Abstract
Foster children exhibit high rates of atypical neuroendocrine functioning compared to children in the general population. In particular, alterations in the daytime diurnal activity of the hypothalamic-pituitary-adrenal (HPA) axis have been observed in foster children, often characterized by blunted salivary cortisol levels (i.e., low morning levels that remain low throughout the day). There is emerging evidence that therapeutic interventions for foster children can affect this pattern of HPA axis activity, but the specific intervention components responsible for change have not been fully explicated. Within a randomized trial to evaluate a therapeutic intervention for foster preschoolers (n = 57 intervention condition; n = 60 comparison condition; n = 60 community comparison condition), the present study examined whether diurnal cortisol activity was associated with caregiver self-reported stress in response to child problem behavior. Results showed immediate reductions in caregiver stress that were sustained through 12 months postbaseline in the intervention condition. In contrast, caregivers in the regular foster care condition showed higher rates of stress across time and increased stress sensitivity to child problem behaviors. In addition, among caregivers in regular foster care, higher self-reported stress was associated with lower morning cortisol levels and more blunted diurnal cortisol activity. These results provide evidence that interventions can simultaneously impact caregiver stress and buffer children from the negative impacts of caregiver stress on HPA axis regulation
Keywords: foster care, cortisol, problem behavior, caregiver stress, preschool
Prevention science has great potential to improve outcomes of children in the foster care system. This is an issue of considerable public health significance given that there are more than 500,000 children in foster care in the United States, one third of whom are age 5 years or younger (U.S. Department of Health and Human Services, Administration on Children, Youth and Families, 2005). Moreover, foster care is increasingly being employed as an alternative to institutional care in developing countries (George, van Oudenhoven, & Wazir, 2001). Studies of foster children have consistently found high rates of psychosocial maladjustment and emotional/behavioral disorders (Landsverk, Garland, & Leslie, 2002). Among infants and young children in foster care, developmental delays are also very prevalent (Klee, Kronstat, & Zlotnick 1997).
That such disparities exist in foster care is not surprising. In addition to maltreatment (e.g., neglect, physical abuse, and sexual abuse), foster children undergo at least one and often numerous caregiver transitions. Many of these transitions occur without warning to the child and result in the immediate loss of contact with prior caregivers, sometimes permanently (Fisher & Kim, 2007). Compounding this situation, some of these children have lacked a basic attachment figure from birth.
Although poor outcomes are common, not all foster children fare poorly in the long term. Indeed, many children are remarkably resilient in the face of these difficult circumstances. Differential pathways towards risk and resiliency among foster children can be accounted for in part by the experiences the child has with his/her caregivers (i.e., biological parents and foster caregivers) prior to and following placement in foster care. Inadequate early caregiving environments can increase the risk for poor outcomes, and more supportive foster family environments has the potential mitigate some of these risks.
For the purposes of prevention, identifying specific aspects developmental processes such as caregiving, that are associated with positive and negative outcomes for foster children, can provide targets for intervention. Indeed, the utility of applying empirically and theory-driven constructs from developmental psychology to the science of prevention has been strongly advocated for in prior discussions in the literature (Cicchetti & Hinshaw, 2002; Cicchetti & Rogosch, 1999). Perspectives from attachment theory and social learning theory have been particularly useful in this area. Dozier, Albus, Fisher, and Sepulveda (2002) noted that the attachment dimensions of warmth/sensitivity and the social learning dimensions of contingency/predictability in the family of origin and in foster care appear to be key elements of caregiving associated with child outcomes. The relative influence of these two dimensions might be in part a function of the age of the child, with attachment-related dimensions being more critical in infancy/toddlerhood and social learning dimensions being more important beginning in preschool.
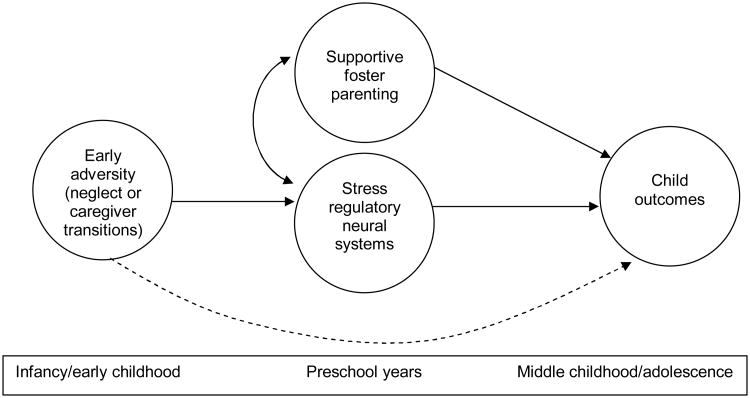
As is shown in Figure 1 (the conceptual model guiding the present study) these caregiving processes are known to have direct effects on child psychosocial wellbeing. Notably, these processes also appear integral to the development and ongoing functioning of the hypothalamic-pituitary-adrenal (HPA) axis. Indeed, an extensive body of literature involving studies of humans and animals has documented an association between disruptions in early caregiving, altered HPA axis functioning, and subsequent negative psychosocial outcomes—especially anxiety and affective disorders (see Levine, 2005). However, there is emerging evidence of the corrective effects of optimal environmental conditions on the HPA axis following early adversity (Bruce, Kroupina, Parker, & Gunnar, 2000; Gunnar, Morrison, Chisholm, & Schuder, 2001; Kertes, Gunnar, & Madsen, 2007; Turner & Greenough, 1985), and of the potential for psychotherapeutic interventions to positively impact HPA axis functioning (Gaab et al., 2003; Gaab, Sonderegger, Scherrer, & Ehlert, 2006; Hammerfald et al., 2005; Mommersteeg, Keijsers, Heijnen, Verbraak, & van Doornen, 2006). Below we describe the physiology of the HPA axis, the implications for foster children of prior research on the effects of early stress on the HPA axis, and how caregiver stress may be a critical target for interventions aimed at improving foster children's HPA axis activity and subsequent psychosocial adjustment.
Figure 1.
Original heuristic model for children in foster care.
HPA Axis Physiology
The HPA axis can be activated by physical or psychological stressors. This activation consists of a hormonal cascade: (1) corticotrophin releasing hormone (CRH) is released in the paraventricular nucleus of the hypothalamus, (2) adrenocorticotropic hormone (ACTH) is released in the interior region of the pituitary, and (3) glucocorticoids (cortisol in humans and nonhuman primates; corticosterone in rodents) are released in the adrenal cortex. The HPA axis maintains a homeostatic balance through negative feedback, whereby the release of glucocorticoids slows the release of ACTH and CRH. In addition, glucocorticoids have numerous effects on bodily systems, including the stimulation of the immune system and the metabolism of stored energy. The HPA axis, by no means the only neurobiological system involved in the response to stress, is connected by structural and functional pathways to a number of other key brain structures, including the central nucleus of the amygdala (involved in the detection of threat), the medial prefrontal cortex (involved in inhibitory control), and the hippocampus (involved in the translation of experience into memory; Herman & Cullinan, 1997).
As is noted elsewhere (Fisher, Gunnar, Dozier, Bruce, & Pears, 2006; Gunnar, Fisher, & the Early Experience, Stress, and Prevention Science Network, 2006), studies using rodent models of maternal separation have documented long-term effects of disruptive caregiving on HPA axis functioning. This research points to three areas relevant to foster care populations. First, the specific timing at which the disruption of maternal care occurs exerts a strong influence on subsequent outcomes (Meany & Szyf, 2005). Early separation, in particular, appears to have the most profound effects on subsequent HPA axis development. Second, there is an interval in the course of development (observed in rodent studies) known as the stress hyporesponsive period (SHRP), during which there is a notable lack of response of the HPA axis to external stressors (Sapolsky & Meany, 1986). This period is believed to coincide with the development of extensive neural circuitry involved in learning, memory, and stress responsivity. The presence of high levels of glucocorticoids during this period might negatively impact development in these systems. In humans, the SHRP may last until the onset of adolescence. Third, and most germane to the present study, the buffering of the HPA axis response that occurs during the SHRP is thought to be largely caregiver mediated (Rosenfeld, Suchecki, & Levine, 1992; Smotherman & Bell, 1980). That is, caregiver attention to and regulation of the infant's arousal and distress are associated with less HPA axis response to external stressors. Conversely, the absence of a responsive caregiver can lead to a significant dysregulation of the HPA axis response. For example, Gunnar and colleagues (Gunnar & Donzella, 2002; Gunnar et al., 2006) reported that children showing insecure attachment relationships were more likely to exhibit elevations in cortisol when they encountered moderately stressful events (e.g., inoculations or the approach of a stranger) compared to children with more secure attachment relationships.
