidase activity of M1 and M2 membranes (100 ng of membrane proteins each). (C) KCN titration of membrane oxidase activity. The NADH-oxidase activities of membranes are reported as percent activity in response to addition of increasing amounts of KCN (0–5 μM).
We tested the NADH-oxidase activity with the oxygen as terminal electron acceptor in order to give a functional significance to the decrease of heme a in CL-enriched membranes. Fig. 3B shows that the NADH-oxidase activity was reduced in solubilized membranes having higher CL-content, i.e. in M2 compared to M1 samples. By analysing the KCN inhibition of respiratory activity, we could obtain some information on the functional arrangement of the respiratory chain as well as the kind of terminal oxidases present in the CL-enriched membranes. The NADH-oxidase activities of the two kinds of membranes were also examined by KCN titration (Fig. 3C); the rapid decrease of oxidase activities of both the membranes, following an addition of 0.5–1 μM KCN, was suggestive of inhibition of copper–heme oxidases activity. On the contrary the bd-oxidase activity was inhibited for KCN values higher than 1 mM (not shown). By comparing the two curves reported in Fig. 3C, it can be seen that the cyanide inhibition was minor in membranes having a higher CL-content. The reported data suggest that about 50% of the maximal electron flux from NADH to oxygen is supported by copper–heme oxidases in B. subtilis membranes having a lower CL-level; at higher CL-level the functional importance of these enzymes is reduced and in the 0.5–1.0 μM of KCN concentration range only the 25% of activity is lost. This result suggests that the bd-oxidases, typically resistant to cyanide, are dominant in these conditions.
3.3. Effect of cyanide on CL-levels
We found that CL may also accumulate in B. subtilis cells if energy metabolism is impaired with cyanide. In particular, cells were incubated under aeration in the presence of 0.5 or 10.5 mM KCN for 30 min; then total lipids were extracted from cell suspensions. The TLC lipid patterns presented in Fig. 4 show that the respiratory poison caused a CL increase paralleled with a PG decrease in both the cyanide concentrations, and that the lipid changes are concentration-dependent. Thus the inhibition of the terminal respiration caused an increase in CL-content. The CL-enrichment is also evident if the MALDI-TOF mass spectra (negative mode) of the total lipid extracts of control cells and cells stressed by the presence of cyanide are compared. A list of peaks present in the spectra is given in Table S1 (Supplementary material). The main clusters of signals are diagnostic for the phospholipids previously observed on TLC. The comparison of MALDI-TOF/MS lipid profiles shows that the ratio between PG and CL peaks is reversed after CN incubation (Fig. 4).
Fig. 4.
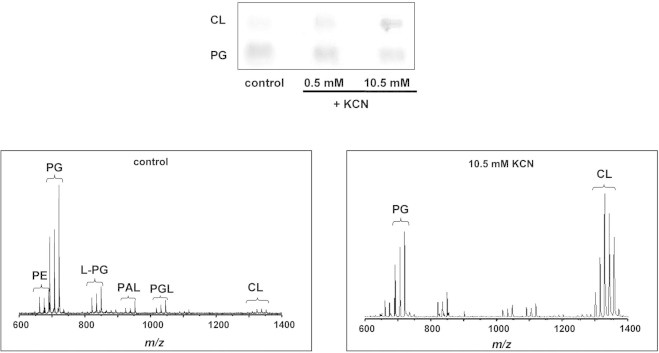
KCN increases CL level. Equivalent aliquots of cells were incubated in an orbital shaker at 37 °C for 30 min (aerated cells) in the absence and in the presence of either 0.5 or 10.5 mM KCN before lipid extraction. Top: TLC bands of PG and CL. Bottom: MALDI-TOF mass spectra (negative mode) of lipid extracts of cells incubated in the absence (left) and in the presence of 10.5 mM KCN (right). Detailed lipid assignments in Table S1.
Finally KCN-exposed cells have been washed to remove the inhibitor and then suspended in LB medium in order to measure the endogenous respiration of CL-enriched cells using an oxygen electrode. The bar graph in Fig. 5 shows that the O2 consumption rates of cells having higher CL content is significantly lower than that of control one (see black bars). In the presence of KCN, inhibition of respiratory activity was stronger in control cells, while CL-enriched cells exhibited the typical cyanide-resistance (see light- and dark-grey bars).
