Abstract
Some ligand–receptor couples involve spare receptors, which are apparent when a maximal response is achieved with only a small fraction of the receptor population occupied. This situation favours cross-reactions with low-affinity ligands, which may be detrimental for cell signaling. In the case of the adenosine A2A receptors (A2AR), which have an immunosuppressive effect on lymphocytes through cAMP production, the presence of spare A2AR remains to be established. We examined the situation using patients over-expressing lymphocyte A2AR and an agonist-like mAb to A2AR. We found that maximal mAb binding and functional response varied among the patients whereas the dissociation constant and half-maximal effective concentration had similar mean values (0.19 and 0.18 μM, respectively). Lymphocyte A2AR expression was correlated to plasma adenosine level and A2AR occupation but not to A2AR response. These results are consistent with a lack of a reserve of functional A2AR on human lymphocytes as a general rule and suggest that the amount and functional state of the expressed A2AR determine the maximal level of the lymphocyte response to adenosine.
Keywords: Adenosine A2A receptor, Agonist, Lymphocyte, mAb, Spare receptor
Abbreviations: A2AR, adenosine A2A receptors; Emax, maximal ligand response; Bmax, maximal occupation of the receptors; EC50, half-maximal effective concentration; KD, dissociation constant; NMS, neurally-mediated syncope; APC, adenosine plasma concentration; PBMC, peripheral blood mononuclear cells; AU, arbitrary units
Highlights
▸ We examined the presence of a reserve of A2A receptors (A2AR) on human lymphocytes. ▸ We used patient's lymphocytes overexpressing A2AR and an agonist-like mAb to A2AR. ▸ We studied the occupation, function and expression of A2AR. ▸ We found no reserve of A2AR in most patients we examined. ▸ A2AR expression was not correlated to the efficiency of the functional response.
1. Introduction
Adenosine, an endogenous purine nucleoside, acts through four classes of G protein-coupled receptors (A1R, A2AR, A2BR and A4R) to exert various physiologic effects [1]. Most of these actions affect cardiovascular, neuronal and immune cells [2–7]. In lymphocytes, adenosine regulates multiple physiologic processes including inflammation [8–14] and congenital defect of adenosine deaminase – an enzyme that degrades the nucleoside and hence regulates its plasma level – associated with aberrant signaling through adenosine receptors contributes to the severe combined immunodeficiency syndrome [15]. CD4+ and CD8+ T-cells express A2AR, A2BR, and A3R [16–21]. Numerous in vivo and in vitro studies suggest that A2AR selectively inhibit T-cell receptors of activated T-cells, thereby inhibiting lymphocyte inflammatory activity [18,22–25]. Activation of the A2AR on CD4+ T-cells prevents myocardial ischemia–reperfusion injury by inhibiting the lymphocyte accumulation and activation in the reperfused heart [26]. A2AR can also prevent Th1/Th2 development [27] as well as T-cell apoptosis [28]. Finally, it was reported that adenosine produced by regulatory T-cells mediates immune suppression activity through A2AR [29,30]. Thus, the study of the expression and function of A2AR on lymphocytes appears to be of pivotal importance to evaluate their immuno-regulatory role.
Various ligand–receptor models are available in pharmacology and may be applicable to agonist–A2AR interactions in order to study the role of A2AR in lymphocyte regulation. Among them, the spare receptor theory [31] postulates that a given ligand can exert maximum biological effect while occupying only a small amount of the available receptors. Such a reserve of receptors may allow for a response to low, transient ligand concentrations and low-affinity interactions which are compatible with several T-cells properties, hence supporting the presence of spare receptors for T-cell receptor activation [32]. From a pharmacological point of view, the presence of spare receptors is evidenced if the maximal ligand response (Emax) is obtained at less than the maximal occupation of the receptors (Bmax). This pattern is usually determined by comparing the half-maximal effective concentration (EC50) that refers to the ligand concentration necessary to reach 50% of the maximal effect with the dissociation constant (KD) that refers to the ligand concentration necessary to reach 50% of the maximal binding. If the EC50 is less than the KD, spare receptors are existing.