Research on the role of the caregiver in the development and regulation of the HPA axis has clear implications for foster children. Experiences associated with placement in foster care, including chronic neglect and maternal separation, represent extreme forms of nonresponsive caregiving that have been found in prior animal and human research to exert negative influences on HPA axis development. Thus, high rates of dysregulation in HPA axis functioning might be expected among foster children in comparison to children in the general population. Moreover, once in foster care, ongoing exposure to nonresponsive caregiving could be associated with ongoing HPA dysregulation or even deterioration in the system's functioning; in contrast, however, an increase in foster caregiver responsiveness might lead to more typical HPA axis function over time.
These issues have been investigated in a number of recent studies of maltreated children focusing on diurnal activity of the HPA axis during the waking hours (hereafter referred to simply as “diurnal HPA axis activity”). In individuals exposed to substantial early adversity, the characteristic pattern is blunted diurnal HPA axis activity (e.g., low or absent morning peak in salivary cortisol and low levels continuing throughout the day; Gunnar & Vazquez, 2001). This suggests a downregulation in this system due to the chronicity of the early adversity and to the lack of adaptive value in elevated HPA axis function (Friese, Hesse, Hellhammer, & Hellhammer, 2005). (Note: It is important to distinguish diurnal HPA axis activity from the HPA stress response, which has different sources of activation and regulation.)
Studies of children who have experienced severe caregiver neglect in institutional settings overseas have reported blunted diurnal HPA axis activity among young children (Carlson & Earls, 1997; Gunnar & Vazquez, 2001). Similarly, in two samples of preschool-aged foster children, blunted diurnal HPA axis activity has been observed (Gunnar et al., 2006). Consistent with the animal literature on maternal deprivation effects on the development of the HPA axis, neglectful caregiving appears to be highly predictive of such blunting (Bruce, Fisher, Pears, & Levine, 2007).
Beyond these issues is the potential for HPA axis plasticity in response to psychotherapeutic interventions. Fisher, Stoolmiller, Gunnar, and Burraston (in press) noted that a caregiver-based intervention for foster children produced differential patterns of HPA axis activity over time relative to children in regular foster care. Notably, the regular foster care group showed increased blunting in diurnal HPA axis activity, whereas the intervention group showed relatively typical diurnal HPA axis activity.
Caregiver Stress and Foster Children's HPA Axis Activity
There is a large body of literature (e.g., Keller, Spieker, & Gilchrist, 2005; Malik et al., 2007) on the impact of caregiver stress upon child and family outcomes. Research has been conducted in a variety of high-risk populations, including families with domestic violence (Owen, Thompson, & Kaslow, 2006) and children with developmental disabilities (Secco et al., 2006), sleep disturbances (Meltzer & Mindell, 2007), and attention deficit disorders and disruptive behavioral problems (Danforth, Harvey, Ulaszek, & McKee, 2006). Studies have found that the ability of caregivers to employ efficacious strategies for supporting positive development is often compromised by high levels of stress (Halme, Tarkka, Nummi, & Åstedt-Kurki, 2006). On a positive note, parent training interventions (Danforth et al., 2006; Sharry, Guerin, Griffin, & Drumm, 2005) have been found to reduce caregiver stress. (Note that the term “caregiver stress” in this article refers to self-report measures of caregiver stress rather than to observed or biological stress measures.)
Perhaps most relevant to the present study, caregiver stress has been found to be associated with infant psychosocial wellbeing and HPA axis functioning. Tu et al. (2007), for example, observed significant associations among maternal reports of stress, child stress, and child salivary cortisol levels in a sample of preterm infants. Less clear, however, is whether the intervention effects that impact caregiver stress show concordant effects on child cortisol levels.
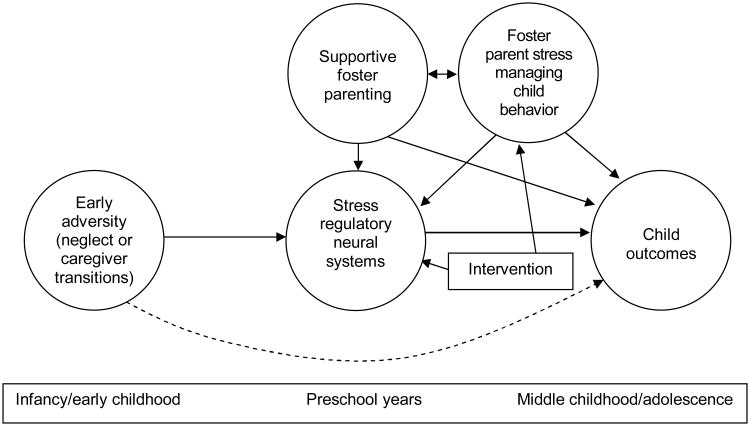
These issues are particularly salient to the context of foster care. High levels of foster parent stress—especially stress associated with managing child problem behavior—might limit a foster caregiver's ability to employ efficacious parenting strategies and might limit the foster parents' ability to provide the foster child with a sufficiently sensitive and responsive environment. This in turn might lead to lack of bonding in the foster home, placement failures, and other negative outcomes (Soliday, McCluskey-Fawcett, & Meck, 1994). Thus, as is shown in the model in Figure 2, the present study examined longitudinal intervention effects on caregiver stress managing child problem behavior and the associations between caregiver stress and child diurnal HPA axis activity.
Figure 2.
Model incorporating caregiver stress associated with child behavior problems.
The context for this study was a randomized efficacy trial of the Multidimensional Treatment Foster Care for Preschoolers (MTFC-P) intervention (Fisher, Burraston, & Pears, 2005; Fisher, Ellis, & Chamberlain, 1999). There were three primary areas of investigation in the current study:
The main effects of the intervention on caregiver stress when managing child problem behavior were examined. Given the intervention's high level of support and consultation provided to foster parents and the empirically-based parenting techniques that are employed (see intervention description in Methods section), it was hypothesized that there would be immediate and lasting intervention effects on caregiver stress when managing child problem behavior.
Whether caregiver stress levels increased as child problem behavior increased was investigated. Based on evidence of intervention effects in this area with a similar intervention program (Chamberlain et al., 2006), it was hypothesized that caregiver stress levels would be directly related to child problem behavior in regular foster care. In contrast, it was anticipated that there would be a buffering effect of the intervention on the caregiver stress–child problem behavior association, with caregiver stress levels being lower in general and not as strongly associated with child problem behavior.
The longitudinal associations between caregiver stress and children's HPA activity were examined. As is noted above, MTFC-P intervention main effects on HPA axis activity found to date have been characterized by stable diurnal HPA axis activity over time for the intervention group and increasingly blunted diurnal HPA axis activity for the regular foster care group (Fisher et al., in press). The present study examined whether this blunting resulted an increased sensitivity to caregiver stress over time. Such increased sensitivity might be inferred to the extent that diurnal HPA axis activity and caregiver stress show increased covariation over time. Thus, it was hypothesized that, in the regular foster care condition, caregiver stress levels over time would be associated with diurnal HPA axis activity in later months of the study. In particular, it was hypothesized that higher levels of caregiver stress on a given day would be increasingly likely to predict greater blunting of diurnal HPA activity the next day and that lower levels of caregiver stress on a given day would predict more typical diurnal HPA axis activity the next day. These associations were not anticipated in the intervention condition or in the participating community comparison group of nonmaltreated children.
Methods
Participants
Foster preschoolers (ages 3–6 years) who were entering new placements (N = 117) in a county child welfare system in the Pacific Northwest were recruited to participate in the study. For a child to be eligible, his/her planned placement had to be at least 3 months long. Participants were recruited continuously over 3.5 years.
Eligible foster children were randomly assigned to the MTFC-P intervention condition or to a regular foster care (RFC) comparison condition. Once group membership was determined, a staff member contacted the child's caseworker (i.e., the legal guardian while the child is in care) and requested consent for the child to participate. If consent was obtained, a staff member contacted the foster caregiver(s). Consent was obtained for 89% of the MTFC-P children (n = 57) and 82% of the RFC children (n = 60). Acceptance rates did not differ between conditions, χ2 = 1.29, df = 1, p = .26. Staff members involved in data collection were blind to the study conditions.