Fig. 5.
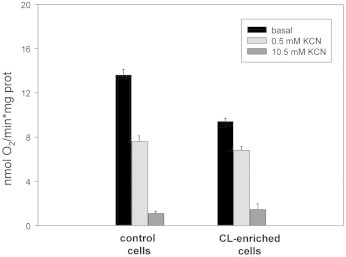
Endogenous respiration of CL-enriched cells after KCN exposure. O2 consumption rates of control cells (left) and CL-enriched cells after KCN exposure (right) were compared. Cells after exposure to 10.5 mM KCN, as described in Fig. 4, were harvested, washed and then 20-fold diluted in LB medium. Then O2 consumption rate was measured in the absence (black bars) and in the presence of 0.5 or 10.5 mM KCN (light- and dark-grey bars), as described in Section 2. Each experimental data point has been repeated at least three times starting from at least three independent cultures.
4. Discussion
CL is the key lipid of respiratory chain in mitochondria as well as in bacteria [15,16]. In this report we document parallel changes in CL content and functioning of respiratory chain in B. subtilis cells under stress conditions.
Generally aerobic bacteria can adapt to changes in their environment by using different types of respiratory pathways [17]. Usually, bacteria possess more than one terminal oxidase. In particular, the electron transport chain in B. subtilis contains two major branches, one quinol oxidase branch and one cytochrome c oxidase one. Three terminal oxidases are usually used by B. subtilis under aerobic growth conditions: a cytochrome c oxidase (cytochrome caa3) and two quinol oxidases (cytochrome aa3 and cytochrome bd) [18]. The bd-type terminal oxidases are a group of enzymes, not related to the heme–copper oxidases, which do not pump protons or contain copper as redox group. At least one quinol oxidase is essential for aerobic growth of B. subtilis, and at least one of the heme–copper oxidases, cytochrome caa3 or cytochrome aa3, is required for normal sporulation; this last oxidase is the most important terminal one during the exponential growth phase of B. subtilis [18]. It is known that under O2 limitation (hypoxic conditions), many bacteria induce high-affinity oxidases to respire traces of molecular oxygen; cytochrome bd is produced under conditions of low O2 tension in B. subtilis [19].
According to above literature reports, our analysis of solubilized membranes isolated from hypoxic cultures of B. subtilis shows evidences for the presence of bd-type oxidase together with a decrease of heme a content, indicating a substantial drop of copper–heme oxidase activity upon hypoxic stress. In correspondence of these changes in terminal oxidase activities, a CL-increase occurs at expense of PG. Either suspension in a non-growth medium without aeration or exposure to cyanide of aerated BS168 cells resulted in an immediate conversion of PG to CL. Furthermore, changes in B. subtilis cell morphology corresponded to the rise of CL-content under oxygen-limiting conditions. Parallel changes in CL level and membrane morphology have been recently described in mitochondria [20,21].
Our results on respiration in CL-enriched membranes and cells indicate that the CL accumulation is associated with cyanide-resistant terminal oxidases. This phenomenon could depend on the bd-type oxidase branch prevalence under stress conditions or on membrane CL-level increase per se or on the combination of both the factors.
Importance of CL in heme–copper terminal oxidase activity, as well as for others membrane-linked enzymes involved in bioenergetics functions, is well described in the literature [15,22]. X-ray studies of the yeast cytochrome bc1 complex indicated that CL is positioned at the entrance to one possible proton uptake pathway at the ubiquinone reduction site; thus it has been proposed that CL ensures structural integrity of the proton-conducting protein environment and takes part directly in H+ uptake [23]. Even in membranes of archaeal organisms a functional association of CL with a terminal oxidase has been reported [24].
It has been suggested that high CL levels would buffer protons over the membrane surface [25]; under conditions in which function of terminal oxidases is impaired this might be useful because would reduce the proton gradient dissipation. The importance of proton trapping over the membrane surface in the working operation of the respiratory chain has been widely discussed [26,27].
