Abstract
EphA2 is activated through phosphorylation on serine 897 (S897) by Akt to promote cancer cell motility and invasion, independently of stimulation by ephrin, its ligand. Here we show that S897 phosphorylation of EphA2 strengthens the interaction between EphA2 and Ephexin4, a guanine nucleotide exchange factor for the small GTPase RhoG. S897A mutation of EphA2 abolished the EphA2/Ephexin4-mediated RhoG activation, promotion of cell migration, and resistance to anoikis. Our results suggest that S897-phosphorylated EphA2 recruits Ephexin4 to promote cell migration and anoikis resistance, providing a molecular link between S897 phosphorylation of EphA2 and tumor progression.
Keywords: RhoG, Cell migration, Anoikis resistance, EphA2, Ephexin, RhoGEF
Abbreviations: GEF, guanine nucleotide exchange factor; GAP, GTPase-activating protein; SAM, sterile-α-motif; poly-HEMA, poly-hydroxyethylmethacrylate.
Highlights
▸ S897 phosphorylation of EphA2 strengthens the interaction between EphA2 and Ephexin4. ▸ S897A mutation of EphA2 suppresses EphA2-mediated RhoG activation. ▸ S897A mutation blocks EphA2/Ephexin4-mediated cell migration and anoikis resistance.
1. Introduction
Eph receptors constitute the largest family of receptor tyrosine kinases. Their ligands, ephrins, are membrane-anchored proteins, which are divided into two subclasses: class A and class B ephrins, and there are also two classes of Eph receptors, EphA and EphB, based on homology and binding affinity for class A and class B ephrins. Ephrin/Eph receptor signaling pathways have many important functions during development and in tissue homeostasis, and many studies have shown that dysregulation of ephrin/Eph receptor signaling contributes to cancer progression [1–3]. Among them, EphA2 is frequently overexpressed in a variety of human cancers, and a number of studies have reported that overexpression of EphA2 is associated with an aggressive and metastatic cellular phenotype in different types of cancer cells. EphA2 is phosphorylated by Akt on S897 downstream of growth factor receptors and promotes cancer cell migration independently of ligand ephrin stimulation. Furthermore, S897 phosphorylation of EphA2 is correlated with malignant progression of human astrocytoma [4]. However, the mechanisms underlying the oncogenic effects of S897-phosphorylated EphA2 remain poorly understood.
It is already well known that members of the Rho family of small GTPases play pivotal roles in the regulation of cell morphology, migration, proliferation, and survival. Among them, RhoG is a key upstream regulator of another Rho family member Rac to induce diverse cellular functions, including promotion of cell migration, neurite outgrowth in neuronal cells, and stimulation of phagocytosis [5–7]. ELMO is an effector for RhoG that mediates activation of Rac through the interaction with Rac GEF Dock180 or Dock4. When RhoG is activated, it binds to ELMO to induce translocation of the ELMO–Dock180 or ELMO–Dock4 complex from the cytoplasm to the plasma membrane, leading to activation of Rac [7–9]. On the other hand, RhoG binds to PI3K p85α regulatory subunit and activates the PI3K/Akt signaling pathway to promote cell proliferation and survival independently of the activation of Rac [10–12]. RhoG-mediated activation of PI3K and Akt also leads to suppression of anoikis, a subtype of apoptosis induced by detachment of adherent cells from the extracellular matrix. Anoikis contributes to the regulation of tissue homeostasis and development, and resistance to anoikis has been suggested to be a prerequisite for cancer cells to metastasize [13].
Eph receptors contain a protein kinase domain and a sterile-α-motif (SAM) domain in the cytoplasmic region and bind to diverse signaling effectors that regulate the activities of kinases and small GTPases. Among them, Ephexin was identified as a subfamily of Dbl type Rho GEFs that interacts directly with Eph receptors [14,15]. At least five members of the Ephexin subfamily (Ephexin1–5) have been reported, and we recently identified Ephexin4 as a GEF for the Rho family small GTPase RhoG. Ephexin4 interacts with EphA2 and mediates ephrin-independent promotion of cell migration and suppression of anoikis through activation of RhoG [16,17]. In this study, we have investigated the relationship between S897 phosphorylation of EphA2 and Ephexin4-mediated signaling pathway. We show that phosphorylation of EphA2 on S897 by Akt regulates the EphA2–Ephexin4 interaction and EphA2-mediated RhoG activation to promote cell migration. We also provide evidence that S897 phosphorylation of EphA2 is crucial for EphA2-mediated resistance to anoikis.
