Summary
Methicillin-resistant Staphylococcus aureus (MRSA) induces a pro-thrombotic and pro-inflammatory milieu. Although timely antibiotic administration in MRSA sepsis may improve outcomes by arresting bacterial growth, the effects of antibiotics on mitigating injurious thrombo-inflammatory cellular responses remains unexplored. Using a newly developed human whole blood model and an in vivo mouse model of MRSA infection, we examined how antibiotics inhibit MRSA induced thrombo-inflammatory pathways. Human whole blood was inoculated with MRSA. Thrombin generation and inflammatory cytokine synthesis was measured in the presence or absence of linezolid and vancomycin. C57BL/6 mice were injected with MRSA and the effect of vancomycin administration was examined. MRSA accelerated thrombin generation in a time- and concentration-dependent manner and induced the release of cytokines, including interleukin (IL)-6, IL-8, and monocyte chemotactic protein (MCP)-1. The increase in thrombin generation and inflammatory responses was mediated through the synthesis of tissue factor and cytokines, respectively, and the release of microparticles. The early administration of antibiotics restored normal thrombin generation patterns and significantly reduced the synthesis of cytokines. In contrast, when antibiotic administration was delayed, thrombin generation and cytokine synthesis were not significantly reduced. In mice infected with MRSA, early antibiotic administration reduced thrombin anti-thrombin complexes and cytokine synthesis, whereas delayed antibiotic administration did not. These data provide novel mechanistic evidence of the importance of prompt antibiotic administration in infectious syndromes.
Keywords: Thrombin, tissue factor, cytokines, methicillin-resistant Staphylococcus aureus, antibiotics
Introduction
Staphylococcus aureus (S. aureus) is a common cause of Gram-positive sepsis associated with increased rates of micro- and macrovascular thrombosis leading to organ failure and death (1–7). The pathogenicity of S. aureus is due, in part, to numerous toxins, adhesions, and cell-surface components produced by the organism promoting bacterial invasion with robust pro-coagulant and pro-inflammatory responses (8–10). The thrombotic complications due to S. aureus infections are common and include both macrovascular and microvascular thrombosis as well as disseminated intravascular coagulation (DIC). DIC can result from monocytes exposed to bacterial toxins such as peptidoglycan (8), a component of the outer cell wall of Gram-positive bacteria, and lipoteichoic acid from S. aureus (10). The pro-inflammatory milieu induced by S. aureus infection is multifactorial, but includes direct interaction of the bacteria or its toxins with monocytes and macrophages inducing the release of cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, IL-6, and IL-8 (9, 11–13).
Currently approved antibiotics for the treatment of S. aureus infections include linezolid and vancomycin. Linezolid may have greater efficacy in patients with methicillin-resistant S. aureus (MRSA) pneumonia, although clinical outcome data remain controversial (14, 15). In addition to the type of antibiotics, the timing of antibiotic administration has significant consequences for efficacy. The early administration of appropriate antibiotics reduces the risk of subsequent adverse events in S. aureus infection. For example, in septic shock and MRSA bacteraemia, delays in antibiotic administration are associated with significant increases in mortality (16). Similarly, in patients with ventilator-associated pneumonia, where MRSA is a common pathogen (17), the prompt administration of adequate antibiotics reduces mortality by 50% (17, 18).
Although timely antibiotic administration offers the obvious benefit of arresting bacterial growth at an earlier phase, additional molecular mechanisms underlying these benefits remain largely unexplored. In addition, antibiotics themselves have been shown to reduce inflammation by directly acting on inflammatory cells (19–22). To examine these mechanisms and the role of antibiotic treatment in modulating these responses, we developed an in vitro human whole blood and mouse model of infection using whole bacterial organisms to determine how the timing of antibiotic administration influences prothrombotic and pro-inflammatory responses. In contrast to previous studies, which have commonly employed the use of bacterial toxins (9, 20, 23–25), the use of whole organisms allowed us to determine the effects of antibiotic treatment and to determine how antibiotics modulate the thrombo-inflammatory response. By employing both model systems, we demonstrate early administration of antibiotics reduces the thrombo-inflammatory “cytokine storm” associated with MRSA-induced infection.
Materials and methods
Bacteria and toxins
For all experiments, an isolate of MRSA obtained from a patient with documented MRSA bacteraemia was used. Other clinical strains of MRSA and methicillin-sensitive S. aureus (MSSA) were studied in selected experiments for comparisons. For each experiment, the bacteria were expanded on blood agar plates (Hardy Diagnostics, Santa Maria, CA, USA) overnight at 37°C in 5% CO2. Single colonies of bacteria were then suspended in phosphate-buffered saline (pH 7.4) (PBS) and the concentration was confirmed by colourimetry (VITEK Colorimeter, bioMerieux, Inc., Durham NC, USA). To quantify the colony growth of the bacteria, inoculated blood was serially diluted and 100 μl of each dilution was plated on blood agar overnight at 37°C in 5% CO2. Colony forming units (CFU) were counted the following day. S. aureus-derived alpha toxin (AT) was obtained from List Biological Laboratories (Campbell, CA, USA) and lipopolysaccharide (LPS) was purchased from Invivogen (San Diego, CA, USA)
Antibiotics
Linezolid (Pfizer, New York, NY, USA) and vancomycin (Hospira, Inc., Lake Forest, IL, USA) were re-suspended according to manufacturer’s recommendations. Minimum inhibitory concentrations (MIC) of these antibiotics for all S. aureus strains were determined by a microdilution broth method used according to Clinical Laboratory Standards Institute guidelines (17). These assays were performed by ARUP Laboratories (Salt Lake City, UT, USA), a national reference laboratory (www.aruplab.com). The MIC for both linezolid and vancomycin was 1.0 μg/ml. In all in vitro experiments, linezolid and vancomycin were used at a concentration of five times the MIC (i.e. final concentration of 5.0 μg/ml).
Whole blood collection and plasma isolation
The University of Utah Institutional Review Board approved this study and all subjects provided informed consent. Human peripheral venous blood (25–50 ml) from healthy, medication-free, fasting adult subjects was drawn into acid-citrate-dextrose (1.4 ml ACD/8.6 ml blood) through standard venipuncture technique and used immediately upon collection. S. aureus isolates were incubated in whole blood with linezolid or vancomycin at various concentrations and times. The growth of MRSA in whole blood appeared to only occur in logarithmic phase (see Suppl. Figure 1, available online at www.thrombosis-online.com). Plasma was harvested by centrifuging the whole blood at 500 x g for 20 minutes (min) and then once more at 13,000 x g for 2 min to remove remaining cell contaminants.
Monocyte isolation
Human monocytes were isolated by drawing human peripheral venous blood (500 ml) from healthy, medication-free, fasting adult subjects. Blood was centrifuged at 150 x g for 20 min at 20°C to separate platelet-rich plasma (PRP) from red and white blood cells (RBC/WBC). The PRP was removed and the remaining RBC/ WBC mixture was resuspended with 0.9% sterile saline back to the original volume and layered over an equal volume of Ficoll-Paque Plus (GE Healthcare Biosciences, Piscataway, NJ, USA). The layered cells were then centrifuged for 30 min at 400 x g at 20°C. After 30 min, the mononuclear leukocyte layer was removed and washed with Hank’s Balance Salt Solution (Sigma-Aldrich, St. Louis, MO, USA) with 1% human serum albumin (HBSS/A) (University of Utah Hospital, Salt Lake City, UT, USA) and centrifuged for 10 min at 400 x g at 20°C. The cell pellet was then resuspended in 1 ml of HBSS/A and 500 μl of CD14 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and incubated at 4°C for 15 min. The cells were then washed with HBSS/A to remove any free CD14 microbeads and then resuspended in 500 μl of HBSS/A. The monocytes were then isolated by running the cell solution through an autoMACs cell separator (Miltenyi Biotec) using the PosselD2 program. Cells were then washed with HBSS/A and resuspended in M199 (BioWhitaker, Walkersville, MD, USA) and counted. The purity of monocytes was > 95% (data not shown).