Spare A2AR were reported in cardiac tissue of guinea pig using an irreversible A2AR antagonist to block receptor response to various agonists [33]. In other study on mouse T-cells, using gene targeting to generate mice lacking one or two alleles of A2AR, the authors showed that the decrease in the number of A2AR in thymocytes from A2AR+/− and A2AR−/− mice versus the A2AR+/+ control mice resulted in proportional decrease in the adenosine-induced cAMP and apoptosis responses of their respective T-cells [34]. No investigations were conducted on human lymphocyte A2AR expression, occupation and function because of some experimental constraints, mainly low A2AR expression in normal individuals and lack of efficient ligand to stably bind to the receptor. Here, we took advantage of Adonis, an agonist-like mAb which steadily binds to an extra-cellular part of the A2AR [35], to investigate the presence of a reserve of A2AR in peripheral lymphocytes of patients with neurally-mediated syncope (NMS) who generally display high adenosine plasma concentrations (APC) and increased lymphocyte A2AR expressions [36–38].
2. Materials and methods
2.1. Selection of patients
Ten patients with clinical symptoms of NMS [38] were enrolled. NMS patients were chosen because they frequently exhibit high APC [36,39] associated with high expression levels of A2AR on peripheral lymphocytes [37], which was found to mirror the increase in A2AR expression on disease target organs [40]. This study was conducted in compliance with the principles of the Declaration of Helsinki and approved by the Ethics Committee for Human Research of our university hospital. All patients provided written informed consent to participate.
2.2. Adenosine assay
Blood sampling was processed as described using laboratory-prepared tubes containing 3 ml of cold-stop solution composed of inhibitors of degradation and red blood cell uptake of adenosine [36,39,41]. Samples were then maintained on ice until centrifugation. After deproteinization, adenosine was quantified by HPLC (ChromSystems, Munich, Germany), adenosine being identified by its elution time and spectrum [39]. Measurement was made by comparison of peak areas with those obtained using standards. The intra-assay and inter-assay coefficients of variation ranged from 1% to 3%.
2.3. Incubation of Adonis with lymphocytes
Blood samples were collected from brachial vein into 8 ml tubes containing sodium citrate/Ficoll (BD Vacutainer CPT, Beckton Dickinson, Franklin lakes, NJ). Peripheral blood mononuclear cells (PBMC) were prepared according to the manufacturer's instructions. Viable cell recovery was consistently over 98% with more than 95% of lymphocytes and less than 3% granulocytes. 0.25 × 106 cells were mixed with Adonis (0–1.2 μM in 1 ml culture medium), an IgM mAb directed against a linear part of the second external loop of A2AR [35]. After 90 min incubation at room temperature under shaking, PBMC were either washed twice with phosphate-buffered saline, pH 7.3 for binding and A2AR expression tests or only centrifuged without washing for functional test. Cell pellets were kept at −20 °C until use.
2.4. Western blotting
To quantify both Adonis binding to lymphocytes and the lymphocyte A2AR expression, we used the Western blotting procedure previously described [42]. PBMC pellets previously incubated with or without Adonis were lysed by a 3 min sonication treatment at 47 kHz with the SDS–PAGE loading buffer containing 5% mercaptoethanol. Samples were then submitted to 12% SDS–PAGE analysis followed by transfer onto a PVDF membrane. Filters were incubated 20 min with Adonis and then with horseradish peroxidase-labeled anti-mouse IgG Fab specific antibodies prior to chemiluminescent staining with SuperSignal West Femto (Pierce Biotechnology, Rockford, IL).
In SDS–PAGE analysis in reducing condition, Adonis resolved in heavy (65 kDa) and light (25 kDa) IgM chains bands. Quantification of Adonis bound to A2AR was based on the detection of the light chain of the IgM because it is readily detected by the labeled second antibody directed partly to the light chain of Fab portion of mouse IgG shared by mouse IgM. Densitometry analysis used ImageJ 1.42q (National Institutes of Health, USA) and results from duplicates were expressed as arbitrary units (AU) defined as pixels of the light chain band versus blot background.
The lymphocyte A2AR expression was similarly quantified except that AU were defined as pixels of the canonical A2AR band versus blot background. A2AR from PBMC not previously incubated with Adonis migrated at 45 kDa and the corresponding band was detected by Adonis binding during the Western blot procedure.
Adonis binding curves were established using six concentrations of Adonis and non linear regression analysis were performed with a one site specific binding equation Y = Bmax × X /(KD + X) using Prism 5 (GraphPad Software, Inc La Jolla, CA). Bmax expressed using the same units as Y values and KD expressed using the same units as X values were estimated for Adonis binding to each PBMC preparation.