2. Materials and methods
2.1. Plasmids and antibodies
The expression plasmid pCAG encoding YFP was a generous gift from Drs. J. Miyazaki (Osaka University, Osaka, Japan) and T. Saito (Chiba University, Chiba, Japan). Plasmids expressing Myc-tagged EphA2, Flag-tagged Ephexin4, HA-tagged Akt fused with the Src myristoylation signal at the N terminus, and Myc-tagged RhoG-V12 were obtained as described previously [5,16,18,19]. EphA2-SA (S897A) was constructed by PCR-mediated mutagenesis. The shRNAs for control luciferase, human Ephexin4, and EphA2 were designed to target 19 nucleotides and expressed by using an shRNA expression vector pSilencer-hygro (Ambion) as described previously [16].
The following antibodies were used in this study: a mouse monoclonal antibody against Myc, a rabbit polyclonal antibody against EphA2 (C-20), and a rat monoclonal antibody against RhoG (1F3 B3 E5) (Santa Cruz Biotechnology); mouse monoclonal antibodies against Flag (M2) and α-tubulin (Sigma); rabbit polyclonal antibodies against Akt and phosphor-(Ser/Thr) Akt substrate (Cell Signaling); a mouse monoclonal antibody against EphA2 (clone D7, Millipore); a rabbit polyclonal antibody against phospho-EphA2 (Ser-897) (Cell Applications); secondary antibodies conjugated to horseradish peroxidase (DAKO). Anti-Ephexin4 antibody was described previously [16].
2.2. Cell culture and transfection
HeLa, MCF-7, and HEK293T cells were grown in DMEM containing 10% FBS, 4 mM glutamine, 100 units/ml of penicillin, and 0.1 mg/ml of streptomycin under humidified air containing 5% CO2 at 37 °C. Cells were transfected with the indicated plasmids using LipofectAMINE Plus (for HEK293T cells, Invitrogen) or LipofectAMINE 2000 (for HeLa and MCF-7 cells), according to the manufacturer's instructions. To examine the involvement of Akt, cells were treated with Akt inhibitor IV (an Akt specific inhibitor, Calbiochem, 1 μM).
2.3. Transwell cell migration assay
Migration of HeLa or MCF-7 cells was evaluated by Transwell migration assays as described previously [16]. Relative cell migration was determined by the number of the YFP-positive migrated cells normalized to the total number of the YFP-positive cells adhering to the plate. For each experiment, the number of cells in at least eight random fields on the underside of the filter was counted, and four or five independent filters were analyzed.
2.4. Anoikis assay
Cells were detached from tissue culture plates with 0.01% EDTA in PBS and cultured in complete medium in 24-well plates that had been coated with poly-hydroxyethylmethacrylate (poly-HEMA) at a density of 5 × 104 cells/well. They were then collected and fixed in 4% paraformaldehyde in PBS for 15 min. After washing once with PBS, they were incubated with Hoechst 33258 (Molecular Probes) in PBS. Hoechst staining in YFP-positive cells was analyzed with a Leica DC350F digital camera system equipped with a Nikon Eclipse E800 microscope.
2.5. Measurement of RhoG activity
Measurement of RhoG activity in cells was performed as described previously [16]. Densitometry analysis was performed with ImageJ software, and relative RhoG activity was determined by the amount of RhoG bound to GST-ELMO-NT (amino acids 1–362) normalized to the amount of RhoG in cell lysates.