Microparticle analysis
Whole blood stimulated with MRSA (9 x 102 cfu/ml) was incubated for 18 hours (h). The blood was initially centrifuged at 400 x g f or 15 min followed by a 13,000 x g spin for 2 min. Microparticles (MP) were isolated by centrifuging the resulting supernatant at 20,000 x g for 20 min at 4°C. MPs were then washed twice with PBS (pH 7.4) before being resuspended in PBS. MP analysis was performed using monodisperse fluorescent beads (Megamix, BioCytex, Marseille, France) of three diameters (0.5, 0.9 and 3 μm) (26–29). Forward and side scatter (SS) parameters were plotted on logarithmic scales to best cover a wide size range. MPs were incubated with antibodies in Annexin V Binding Buffer. FITC-Annexin V (Beckman Coulter, Brea, CA, USA) was used to detect phosphatidylserine (PS). PE-anti-CD41 was used as a cell marker for platelet MPs (BD Biosciences, San Jose, CA, USA). PE-anti-glycophorin A was a cell marker for red blood cell MPs (Immunotech, Marseille, France). PE-anti-CD15 was used as a cell marker for neutrophil-derived MPs (BD Biosciences). Brilliant Violent 421-anti-human CD11b was used as a cell marker for monocytes derived MPs (BD Biosciences). Single staining controls and control IgG antibodies were used to check fluorescence compensation settings and to set up positive regions on a FACSCanto II flow cytometer (BD Biosciences). The total MP population was examined first to determine which percentage of MPs were PS-positive. The different cell populations were then determined based on the percent PS-positive MP since these MP can contribute to procoagulant activity.
Cytokine expression analyses
Commercially available sandwich ELISAs were used to study IL-6, IL-8, MCP-1, TNF-α and IL-1β concentrations as well as mouse MCP-1, TNF-α and IL-1β levels, according to the manufacturer’s guidelines (R&D, Minneapolis, MN, USA). LPS and AT were used as known agonists for inducible cytokine expression. Control experiments confirmed that detection antibodies for IL-6, IL-8, MCP-1, TNF-α and IL-1β did not exhibit non-specific binding to any strain of S. aureus used (data not shown).
Thrombin and FXa generation assays
Falcon PRO-BIND™ 96-well flat bottom plates (Becton Dickinson, Franklin Lakes, NJ, USA) were used to perform the fluorogenic reactions. Thrombin generation was measured with a Synergy HT multi Detection Microplate Reader (Bio-Tek Instruments, Winooski, VT, USA) at excitation/emission wavelength of 360 nm/460 nm. Fluorogenic substrate (Z-Gly-Gly-Arg-AMC; 0.5 mM, final), and RC high reagent (7.16 pM TF and 0.32 μM phospholipid micelles, final) were obtained from a Technothrombin® TGA kit (Technoclone, Vienna, Austria). Additional CaCl2 was added to the substrate reagent to reach a final concentration of 15 mM CaCl2. Reagents were combined with sample plasma in duplicate according to manufacturer’s instructions in a 96-well plate and read every minute for 90 min. Thrombin calibration curves were performed and analysed according to manufacturer’s instructions. In some assays, a mouse anti-human TF antibody (Cat 550252, BD Pharmingen, San Jose, CA, USA), or control mouse IgG (Cat 555746, BD Pharmingen) was pre-incubated at 37°C with the plasma before initiating the reaction (10 μg/ml, final).
For the factor (F)Xa assays, isolated monocytes were incubated with vehicle, LPS (100 ng/ml, final), or MRSA (9 x 102 cfu/ml, final) for 18 h in M199 at 37°C. In some experiments, monocytes and monocytes MPs were removed by centrifuging the supernatant at 20,000 x g for 20 min after which FXa activity was measured. FVIIa (100 pg/ml, final) was incubated with the monocytes or monocyte-derived MPs for 5 min before addition of FX (10 μg/ml, final). Thirty minutes later, FXa generation was measured using a chromogenic substrate, as previously described (30, 31). To examine red blood cell (RBC) MP activity, purified RBC were isolated from one-day old expired blood generously donated by Robert C. Blaylock and ARUP Laboratories (Salt Lake City, UT, USA). RBCs (50% haematocrit, final) were washed twice with PBS and incubated with MRSA (9 x 102 cfu/ml, final) to generate MPs. To examine platelet derived MPs, platelets (2 x 108/ml, final) were isolated as previously described (32) and incubated with MRSA (9 x 102 cfu/ml, final) to generate MPs. RBC and platelet MP tissue factor expression was measured as described above.
Mouse model of MRSA infection
All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC). A mouse model of MRSA infection was modified from previous studies as described below (33): Seventy-six C57BL/6 mice (weighing 20–25 g) were randomly assigned to one of four groups: 1) control intraperitoneal (i.p.) saline injection (PBS 250 μl); 2) MRSA re-suspended in PBS at 9 x 108/ml i.p. injection (280 μl or 2.5 x 108 total bacteria); 3) MRSA re-suspended in PBS at 9 x 108/ml i.p. injection (280 μl or 2.5 x 108 total bacteria) followed by vancomycin injections (60 mg/kg in sterile water, 200 μl) at 2 and 8 h post infection (early antibiotic treatment); 4) MRSA re-suspended in PBS at 9 x 108/ml i.p. injection (280 μl or 2.5 x 108 total bacteria) followed by vancomycin i.p. injections (60 mg/kg in sterile water, 200 μl) at 8 and 14 h post infection (late antibiotic treatment). At 24 h after infection, mice were euthanised using CO2 asphyxiation followed by cervical dislocation, and peritoneal lavage was performed with 5 ml of HBSS. To quantify the colony growth of the MRSA, peritoneal lavage fluid was diluted 1:200 and 100 μl was plated on blood agar overnight at 37°C in 5% CO2. CFU were counted the following day. MCP-1, IL-1β, and TNF-α in peritoneal lavage fluid was measured by ELISA after the lavage fluid was centrifuged at 13,000 x g for 2 min. After 24 h, i.p. injection of bacteria resulted in little bacteraemia (data not shown).
To address changes in procoagulant activity in MRSA-infected animals, 3.3 x 106 cfu total bacteria were injected intravenously (i.v.) through the tail vein in C57BL/6 mice. Antibiotics were administered at a dose of 60 mg/kg through the tail vein. Early antibiotic administration was performed at 3 h post-infection and late antibiotic administration was performed at 9 h post-infection. Whole blood was drawn from the carotid artery 24 h after initial bacterial injection into ACD. Thrombin anti-thrombin levels were measured by ELISA (Enzygnost TAT micro ELISA; Siemens, Erlangen, Germany). Samples showing gross haemolysis were excluded.
Statistical analyses
Experimental results reflect ≥ 3 experiments with data expressed as mean ( standard error of the mean (SEM). For all analyses, continuous variables were assessed for normality and if distributions were normal, parametric t-tests were used. If distributions were not normal, Wilcoxon Rank Sum tests were used. Categorical variables were compared using the Fisher’s Exact test and continuous variables were compared using t-tests. For comparisons of thrombin generation between groups, a one-way ANOVA was completed. Since the distributions were normal, a Tukey’s post-hoc analysis was used to detect significant differences. For all comparisons, significance was predetermined at p ≤ 0.05.