CL is considered the most effective phospholipid to restore the activity of some purified respiratory complex of bacteria such as the NADH dehydrogenase, the lactate dehydrogenase, the succinate dehydrogenase, the cytochrome bo3-ubiquinol oxidase [28]. Recently the X-ray crystal structures have reported the presence of a tightly bound CL molecule to the formate dehydrogenase and nitrate reductase A, two anaerobic respiratory complexes from E. coli. In particular the three dimensional structure of the nitrate reductase A suggested the engulfment of two acyl chains of CL within a large hydrophobic groove of the protein and the proximity of the cavity at which quinol substrate binding occurs [16,28].
Data here reported point out a possible interdependence between the effect of hypoxia (or CN) on CL metabolism and the CL role on the effectiveness of CN inhibition (or oxygen interaction with heme). We propose that CL specific binding might affect the quinone stability and mobility in the bacterial membrane with significant changes in the operation mode of the respiratory chain of B. subtilis.
Acknowledgements
We thank Domenico Grieco for experiments on isolated membranes, Maristella Baronio and Pietro Luca Martino for endogenous respiration measurements. This work was funded by the Italian Minister of Defence (Contract No. 1353/28.12.2010).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplementary material
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.fob.2013.02.002.
Appendix. Supplementary Materials
MALDI-TOF signals of phospholipids of B. subtilis control cells.
References
- 1.Ventosa A., Nieto J.J., Oren A. Biology of moderately halophilic aerobic bacteria. Microbiol. Mol. Biol. Rev. 1998;62:504–544. doi: 10.1128/mmbr.62.2.504-544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kempf B., Bremer E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 1998;170:319–330. doi: 10.1007/s002030050649. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y.M., Rock C.O. Transcriptional regulation in bacterial membrane lipid synthesis. J. Lipid Res. 2009;50(Suppl):S115–S119. doi: 10.1194/jlr.R800046-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cronan J.E. Phospholipid alterations during growth of Escherichia coli. J. Bacteriol. 1968;95:2054–2061. doi: 10.1128/jb.95.6.2054-2061.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romantsov T., Guan Z., Wood J.M. Cardiolipin and osmotic stress responses of bacteria. Biochim. Biophys. Acta. 2009;1788:2092–2100. doi: 10.1016/j.bbamem.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okabe A., Hirai Y., Hayashi H., Kanemasa Y. Alteration in phospholipid composition of Staphylococcus aureus during formation of autoplast. Biochim. Biophys. Acta. 1980;617:28–35. doi: 10.1016/0005-2760(80)90221-0. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Rivas C., Bohin J.P. Cardiolipin content and protoplast fusion efficiency in Bacillus subtilis. FEMS Microbiol. Lett. 1983;19:137–141. [Google Scholar]
- 8.Catucci L., Depalo N., Lattanzio V.M.T., Agostiano A., Corcelli A. Neosynthesis of cardiolipin in Rhodobacter sphaeroides under osmotic stress. Biochemistry. 2004;43:15066–15072. doi: 10.1021/bi048802k. [DOI] [PubMed] [Google Scholar]
- 9.Lopez C.S., Heras H., Ruzal S.M., Sanchez-Rivas C., Rivas E.A. Variations of the envelope composition of Bacillus subtilis during growth in hyperosmotic medium. Curr. Microbiol. 1998;36:55–61. doi: 10.1007/s002849900279. [DOI] [PubMed] [Google Scholar]
- 10.Petersohn A., Brigulla M., Haas S., Hoheisel J.D., Völker U., Hecker M. Global analysis of the general stress response of Bacillus subtilis. J. Bacteriol. 2001;183:5617–5631. doi: 10.1128/JB.183.19.5617-5631.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angelini R., Babudri F., Lobasso S., Corcelli A. MALDI-TOF/MS analysis of archaebacterial lipids in lyophilized membranes dry-mixed with 9-aminoacridine. J. Lipid Res. 2010;51:2818–2825. doi: 10.1194/jlr.D007328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petit J.M., Maftah A., Ratinaud M.H., Julien R. 10-N-nonyl acridine orange interacts with cardiolipin and allows the quantification of this phospholipid in isolated mitochondria. Eur. J. Biochem. 1992;209:267–273. doi: 10.1111/j.1432-1033.1992.tb17285.x. [DOI] [PubMed] [Google Scholar]