2.6. Immunofluorescence microscopy
Cells on coverslips were fixed with 4% paraformaldehyde in PBS for 15 min and washed with PBS five times. Cells were permeabilized with 0.2% Triton X-100 in PBS for 10 min and incubated with 10% FBS in PBS for 30 min to block nonspecific antibody binding. Then cells were incubated with primary antibodies in PBS for 24 h. After wash with PBS at once, cells were incubated with secondary antibodies conjugated with Alexa Fluor 488 or 594 in PBS for 1 h, washed with PBS for 30 min, and mounted in 90% glycerol containing 0.1% p-phenylenediamine dihydrochloride in PBS. Images were captured using IM50 software (Leica) and a microscope (Eclipse E800, Nikon) with a 40 × 0.95 objective (Nikon) and a digital camera (DC350F, Leica).
3. Results
We previously reported that overexpression of EphA2 induced ligand-independent promotion of cell migration in breast cancer cell line MCF-7 and cervical carcinoma HeLa cells through Ephexin4-mediated RhoG activation [16]. To investigate the relationship between EphA2 S897 phosphorylation and the Ephexin4-dependent signaling pathway, we overexpressed wild-type EphA2 (EphA2-WT) or EphA2 containing S897A substitution (EphA2-SA) in these cell lines and performed Transwell cell migration assays by using FBS as a chemoattractant. Overexpression of EphA2-WT promoted cell migration in both MCF-7 (Fig. 1A) and HeLa cells (Fig. 1B), whereas overexpression of EphA2-SA did not. To confirm that overexpressed EphA2-WT, but not EphA2-SA, was phosphorylated on S897, we used an antibody against phosphorylated S897 of EphA2 (anti-pS897-EphA2 antibody). Immunoprecipitated EphA2-WT was detected with anti-pS897-EphA2 antibody, but EphA2-SA was not (Fig. 1C). These results suggest that S897 phosphorylation of EphA2 plays a role in the EphA2/Ephexin4-mediated cell migration.
Fig. 1.
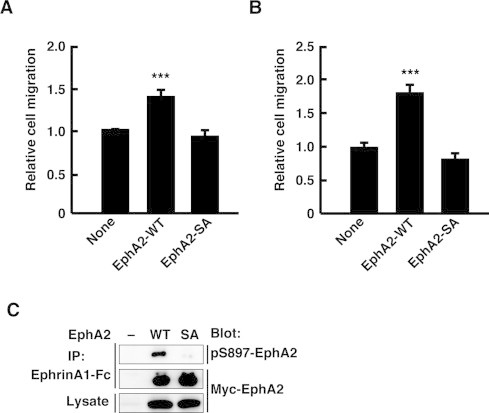
S897A mutation abolishes ligand-independent promotion of cell migration by EphA2 in MCF-7 and HeLa cells. (A and B) Motility of MCF-7 (A) or HeLa cells (B) was evaluated by Transwell cell migration assays. Cells transfected with YFP alone or together with EphA2-WT or EphA2-SA were plated in the upper chamber of the filters, and at 4 h after plating, cells that had migrated to the underside of the filters were fixed. Relative cell migration was determined by the number of the YFP-positive migrated cells normalized to the total number of the YFP-positive attached cells. Data are presented as the means ± SEM from four or five independent experiments (***, P < 0.001; t-test). (C) The cell lysates of HeLa cells transfected with Myc-EphA2-WT or -SA were immunoprecipitated with ephrinA1-Fc, and bound proteins and total cell lysates were analyzed by immunoblotting with the indicated antibodies.
EphA2 also mediates ligand-independent suppression of anoikis through Ephexin4 and RhoG [17]. To examine the role of EphA2 S897 phosphorylation in the EphA2/Ephexin4-mediated suppression of anoikis, we performed an anoikis assay using HeLa cells transfected with EphA2-WT or EphA2-SA. After transfection, HeLa cells were cultured in suspension for 24 h, and apoptotic cell death was analyzed by staining of nuclei with Hoechst 33258. In apoptotic cells, nuclear DNA condensation was observed (Fig. 2A). As we observed previously [17], overexpression of EphA2-WT did not cause a further decrease in the number of apoptotic cells cultured in suspension compared with untransfected cells, because HeLa cells acquire resistance to anoikis and undergo low levels of apoptosis upon detachment from substratum. Therefore, we performed knockdown and rescue experiments. Transfection of HeLa cells with EphA2 shRNA (shEphA2), which effectively reduced the protein level of EphA2 in HeLa cells (Fig. 2B), significantly increased the number of apoptotic cells cultured in suspension for 24 h compared with transfection with control shRNA (shControl). Because shEphA2 used in this study targets human EphA2 mRNA 3′-untranslated region [16], we used a plasmid expressing Myc-tagged EphA2, which contains only the coding region of EphA2. Expression of EphA2-WT completely suppressed the shEphA2-induced promotion of anoikis, whereas expression of EphA2-SA did not (Fig. 2C). In these experiments, we confirmed that there is no significant difference in expression levels between EphA2-WT and EphA2-SA by immunoblotting (data not shown). These results suggest that phosphorylation of EphA2 on S897 is also important for the EphA2/Ephexin4-mediated suppression of anoikis.