2.5. cAMP test
The agonist properties of Adonis allow its use as a specific effector for cAMP production [35]. Briefly, cellular cAMP measurement was achieved on PBMC pellets incubated with Adonis by ELISA using the Amersham Biotrak Kit (GE healthcare Bio-Sciences, Upsala, Sweden). Stimulation was stopped by adding dodecyltrimethylammonium bromide acetate buffer. Results of duplicates were expressed as AU defined as cAMP production generated in the presence versus in the absence of Adonis. We examined the results obtained using six concentrations of Adonis with Prism 5 (GraphPad Software) and the sigmoidal dose–response curve: Y = Bottom + (Top − Bottom)/(1 + 10[(Log EC50 − X) × Hill Slope ]). Top (Emax here) and Bottom are plateau values in the units of Y and EC50 is the concentration of Adonis that gives a response half way between Top and Bottom values. Hill slope describes the steepness of the family of curves.
2.6. Statistical analysis
Quantitative variables were expressed using means ± SEM. Paired t-test was used to compare KD and EC50 values and correlation tests were made between adenosine, Bmax and Emax values. All the tests were two-tailed and P-values less than 0.05 were considered as statistically significant. Analyses were performed with Prism 5 (GraphPad Software).
3. Results
3.1. Adenosine levels
The patients listed in Table 1 (7 women and 3 men; mean age: 52 years) were selected on the basis of NMS clinical symptoms. They had APC ranging from 0.29 to 2.91 μM with a mean value ± SEM of 1.29 ± 0.27 μM. As expected from the literature on NMS, most patients (7 out of 10) had higher APC than control subjects (0.20 to 0.70 μM; data from the Biochemistry Laboratory of the Timone Hospital, Marseille, France).
Table 1.
List of patients with results.
| Patients | Sex | Age | APC (μM) | Bmax (AU) | KD (μM) | Emax (AU) | EC50 (μM) | A2AR (AU) |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 62 | 1.55 | 63.14 | 0.12 | 22.57 | 0.48 | 19.56 |
| 2 | Female | 18 | 0.99 | 26.66 | 0.15 | 32 | 0.1 | 19.53 |
| 3 | Female | 39 | 2.37 | 68.13 | 0.09 | 16.5 | 0.08 | 32.02 |
| 4 | Female | 49 | 1.82 | 59.16 | 0.08 | 28.29 | 0.11 | 12.12 |
| 5 | Female | 74 | 0.77 | 27.33 | 0.33 | 34.62 | 0.36 | 6.41 |
| 6 | Male | 38 | 2.91 | 61.49 | 0.26 | 41.73 | 0.23 | 17.97 |
| 7 | Male | 62 | 0.29 | 12.39 | 0.42 | 49.89 | 0.09 | 8.69 |
| 8 | Female | 49 | 0.66 | 8.92 | 0.23 | 21.56 | 0.17 | 7.29 |
| 9 | Female | 52 | 1.01 | 3.05 | 0.08 | 31.57 | 0,11 | 9.24 |
| 10 | Male | 71 | 0.5 | 12.37 | 0.13 | 36 | 0.06 | 8.61 |
| Mean | 51.7 | 1.29 | 34.26 | 0.19 | 31.47 | 0.18 | 14.14 | |
| ± SEM | 5.43 | 0.27 | 8.18 | 0.04 | 3.14 | 0.04 | 2.56 |
3.2. Saturation curves and dose–response curves
Adonis binding to PBMC from patients was assessed by Western blotting visualizing the light chain of bound Adonis (Fig. 1(A)). After densitometry analysis of the bands, the resulting Adonis binding curves exhibited various plateau values ranging from 3.05 to 68.13 AU (pixels of the light chain band versus blot background) with a mean value ± SEM of 34.26 ± 8.18 AU (Fig. 1(B) and Table 1). Representative curves from Patients 1 and 2 were highlighted in black in Fig. 1 (B). Binding reached a plateau value at 63.14 AU of Adonis binding for Patient 1 and at 26.66 AU for Patient 2, both patients exhibiting similar KD value (0.12 and 0.15 μM, respectively; Table 1).
Fig. 1.