- 13.Kawai F., Shoda M., Harashima R., Sadaie Y., Hara H., Matsumoto K. Cardiolipin domains in Bacillus subtilis marburg membranes. J. Bacteriol. 2004;186:1475–1483. doi: 10.1128/JB.186.5.1475-1483.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan P., Kucera I. Relationship between cardiolipin content and cytochrome c oxidase activity of cytochrome aa3 in Paracoccus denitrificans. Biochem. Int. 1992;28:137–142. [PubMed] [Google Scholar]
- 15.Mileykovskaya E., Dowhan W. Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochim. Biophys. Acta. 2009;1788:2084–2091. doi: 10.1016/j.bbamem.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arias-Cartin R., Grimaldi S., Pommier J., Lanciano P., Schaefer C., Arnoux P., Giordano G., Guigliarelli B., Magalon A. Cardiolipin-based respiratory complex activation in bacteria. Proc. Natl. Acad. Sci. USA. 2011;108:7781–7786. doi: 10.1073/pnas.1010427108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anraku Y. Bacterial electron transport chains. Annu. Rev. Biochem. 1988;57:101–132. doi: 10.1146/annurev.bi.57.070188.000533. [DOI] [PubMed] [Google Scholar]
- 18.Winstedt L., von Wachenfeldt C. Terminal oxidases of Bacillus subtilis strain 168: one quinol oxidase, cytochrome aa3 or cytochrome bd, is required for aerobic growth. J. Bacteriol. 2000;182:6557–6564. doi: 10.1128/jb.182.23.6557-6564.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winstedt L., Yoshida K., Fujita Y., von Wachenfeldt C. Cytochrome bd biosynthesis in Bacillus subtilis: characterization of the cydABCD operon. J. Bacteriol. 1998;180:6571–6580. doi: 10.1128/jb.180.24.6571-6580.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acehan D., Malhotra A., Xu Y., Ren M., Stokes D.L., Schlame M. Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophys. J. 2011;100:2184–2192. doi: 10.1016/j.bpj.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corcelli A., Sublimi Saponetti M., Zaccagnino P., Lopalco P., Mastrodonato M., Liquori G.E., Lorusso M. Mitochondria isolated in nearly isotonic KCl buffer: focus on cardiolipin and organelle morphology. Biochim. Biophys. Acta. 2010;1798:681–687. doi: 10.1016/j.bbamem.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Schlame M. Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. J. Lipid Res. 2008;49:1607–1620. doi: 10.1194/jlr.R700018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lange C., Nett J.H., Trumpower B.L., Hunte C. Specific roles of protein–phospholipid interactions in the yeast cytochrome bc1 complex structure. EMBO J. 2001;20:6591–6600. doi: 10.1093/emboj/20.23.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corcelli A., Lobasso S., Palese L.L., Sublimi Saponetti M., Papa S. Cardiolipin is associated with the terminal oxidase of an extremely halophilic archaeon. Biochem. Biophys. Res. Commun. 2007;354:795–801. doi: 10.1016/j.bbrc.2007.01.060. [DOI] [PubMed] [Google Scholar]
- 25.Haines T.H., Dencher N.A. Cardiolipin: a proton trap for oxidative phosphorylation. FEBS Lett. 2002;528:35–39. doi: 10.1016/s0014-5793(02)03292-1. [DOI] [PubMed] [Google Scholar]
- 26.Cherepanov D.A., Junge W., Mulkidjanian A.Y. Proton transfer dynamics at the membrane/water interface: dependence on the fixed and mobile pH buffers, on the size and form of membrane particles, and on the interfacial potential barrier. Biophys. J. 2004;86:665–680. doi: 10.1016/S0006-3495(04)74146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulkidjanian A.Y. Proton in the well and through the desolvation barrier. Biochim. Biophys. Acta. 2006;1757:415–427. doi: 10.1016/j.bbabio.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 28.Arias-Cartin R., Grimaldi S., Arnoux P., Guigliarelli B., Magalon A. Cardiolipin binding in bacterial respiratory complexes: structural and functional implications. Biochim. Biophys. Acta. 2012;1817:1937–1949. doi: 10.1016/j.bbabio.2012.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MALDI-TOF signals of phospholipids of B. subtilis control cells.