Fig. 2.
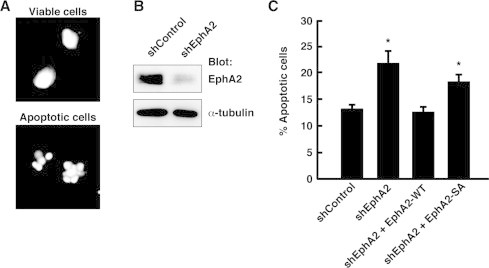
S897A mutation abolishes ligand-independent suppression of anoikis by EphA2 in HeLa cells. (A) At 3 days after transfection, HeLa cells were detached from tissue culture plates and cultured in suspension for 24 h. Then they were collected, and apoptotic cell death was analyzed by staining of nuclei with Hoechst 33258. Examples of scored viable (upper panel) and apoptotic (lower panel) HeLa cells are shown. (B) Lysates from HeLa cells transiently transfected with control luciferase (shControl) or EphA2 shRNA (shEphA2) were analyzed by immunoblotting with antibodies against EphA2 and α-tubulin. (C) Apoptotic cells were scored as a percentage of the total number of transfected cells, and data are the means ± SEM from four independent experiments (*, P < 0.05; t-test). In one experiment, at least 100 cells were counted in randomly selected fields.
Ephexin4 mediates EphA2-induced promotion of cell migration and resistance to anoikis through activation of RhoG [16,17]. We next measured RhoG activity in cells expressing EphA2-WT or EphA2-SA by a pull-down assay with GST-fused N-terminal RhoG-binding region of ELMO, which could specifically interact with GTP-bound active RhoG [8,16]. Expression of EphA2-WT in HeLa cells increased the amount of active RhoG about 6-fold over the basal level. However, expression of EphA2-SA had little effect on the RhoG activity (Fig. 3). These results suggest that EphA2-mediated RhoG activation is regulated by S897 phosphorylation of EphA2. We also measured Rac activity in HeLa cells by the same pull-down assay using GST-fused CRIB domain of Pak. However, we could not show a significant increase in Rac activity in HeLa cells expressing EphA2-WT compared to that in the control cells. A possible reason for this result is that EphA2 binds to a Rac-specific GAP β-chimaerin in HeLa cells [18], which may inactivate another pool of Rac. Therefore total Rac activity in whole cells may not change when EphA2 is overexpressed.
Fig. 3.
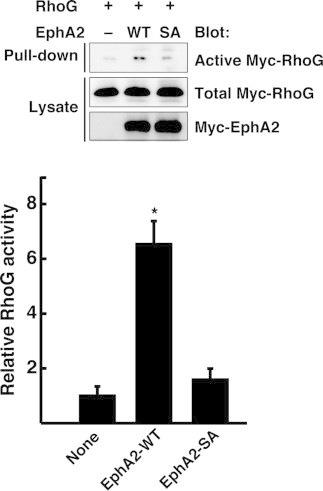
S897A mutation abolishes EphA2-induced RhoG activation. Cell lysates from HeLa cells co-transfected with Myc-RhoG and Myc-EphA2-WT or EphA2-SA were incubated with GST-ELMO, and bound Myc-RhoG (Pull-down) and total cell lysates (Lysates) were analyzed with anti-Myc antibody. Relative RhoG activity was determined by the amount of GTP-bound active Myc-RhoG normalized to the amount of Myc-RhoG in cell lysates analyzed by ImageJ software, and the data shown are relative to the basal activity (None). Data are presented as the means ± SEM from three independent experiments (*, P < 0.05; t-test).