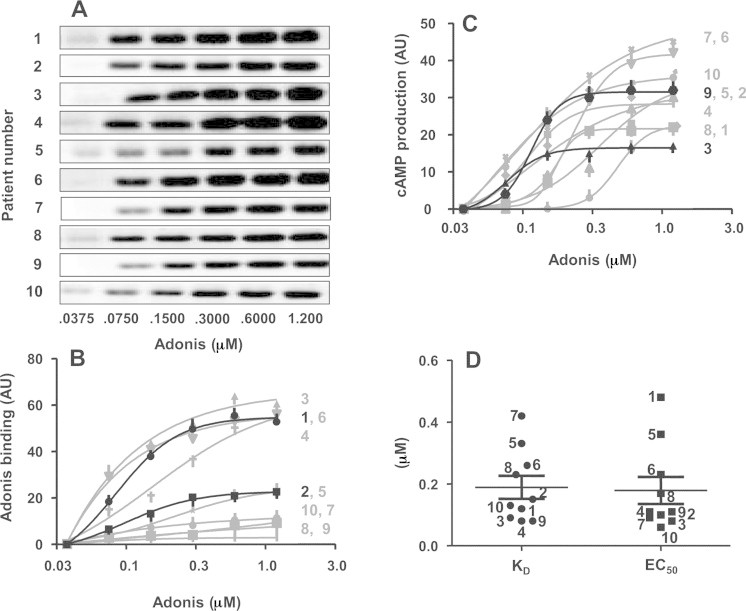
(A) Western blots of Adonis (25 kDa light chain) binding to PBMC from the 10 patients. For each patient, 6 doses of Adonis were tested in duplicate on separate gels and one representative set of blots is shown. (B) Binding curves giving Bmax and KD values. Curves derived from the pixel analysis of the Adonis light chain blots. (C) Dose–response curves giving Emax and EC50 values for the 10 patients. For each set of curves, 2 representative curves are in black, the 8 other ones are in gray. Patient numbers are at right of the curves. Results expressed in arbitrary units (AU) as defined in Materials and methods are means ± SD of duplicates. (D) Comparison of KD (black circles) and EC50 (black squares) values interpolated from complete binding and dose–response curves with Adonis. Results are expressed in μM. Each symbol with the corresponding patient number represents individual mean value. Horizontal bars are means ± SEM for the 10 patients.
Adonis response curves for cAMP production also exhibited various plateau values ranging from 16.50 to 49.89 AU (cAMP production generated by Adonis versus basal cAMP level in the absence of Adonis) with a mean value ± SEM of 31.47 ± 3.14 AU (Fig. 1(C) and Table 1). Representative curves from Patients 9 and 3 were highlighted in black in Fig. 1 (C). A maximal effect at 31.57 AU of cAMP production was obtained for Patient 9 whereas only 16.5 AU were obtained for Patient 3, both patients exhibiting similar EC50 values (0.11 and 0.08 μM, respectively; Table 1).
Fig. 1(D) shows that, taken as a whole, the distribution of KD and EC50 values extrapolated from the above curves was not statistically different: KD values ranged from 0.08 to 0.42 μM with a mean value ± SEM of 0.19 ± 0.04 μM and EC50 values ranged from 0.06 to 0.48 μM with a mean value ± SEM of 0.18 ± 0.04 μM. We noticed 2 exceptions, Patient 7 displaying the highest KD (0.42 μM) had a 5-fold lower EC50 (0.09 μM) and, conversely, Patient 1 displaying the highest EC50 (0.48 μM) had a 4-fold lower KD (0.12 μM) (Fig. 1(D) and Table 1).
3.3. Expression of A2AR
We found various lymphocyte A2AR expression levels with values ranging from 6.41 to 32.02 AU (pixels of the A2AR band versus blot background) with a mean value ± SEM of 14.14 ± 2.56 AU. Correlation studies were performed to compare lymphocyte A2AR expressions to APC, Bmax and Emax. Lymphocyte A2AR expressions were found to be correlated with APC (Fig. 2(A)) and Bmax (Fig. 2(B)) but not Emax (Fig. 2(C)) values. Once more, we noticed 2 exceptions: Patient 7 displaying the highest Emax (49.89 AU) had low A2AR level (8.69 AU) while Patient 3 displaying the lowest Emax (16.5 AU) expressed the highest A2AR level (32.02 AU).
Fig. 2.
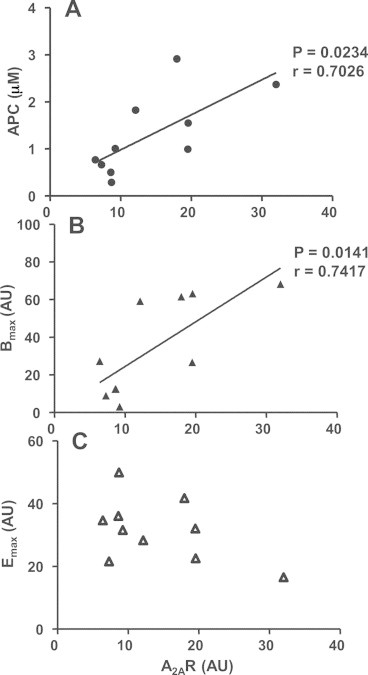
Correlation study between lymphocyte A2AR expression levels and (A) APC, (B) Bmax and (C) Emax. Results are expressed in μM for adenosine plasma levels and arbitrary units (AU) as defined in Materials and methods for A2AR expression, Bmax and Emax levels. A2AR expressions were measured using Western blotting. Each symbol represents individual mean value. Significant correlation (P < 0.05) for the 10 patients is indicated at right.