At entry into the study, placement types included first-time foster placements, moves between foster homes, and reentries into foster care following failed permanent placements. Foster children in the two conditions did not differ on placement type at baseline, χ2(3) = 2.24, p = .52. There was a trend for MTFC-P children to have spent a greater number of days in care prior to baseline (RFC M = 139.20, SD = 141.03; MTFC-P M = 204.40, SD = 221.19; t(114) = −1.9, p = .06).
A comparison group of low-income, age-matched, nonmaltreated community children (CC) and their families was also recruited (n = 60). Median income for the CC sample was $15,000–19,999. To verify that no prior maltreatment had occurred, the CC families consented to a screening process using child welfare system records. No previous reports of maltreatment or involvement in child welfare services were found.
The three groups were equivalent in terms of child age and gender. Mean age at baseline was 4.4 years (M range = 4.3–4.5; SD range = .79–.86), F(2, 174) = 1.13, p = .33. Boys made up 49% (n = 28) of the MTFC-P group, 58% (n = 35) of the RFC group, and 53% (n = 32) of the CC group, χ2(2) = .10, p = .61. Ethnicity did not differ between groups, χ2(8) = 9.07, p = .34. Overall, the sample was 89% European American, 5% Latino, 5% Native American, and 1% African American. These proportions are representative of the community in which the sample was recruited. Retention was high across all study conditions: 97% at baseline and 80% at 12 months postbaseline. Treatment of missing data is described near the end of the Method section.
MTFC-P Group Procedures
MTFC-P is a caregiver-based preventive intervention designed to address the developmental and social-emotional needs of preschool aged foster children. It is delivered via a treatment team approach. In the present study, MTFC-P services were provided to foster children, their foster caregivers, and their permanent placement resources (birthparents or adoptive relatives/nonrelatives). Prior to placement, foster caregivers completed 12 hours of intensive training. After the placement, foster caregivers received support and supervision via daily telephone contacts, weekly group meetings, and 24-hour staff availability. These services were intended to facilitate the maintenance of a warm, responsive, consistent environment in which positive behavior was encouraged and problem behavior was limited and to reduce caregiver stress in managing child problem behavior. Children received individualized treatment with child therapists to facilitate the acquisition of prosocial skills and to improve functioning in preschool/daycare and home settings. Children also participated in weekly therapeutic playgroup sessions focused on facilitating school readiness. The playgroup emphasized social/emotional functioning and early literacy skills. When children transitioned to permanent placements, family therapists worked to familiarize these families with the intervention parenting techniques. This helped to facilitate consistency between settings.
Services were provided typically for 6–9 months, including the transition to a permanent placement. Some children remained in long-term foster care; for these individuals, services lasted until their behavior stabilized and the risk of placement disruption appeared to have been mitigated. To ensure treatment fidelity for all MTFC-P components, progress notes and checklists regarding services received were completed by the clinical staff and monitored by the research team. Additional information about MTFC-P program and its theoretical underpinnings can be found in Fisher et al. (1999) and in Fisher and Chamberlain (2000).
RFC Group Procedures
Children in the RFC group received foster care services as usual. As is typical in this county child welfare system, these services included monthly or more frequent contact with caseworkers to monitor progress in the foster home and to identify issues in need of attention (e.g., weekly individual psychotherapy to address trauma and/or behavioral issues, medication prescribed by a primary care physician or child psychiatrist for extreme behavioral and emotional problems, and developmental screening and early childhood special education services as necessary).
Measures
Caregiver self-reported stress in managing child problem behavior
Caregiver stress was computed from the Parent Daily Report (PDR; Chamberlain & Reid, 1987), a measure of child problem behaviors designed to be collected at multiple intervals via 5- to 10-min telephone interviews. During each call, a trained interviewer asked the foster parent, “Thinking about (child's name), during the past 24 hours, did any of the following behaviors occur?” Using a yes/no format, caregivers responded to each behavior. For any behavior that occurred, also using a yes/no format, caregivers indicated whether they experienced that behavior as stressful.
The current study used the 52-item version of the PDR, which was designed for the preschool age range (see Appendix). PDR calls were administered on the evenings of days when saliva was collected. Thus, the PDR provided a measure of problem behavior concordant with the monthly measure of diurnal HPA axis activity. The PDR caregiver stress self-report measure employed for the current study was obtained by computing the proportion of child behavior problems that occurred and were rated as being stressful. As such, it represented the conditional probability of caregiver stress given child problem behavior.
Use of the PDR made it possible to avoid the need for aggregate recall over longer intervals (e.g., the past week or month) for estimates of the frequency with which specific behaviors occurred. Previous studies have documented that a respondent's emotional state at the time of reporting might bias this/her responses (Bower, 1981). In particular, respondents might give more weight to recent and peak levels of experiences rather than giving equal weight to each instance (Stone, Broderick, Kaell, DelesPaul, & Porter, 2000). The structure of the PDR (i.e., repeated administrations focusing on only the past 24 h) reduced these potential sources of measurement error and increased the validity and reliability of the caregiver reports.
The PDR has been employed in prior studies to assess treatment outcomes for families referred due to child disruptive problem behavior (McClowry, Snow, & Tamis-LeMonda, 2005). The measure has also been employed for children and adolescents returning to community placements following psychiatric hospitalization (Chamberlain & Reid, 1991). Several studies involving foster children have employed the PDR. Chamberlain, Moreland, and Reid (1992) used the PDR to assess the functioning of youth in regular foster care who were placed with foster caregivers receiving behavioral parent management training. More recently, Chamberlain et al. (2006) used the PDR to predict the risk of placement failures for children in regular foster care. The concurrent validity of the PDR has been documented in association with a number of measures of child and family functioning, including live observations of family interactions coded in the home (Forgatch & Toobert, 1979; Patterson, 1976) and parents' global ratings of child problem behavior (Becker, Madsen, Arnold, & Thomas, 1967). The stability and interrater reliability of the PDR has been found to be adequate in previous studies (Chamberlain & Reid, 1991; Weinrott, Bauske, & Patterson, 1979).
Salivary cortisol collection, assay, and measures
Monthly salivary cortisol samples were gathered on 2 consecutive days for 12 months (M1–M12). However, as is described below, to examine the sequential effects of caregiver stress on children's cortisol, only the cortisol samples from Day 2 of sampling and the PDR measures from Day 1 of sampling were included in the present study. For foster children, M1 (baseline) assessments occurred 3–5 weeks postplacement. Saliva collections occurred 30 min after the child awoke and before eating or drinking (AM) and 30 min before bedtime (PM). Caregivers were trained by research staff members to complete saliva collection at home, following procedures described in Schwartz, Granger, Susman, Gunnar, and Laird (1998). The child chewed a piece of Trident Original Flavor Gum (Cadbury Adams USA, Plano, TX) for 1 min to stimulate saliva flow. The child then spat the gum out, and the caregiver tipped a Salivette (Sarstedt, Newton, NC) absorbent roll from a protective plastic tube into the child's mouth without touching the roll. The child kept the roll in his/her mouth for 1 min and was instructed not to touch it with his/her fingers. The caregiver then assisted the child in placing the roll into the protective tube. Caregivers recorded the date and time of the collection on the tube label.
Saliva samples were stored in the participants' freezers until collected for assay by research staff members. Samples were assayed using the High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit (Salimetrics, State College, PA). Both samples from each child were included in the same assay batch to minimize within-subject variability. Samples were assayed in duplicate and were averaged. Duplicates varying by more than 15% were reassayed. The intraassay and interassay coefficients of variance were 2.69% and 10.98%, respectively.
A variety of extraneous variables (e.g., certain medications, general health, food intake, and sleep patterns) have been shown to affect cortisol levels (de Kloet, 1991). In the present study, any child using steroid-based medications on a regular basis (e.g., asthma inhalers or hydrocortisone cream) was excluded from the study. Each caregiver was instructed at each monthly collection to avoid sampling if the child was currently using steroid-based medications or was ill. As an additional safeguard, caregivers completed brief questionnaires regarding sampling times and their children's eating and sleeping behaviors on sampling days. These questionnaires were inspected to ensure compliance with sampling guidelines.