To determine whether S897 phosphorylation of EphA2 affects the interaction between EphA2 and Ephexin4, HEK293T cells were co-transfected with Flag-tagged Ephexin4 and Myc-tagged EphA2-WT or EphA2-SA, and EphA2 was immunoprecipitated from the cell lysates with recombinant ephrinA1-Fc chimera. Ephexin4 was co-immunoprecipitated with EphA2-WT. However, S897A mutation reduced the interaction (Fig. 4A). In this experiment, immunoprecipitated EphA2-WT was detected with an antibody that recognized phosphorylated Akt substrates at Akt consensus phosphorylation sequence (p-Akt-Sub), but the antibody failed to detect a band on the EphA2-SA immunoprecipitate. Treatment of the cells with an Akt inhibitor also reduced the level of EphA2 phosphorylation and the interaction between Myc-EphA2-WT and Flag-Ephexin4 (Fig. 4B). On the other hand, co-expression of a constitutively active form of Akt (HA-myr-Akt, Akt fused with the Src myristoylation signal at the NH2 terminus [19]) promoted the EphA2–Ephexin4 interaction (Fig. 4C). To examine whether endogenous interaction between EphA2 and Ephexin4 was also regulated by Akt-dependent phosphorylation of EphA2, MCF-7 cells were treated with the Akt inhibitor, and the cell lysates were immunoprecipitated with anti-EphA2 antibody. Immunoblot analysis with anti-Ephexin4 antibody demonstrates a reduced interaction between endogenous EphA2 and Ephexin4 in MCF-7 cells in the presence of the Akt inhibitor (Fig. 4D). We used anti-pS897-EphA2 antibody to show the effect of the Akt inhibitor on S897 phosphorylation of EphA2. These results suggest that phosphorylation of EphA2 on S897 by Akt promotes the interaction between EphA2 and Ephexin4.
Fig. 4.
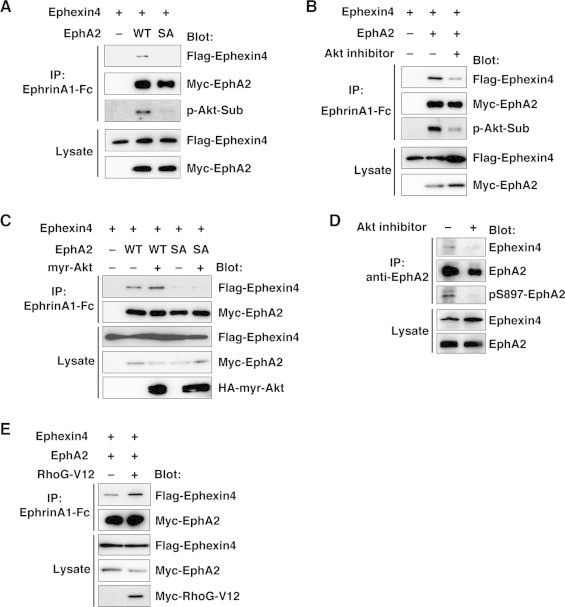
Phosphorylation of EphA2 on S897 regulates the interaction between EphA2 and Ephexin4. (A–C and E) The cell lysates of HEK293T cells transfected indicated plasmids were immunoprecipitated with ephrinA1-Fc, and bound proteins and total cell lysates were analyzed by immunoblotting with the indicated antibodies (p-Akt-Sub, an antibody that recognized phosphorylated Akt substrates at Akt consensus phosphorylation sequence; myr-Akt, Akt fused with the Src myristoylation signal at the NH2 terminus). (D) The lysates of MCF-7 cells were immunoprecipitated with anti-EphA2 antibody, and bound proteins and total cell lysates were analyzed by immunoblotting with the indicated antibodies (pS897-EphA2, an antibody against phosphorylated S897 of EphA2).