4. Discussion
We found that Bmax of Adonis to A2AR varied from subject to subject whereas KD values were similar. Various Emax were also found whereas the EC50 values were similar. More importantly, the KD values were found to be statistically not different from the EC50 values, suggesting a lack of a reserve of receptors according to the criteria previously defined. In 8 out of the 10 patients tested, the highest cAMP production occurred when all the receptors were occupied by Adonis. Results from 2 patients, however, illustrate opposite situations: Patient 7 exhibited a KD which was 5-fold higher than EC50, consistent with the situation where a few occupied receptors produce a maximal effect (presence of spare receptors), and Patient 1 exhibited a KD which was 4-fold lower than EC50, indicating a situation where a low effect is obtained while all receptors are occupied. The latter case suggests that many A2AR of this subject are not functional.
We also performed experiments to address the lymphocyte A2AR expression using Western blotting. As previously reported in patients with intradialytic hypotension during hemodialysis [43] and spontaneous and head-up-tilt-induced syncope [37,38], we found an over-expression of lymphocyte A2AR which correlated with the increase in APC and therefore with the maximal level of Adonis binding. This suggests that adenosine and A2AR are upregulated concomitantly and/or interdependently. More intriguingly, lymphocyte A2AR expression was generally not correlated with Emax suggesting a non linear relation between the amount of receptors and their overall efficiency. Therefore, adenosine appeared necessary but not sufficient to modulate its immunosuppressive effect via the A2AR. Two patients illustrate opposite situations regarding Emax: Patient 7, previously suspected with spare receptors, who expressed a few but highly efficient receptors and, conversely, Patient 3 who expressed high level of receptors exhibiting low overall efficiency. The former, displaying a KD higher than EC50, had probably some receptors engaged in an hyper-active state with an efficient coupling to the effector (Gs for cAMP production) whereas the latter, displaying a KD similar to EC50, likely expressed mostly inactive receptors, unmounted to cell-surface and constituting a putative reserve of intra-cellular standby receptors.
T-cells possess a T-cell receptor reserve to increase the flexibility of cell response to ligands of weaker potency [44]. Accordingly, we suggest that a reserve of A2AR would promote cross-reactions with low affinity ligands such as weak and partial agonists/antagonists. Consequently, stimulating or blocking ligands other than adenosine would impede the fine tuning of adenosine-induced immune regulation by occupying more receptors to respond. However, absence of spare receptors does not systematically mean that the amount of expressed A2AR on lymphocytes is the limiting factor to determine the extent of the effector response. Regarding the NMS situation, A2AR are frequently over-expressed in peripheral lymphocytes and some patients having a specific A2AR gene polymorphism express more receptors [45]. However, the resulting efficiency of the A2AR response can also depend on the functional state of the receptors. The multimerization process of G protein-coupled receptors [46] modulates agonist efficiency when occupancy of a single receptor is needed to obtain a full agonist action. For a recent example, TSH receptor signals via activation of Gs to stimulate cAMP production when TSH binds to one protomer of the TSH receptor homodimer [47]. Therefore, the presence of spare receptors may also refer to the functional state of the multimerized receptors.
Together, these data are consistent with a lack of spare A2AR on lymphocytes as a general rule and their putative presence in some patients appeared to be dependent on the functional state of the targeted receptors. Therefore, the adenosinergic signaling efficiency in lymphocytes likely involves many but not a few A2AR expressed on lymphocytes and this is the amount and functional state of these expressed receptors that determine the maximal level of the lymphocyte response to adenosine.