Of nearly 8,000 saliva samples in the overall study, 572 samples (7%; 315 AM samples and 257 PM samples) were missing because the caregivers either did not return the samples or did not collect data at one of the sampling times. In addition, 9 samples were excluded due to out-of-range cortisol values (> 2.0 μg/dl; 2 AM samples and 7 PM samples), and 72 samples were excluded due to incorrect sampling time (i.e., collection time recorded on sample tube and in diary differed by more than 30 min or did not correspond to the sampling window; 36 AM samples and 36 PM samples).
The primary cortisol measure was a difference score computed by subtracting the daily PM cortisol level from the daily AM cortisol level (AM–PM). This provided a rough index of the diurnal HPA axis activity on a given day. Although additional time points (e.g., midmorning and afternoon) would have better defined the diurnal pattern, the highly vulnerable nature of these children and the emphasis on limiting the assessment burden on foster children and their caregivers guided the selection of only two time points. The AM–PM cortisol levels were analyzed first and were then decomposed into AM and PM levels to determine whether AM cortisol, PM cortisol, or both were related to caregiver stress.
Data Analyses and Statistical Modeling
The primary outcome measure for evaluating intervention effects was the PDR caregiver stress measure. We used a three-level linear growth model: level one consisted of repeated assessments on 2 consecutive days within a month, level two consisted of repeated assessments over 12 months within a family, and level three consisted of the family (N = 177). An initial inspection of the data revealed that the pattern of change across the groups was not a simple difference in linear trend over 12 months but rather an immediate impact from M1 to M2 followed by maintenance from M3 to M12. This pattern was most easily captured by fitting two sets of intercepts and slopes, one intercept and slope for the first 2 months and another intercept and slope for the last 10 months. Residual variance at the month level within families for M1 and M2 were constrained to zero for model identification but were constrained to be equal from M3 to M12. Residual variances at the day level within months within families were constrained to be equal from M1 to M12.
Child problem behavior was included as a time-varying covariate of caregiver stress for three reasons. First, it was related to missingness of caregiver stress scores because, without any child problem behavior, the caregiver stress score was indeterminate. Second, on days when child problem behavior occurred, it was a strong positive predictor of caregiver stress. Third, it tested the possibility that the intervention would lower or prevent increases in caregiver stress sensitivity to child problem behavior. It seemed overly simplistic to assume that, regardless of the intervention, all caregivers would be equally stress sensitive to child problem behavior; to address this problem, a random effect for child problem behavior was specified to allow for individual caregiver differences.
Fixed effects included the means of the intercepts (initial status) and the slopes (linear change over time). Group membership was included by dummy-coded contrasts, with the CC and RFC groups contrasted against the MTFC-P group. Change Over Time × Group interaction effects represented CC and RFC group differences from the MTFC-P group; thus the Change Over Time × RFC interaction effect captured the intervention effect, and the Change Over Time × CC interaction effect indicated whether the MTFC-P group changed at the same linear rate as the CC group. The correlations among the subject-level random effects were freely estimated (not shown).
To test the relationship between caregiver stress and diurnal HPA axis activity, we fit separate two-level linear growth models to the AM, PM, and AM–PM cortisol data but used caregiver stress on Day 1 within a month as a time-varying, prospective covariate of child cortisol levels on Day 2 within a month. In other words, caregiver stress levels on a given day were used to predict diurnal HPA axis activity the next day. We did this to avoid ambiguity about the temporal sequencing of events and to strengthen the case for an effect of caregiver stress on diurnal HPA axis activity rather than the reverse. This also simplified the model down to two levels, repeated assessments (12) within a family at level one and families at level two (177).
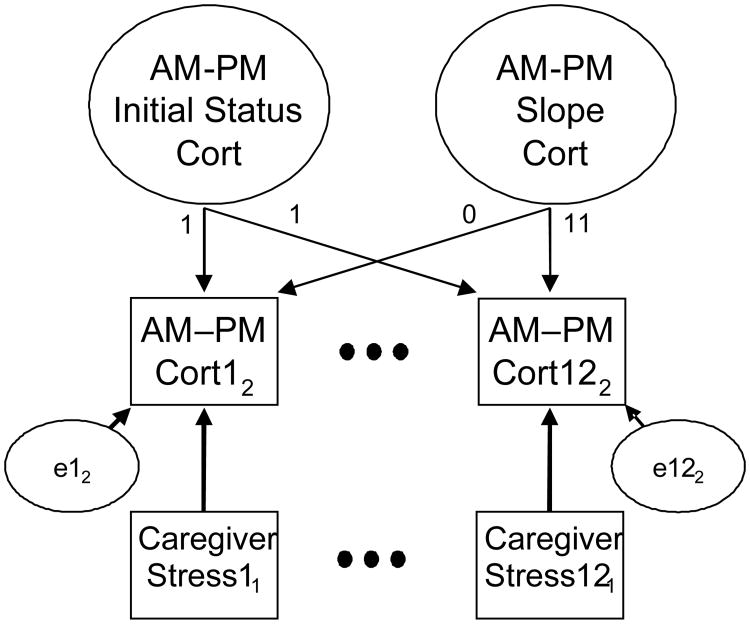
A number of additional effects were omitted because they were not significant in preliminary analyses and/or because they did not alter the effects of main interest. Omitted effects included intervention (group) effects, child problem behavior, the time of AM cortisol collection, and the time lag between child awakening and AM cortisol collection. A simplified schematic diagram of the model is shown in Figure 3. It depicts the cortisol growth model and the time-varying predictor of caregiver stress for M1 and M12 (M2 through M11 repeat the same pattern as is indicated by the ellipses). The models for AM and PM cortisol levels were identical to the AM–PM cortisol change model.
Figure 3.
Schematic diagram for longitudinal growth model examining time-varying associations between caregiver stress and child AM–PM cortisol levels. Note. Day of assessment within a month is indicated by subscripts. Ellipses indicate repetition of the pattern of assessment.
All models were estimated using the lme function (Pinheiro & Bates, 2000) of the R (R Development Core Team, 2005) and S-PLUS (Insightful Corporation, 2001) statistical packages. Model specifications (normality of random effects and invariance of month- and day-level residuals across time and groups) were thoroughly checked through residual diagnostic plots, and corrective action was taken as necessary (noted below).
Missing data
All models were estimated using all available data for all participants at all time points, even when only partial data was available. This approach is the recommended standard for growth models (Schafer & Graham, 2002) and performs better than alternative approaches (e.g., use of only participants with complete data or single imputation of missing values) by minimizing potential bias due to attrition relative to the baseline (M1) sample and by maximizing power. The models are estimated assuming ignorable missingness; that is, conditional on covariates, a particular outcome value on a given day (cortisol level or caregiver stress) does not interfere with the collecting and recording of that particular outcome value. As is noted above, child problem behavior was included as a time-varying covariate of caregiver stress because it is predictive of missingness.
The total amount of missing data was not equally distributed across groups. The amount of missing AM–PM, AM, and PM cortisol data was highest for the RFC group, followed by the MTFC-P group and then the CC group. The differences between the MTFC-P and CC groups were not significant, but the two groups had significantly fewer missing data than the RFC group. There were no individuals for whom more than 35% of the data were missing. Missing data did not necessarily indicate attrition from the study; rather, there were instances when it was not possible to collect cortisol for one or more monthly intervals. Given the robustness of the analytic procedures for dealing with missing data, group differences in missing data, though noteworthy, were unlikely to have affected the results.