Because activation of RhoG triggers the PI3K/Akt signaling pathway [10–12], we next examined whether activation of RhoG regulates the EphA2–Ephexin4 interaction. HEK293T cells were co-transfected with Flag-Ephexin4 and Myc-EphA2-WT together with a constitutively active form of RhoG (Myc-RhoG-V12), and EphA2 was immunoprecipitated from the cell lysates with recombinant ephrinA1-Fc chimera. We found that co-expression of RhoG-V12 increased the interaction between EphA2 and Ephexin4 (Fig. 4E), suggesting the existence a positive feedback mechanism of regulating RhoG activity.
To examine whether S897 phosphorylation of EphA2 regulates the subcellular localization of Ephexin4, MCF-7 cells were transfected with EphA2-WT or EphA2-SA, and endogenous Ephexin4 was visualized with anti-Ephexin4 antibody. Consistent with previous reports [4], EphA2-WT was localized to cell protrusions at the leading edge in addition to the cell–cell junctions, and it was also observed in the perinuclear region in MCF-7 cells. Immunofluorescence staining with anti-Ephexin4 antibody revealed that Ephexin4 was mainly localized in the cytoplasm but partly co-localized with EphA2-WT at the leading edge in the cells expressing EphA2-WT (Fig. 5A). However, little Ephexin4 staining was observed at the cell–cell junctions. On the other hand, EphA2-SA was localized to the cell–cell junctions and the perinuclear region, and expression of EphA2-SA had no effect on the subcellular localization of Ephexin4. We verified the specificity of Ephexin4 staining in MCF-7 cells transfected with control luciferase shRNA (shControl) or Ephexin4 shRNA (shEphexin4), showing that anti-Ephexin4 antibody staining was reduced by Ephexin4 knockdown (Fig. 5B). These results suggest that phosphorylation of EphA2 on S897 regulates EphA2–Ephexin4 interaction in intact cells.
Fig. 5.
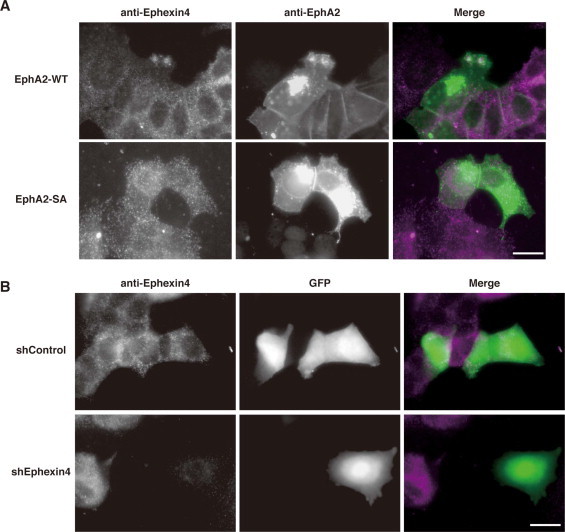
Phosphorylation of EphA2 on S897 regulates the subcellular localization of Ephexin4. (A) MCF-7 cells were transfected with EphA2-WT or EphA2-SA. At 24 h after transfection, they were fixed and subjected to immunofluorescent staining with anti-EphA2 and anti-Ephexin4 antibodies. The right panels show the merge of the two images with Ephexin4 (magenta) and EphA2 (green). (B) MCF-7 cells were transfected with GFP together with control luciferase shRNA (shControl) or Ephexin4 shRNA (shEphexin4). At 72 h after transfection, they were fixed and subjected to immunofluorescent staining with anti-Ephexin4 antibody. The right panels show the merge of the two images with Ephexin4 (magenta) and GFP (green). Bars, 20 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
EphA2 is phosphorylated on S897 by Akt, and this phosphorylation is required for ligand-independent promotion of cell migration by EphA2. In addition, S897 phosphorylation of EphA2 is correlated with malignant progression of human astrocytoma [4]. In this study, we report a mechanism by which phosphorylation of EphA2 on S897 induces promotion of cell migration and suppression of anoikis in cancer cells. We previously described the role of Ephexin4 in the ligand-independent promotion of cell migration and suppression of anoikis by EphA2. Ephexin4 interacts with EphA2 to induce activation of RhoG. RhoG binds to its effector ELMO2 and recruits ELMO2 and its binding partner Dock4, a GEF for the small GTPase Rac, from the cytoplasm to the plasma membrane. The recruitment of the ELMO2–Dock4 complex triggers a local activation of Rac by Dock4, which can cause the formation of cortactin-rich protrusion, leading to promotion of cell polarization and migration [16]. On the other hand, EphA2/Ephexin4-mediated RhoG activation also promotes resistance to anoikis in cancer cells through PI3K/Akt signaling pathway [17]. In the present study, we show that S897 phosphorylation of EphA2 by Akt promotes the interaction between EphA2 and Ephexin4 and the Ephexin4-dependent RhoG activation, leading to promotion of cell migration and suppression of anoikis. Thus, our data provide evidence for Ephexin4 as a molecular link between S897 phosphorylation of EphA2 and EphA2-mediated tumor progression.