Acknowledgments
Y. By is a recipient of grant from the Assistance Publique, Hôpitaux de Marseille, France
References
- 1.Olah ME, Stiles GL. Adenosine receptor subtypes: characterization and therapeutic regulation. Annu. Rev. Pharmacol. Toxicol. 1995;35:581–606. doi: 10.1146/annurev.pa.35.040195.003053. [DOI] [PubMed] [Google Scholar]
- 2.Cohen MV, Yang XM, Downey JM. Acidosis, oxygen, and interference with mitochondrial permeability transition pore formation in the early minutes of reperfusion are critical to postconditioning's success. Basic Res. Cardiol. 2008;103:464–471. doi: 10.1007/s00395-008-0737-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng J, Wang R, Zambraski E, Wu D, Jacobson KA, Liang BT. Protective roles of adenosine A1, A2A, and A3 receptors in skeletal muscle ischemia and reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H3685–H3691. doi: 10.1152/ajpheart.00819.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorklund O, Shang M, Tonazzini I, Dare E, Fredholm BB. Adenosine A1 and A3 receptors protect astrocytes from hypoxic damage. Eur. J. Pharmacol. 2008;596:6–13. doi: 10.1016/j.ejphar.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Fredholm BB, Chen JF, Masino SA, Vaugeois JM. Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu. Rev. Pharmacol. Toxicol. 2005;45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- 6.Chen GJ, Harvey BK, Shen H, Chou J, Victor A, Wang Y. Activation of adenosine A3 receptors reduces ischemic brain injury in rodents. J. Neurosci. Res. 2006;84:1848–1855. doi: 10.1002/jnr.21071. [DOI] [PubMed] [Google Scholar]
- 7.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bours MJ, Swennen EL, Di VF, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Marone G, Vigorita S, Triggiani M, Condorelli M. Adenosine receptors on human lymphocytes. Adv. Exp. Med. Biol. 1986;195 Pt B:7–14. doi: 10.1007/978-1-4684-1248-2_2. [DOI] [PubMed] [Google Scholar]
- 10.Marone G, Petracca R, Vigorita S, Genovese A, Casolaro V. Adenosine receptors on human leukocytes. IV. Characterization of an A1/Ri receptor. Int. J. Clin. Lab Res. 1992;22:235–242. doi: 10.1007/BF02591429. [DOI] [PubMed] [Google Scholar]
- 11.Priebe T, Kandil O, Nakic M, Pan BF, Nelson JA. Selective modulation of antibody response and natural killer cell activity by purine nucleoside analogues. Cancer Res. 1988;48:4799–4803. [PubMed] [Google Scholar]
- 12.Priebe T, Platsoucas CD, Nelson JA. Adenosine receptors and modulation of natural killer cell activity by purine nucleosides. Cancer Res. 1990;50:4328–4331. [PubMed] [Google Scholar]
- 13.Priebe T, Platsoucas CD, Seki H, Fox FE, Nelson JA. Purine nucleoside modulation of functions of human lymphocytes. Cell Immunol. 1990;129:321–328. doi: 10.1016/0008-8749(90)90208-9. [DOI] [PubMed] [Google Scholar]
- 14.Priebe T, Ruiz L, Nelson JA. Role of natural killer cells in the modulation of primary antibody production by purine nucleosides and their analogs. Cell Immunol. 1990;130:513–519. doi: 10.1016/0008-8749(90)90291-x. [DOI] [PubMed] [Google Scholar]
- 15.Apasov SG, Sitkovsky MV. The extracellular versus intracellular mechanisms of inhibition of TCR-triggered activation in thymocytes by adenosine under conditions of inhibited adenosine deaminase. Int. Immunol. 1999;11:179–189. doi: 10.1093/intimm/11.2.179. [DOI] [PubMed] [Google Scholar]
- 16.Gessi S, Varani K, Merighi S, Cattabriga E, Pancaldi C, Szabadkai Y, Rizzuto R, Klotz KN, Leung E, Mac LS, Baraldi PG, Borea PA. Expression, pharmacological profile, and functional coupling of A2B receptors in a recombinant system and in peripheral blood cells using a novel selective antagonist radioligand, [3H]MRE 2029-F20. Mol. Pharmacol. 2005;67:2137–2147. doi: 10.1124/mol.104.009225. [DOI] [PubMed] [Google Scholar]
- 17.Gessi S, Varani K, Merighi S, Cattabriga E, Avitabile A, Gavioli R, Fortini C, Leung E, Mac LS, Borea PA. Expression of A3 adenosine receptors in human lymphocytes: up-regulation in T cell activation. Mol. Pharmacol. 2004;65:711–719. doi: 10.1124/mol.65.3.711. [DOI] [PubMed] [Google Scholar]