Results
Intervention Effects on Caregiver Stress Managing Child Problem Behavior
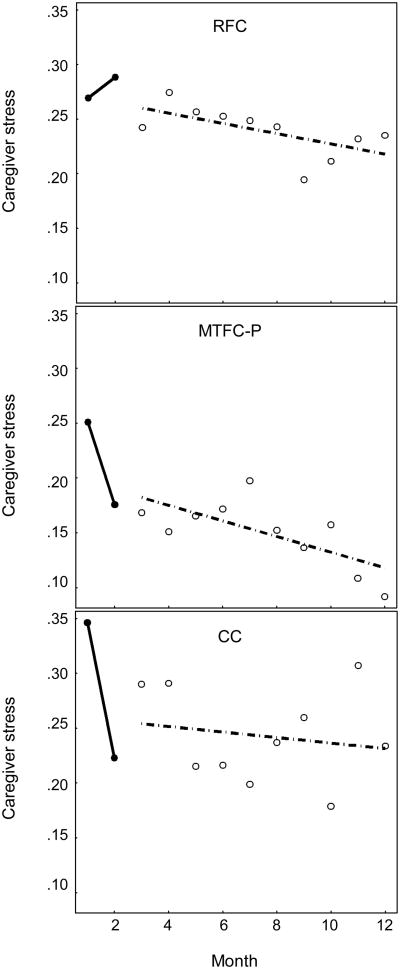
Change in caregiver stress over time for the three groups is shown in Figure 4; detailed results are shown in Table 1. As is shown in Table 1, the fixed effects for intercepts and slopes indicated that MTFC-P group decreased significantly from M1 to M2 (Slope M1–M2) and was stable from M3 to M12 (Slope M3–M12). In contrast, the RFC group increased significantly from M1 to M2, and the RFC group versus MTFC-P group difference in slope was significant (Slope M1–M2 × RFC). The CC group started initially higher than the MTFC-P group (Intercept M1–M2 × CC) but decreased from M1 to M2 and was not significantly different from the MTFC-P group in change (Slope M1–M2 × CC).
Figure 4.
Change in caregiver stress over time, by group.
Table 1. Intervention Effects on Caregiver Stress.
| Effect | Estimate | SE | t | p |
|---|---|---|---|---|
| Intercept (M1–M2) | 0.3227 | 0.0429 | 7.5275 | 0.0000 |
| Slope (M1–M2) | −0.1068 | 0.0407 | −2.6275 | 0.0087 |
| Intercept (M3–M12) | 0.2142 | 0.0319 | 6.7235 | 0.0000 |
| Slope (M3–M12) | 0.0026 | 0.0041 | 0.6338 | 0.5263 |
| Child behavior problems | 0.1222 | 0.0152 | 8.0500 | 0.0000 |
| Intercept (M1–M2) × RFC | 0.0216 | 0.0605 | 0.3571 | 0.7210 |
| Slope (M1–M2) × RFC | 0.1666 | 0.0581 | 2.8675 | 0.0042 |
| Intercept (M3–M12) × RFC | 0.1380 | 0.0456 | 3.0258 | 0.0025 |
| Slope (M3–M12) × RFC | −0.0021 | 0.0062 | −0.3400 | 0.7339 |
| Child behavior problems × M1–M6 × RFC | 0.0222 | 0.0231 | 0.9625 | 0.3359 |
| Child behavior problems × M7–M12 × RFC | 0.0667 | 0.0257 | 2.5927 | 0.0096 |
| Intercept (M1–M2) × CC | 0.1783 | 0.0594 | 3.0000 | 0.0027 |
| Slope (M1–M2) × CC | −0.0454 | 0.0571 | −0.7941 | 0.4272 |
| Intercept (M3–M12) × CC | 0.1947 | 0.0450 | 4.3299 | 0.0000 |
| Slope (M3–M12) × CC | −0.0085 | 0.0059 | −1.4323 | 0.1523 |
| Child behavior problems × CC | 0.0658 | 0.0239 | 2.7513 | 0.0060 |
Note. M1–M12 = monthly assessments 1–12. CC = community comparison group. RFC = regular foster care comparison group.
The intercepts for M3 to M12 were centered at M7, the middle of the time period. Both contrasts of the CC group and the RFC group versus the MTFC-P group for intercepts were positive and significant, indicating that both groups were significantly higher on caregiver stress on average than the MTFC-P group. The slope for the MTFC-P group during this period was not significantly different from zero, and none of the slope contrasts with the RFC or CC groups were significant, indicating maintenance of group differences during this time period.
The fixed effect of child problem behavior was highly significant in the MTFC-P group and was significantly lower than the corresponding effect in the CC group. For both groups, the effects were stable over time. For the first 6 months, the RFC group was not significantly different from the MTFC-P group, but the effect of child problem behavior was significantly stronger in the RFC group than in the MTFC-P group from M7 to M12. Follow-up tests (not shown) indicated that the effect of child problem behavior in the RFC group increased significantly (z = 2.17, p = .03) and was not significantly different from that in the CC group from M7 to M12 (z = −0.055, p = .96). The shift in the RFC group suggested that, over time, the RFC caregivers became more stress sensitive to child problem behavior compared to the MTFC-P caregivers.
For the random effects (not shown), all variance components were highly significant, indicating individual differences in growth and stress sensitivity over and above the effects of group membership. To obtain a proper solution, correlations between the random effect for child problem behavior and the intercepts and slopes were forced to zero. These correlations were small, and setting them to zero did not significantly degrade the fit of the model, χ2 = 1.08, df = 4, p = .90, or change any of the patterns of significance for the fixed effects. Only two correlations among the random effects were significant: the .58 correlation between the intercepts (indicating a high level of stability in caregiver stress in the two periods) and the –.53 correlation between intercept and slope from M1 to M2 (indicating regression towards the mean). It should be noted, however, that this is not an artifact of measurement error, which is eliminated by having two repeated assessments within each month. Day-level residual variance was allowed to change over time from M3 to M12 differently for each group by estimating a per month variance multiplier for each group; for the MTFC-P group, it was significantly less than 1 (indicating shrinkage); for the CC group, it was significantly greater than 1 (indicating expansion); and for the RFC group, it was not significantly different from 1 (indicating stability).
Residual diagnostics indicated that the day- and month-level residuals were mildly positively skewed but that there were no extreme outliers that appeared to unduly influence the results. The family-level random effects were reasonably normally distributed. Day- and month-level residual variances were not consistent over months but showed no interpretable patterns. No additional group differences in mean level or variance showed up for any of the random effects at any of the three levels.
In summary, the results indicated that the MTFC-P intervention lowered mean-level caregiver stress over time. In addition, and perhaps most remarkably, the MTFC-P intervention appeared to prevent increased caregiver stress sensitivity to child problem behavior over time, which occurred in the RFC group. Finally, day-level residual variance shrank in the MTFC-P group compared to the RFC group, suggesting more consistent day-to-day caregiver stress levels in the intervention condition.
Associations between Caregiver Stress and Child HPA Axis Activity
The results for caregiver stress associations with AM–PM cortisol are presented in Table 2. Preliminary analyses suggested that the caregiver stress effect was negative and significant, though only in the RFC group (not shown). Because the results discussed previously for intervention effects on caregiver stress were primarily in M1–M2, the caregiver effect in the RFC group was examined to see if it remained constant in M3–M12. Using the M1–M2 and M3–M12 periods, the RFC group was contrasted against the TFC and CC groups, which were not significantly different from each other and not significantly different from zero. For M1–M2, the effect of caregiver stress on AM cortisol for all three groups was negative but not significant and was close to zero. The TFC and CC versus RFC contrast was also not significant, indicating that, for all three groups, caregiver stress on Day 1 was not significantly associated with AM–PM cortisol on Day 2 for M–M2. For M3–M12, there was very little change for TFC and CC, and the caregiver stress effect was negative but nonsignificant and was close to zero. For M3–M12, however, the caregiver stress effect in the RFC group was negative, significantly different from zero, and significantly different from the corresponding effect in the TFC and CC groups. Thus, it would appear that all three groups started about the same, with no effect of caregiver stress on AM–PM cortisol; however, whereas the TFC and CC groups remained that way, a significant negative relation developed in the RFC group starting M3 such that increased caregiver stress on Day 1 was associated with lower AM–PM cortisol levels on Day 2. The results for caregiver stress associations with AM cortisol levels (see Table 3) were similar to results for AM–PM cortisol levels, although they were somewhat weaker. The results for caregiver stress associations with PM cortisol levels (see Table 3) showed no associations in any group during any time period.
Table 2. Caregiver Stress Effects on AM–PM Cortisol Levels.
| Effect | Estimate | t | p |
|---|---|---|---|
| Fixed effects | |||
| Intercept | 0.384 | 30.911 | 0.000 |
| Caregiver stress (M1–M2) | 0.033 | 0.515 | 0.607 |
| Caregiver stress (M3–M12) | −0.019 | −0.533 | 0.594 |
| Caregiver stress (M1–M2) × RFC | −0.036 | −0.339 | 0.734 |
| Caregiver stress (M3–M12) × RFC | −0.136 | −2.143 | 0.032 |
| Random effects | |||
| Intercept | 0.506 | 7.474 | 0.000 |
| Slope | 0.911 | 3.735 | 0.000 |
| Cor(intercept, slope) | −0.913 | −4.525 | 0.000 |
| Residual SD | 0.267 | 50.371 | 0.000 |
Note. M1–M12 = monthly assessments 1–12. RFC = regular foster care comparison group.