The cytoplasmic domains of Eph receptors contain several phosphorylation sites [20,21]. We show here that phosphorylation of EphA2 on S897 regulates the recruitment of Ephexin4. S897 of EphA2 is located in the linker region between the kinase and SAM domains. We previously demonstrated that EphA2 interacted with Ephexin4 through the kinase domain, which was enhanced by deletion of the C-terminal SAM domain and the linker region containing S897 [16]. Therefore, the linker region and/or the SAM domain have an inhibitory effect on the interaction of EphA2 with Ephexin4, and phosphorylation of EphA2 on S897 may trigger a conformational change within the cytoplasmic region and expose the Ephexin4 binding site to promote the interaction with Ephexin4 and the Ephexin4-mediated RhoG activation.
Because activation of RhoG triggers the PI3K/Akt signaling pathway [10–12], the EphA2–Ephexin4 interaction might be regulated downstream of RhoG through Akt. There have been some previous reports showing positive feedback regulations of the Rho family GTPase activities. For example, Dia1, in addition to its role as a downstream effector for RhoA, can bind to the leukemia-associated Rho-GEF (LARG) and stimulates its GEF activity on RhoA, and this positive feedback loop toward RhoA activity is necessary for LPA-stimulated Rho/ROCK signaling for tumor cell morphology and invasion [22]. On the other hand, RhoG is a potent activator of the PI3K/Akt signaling pathway [10–12], and we show here that expression of constitutively active RhoG or Akt promotes the interaction between EphA2 and Ephexin4 through S897 phosphorylation of EphA2. Based on these findings, it is conceivable that activation of RhoG downstream of EphA2 and Ephexin4 may promote the interaction between EphA2 and Ephexin4 through the activation of PI3K and Akt, which in turn activates additional RhoG, providing a positive feedback mechanism of RhoG activation by EphA2 and Ephexin4.
Acknowledgements
We thank Dr. Tetsuichiro Saito (Chiba University, Chiba, Japan) for providing an YFP expression vector, and Dr. Junichi Miyazaki (Osaka University, Osaka, Japan) for a CAG promoter-containing vector. This work was supported in part by Grants-in-aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Pasquale E.B. Eph–Ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Pasquale E.B. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat. Rev. Cancer. 2010;10:165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merlos-Suarez A., Batlle E. Eph–ephrin signalling in adult tissues and cancer. Curr. Opin. Cell Biol. 2008;20:194–200. doi: 10.1016/j.ceb.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Miao H., Li D.Q., Mukherjee A., Guo H., Petty H., Cutter J., Basilion J.P., Sedor J., Wu J., Danielpour D., Sloan A.E., Cohen M.l., Wang B. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell. 2009;16:9–20. doi: 10.1016/j.ccr.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katoh H., Yasui H., Yamaguchi Y., Aoki J., Fujita H., Mori K., Negishi M. Small GTPase RhoG is a key regulator for neurite outgrowth in PC12 cells. Mol. Cell. Biol. 2000;20:7378–7387. doi: 10.1128/mcb.20.19.7378-7387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.deBakker C.D., Haney L.B., Kinchen J.M., Grimsley C., Lu M., Klingele D., Hsu P.K., Chou B.K., Cheng L.C., Blangy A., Sondek J., Hengartner M.O., Wu Y.C., Ravichandran K.S. Phagocytosis of apoptotic cells is regulated by a UNC-73/TRIO-MIG-2/RhoG signaling module and armadillo repeats of CED-12/ELMO. Curr. Biol. 2004;14:2208–2216. doi: 10.1016/j.cub.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 7.Katoh H., Hiramoto K., Negishi M. Activation of Rac1 by RhoG regulates cell migration. J. Cell Sci. 2006;119:56–65. doi: 10.1242/jcs.02720. [DOI] [PubMed] [Google Scholar]