Results
MRSA enhances thrombin generation
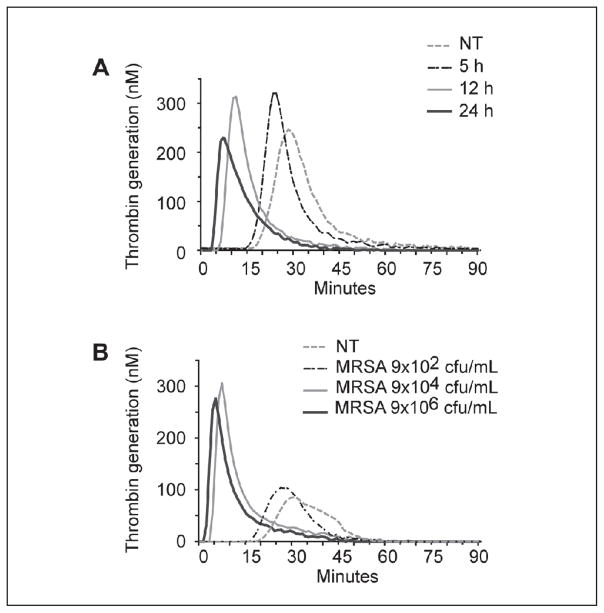
Since MRSA induces a hypercoagulable state via the extrinsic coagulation pathway (34, 35), we tested the hypothesis that MRSA would amplify thrombin generation. We found that MRSA significantly shortened the lag time (LT) and time to peak (TTP) thrombin generation and accelerated the rate of thrombin generation in a concentration- and time-dependent effect (Figure 1, Table 1 A and B, and data not shown). However, the addition of MRSA to whole blood did not significantly alter the area under the curve (AUC) or peak thrombin concentration. These data suggest that MRSA primarily effects the initiation and rate of thrombin generation but does not increase the total amount of thrombin generated. Strains of MSSA induced a response similar to MRSA strains (data not shown).
Figure 1. MRSA induces a pro-coagulant response in whole blood.
MRSA was incubated in whole blood for up to 24 hours. Thrombin generation was initiated in harvested, platelet-poor plasma by the addition of 7.16 pM recombinant TF, 0.32 M phospholipid micelles, and 15 mM CaCl2 (final). Thrombin generation was measured in duplicate every minute for ≥ 90 minutes following initiation. A) Increasing MRSA incubation times: 5, 12, and 24 hours with 9 x 104 cfu/ml or baseline untreated sample (NT). Samples left untreated for 5 and 12 hours had similar curves to baseline, while the 24 hours untreated sample had a slightly prolonged lag time (data not shown). B) Effect of increasing MRSA concentrations. Increased thrombin generation is demonstrated by a shortened lag time and time to peak thrombin concentration as well as increased rate and peak thrombin concentration with increasing MRSA incubation times (A) and concentrations (B). These data are representative of ≥3 individual experiments.
Table 1.
MRSA induces a time-dependent (A) and concentration-dependent (B), pro-coagulant response in whole blood.
| A | NT | 5 h | 12 h | 24 h |
|---|---|---|---|---|
| Lag (min) | 21.0 ± 1.9 | 16.3 ± 0.8 | 6.2 ± 0.6* | 4.7 ± 0.7* |
| Peak (nM) | 196.8 ± 22.7 | 279.3 ± 86.4 | 318.5 ± 9.0 | 256.1 ± 20.4 |
| Time to Peak (min) | 31.3 ± 2.0 | 28.2 ± 5.4 | 9.0 ± 1.0* | 6.2 ± 1.1* |
| Rate (nM/min) | 21.2 ± 4.2 | 36.6 ± 16.8 | 118.8 ± 18.0 | 186.0 ± 45.2* |
| AUC (nM* min) | 2999.8 ± 449.8 | 2762.0 ± 900.3 | 3162.6 ± 122.1 | 2579.6 ± 115.7 |
| B | NT | 9x102 cfu/ml | 9x104 cfu/ml | 9x106 cfu/ml |
| Lag (min) | 20.0 ± 0.8 | 19.0 ± 1.4 | 6.2 ± 0.6* | 4.0 ± 0.6* |
| Peak (nM) | 192.2 ± 33.4 | 159.7 ± 24.3 | 318.5 ± 9 | 342.0 ± 34.5 |
| Time to Peak (min) | 32.3 ± 0.9 | 29.8 ± 1.8 | 9.0 ± 1* | 6.2 ± 0.7* |
| Rate (nM/min) | 16.8 ± 4.1 | 15.8 ± 3.6 | 118.8 ± 18 | 161.6 ± 17.7* |
| AUC (nM* min) | 2379.7 ± 443.6 | 2241.0 ± 314 | 3162.6 ± 122.1 | 3276.8 ± 398.3 |
p<0.05 vs. NT.
As thrombin generation can be dependent on TF activity, we next pre-incubated plasma from MRSA-treated blood with anti-TF or control antibodies to investigate the mechanism of increased thrombin generation. The addition of the anti-TF antibody, but not control antibody, significantly increased the LT and the TTP and reduced the rate of thrombin generation. The addition of an anti-TF antibody to untreated plasma had no effect on thrombin generation (data not shown). We next examined whether MPs, small lipid vesicles less than one micron in size (27–29), which can carry TF, contribute to increased thrombin generation in MRSA-treated blood. Plasma isolated from MRSA- or un-treated whole blood was centrifuged at 20,000 x g for 20 min to reduce the concentration of MP in the plasma. Removal of MPs from MRSA treated blood significantly increased the LT and TTP and reduced the rate of thrombin generation (Table 2), while removal of MPs from untreated plasma had little effect on thrombin generation parameters (data not shown). These data suggest that higher rates of thrombin generation in MRSA-treated blood are due to increased TF expression and MP generation.
Table 2.
Tissue factor mediates MRSA-induced thrombin generation.
| MRSA + MP | MRSA − MP | MRSA + IgG | MRSA + TF Ab | |
|---|---|---|---|---|
| Lag (min) | 6.8 ± 1.1 | 18.3 ± 4.9* | 6.8 ± 1.1 | 20.0 ± 6.8** |
| Peak Height (nM) | 256 ± 44 | 133 ± 28 | 258 ± 44 | 198 ± 45 |
| Time to Peak (min) | 10.7 ± 1.8 | 28.0 ± 6.0* | 10.7 ± 1.8 | 27.3 ± 8.2** |
| Rate (nM/min) | 78.1 ± 28.0 | 15.3 ± 4.4* | 78.1 ± 26.7 | 32.5 ± 12.5** |
| AUC (nm* min) | 2665 ± 413 | 1980 ± 363 | 2370 ± 399 | 2202 ± 392 |
p<0.05 vs. MRSA + MP.
p<0.05 vs. MRSA + IgG.
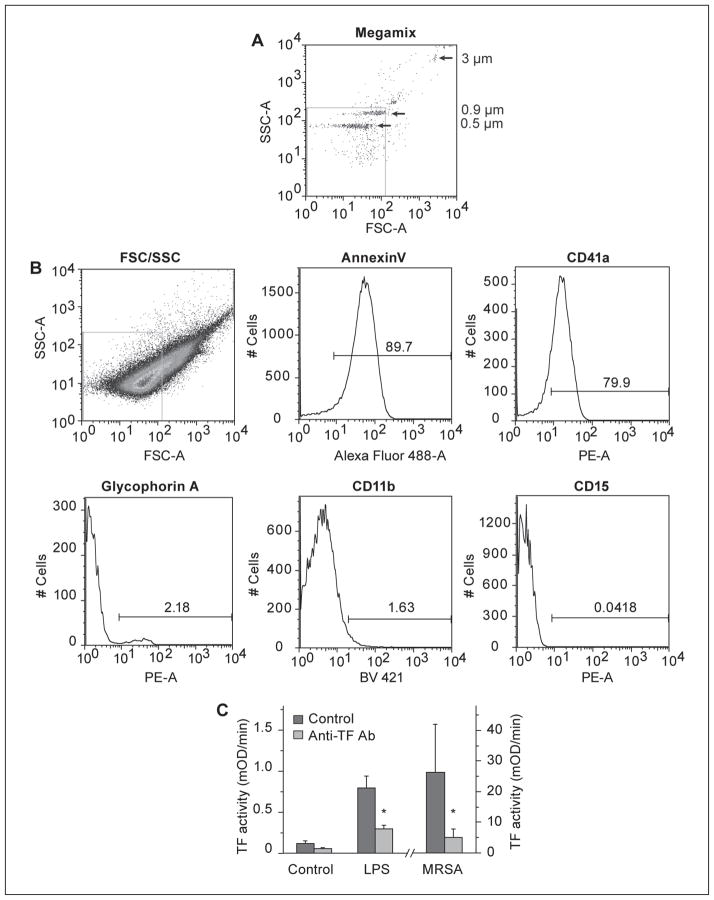
Since TF-bearing MPs are integral to pro-coagulant responses in MRSA-treated blood, we next determined the cellular characteristics of the MPs generated. MPs from MRSA-treated whole blood were examined using flow cytometry in accordance with published guidelines (26–29) with a gate set below 0.9 micron as determined with fluorescently labelled microspheres (Figure 2 A). MRSA-generated MPs stained brightly for Annexin V, indicating a significant fraction (>80%) had exposed PS on their surface. Of the PS-positive MPs, monocytes, platelet, and RBC-derived MPs were all detected. Minimal neutrophil-derived MPs were detected (Figure 2 B). To assess the TF-activity of the MRSA-generated MPs, a TF activity assay was performed. LPS-stimulated and MRSA-stimulated whole blood generated significant quantities of TF-active MPs, which were inhibited by a specific anti-TF antibody. Untreated whole blood had minimal TF-active MPs (Figure 2 C).