Table 3. Caregiver Stress Effects on AM Cortisol Levels (Root Transformed).
| Effect | Estimate | t | p |
|---|---|---|---|
| Fixed effects | |||
| Intercept | 0.602 | 60.964 | 0.000 |
| Caregiver stress (M1–M2) | 0.002 | 0.046 | 0.964 |
| Caregiver stress (M3–M12) | −0.021 | −0.740 | 0.460 |
| Caregiver stress (M1–M2) × RFC | −0.048 | −0.545 | 0.586 |
| Caregiver stress (M3–M12) × RFC | −0.099 | −1.980 | 0.048 |
| Random effects | |||
| Intercept | 0.577 | 8.624 | 0.000 |
| Slope | 0.863 | 4.171 | 0.000 |
| Cor(intercept, slope) | −0.920 | −5.384 | 0.000 |
| Residual SD | 0.216 | 50.837 | 0.000 |
Note. M1–M12 = monthly assessments 1–12. RFC = regular foster care comparison group.
Discussion
This study investigate three topics relevant to prevention in foster care: (a) the main effects of a caregiver-based intervention on foster caregiver stress in response to child problem behavior, (b) whether caregiver stress increased as child problem behaviors increased and whether this association was impacted by the intervention, and (c) whether previously observed blunting of diurnal HPA axis activity among children in regular foster care (Fisher et al., in press) was associated with higher caregiver stress levels and an increased caregiver stress sensitivity over time.
The data analyses yielded a number of noteworthy results. Participation in the MTFC-P intervention appeared to be associated with an immediate and lasting decrease in mean-level and day-to-day variability of caregiver stress related to child problem behavior. Moreover, the intervention appeared to prevent an increase in the likelihood and sensitivity of caregiver stress related to child problem behavior. In contrast, increased sensitivity was observed among caregivers in regular foster care from months 6-12 of the study. In addition, and most relevant to the neurobiologically informed conceptual model guiding the research, caregiver stress in conjunction with high levels of child problem behavior in the RFC group proved to be a significant time-varying covariate of diurnal HPA axis activity over time. In particular, higher caregiver stress in response to child problem behavior was associated with more blunted diurnal HPA axis activity. These results and related issues are discussed below.
Main Effects of MTFC-P on Caregiver Stress
MTFC-P caregivers reported an immediate decrease of stress in response to child problem behavior from months 1-2 of the study. Caregiver stress then remained relatively stable, continuing to decrease slightly from months 3-12. In contrast, there was an immediate increase in RFC caregiver stress from month 1-2, and caregiver stress remained high from months 3-12.
These results are consistent with the intervention's aim to provide a high level of support and consultation to caregivers. Such support is typically less available to RFC caregivers as child welfare social workers often carry very large caseloads and may find it more difficult to provide individualized, proactive support to foster families. Although implementing the MTFC-P program requires financial and personnel resources over and above what is typically available in the child welfare system, these results suggest that the implementation of such resources may be a worthwhile investment in the long-term well-being of foster children (especially foster preschoolers with significant behavioral problems).
The emphasis on caregiver support in the MTFC-P intervention might affect caregiver stress in several ways. First, it might increase caregivers' access to specific information about effective techniques for managing child problem behavior. Second, it might decrease the extent to which caregivers feel isolated when managing child problem behavior. Of course, the present study did not examine which aspects of the intervention led to lower stress levels. Perhaps some combination of these two factors was responsible, or perhaps additional variables came into play. This will be an important topic for future research.
Perhaps most noteworthy in these results is the fact that the intervention effect on caregiver stress was immediate and was sustained across time, whereas that RFC caregivers reported an immediate and sustained increase in caregiver stress. Inasmuch as intervention effects often accrue additively over time and usually take time to become statistically significant, these results are fairly remarkable.
Intervention effects were also observed in the area of stress sensitivity to child problem behavior. In the RFC and MTFC-P groups, stress sensitivity was relatively equal and was significantly lower than in the CC group from months 1-6 of the study. However, from months 7-12, RFC caregivers became significantly more stress sensitive to child problem behavior (significantly higher than MTFC-P caregivers and nonsignificantly different from CC caregivers). The results for the RFC caregivers are consistent with discussions of the bidirectional processes of reciprocal influence described in social learning (Patterson, 1982) and transactional models of family interaction (Sameroff, 1975; Sameroff & Chandler, 1975). Over time, child problem behavior appears to exert a negative influence on RFC caregiver stress levels. In contrast, MTFC-P appears to buffer against the long-term effects of child problem behavior on caregiver stress.
The results for the two foster care groups need to be considered within the context of the CC group, which exhibited consistently high levels of stress sensitivity to child problem behavior throughout the study. Although this result might be somewhat counterintuitive, one must consider the basic expectations of caregivers in typical versus foster families. In typical families, caregivers might expect low rates of child problem behavior; to the extent that child behavior is not consistent with these expectations, caregiver stress levels might increase. In contrast, foster caregivers might expect at least some behavioral difficulties and might be somewhat resilient in the face of these behaviors (at least initially). However, as is indicated by the results for the RFC group, over time and without proper support, foster caregivers might lose this resilience.
The risk for foster children in this context might lie in the area of foster caregiver commitment to the child (Dozier & Lindheim, 2006). In typical families, higher levels of stress in response to child problem behavior might predict long-term outcomes of coercive interaction, harsh parenting, and child antisocial behavior. In contrast, in foster families, especially those in which the commitment to the child is weak or limited, increased stress sensitivity might have major consequences (e.g., disengagement from the child and foster placement disruptions).
It is important to acknowledge the speculative nature of the interpretation of differences in stress sensitivity between family types. These issues are clearly outside the scope of the present study and are offered in an effort to generate hypotheses. Extensive additional research would be required to investigate these issues properly.
Associations between Caregiver Stress and Child HPA Axis Activity
Analyses linking caregiver stress to child cortisol levels revealed a number of interesting results. Increased caregiver stress was associated with less change in AM–PM cortisol levels and with lower AM cortisol (though only in the RFC group) from months 3-12 of the study. Caregiver stress was not associated with PM cortisol in any of the groups at any month. Thus, the AM–PM variation seems to have resulted from lower AM cortisol alone. The results also suggest that the association is not initially present in foster children but emerges later, perhaps as a result of the increase in caregiver stress during months 1-2. In other words, starting at month 3 of the study in the RFC group, diurnal HPA axis activity was further impacted by increased caregiver stress in response to child problem behavior. Although this is speculative, the drop in caregiver stress from month 1 to month 2 in the MTFC-P group might have prevented a similar change.
These results provide important evidence of a connection between a caregiver's psychological state and a child's stress neurobiology. Similar effects have been observed elsewhere in terms of the association between maternal depression and alterations in child HPA axis activity (O'Connor et al., 2005). What is particularly interesting in the present study is the association between increased caregiver stress and blunted diurnal HPA axis activity in the RFC group. This blunted pattern is consistent with the lower basal levels of diurnal activity that have been observed in foster children (Gunnar et al., 2006) and children adopted following institutional rearing (Carlson & Earls, 1997). It is suggestive of an ongoing vulnerability to downregulation of the HPA axis in conditions of stress for children who have experienced significant early adversity. Such downregulation might be an adaptive response to nonresponsive environmental conditions; less clear is whether it increases risk for subsequent psychopathology in these populations, although there is evidence that altered HPA axis functioning is associated with anxiety and affective disorders (see Gunnar & Vazquez, 2001).
Taken together with the MTFC-P intervention effects on caregiver stress described in this paper and on diurnal HPA axis activity reported previously (Fisher et al., 2006), these results provide new evidence of the manner in which interventions to support foster parents in managing child problem behavior might reduce overall stress, might mitigate the tendency for higher levels of behavior problems to increase caregiver stress over time, and might ultimately translate into greater physiological regulation for foster children. In terms of the overarching conceptual model, it provides validation of the hypothesized link between caregiver functioning and child neurobiology. In terms of policy and practice, it suggests that, at least for children with high rates of problem behavior, providing support and resources to foster caregivers is a key component of improving child outcomes.