- 8.Katoh H., Negishi M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature. 2003;424:461–464. doi: 10.1038/nature01817. [DOI] [PubMed] [Google Scholar]
- 9.Hiramoto K., Negishi M., Katoh H. Dock4 is regulated by RhoG and promotes Rac-dependent cell migration. Exp. Cell Res. 2006;312:4205–4216. doi: 10.1016/j.yexcr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Murga C., Zohar M., Teramoto H., Gutkind J.S. Rac1 and RhoG promote cell survival by the activation of PI3K and Akt, independently of their ability to stimulate JNK and NF-kB. Oncogene. 2002;21:207–216. doi: 10.1038/sj.onc.1205036. [DOI] [PubMed] [Google Scholar]
- 11.Yamaki N., Negishi M., Katoh H. RhoG regulates anoikis through a phosphatidylinositol 3-kinase-dependent mechanism. Exp. Cell Res. 2007;313:2821–2832. doi: 10.1016/j.yexcr.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Fujimoto S., Negishi M., Katoh H. RhoG promotes neural progenitor cell proliferation in mouse cerebral cortex. Mol. Biol. Cell. 2009;20:4941–4950. doi: 10.1091/mbc.E09-03-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simpson C.D., Anyiwe K., Schimmer A.D. Anoikis resistance and tumor metastasis. Cancer Lett. 2008;272:177–185. doi: 10.1016/j.canlet.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 14.Shamah S.M., Lin M.Z., Goldberg J.L., Estrach S., Sahin M., Hu L., Bazalakova M., Neve R.L., Corfas G., Debant A., Greenberg M.E. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;312:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- 15.Sahin M., Greer P.L., Lin M.Z., Poucher H., Eberhart J., Schmidt S., Wright T.M., Shamah S.M., O’Connell S., Cowan C.W., Hu L., Goldberg J.L., Debant A., Corfas G., Krull C.E., Greenberg M.E. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron. 2005;46:191–204. doi: 10.1016/j.neuron.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 16.Hiramoto-Yamaki N., Takeuchi S., Ueda S., Harada K., Fujimoto S., Negishi M., Katoh H. Ephexin4 and EphA2 mediate cell migration through a RhoG-dependent mechanism. J. Cell Biol. 2010;190:461–477. doi: 10.1083/jcb.201005141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harada K., Hiramoto-Yamaki N., Negishi M., Katoh H. Ephexin4 and EphA2 mediate resistance to anoikis through RhoG and phosphatidylinositol 3-kinase. Exp. Cell Res. 2011;317:1701–1713. doi: 10.1016/j.yexcr.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi S., Yamaki N., Negishi M., Katoh H. β2-Chimaerin binds to EphA receptors and regulates cell migration. FEBS Lett. 2007;583:1237–1242. doi: 10.1016/j.febslet.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 19.Ito Y., Oinuma I., Katoh H., Kaibuchi K., Negishi M. Sema4D/plexin-B1 activates GSK-3β through R-Ras GAP activity, inducing growth cone collapse. EMBO Rep. 2006;7:704–709. doi: 10.1038/sj.embor.7400737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kullander K., Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat. Rev. Mol. Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- 21.Pasquale E.B. Eph receptor signalling casts a wide net on cell behavior. Nat. Rev. Mol. Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 22.Kitzing T.M., Sahadevan A.S., Brandt D.T., Knieling H., Hannemann S., Fackler O.T., Großhans J., Grosse R. Positive feedback between Dia1, LARG, and RhoA regulates cell morphology and invasion. Genes Dev. 2007;21:1478–1483. doi: 10.1101/gad.424807. [DOI] [PMC free article] [PubMed] [Google Scholar]