Figure 2. Phosphatidylserine and TF-positive microparticles are generated after MRSA inoculation of whole blood.
Fluorescent microspheres of 0.5, 0.9, and 3.0 microns were used to determine the MP gate (A). MRSA-treated whole blood (9 x 102 cfu/ ml) was allowed to incubate overnight at 37°C. The next day plasma was isolated by centrifuging whole blood for 1500 x g for 15 minutes followed by a 13,000 x g for 2 minutes spin. MPs were isolated from the resulting plasma by centrifuging plasma at 20,000 x g for 20 minutes at 4°C and re-suspended in PBS. MPs were stained as described in Methods (B). TF activity was measured on MPs derived from control, LPS-, or MRSA-stimulated whole blood as described in Methods. These data are representative of four individual experiments.
MRSA induces the release of cytokines
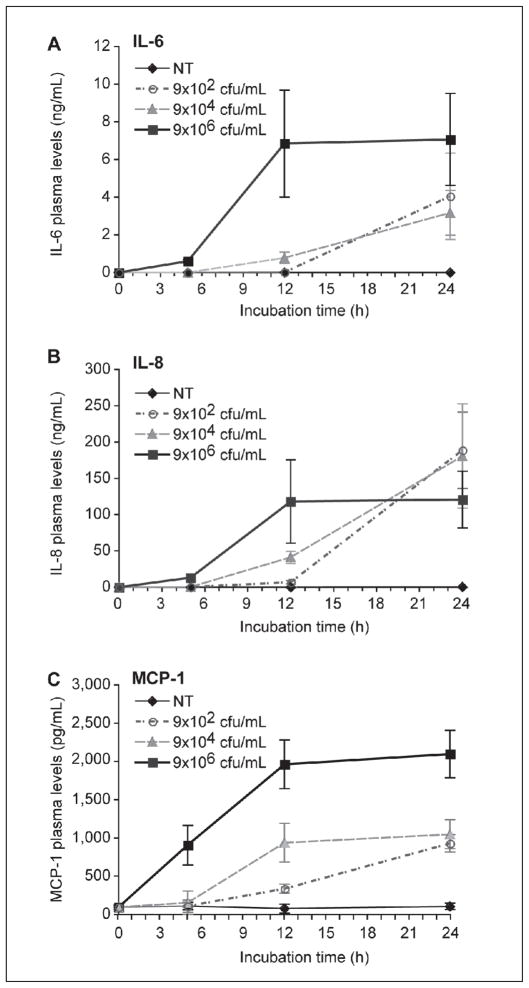
As there is growing appreciation for the cross-talk between pro-inflammatory and pro-coagulant responses (36, 37), especially during acute infections, we next determined if inoculation of whole blood with MRSA induced cytokine synthesis after 24 h. MRSA robustly induced the synthesis of IL-6, IL-8, and, MCP-1 in a time- and concentration-dependent manner (Figure 3). Synthesis of IL-1β and TNF-α, cytokines implicated in S. aureus sepsis (9, 11–13), also increased in a time- and concentration-dependent manner (see Suppl. Figure 2, available online at www.thrombosis-online.com). We observed a plateau effect that was more prominent with higher bacterial concentrations (9x106 cfu/ml of MRSA) and periods ≥12 h, suggesting maximal stimulation of immune cells or MRSA-induced apoptosis of immune cells with high concentrations of bacteria. As early as 5 h after inoculation, plasma levels of MCP-1 rose dramatically and achieved concentrations approaching 50% of their peak concentration at 24 h (Figure 3 C). In contrast, while both IL-6 and IL-8 were also robustly synthesised at 5 h, their plasma concentrations were much less, relative to peak concentrations at 24 h (Figure 3 A and B). High concentrations of heat-inactivated bacteria (9 x 106 cfu/ml) did not induce cytokine synthesis, suggesting bacteria growth and/or toxin production are necessary for these thrombo-inflammatory responses (data not shown). Similar responses were observed with various clinical strains of MSSA (Suppl. Figure 3, available online at www.thrombosis-online.com).
Figure 3. MRSA induces the release of IL-6, IL-8, and MCP-1 in whole blood.
MRSA was grown overnight and then inoculated in 2 ml of whole blood at a starting concentration of 9x102, 9x104, 9x106 cfu/ml, or not treated (NT). Whole blood inoculated with MRSA was incubated at 37°C for 24 hours and plasma levels of each cytokine were measured over time by ELISA. Each point reflects the mean (± SEM) of 3–5 experiments.
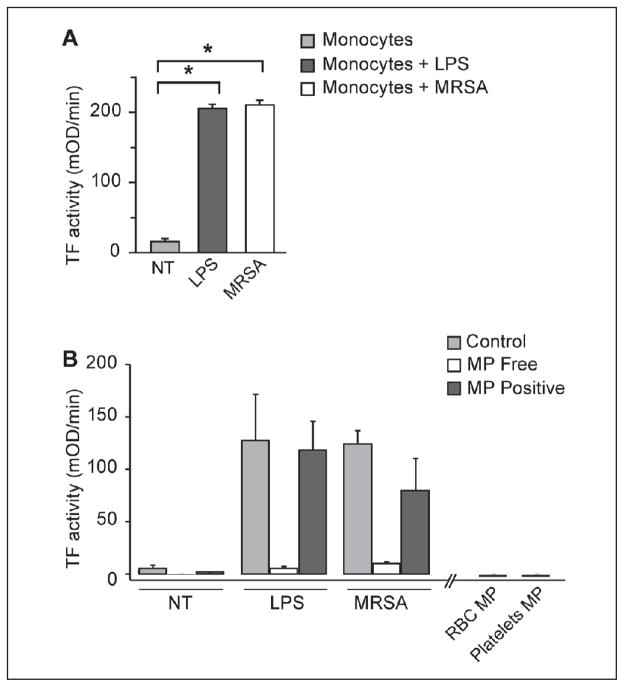
Isolated monocytes synthesise TF and pro-inflammatory cytokines in response to MRSA
Since monocyte-derived MPs were detected in MRSA-stimulated whole blood and are key effector cells for both TF and cytokine synthesis, we incubated freshly-isolated human monocytes with MRSA to determine the role of monocytes in MRSA infection. Purified monocytes incubated with MRSA synthesised robust amounts of TF similar to levels observed to LPS-stimulated monocytes (Figure 4 A). Interestingly, removal of MPs associated with MRSA-stimulated monocytes completely removed all TF activity for the monocyte supernatants. However, TF-activity was retained in the removed MP fraction (Figure 4 B), consistent with our MRSA-treated whole blood results (Table 2). Furthermore, RBC- and platelet-derived MP had little TF activity associated with them, suggesting that monocytes are the primary source of TF-positive MPs (Figure 4 B). Similar to our whole blood studies, isolated monocytes incubated with MRSA for 18 h also synthesised IL-6, IL-8, and MCP-1 (Suppl. Figure 4, available online at www.thrombosis-online.com).