Limitations and Conclusions
The current study has a number of limitations. First, although this study focused on diurnal HPA axis activity, only morning and evening cortisol samples were obtained. Optimally, an examination of the diurnal cycle would involve additional sampling times throughout the day (e.g., midmorning and afternoon) to provided additional information about diurnal variation. Second, the present study did not gather information about circadian cortisol levels. Altered diurnal levels might reflect disturbances to nighttime HPA axis activity as well. Low morning cortisol levels, for instance, might indicate a decrease in peak daily cortisol production or might indicate a phase shift to an earlier peak. Third, the study did not measure HPA stress response. Although this decision is justifiable in terms of the age and vulnerability of the study sample, it is important to differentiate the results obtained from those that focus on HPA axis responses to a stressor.
Fourth, the study only one element of the larger conceptual model guiding the research. Given the complexity of the model, there are limits to how much of it can be examined within a single study. Rather, our strategy was to explore and validate a series of subcomponents, such as the association between caregiver stress and diurnal HPA axis activity. Although this strategy has been an effective way to advance knowledge, it provides somewhat less robust evidence than a single comprehensive test of a model.
These limitations indicate that caution should be exercised when evaluating the evidence of associations between caregiver stress and diurnal HPA axis activity. Nevertheless, the results provide fairly strong evidence of intervention effects on reducing caregiver stress and stress sensitivity in response to child problem behavior. Moreover, the caregiver stress–diurnal HPA axis activity connection that was observed in this study suggests many directions for future research, both to confirm and clarify the nature of this relationship. In general, this study serves as a model for how neurobiological measures can be meaningfully integrating into the theory and design of prevention research intended to reduce risk for psychopathology and improve long-term psychosocial maladjustment.
Table 4. Caregiver Stress Effects on PM Cortisol Levels (Transformed).
| Effect | Estimate | t | p |
|---|---|---|---|
| Fixed effects | |||
| Intercept | 5.102 | 92.171 | 0.000 |
| Caregiver stress (M1–M2) | −0.171 | −0.626 | 0.531 |
| Caregiver stress (M3–M12) | −0.033 | −0.227 | 0.821 |
| Caregiver stress (M1–M2) × RFC | −0.253 | −0.554 | 0.580 |
| Caregiver stress (M3–M12) × RFC | 0.103 | 0.396 | 0.692 |
| Random effects | |||
| Intercept | 0.773 | 11.440 | 0.000 |
| Slope | 0.821 | 8.052 | 0.000 |
| Cor(intercept, slope) | −0.891 | −7.659 | 0.000 |
| TFC M6–M12 residual SD shrinkage | 0.880 | 2.769 | 0.006 |
| Residual SD | 1.082 | 46.061 | 0.000 |
Note. M1–M12 = monthly assessments 1–12. RFC = regular foster care comparison group.
Acknowledgments
Support for this research was provided by the following grants: MH059780, NIMH, U.S. PHS; HD045894, NICHD, U.S. PHS; and DA021424 and DA017592, NIDA, U.S. PHS. The authors thank Matthew Rabel for editorial assistance and Kristen Greenley and the staff and families of the Multidimensional Treatment Foster Care for Preschoolers project for their ongoing dedication and participation
Appendix.
Items from the Parent Daily Report Checklist.
| Arguing | Inappropriate sexual behavior | Short attention span |
| Baby talk | Interrupting | Skipping meals |
| Back talking | Irresponsibility | Sluggishness |
| Bedwetting | Irritability | Soiling floor/other location |
| Biting | Jealousy | Soiling in pants/pajamas |
| Boisterous/rowdy | Loudness | Stealing |
| Competitive | Lying | Swearing/other obscenities |
| Complaining | Negativism | Tantrums |
| Cruel to animals | Nervousness (jittery) | Teasing/provoking |
| Crying | Nightmares/night terrors | Threatening |
| Daydreaming | Not minding | Urinating on floor/other location |
| Defiance | Pouting | Waking at night |
| Depression/sadness | Repetitive questions | Wetting pants |
| Fearfulness | Runaway | Worried/anxious |
| Fighting | School problems | Yelling |
| Hitting others | Self-destructiveness | |
| Hyperactivity | Sexual talk |
Contributor Information
Philip A. Fisher, Oregon Social Learning Center & Center for Research to Practice
Mike Stoolmiller, University Of Oregon.
References
- Becker WC, Madsen CH, Arnold CR, Thomas DR. The contingent use of teacher attention and praise in reducing classroom behavior problems. Journal of Special Education. 1967;1:287–307. [Google Scholar]
- Bower GH. Mood and memory. American Psychologist. 1981;36:129–148. doi: 10.1037//0003-066x.36.2.129. [DOI] [PubMed] [Google Scholar]
- Bruce J, Kroupina M, Parker S, Gunnar M. The relationships between cortisol patterns, growth retardation, and developmental delays in postinstitutionalized children; Paper presented at the International Conference on Infant Studies; Brighton, UK. 2000. Jul, [Google Scholar]
- Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol levels in preschool-aged foster children: differential effects of maltreatment type. 2007 doi: 10.1002/dev.20333. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of Neuroscience Abstracts. 1997;218:12. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Chamberlain P, Moreland S, Reid K. Enhanced services and stipends for foster parents: Effects on retention rates and outcomes for children. Child Welfare. 1992;5:387–401. [PubMed] [Google Scholar]
- Chamberlain P, Price JM, Reid JB, Landsverk J, Fisher PA, Stoolmiller M. Who disrupts from placement in foster and kinship care? Child Abuse and Neglect. 2006;30:409–424. doi: 10.1016/j.chiabu.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Chamberlain P, Reid JB. Parent observation and report of child symptoms. Behavioral Assessment. 1987;9:97–109. [Google Scholar]
- Chamberlain P, Reid JB. Using a specialized foster care community treatment model for children and adolescents leaving the state mental hospital. Journal of Community Psychology. 1991;19:266–276. [Google Scholar]
- Cicchetti D, Hinshaw SP. Editorial: Prevention and intervention science: Contributions to developmental theory. Development and Psychopathology. 2002;14:667–671. doi: 10.1017/s0954579402004017. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Psychopathology as risk for adolescent substance use disorders: A developmental psychopathology perspective. Journal of Clinical Child Psychology. 1999;28:355–365. doi: 10.1207/S15374424jccp280308. [DOI] [PubMed] [Google Scholar]
- Danforth JS, Harvey E, Ulaszek WR, McKee TE. The outcome of group parent training for families of children with attention-deficit hyperactivity disorder and defiant/aggressive behavior. Journal of Behavior Therapy and Experimental Psychiatry. 2006;37:188–205. doi: 10.1016/j.jbtep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- de Kloet ER. Brain corticosteroid receptor balance and homeostatic control. Frontiers in Neuroendocrinology. 1991;12:95–164. doi: 10.1016/j.yfrne.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Dozier M, Albus K, Fisher PA, Sepulveda S. Interventions for foster parents: Implications for developmental theory. Development and Psychopathology. 2002;14:843–860. doi: 10.1017/s0954579402004091. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Burraston B, Pears KC. The Early Intervention Foster Care Program: Permanent placement outcomes from a randomized trial. Child Maltreatment. 2005;10:61–71. doi: 10.1177/1077559504271561. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Chamberlain P. Multidimensional Treatment Foster Care: A program for intensive parent training, family support, and skill building. Journal of Emotional and Behavioral Disorders. 2000;8:155–164. [Google Scholar]
- Fisher PA, Ellis BH, Chamberlain P. Early Intervention Foster Care: A model for preventing risk in young children who have been maltreated. Children's Services: Social Policy, Research, and Practice. 1999;2:159–182. [Google Scholar]
- Fisher PA, Gunnar MR, Dozier M, Bruce J, Pears KC. Effects of a therapeutic intervention for foster children on behavior problems, caregiver attachment, and stress regulatory neural systems. Annals of the New York Academy of Sciences. 2006;1094:215–225. doi: 10.1196/annals.1376.023. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Kim HK. Intervention effects on foster preschoolers' attachment-related behaviors from a randomized trial. Prevention Science. 2007;8:161–170. doi: 10.1007/s11121-007-0066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston B. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2007.06.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgatch MS, Toobert DJ. A cost-effective parent training program for use with normal preschool children. Journal of Pediatric Psychology. 1979;4:129–145. [Google Scholar]