- 18.Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2A extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600–1610. [PubMed] [Google Scholar]
- 19.Hoskin DW, Mader JS, Furlong SJ, Conrad DM, Blay J. Inhibition of T cell and natural killer cell function by adenosine and its contribution to immune evasion by tumor cells (Review) Int. J. Oncol. 2008;32:527–535. [PubMed] [Google Scholar]
- 20.Koshiba M, Rosin DL, Hayashi N, Linden J, Sitkovsky MV. Patterns of A2A extracellular adenosine receptor expression in different functional subsets of human peripheral T cells. Flow cytometry studies with anti-A2A receptor monoclonal antibodies. Mol. Pharmacol. 1999;55:614–624. [PubMed] [Google Scholar]
- 21.Mirabet M, Herrera C, Cordero OJ, Mallol J, Lluis C, Franco R. Expression of A2B adenosine receptors in human lymphocytes: their role in T cell activation. J. Cell Sci. 1999;112 (Pt 4):491–502. doi: 10.1242/jcs.112.4.491. [DOI] [PubMed] [Google Scholar]
- 22.Apasov S, Koshiba M, Redegeld F, Sitkovsky MV. Role of extracellular ATP and P1 and P2 classes of purinergic receptors in T-cell development and cytotoxic T lymphocyte effector functions. Immunol. Rev. 1995;146:5–19. doi: 10.1111/j.1600-065x.1995.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 23.Apasov S, Chen JF, Smith P, Sitkovsky M. A(2A) receptor dependent and A(2A) receptor independent effects of extracellular adenosine on murine thymocytes in conditions of adenosine deaminase deficiency. Blood. 2000;95:3859–3867. [PubMed] [Google Scholar]
- 24.Erdmann AA, Gao ZG, Jung U, Foley J, Borenstein T, Jacobson KA, Fowler DH. Activation of Th1 and Tc1 cell adenosine A2A receptors directly inhibits IL-2 secretion in vitro and IL-2-driven expansion in vivo. Blood. 2005;105:4707–4714. doi: 10.1182/blood-2004-04-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J. Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 26.Yang Z, Day YJ, Toufektsian MC, Xu Y, Ramos SI, Marshall MA, French BA, Linden J. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation. 2006;114:2056–2064. doi: 10.1161/CIRCULATIONAHA.106.649244. [DOI] [PubMed] [Google Scholar]
- 27.Csoka B, Himer L, Selmeczy Z, Vizi ES, Pacher P, Ledent C, Deitch EA, Spolarics Z, Németh ZH, Hasko G. Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function. FASEB J. 2008;22:3491–3499. doi: 10.1096/fj.08-107458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Himer L, Csoka B, Selmeczy Z, Koscso B, Pocza T, Pacher P, Németh ZH, Deitch EA, Vizi ES, Cronstein BH, Hasko G. Adenosine A2A receptor activation protects CD4+ T lymphocytes against activation-induced cell death. FASEB J. 2010;24:2631–2640. doi: 10.1096/fj.10-155192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sitkovsky MV. T regulatory cells: hypoxia-adenosinergic suppression and re-direction of the immune response. Trends Immunol. 2009;30:102–108. doi: 10.1016/j.it.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Stephenson RP. A modification of receptor theory. Br. J. Pharmacol. Chemother. 1956;11:379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNeil LK, Evavold BD. Dissociation of peripheral T cell responses from thymocyte negative selection by weak agonists supports a spare receptor model of T cell activation. Proc. Natl. Acad. Sci. USA. 2002;99:4520–4525. doi: 10.1073/pnas.072673899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shryock JC, Snowdy S, Baraldi PG, Cacciari B, Spalluto G, Monopoli A, Ongini E, Baker SP, Belardinelli L. A2A-adenosine receptor reserve for coronary vasodilation. Circulation. 1998;98:711–718. doi: 10.1161/01.cir.98.7.711. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong JM, Chen JF, Schwarzschild MA, Apasov S, Smith PT, Caldwell C, Chen P, Figler H, Sullivan G, Fink S, Linden J, Sitkovsky M. Gene dose effect reveals no Gs-coupled A2A adenosine receptor reserve in murine T-lymphocytes: studies of cells from A2A-receptor-gene-deficient mice. Biochem. J. 2001;354:123–130. doi: 10.1042/0264-6021:3540123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.By Y, Durand-Gorde JM, Condo J, Lejeune PJ, Mallet B, Carayon P, Guieu R, Ruf J. Production of an agonist-like monoclonal antibody to the human A2A receptor of adenosine for clinical use. Mol. Immunol. 2009;46:400–405. doi: 10.1016/j.molimm.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Saadjian AY, Levy S, Franceschi F, Zouher I, Paganelli F, Guieu RP. Role of endogenous adenosine as a modulator of syncope induced during tilt testing. Circulation. 2002;106:569–574. doi: 10.1161/01.cir.0000023924.66889.4c. [DOI] [PubMed] [Google Scholar]
- 37.Carrega L, Saadjian AY, Mercier L, Zouher I, Berge-Lefranc JL, Gerolami V, Giaime P, Sbragia P, Paganelli F, Fenouillet E, Levy S, Guieu RP. Increased expression of adenosine A2A receptors in patients with spontaneous and head-up-tilt-induced syncope. Heart Rhythm. 2007;4:870–876. doi: 10.1016/j.hrthm.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Deharo JC, Mechulan A, Giorgi R, Franceschi F, Prevot S, Peyrouse E, Condo J, By Y, Ruf J, Brignole M, Guieu R. Adenosine plasma level and A2A adenosine receptor expression: correlation with laboratory tests in patients with neurally mediated syncope. Heart. 2012;98:855–859. doi: 10.1136/heartjnl-2011-301411. [DOI] [PubMed] [Google Scholar]
- 39.Guieu R, Sampieri F, Bechis G, Halimi G, Dussol B, Berland Y, Sampol J, Rochat H. Development of an HPLC diode array detector method for the determination of human plasma adenosine concentrations. J. Liq. Chromatogr. Related Technol. 1999;22:1829–1841. [Google Scholar]
- 40.Varani K, Laghi-Pasini F, Camurri A, Capecchi PL, Maccherini M, Diciolla F, Ceccatelli L, Lazzerini PE, Ulouglu C, Cattabeni F, Borea PA, Abbracchio MP. Changes of peripheral A2A adenosine receptors in chronic heart failure and cardiac transplantation. FASEB J. 2003;17:280–282. doi: 10.1096/fj.02-0543fje. [DOI] [PubMed] [Google Scholar]
- 41.Bali L, Cuisset T, Giorgi R, Monserrat C, Quilici J, Carrega L, Mouret JP, Nait-Saidi L, Mielot C, Lambert M, Guieu R, Bonnet JL. Prognostic value of ischaemia-modified albumin in patients with non-ST-segment elevation acute coronary syndromes. Arch. Cardiovasc. Dis. 2008;101:645–651. doi: 10.1016/j.acvd.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 42.By Y, Durand-Gorde JM, Condo J, Lejeune PJ, Fenouillet E, Guieu R, Ruf J. Monoclonal antibody-assisted stimulation of adenosine A2A receptors induces simultaneous downregulation of CXCR4 and CCR5 on CD4+ T-cells. Hum. Immunol. 2010;71:1073–1076. doi: 10.1016/j.humimm.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Giaime P, Carrega L, Fenouillet E, Mercier L, Gerolami V, Ruf J, Sauze N, Brunet P, Saadjian A, Berland Y, Dussol B, Guieu R. Relationship between A2A adenosine receptor expression and intradialytic hypotension during hemodialysis. J. Investig. Med. 2006;54:473–477. doi: 10.2310/6650.2006.06005. [DOI] [PubMed] [Google Scholar]
- 44.McNeil LK, Evavold BD. TCR reserve: a novel principe of CD4 T cell activation by weak ligands. J. Immunol. 2003;170:1224–1230. doi: 10.4049/jimmunol.170.3.1224. [DOI] [PubMed] [Google Scholar]
- 45.Saadjian AY, Gerolami V, Giorgi R, Mercier L, Berge-Lefranc JL, Paganelli F, Ibrahim Z, By Y, Gueant JL, Levy S, Guieu RP. Head-up tilt induced syncope and adenosine A2A receptor gene polymorphism. Eur. Heart J. 2009;30:1510–1515. doi: 10.1093/eurheartj/ehp126. [DOI] [PubMed] [Google Scholar]
- 46.Gurevich VV, Gurevich EV. GPCR monomers and oligomers: it takes all kinds. Trends Neurosci. 2008;31:74–81. doi: 10.1016/j.tins.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allen MD, Neumann S, Gershengorn MC. Occupancy of both sites on the thyrotropin (TSH) receptor dimer is necessary for phosphoinositide signaling. FASEB J. 2011;25:3687–3694. doi: 10.1096/fj.11-188961. [DOI] [PMC free article] [PubMed] [Google Scholar]