Figure 4. Isolated monocytes synthesise TF in response to MRSA infection.
A) MRSA (9x102 colony cfu/ml) or LPS (100 ng/ml) was incubated with isolated monocytes (2 x 106/ml) at 37ºC for 18 hours and TF-dependent pro-coagulant activity was measured as described. B) To examine the role of RBC, platelets, and monocyte-derived MP, cellular supernatant was centrifuged at 20,000 x g for 20 minutes to remove MP. The supernatant and/or the resultant MP fraction was measured for TF activity as described. Each point reflects the mean (± SEM) of 2–5 experiments.
Linezolid and vancomycin suppress the thrombin generation induced by MRSA
Clinical studies demonstrate that early, but not late antibiotic administration reduces adverse events in patients with MRSA infection (16, 17). We hypothesised that earlier antibiotic administration with linezolid or vancomycin, antibiotics commonly used for the treatment of MRSA infections, would reduce the MRSA-induced procoagulant response, while delayed antibiotic administration would not. When either linezolid or vancomycin at five times the MIC (final concentration 5 μg/ml) was added immediately upon the start of inoculation with MRSA, the LT, TTP, AUC and the rate of thrombin generation were similar to untreated (i.e. no MRSA) controls (Table 3 and Figure 5 A), suggesting that early antibiotic treatment blocked the procoagulant response induced by MRSA. Delaying the administration of antibiotics until 12 h after inoculation with MRSA shortened the LT, TTP, and accelerated the rate of thrombin generation compared to either untreated controls or inoculates where antibiotic administration was not delayed (Table 3). Thus, delays in antibiotic administration resulted in thrombin generation curves similar to conditions where no antibiotics were administered to MRSA inoculated whole blood. There was no significant difference between linezolid and vancomycin-mediated rescue of normal thrombin generation curves. Similar overall thrombin generation results were seen with MSSA strains (Suppl. Table 1, available online at www.thrombosis-online.com).
Table 3.
Antibiotics inhibit MRSA-induced thrombin generation.
| Time (h) | - | 0 | 6 | 12 | ||||
|---|---|---|---|---|---|---|---|---|
| Antibiotic | - | VANC | LZ | VANC | LZ | VANC | LZ | |
| MRSA | − | + | + | + | + | + | + | + |
| Lag (min) | 18.0 ± 1.3 | 4.3 ± 0.3* | 17.0 ± 0.6** | 18.0 ± 1.8** | 12.7 ± 2.4** | 15.0 ± 0.6** | 5.7 ± 0.7* | 7.0 ± 0.6* |
| Peak (nM) | 269.1 ± 45.2 | 470.0 ± 39 | 275.9 ± 27.5 | 359.0 ± 102.9 | 305.4 ± 51.6 | 265.8 ± 9.7 | 408.6 ± 31.1 | 382.6 ± 4.6 |
| Time to Peak (min) | 30.0 ± 3.3 | 7.2 ± 0.4* | 29.2 ± 1.4** | 27.5 ± 4.1** | 21.3 ± 4** | 24.2 ± 1.7** | 10.3 ± 1.6* | 11.8 ± 1.3* |
| Rate (nM/min) | 26.0 ± 7.5 | 178.6 ± 25.3* | 25.9 ± 5.5** | 57.2 ± 34.4** | 43.6 ± 13.3** | 28.4 ± 5.3** | 93.7 ± 14.3** | 83.7 ± 13.3** |
| AUC (nM* min) | 3458.9 ± 516.7 | 3957.9 ± 456.6 | 3539.7 ± 413 | 3816.0 ± 522.3 | 3767.8 ± 477.8 | 3599.2 ± 265 | 4031.2 ± 467.8 | 3810.7 ± 189.4 |
p<0.05 vs. no MRSA.
p<0.05 vs. MRSA alone without antibiotics.
Figure 5. Antibiotics blunt MRSA induced thrombo-inflammatory response in whole blood.
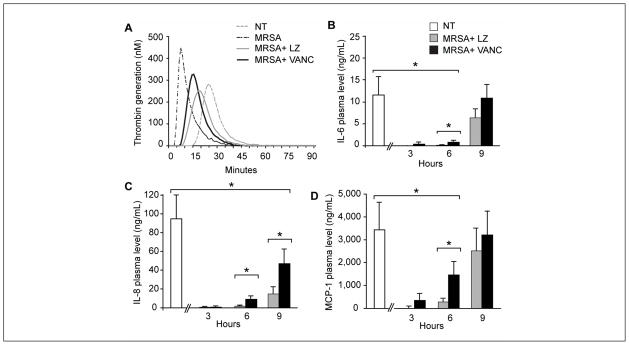
Thrombin generation was measured in duplicate every minute for ≥ 90 minutes following initiation. Increased thrombin generation is demonstrated by a shortened lag time (LT) and time to peak (TTP) thrombin concentration as well as increased rate and peak thrombin concentration (A). MRSA were inoculated in 2 ml of whole blood at a starting concentration of 9x102 cfu/ml. Bacteria were incubated at 37°C in 5% CO2 for 24 hours and linezolid (LZ, 5 g/ml final concentration) or vancomycin (VANC, 5 g/ml final concentration) were added 3, 6, and 9 hours after the inoculation was begun. Plasma levels of IL-6 (B), IL-8 (C), and MCP-1 (D) were measured at 24 hours. The far left bar in each panel reflects the amount of each cytokine produced during 24 hours of inoculation with MRSA in the absence of any antibiotics (control). Each point reflects the mean (± SEM) of 3–5 experiments (*p<0.05 for the comparison of LZ and VANC to no antibiotics).
Early antibiotic administration inhibits MRSA-induced cytokine release
We next examined the effects of linezolid and vancomycin on cy-tokine synthesis induced by MRSA in our whole blood model. The addition of linezolid or vancomycin (five times the MIC, final concentration 5 μg/ml) suppressed cytokine synthesis when the antibiotics were added within 3 h of MRSA inoculation (early administration) (Figure 5 B-D). Linezolid significantly reduced cytokines synthesis compared to vancomycin within 6 h of MRSA inoculation, which may reflect linezolid’s mechanism of action as a bacterial protein synthesis inhibitor (38, 39). However, when antibiotic administration was delayed until nine hours after MRSA inoculation, neither linezolid nor vancomycin significantly reduced MCP-1 or IL-6 production when compared to antibiotic-free MRSA inoculates (Figure 5). This suggests once bacterial growth and toxin production have reached a threshold level, antibiotics therapy becomes ineffective in reducing the inflammatory response. Plating assays with MRSA isolates confirmed there was no difference in bacterial growth inhibition between linezolid- and vancomycin-treated cultures (Suppl. Figure 5A, available online at www.thrombosis-online.com). Both antibiotics appeared to be bacteriostatic in vitro since growth did not increase or decrease after addition of either antibiotic (Suppl. Figure 5B, available online at www.thrombosis-online.com). Recent reports have suggested antibiotics themselves have immunomodulatory effects on immune cells. To ensure the antibiotics were directly effecting bacterial growth and toxin production and not exerting a general effect on immune cells, whole blood was treated with LPS or alpha-toxin in the presence or absence of linezolid or vancomycin. Both LPS and alpha-toxin (Suppl. Figures 6A and B, available online at www.thrombosis-online.com) induced IL-8 synthesis, as expected. Neither linezolid nor vancomycin inhibited IL-8 synthesis. Similar responses were observed with MCP-1 and IL-6 (data not shown). These results suggest antibiotics exert their effects primarily on bacteria rather than immune cells.