- Friese E, Hesse J, Hellhammer J, Hellhammer D. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gaab J, Blättler N, Menzi T, Pabst B, Stoyer S, Ehlert U. Randomized controlled evaluation of the effects of cognitive-behavioral stress management on cortisol responses to acute stress in healthy subjects. Psychoneuroendocrinology. 2003;28:767–779. doi: 10.1016/s0306-4530(02)00069-0. [DOI] [PubMed] [Google Scholar]
- Gaab J, Sonderegger L, Scherrer S, Ehlert U. Psychoneuroendocrine effects of cognitive-behavioral stress management in a naturalistic setting—a randomized controlled trial. Psychoneuroendocrinology. 2006;31:428–438. doi: 10.1016/j.psyneuen.2005.10.005. [DOI] [PubMed] [Google Scholar]
- George S, van Oudenhoven N, Wazir R. Stakeholders in foster care: An international comparative study; Paper presented at the Meeting of the International Federation of Foster Care Organizations; Veldhoven, Netherlands. 2001. Jul, [Google Scholar]
- Gunnar M, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Fisher PA, the Early Experience Stress Prevention Science Network Bringing basic research on early experience and stress neurobiology to bear on preventive intervention research on neglected and maltreated children. Development and Psychopathology. 2006;18:651–677. [PubMed] [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Development and Psychopathology. 2001;13:611–627. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–737. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Halme N, Tarkka MT, Nummi T, Åstedt-Kurki P. The effect of parenting stress on fathers' availability and engagement. Child Care in Practice. 2006;12:13–26. [Google Scholar]
- Hammerfald K, Eberle C, Grau M, Kinsperger A, Zimmermann A, Ehlert U, et al. Persistent effects of cognitive-behavioral stress management on cortisol responses to acute stress in healthy subjects—a randomized controlled trial. Psychoneuroendocrinology. 2005;31:333–339. doi: 10.1016/j.psyneuen.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neurosciences. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Insightful Corporation. S-PLUS 6 for Windows user's guide. Seattle, WA: Author; 2001. [Google Scholar]
- Keller TE, Spieker SJ, Gilchrist L. Patterns of risk and trajectories of preschool problem behaviors: A person-oriented analysis of attachment in context. Development and Psychopathology. 2005;17:249–384. doi: 10.1017/s0954579405050170. [DOI] [PubMed] [Google Scholar]
- Kertes DA, Gunnar M, Madsen NJ. Early deprivation and home basal cortisol levels: A study of internationally-adopted children. 2007 doi: 10.1017/S0954579408000230. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee L, Kronstadt D, Zlotnick C. Foster care's youngest: A preliminary report. American Journal of Orthopsychiatry. 1997;67:290–299. doi: 10.1037/h0080232. [DOI] [PubMed] [Google Scholar]
- Landsverk JA, Garland AF, Leslie LK. Mental health services for children reported to child protective services. In: Meyers J, Briere J, editors. APSAC handbook on child maltreatment. 2nd. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Malik NM, Boris NW, Heller SS, Harden BJ, Squires J, Chazan-Cohen R, et al. Risk for maternal depression and child aggression in Early Head Start families: A test of ecological models. Infant Mental Health Journal. 2007;28:171–191. doi: 10.1002/imhj.20128. [DOI] [PubMed] [Google Scholar]
- McClowry S, Snow DL, Tamis-LeMonda CS. An evaluation of the effects of “INSIGHTS” on the behavior of inner city primary school children. Journal of Primary Prevention. 2005;26:567–584. doi: 10.1007/s10935-005-0015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney M, Szyf M. Environmental programming of stress responses through DNA methylation: Life at the interface between a dynamic environment and a fixed genome. Dialogues in Clinical Neuroscience. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer LJ, Mindell JA. Relationship between child sleep disturbances and maternal sleep, mood, and parenting stress: A pilot study. Journal of Family Psychology. 2007;21:67–73. doi: 10.1037/0893-3200.21.1.67. [DOI] [PubMed] [Google Scholar]
- Mommersteeg PMC, Keijsers GPJ, Heijnen CJ, Verbraak MJPM, van Doornen LJP. Cortisol deviations in people with burnout before and after psychotherapy: A pilot study. Health Psychology. 2006;25:243–248. doi: 10.1037/0278-6133.25.2.243. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biological Psychiatry. 2005;58:211–217. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Owen AE, Thompson MP, Kaslow NJ. The mediating role of parenting stress in the relation between intimate partner violence and child adjustment. Journal of Family Psychology. 2006;20:505–513. doi: 10.1037/0893-3200.20.3.505. [DOI] [PubMed] [Google Scholar]
- Patterson GR. The aggressive child: Victim and architect of a coercive system. In: Mash EJ, Hamerlynck LA, Handy LC, editors. Behavior modification and families I Theory and research II Applications and developments. New York: Brunner/Mazel; 1976. pp. 265–316. [Google Scholar]
- Patterson GR. Coercive family process. Eugene, OR: Castalia; 1982. [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS (Statistics and Computing) New York: Springer; 2000. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2005. [Google Scholar]
- Rosenfeld P, Suchecki D, Levine S. Multifactorial regulation of the hypothalamic-pituitary-adrenal axis during development. Neuroscience and Biobehavioral Reviews. 1992;16:553–568. doi: 10.1016/s0149-7634(05)80196-4. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ. Early influences on development: Fact or fancy? Merrill-Palmer Quarterly. 1975;21:267–294. [Google Scholar]
- Sameroff AJ, Chandler MJ. Reproductive risk and the continuum of caretaking casualty. In: Horowitz FD, editor. Child development research. Vol. 4. Chicago: University of Chicago Press; 1975. pp. 187–244. [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Research Reviews. 1986;11:65–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Schwartz EP, Granger DA, Susman EJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child Development. 1998;69:1503–1513. [PubMed] [Google Scholar]
- Secco ML, Askin D, Yu CT, Garinger J, Mulaire-Cloutier C, Scharf L, et al. Factors affecting parenting stress among biologically vulnerable toddlers. Issues in Comprehensive Pediatric Nursing. 2006;29:131–156. doi: 10.1080/01460860600846867. [DOI] [PubMed] [Google Scholar]
- Sharry J, Guerin S, Griffin C, Drumm M. An evaluation of the Parents Plus Early Years Programme: A video-based early intervention for parents of pre-school children with behavioural and developmental difficulties. Clinical Child Psychology and Psychiatry. 2005;10:319–336. [Google Scholar]
- Smotherman WP, Bell RW. Maternal mediation of early experience. In: Bell RW, Smotherman WP, editors. Maternal influences and early behavior. New York: Spectrum; 1980. pp. 201–210. [Google Scholar]
- Soliday E, McCluskey-Fawcett K, Meck N. Foster mothers' stress, coping, and social support in parenting drug-exposed and other at-risk toddlers. Children's Health Care. 1994;23:15–32. [Google Scholar]
- Stone AA, Broderick JE, Kaell AT, DelesPaul PA, Porter LE. Does the peak-end phenomenon observed in laboratory pain studies apply to real-world pain in rheumatoid arthritics? Journal of Pain. 2000;1:212–217. doi: 10.1054/jpai.2000.7568. [DOI] [PubMed] [Google Scholar]
- Tu MT, Grunau RE, Petrie-Thomas J, Haley DW, Weinberg J, Whitfield MF. Maternal stress and behavior modulate relationships between neonatal stress, attention, and basal cortisol at 8 months in preterm infants. Developmental Psychobiology. 2007;49I:150–164. doi: 10.1002/dev.20204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. I. synaptic and neuronal density and synapses per neuron. Brain Research. 1985;329:195–203. doi: 10.1016/0006-8993(85)90525-6. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, Administration on Children, Youth and Families. Child maltreatment 2003. Washington, DC: U.S. Government Printing Office; 2005. [Google Scholar]
- Weinrott M, Bauske B, Patterson GR. Systematic replication of a social learning approach to parent training. In: Bates SL, Sjoden PO, Dockens WS III, editors. Trends in behavior therapy. New York: Academic Press; 1979. pp. 331–351. [Google Scholar]