To determine if the antibiotic response was strain specific, MSSA-treated whole blood was incubated with linezolid or vancomycin as described above. Similar to our observations with MRSA, inoculation with MSSA also induced significant increases in IL-6, IL-8 and MCP-1 and early administration of antibiotics reduced cytokine synthesis (Suppl. Figure 3, available online at www.thrombosis-online.com, and data not shown).
To determine if antibiotics reduced cytokine synthesis in a purified system, MRSA was inoculated with purified monocytes. In the absence of antibiotics, MRSA induced robust cytokine responses, which were blunted by early administration of antibiotics, suggesting monocytes mediate inflammatory responses to MRSA infection (Suppl. Figure 7, available online at www.thrombosis-online.com).
Early administration of antibiotics in a mouse model of MRSA infection reduces thrombo-inflammatory response
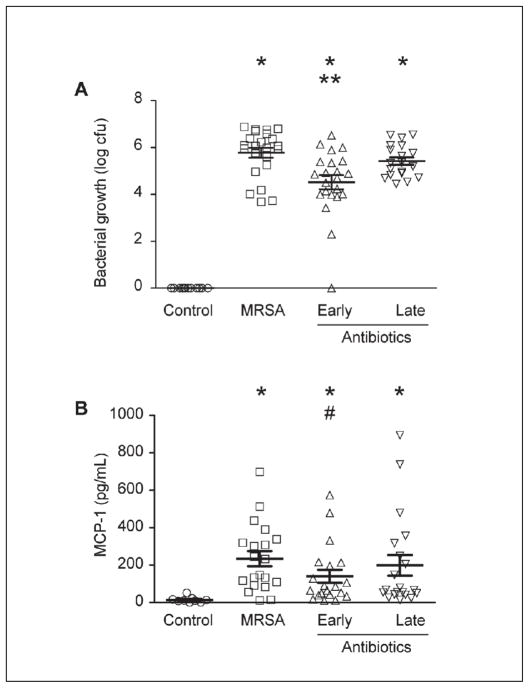
Finally, we examined if early antibiotic administration reduced in vivo inflammatory responses in a mouse model of MRSA infection. C57BL/6 mice were given i.p. injections of MRSA (2.5 x 108 total bacteria) followed by i.p. injections of saline or vancomycin at either 2 and 8 h (early administration) or 8 and 14 h (late administration). MRSA infection, regardless of antibiotic treatment, significantly increased bacteria colony counts in the peritoneal fluid (Figure 6 A), confirming our ability to induce infection in these mice. Early antibiotic administration significantly decreased MRSA bacterial counts in the peritoneal fluid. Late antibiotic administration, however, did not significantly reduce MRSA bacterial load compared to saline-treated mice inoculated with MRSA (Figure 6 A). Mice infected with MRSA had significantly increased levels of MCP-1, but not IL-1β or TNF-α, after 24 h of infection, consistent with the ability of MRSA to induce robust cytokine responses (Figure 6 B and data not shown). Consistent with our in vitro data, early antibiotic administration reduced MCP-1 levels compared to MRSA-infected mice, which received late antibiotic administration (Figure 6 B).
Figure 6. Early antibiotic administration reduces bacterial growth and MCP-1 synthesis in a mouse model of MRSA infection.
C57BL/6 mice (weighing 20–25 g) were randomly assigned to one of four groups: 1) control intraperitoneal (i.p.) saline injection (PBS 250 L); 2) MRSA i.p. injection (2.5 x 108 total bacteria); 3) MRSA i.p. injection (2.5 x 108 total bacteria) followed by vancomycin injections (60 mg/kg) at 2 and 8 hours post infection; 4) MRSA i.p. injection (2.5 x 108 total bacteria) followed by vancomycin i.p. injections (60 mg/kg) at 8 and 14 hours post infection. Twenty-four hours after infection, mice were euthanised using CO2 asphyxiation followed by cervical dislocation and peritoneal lavage was performed with 5 ml of HBSS. To quantify the colony growth of the S. aureus, peritoneal lavage was plated on blood agar overnight at 37°C in 5% CO2. CFU were counted the following day (A). Early vancomycin administration significantly reduced MRSA bacterial load (A) and MCP-1 levels (B) (*p<0.05 compared to control. **p<0.05 compared to MRSA and late antibiotics. #p<0.08 compared to MRSA).
To examine the thrombotic response of MRSA infection, MRSA (3.3 x 106 cfu total) was injected intravenously (i.v.) through the tail vein. Vancomycin (60 mg/kg) was injected i.v. through the tail vein at two time points: 3 h post infection (early administration) and 9 h post infection (late administration). Thrombin anti-thrombin (TAT) complexes were significantly higher in MRSA infected mice compared to saline injected mice. Early antibiotic administration reduced TAT levels similar to saline treated mice while late antibiotic administration had no significant effect on TAT levels (Suppl. Figure 8, available online at www.thrombosis-online.com).
Discussion
S. aureus and specifically MRSA is an increasingly common, virulent cause of septic syndromes, including bacteraemia, abdominal sepsis, and pneumonia (1–7). Thrombosis and DIC often complicate S. aureus sepsis, contributing to organ failure and death (1–7, 40, 41). In clinical studies, the prompt administration of antibiotics to septic patients, where S. aureus is a common cause of infection, reduces mortality (16, 18). Although timely antibiotic administration offers the obvious benefit of arresting bacterial growth at an earlier phase, additional molecular mechanisms underlying these benefits remain largely unexplored.
Our results are the first to demonstrate the benefits of early antibiotic administration in reducing the MRSA-induced thrombo-inflammatory “cytokine storm” using novel in vitro and in vivo models of bacterial infection. S. aureus altered thrombin generation including the rate, lag time and time to peak, resulting in a markedly more procoagulant phenotype. However, early antibiotic treatment returned thrombin generation to normal. To our knowledge, our observations are the first to show increased thrombin generation parameters in the presence of live MRSA bacteria and not just bacterial toxin. Furthermore, previous research using MSSA and MRSA bacteria and toxins have focused on clotting times (9), thromboelastography (42), and consumption of thrombin (35), while our paper is the first to use a global hemostasis assay to demonstrate the effect of S. aureus on thrombin generation. To examine the reason for changes in global haemostasis, we performed high-speed centrifugation (to remove TF-bearing MPs) and/or added anti-TF antibodies to MRSA-treated plasma. In both cases, removal of MPs and an anti-TF antibody rescued thrombin generation parameters, reversing the hypercoagulable response induced by MRSA. In addition, MPs generated after addition of MRSA to whole blood stained positively for Annexin V, were monocyte, platelet, and RBC-derived, and contained significant TF activity. These data suggest TF-bearing MPs are responsible for MRSA-induced procoagulant responses and thus may play an important role in thrombotic complications during S. aureus sepsis.
Purified human monocytes-derived MPs, but not platelet- or RBC-derived MPs, generated in the presence of MRSA supported robust TF activity, indicating that monocyte MPs were the main source of TF induced in MRSA-treated whole blood. While platelets and RBC MPs did not appear to be major contributors of TF activity, these MPs were still PS-positive. Thus, they can contribute to the propagation of thrombin generation and potentially mediate thrombotic responses in MRSA infections. These results provide new mechanistic data supporting the potential benefit of early antibiotic administration in preventing or reducing the thrombo-inflammatory milieu induced by MRSA in septic patients.
The pathophysiology of sepsis and its complications (e.g. microvascular thrombosis, DIC, venous thromboembolism) includes processes that couple the coagulation and inflammatory pathways (40, 43–46). Consistent with the cross-talk between these two key pathways, MRSA in our models induced not only a procoagulant milieu, but also promoted the pro-inflammatory “cytokine storm” implicated in the pathogenesis of sepsis, including the synthesis of IL-6, IL-8, MCP-1, TNF-α, and IL-1β, in a time- and concentration-dependent manner. Higher concentrations of bacteria result in minimal increases in cytokine synthesis at later times, perhaps due to bacteria-induced apoptosis. At lower bacterial loads, cytokine and procoagulant activity increased in a linear fashion, suggesting immune cells are functionally responsive in more moderate conditions. Thrombo-inflammatory responses were similar between MRSA and MSSA strains tested in our experiments, although a more detailed comparison in the future is necessary to fully explore these responses.
Prior reports describe the release of cytokines in human whole blood models of MRSA infection, by using bacterial toxins, not whole bacterial organisms. Using live MRSA in human whole blood and with purified monocytes, we demonstrated that early administration of either linezolid or vancomycin markedly suppresses MRSA-induced cytokine synthesis. In contrast, delays in antibiotic administration were ineffective in blocking cytokine synthesis. Antibiotics did not reduce cytokine synthesis in LPS- or alpha-toxin stimulated whole blood or in purified monocytes, suggesting that the effects of antibiotics were primarily targeted against bacteria rather than on immune cells. Interestingly, linezolid appeared more effective than vancomycin at reducing cytokine synthesis. As bacterial growth inhibition did not differ in linezolid or vancomycin treated blood, this effect may be due to linezolid’s ability to inhibit the synthesis of bacterial toxins (38, 39).
We confirmed these responses using a mouse model of MRSA infection, thus providing in vivo evidence of the importance of timely antibiotic administration in reducing cytokine synthesis and thrombosis risk. Early administration of vancomycin reduced bacterial load, MCP-1 synthesis, and TAT complexes. Late administration had no significant effect on these parameters. Timely administered as well as adequate antibiotic therapy is associated with improved outcome in patients with bacterial meningitis (47) and ventilator-associated pneumonia (17, 18, 48), where S. aureus is a common pathogen. Similarly, in patients with cancer and septic shock (a risk factor for S. aureus infections), in-hospital mortality was higher when antibiotic therapy was started more than 2 h after diagnosis (49). Furthermore, in a larger cohort of patients with septic shock, each hour of delay in antimicrobial administration from the onset of hypotension was associated with an average 8% decrease in survival rate (16). Based on these findings, current guidelines recommended that antibiotics should be administered as early as possible after the recognition of severe sepsis or septic shock (50). Mechanistically, our data suggest the reason patients benefit from early administration of antibiotics is due, in part, to a reduction of monocyte derived TF-bearing MPs as well as a reduction in the monocyte initiated “cytokine storm.” This also suggest that early antibiotic administration may help ensure that the host’s haemostasis and immune pathways are prevented from exaggerated, injurious responses while delayed administration is ineffective, resulting in a dysregulated thrombo-inflammatory response.
In conclusion, we demonstrated that S. aureus induces a prothrombotic and pro-inflammatory response in human whole blood and mice. These data highlight the critical cross-talk between thrombotic and inflammatory pathways during infectious diseases. Furthermore, as we were able to mitigate these responses through the early (but not late) administration of both linezolid and vancomycin, the findings of the current study provide novel mechanistic data supporting the clinical benefit observed with timely antibiotic administration in S. aureus sepsis.
Supplementary Material
What is known about this topic?
Staphylococcus aureus is a common cause of gram-positive sepsis associated with increased rates of micro- and macrovascular thrombosis and increased concentrations of pro-inflammatory cytokines.
Clinically, the early administration of appropriate antibiotics reduces the risk of subsequent adverse events in S. aureus sepsis.
In vitro studies suggest S. aureus toxins and cell wall components can stimulate a thrombo-inflammatory response in blood cells.
What does this paper add?
Live methicillin-resistant S. aureus (MRSA) bacteria induce increased procoagulant activity and cytokine production in whole blood.
MRSA infection in whole blood generates tissue factor-positive, monocyte-derived microparticles, which are responsible for the increased procoagulant response.
Timely administration of antibiotics reduces and/or mitigates the thrombo-inflammatory response observed in whole blood and in mice.
Acknowledgments
Acknowledgements/Funding
This work was funded by the NIH (Grant Numbers 1K23HL092161, 5R01HL092746, 5R01HL091754, 1R03AG040631, and 5T32DK007115-35), a public health service grant ULI-RRO25764 from the National Center for Research Resources, and an Investigator-Initiated Award from Pfizer (GA5951 WK). Pfizer had no role in study design, data interpretation, statistical analyses, or manuscript preparation. We thank Christopher Gibson and Dr. Dean Li for their technical assistant with the in vivo experiments. We thank Jenny Pierce for her editorial assistance, Diana Lim for her assistance with figure preparation, and Dr. Mulvey and Dr. Estelle Harris for kindly providing the MRSA and MSSA strains.
Footnotes
Conflict of Interest
M. Rondina received an Investigator-Initiated Award from Pfizer (GA5951WK). However, Pfizer had no influence on the experimental design, data collection, data analysis, or final content of the manuscript. None of the other authors have any conflict of interest to declare.
References
- 1.JA, Hu H, Rivera J, et al. Staphylococcal superantigen-like protein 5 induces thrombotic and bleeding complications in vivo: Inhibition by an anti-SSL5 antibody and the glycan Bimosiamose. J Thromb Haemost. 2012 doi: 10.1111/jth.12022. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen T, Kyle UG, Jaimon N, et al. Coinfection with Staphylococcus aureus increases risk of severe coagulopathy in critically ill children with influenza A (H1N1) virus infection. Crit Care Med. 2012;40:3246–3250. doi: 10.1097/CCM.0b013e318260c7f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin E, Cevik C, Nugent K. The role of hypervirulent Staphylococcus aureus infections in the development of deep vein thrombosis. Thromb Res. 2012;130:302–308. doi: 10.1016/j.thromres.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Cesar S, Garcia A, Parada E, et al. Cavernous sinus thrombosis due to invasive community-associated methicillin-resistant Staphylococcus aureus infection. Enferm Infecc Microbiol Clin. 2010;28:755–756. doi: 10.1016/j.eimc.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Vardakas KZ, Matthaiou DK, Falagas ME. Comparison of community-acquired pneumonia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus producing the Panton-Valentine leukocidin. Int J Tuberc Lung Dis. 2009;13:1476–1485. [PubMed] [Google Scholar]
- 6.Arai H, Hanai H, Furuta T, et al. A patient who survived total colonic type ulcerative colitis complicated by toxic megacolon, disseminated intravascular coagulation, methicillin-resistant Staphylococcus aureus infection and bilateral femoral phlebothrombosis. J Gastroenterol. 1999;34:395–399. doi: 10.1007/s005350050282. [DOI] [PubMed] [Google Scholar]
- 7.McAdow M, Kim HK, Dedent AC, et al. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog. 2011;7:e1002307. doi: 10.1371/journal.ppat.1002307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattsson E, Herwald H, Bjorck L, et al. Peptidoglycan from Staphylococcus aureus induces tissue factor expression and procoagulant activity in human monocytes. Infect Immun. 2002;70:3033–3039. doi: 10.1128/IAI.70.6.3033-3039.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattsson E, Herwald H, Egesten A. Superantigens from Staphylococcus aureus induce procoagulant activity and monocyte tissue factor expression in whole blood and mononuclear cells via IL-1 beta. J Thromb Haemost. 2003;1:2569–2576. doi: 10.1111/j.1538-7836.2003.00498.x. [DOI] [PubMed] [Google Scholar]
- 10.Mattsson E, Heying R, van de Gevel JS, et al. Staphylococcal peptidoglycan initiates an inflammatory response and procoagulant activity in human vascular endothelial cells: a comparison with highly purified lipoteichoic acid and TSST-1. FEMS Immunol Med Microbiol. 2008;52:110–117. doi: 10.1111/j.1574-695X.2007.00350.x. [DOI] [PubMed] [Google Scholar]
- 11.Kang HJ, Ha JM, Kim HS, et al. The role of phagocytosis in IL-8 production by human monocytes in response to lipoproteins on Staphylococcus aureus. Biochem Biophys Res Commun. 406:449–453. doi: 10.1016/j.bbrc.2011.02.069. [DOI] [PubMed] [Google Scholar]
- 12.Ikejima T, Dinarello CA, Gill DM, et al. Induction of human interleukin-1 by a product of Staphylococcus aureus associated with toxic shock syndrome. J Clin Invest. 1984;73:1312–1320. doi: 10.1172/JCI111334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn JY, Song JY, Yun YS, et al. Protection of Staphylococcus aureus-infected septic mice by suppression of early acute inflammation and enhanced antimicrobial activity by ginsan. FEMS Immunol Med Microbiol. 2006;46:187–197. doi: 10.1111/j.1574-695X.2005.00021.x. [DOI] [PubMed] [Google Scholar]
- 14.Masuta K, Oba Y, Iwata K. Linezolid vs Vancomycin for Methicillin-Resistant Staphylococcus aureus Nosocomial Pneumonia: Controversy continues. Clin Infect Dis. doi: 10.1093/cid/cis331. [DOI] [PubMed] [Google Scholar]
- 15.Wunderink RG, Rello J, Cammarata SK, et al. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2003;124:1789–1797. [PubMed] [Google Scholar]
- 16.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 17.Kollef MH, Shorr A, Tabak YP, et al. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128:3854–3862. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 18.Kollef MH, Ward S. The influence of mini-BAL cultures on patient outcomes: implications for the antibiotic management of ventilator-associated pneumonia. Chest. 1998;113:412–420. doi: 10.1378/chest.113.2.412. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Roca P, Mancilla-Ramirez J, Santos-Segura A, et al. Linezolid diminishes inflammatory cytokine production from human peripheral blood mononuclear cells. Arch Med Res. 2006;37:31–35. doi: 10.1016/j.arcmed.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Lambers C, Burian B, Binder P, et al. Early immunomodulatory effects of linezolid in a human whole blood endotoxin model. Int J Clin Pharmacol Ther. 2010;48:419–424. doi: 10.5414/cpp48419. [DOI] [PubMed] [Google Scholar]
- 21.Kushiya K, Nakagawa S, Taneike I, et al. Inhibitory effect of antimicrobial agents and anisodamine on the staphylococcal superantigenic toxin-induced overproduction of proinflammatory cytokines by human peripheral blood mononuclear cells. J Infect Chemother. 2005;11:192–195. doi: 10.1007/s10156-005-0389-8. [DOI] [PubMed] [Google Scholar]
- 22.Pichereau S, Moran JJ, Hayney MS, et al. Concentration-dependent effects of antimicrobials on Staphylococcus aureus toxin-mediated cytokine production from peripheral blood mononuclear cells. J Antimicrob Chemother. 2012;67:123–129. doi: 10.1093/jac/dkr417. [DOI] [PubMed] [Google Scholar]
- 23.de Vos AF, Pater JM, van den Pangaart PS, et al. In vivo lipopolysaccharide exposure of human blood leukocytes induces cross-tolerance to multiple TLR ligands. J Immunol. 2009;183:533–542. doi: 10.4049/jimmunol.0802189. [DOI] [PubMed] [Google Scholar]
- 24.Kikkert R, de Groot ER, Aarden LA. Cytokine induction by pyrogens: comparison of whole blood, mononuclear cells, and TLR-transfectants. J Immunol Methods. 2008;336:45–55. doi: 10.1016/j.jim.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Dragneva Y, Anuradha CD, Valeva A, et al. Subcytocidal attack by staphylococcal alpha-toxin activates NF-kappaB and induces interleukin-8 production. Infect Immun. 2001;69:2630–2635. doi: 10.1128/IAI.69.4.2630-2635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacroix R, Robert S, Poncelet P, et al. Standardization of platelet-derived microparticle enumeration by flow cytometry with calibrated beads: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb Haemost. 2010;8:2571–2574. doi: 10.1111/j.1538-7836.2010.04047.x. [DOI] [PubMed] [Google Scholar]
- 27.Robert S, Poncelet P, Lacroix R, et al. Standardization of platelet-derived microparticle counting using calibrated beads and a Cytomics FC500 routine flow cytometer: a first step towards multicenter studies? J Thromb Haemost. 2009;7:190–197. doi: 10.1111/j.1538-7836.2008.03200.x. [DOI] [PubMed] [Google Scholar]
- 28.Aleman MM, Gardiner C, Harrison P, et al. Differential contributions of monocyte- and platelet-derived microparticles towards thrombin generation and fibrin formation and stability. J Thromb Haemost. 2011;9:2251–2261. doi: 10.1111/j.1538-7836.2011.04488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang JG, Manly D, Kirchhofer D, et al. Levels of microparticle tissue factor activity correlate with coagulation activation in endotoxemic mice. J Thromb Haemost. 2009;7:1092–1098. doi: 10.1111/j.1538-7836.2009.03448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell RA, Fischer TH, Wolberg AS. A novel approach to improving recombinant factor VIIa activity with a preserved platelet preparation. Br J Haematol. 2007;138:82–93. doi: 10.1111/j.1365-2141.2007.06617.x. [DOI] [PubMed] [Google Scholar]
- 31.Campbell RA, Overmyer KA, Selzman CH, et al. Contributions of extravascular and intravascular cells to fibrin network formation, structure, and stability. Blood. 2009;114:4886–4896. doi: 10.1182/blood-2009-06-228940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwertz H, Koster S, Kahr WH, et al. Anucleate platelets generate progeny. Blood. 2011;115:3801–3809. doi: 10.1182/blood-2009-08-239558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Domenech A, Ribes S, Cabellos C, et al. Experimental study on the efficacy of combinations of glycopeptides and beta-lactams against Staphylococcus aureus with reduced susceptibility to glycopeptides. J Antimicrob Chemother. 2005;56:709–716. doi: 10.1093/jac/dki294. [DOI] [PubMed] [Google Scholar]
- 34.McDevitt D, Nanavaty T, House-Pompeo K, et al. Characterization of the interaction between the Staphylococcus aureus clumping factor (ClfA) and fibrinogen. Eur J Biochem. 1997;247:416–424. doi: 10.1111/j.1432-1033.1997.00416.x. [DOI] [PubMed] [Google Scholar]
- 35.Bokarewa MI, Tarkowski A. Thrombin generation and mortality during Staphylococcus aureus sepsis. Microb Pathog. 2001;30:247–252. doi: 10.1006/mpat.2000.0425. [DOI] [PubMed] [Google Scholar]
- 36.Levi M, van der Poll T, ten Cate H. Tissue factor in infection and severe inflammation. Semin Thromb Hemost. 2006;32:33–39. doi: 10.1055/s-2006-933338. [DOI] [PubMed] [Google Scholar]
- 37.Zimmerman GA, McIntyre TM, Prescott SM, et al. The platelet-activating factor signaling system and its regulators in syndromes of inflammation and thrombosis. Crit Care Med. 2002;30 (5 Suppl):S294–301. doi: 10.1097/00003246-200205001-00020. [DOI] [PubMed] [Google Scholar]
- 38.Bernardo K, Pakulat N, Fleer S, et al. Subinhibitory concentrations of linezolid reduce Staphylococcus aureus virulence factor expression. Antimicrob Agents Chemother. 2004;48:546–555. doi: 10.1128/AAC.48.2.546-555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pichereau S, Pantrangi M, Couet W, et al. Simulated antibiotic exposures in an in vitro hollow-fiber infection model influence toxin gene expression and production in community-associated methicillin-resistant Staphylococcus aureus strain MW2. Antimicrob Agents Chemother. 2012;56:140–147. doi: 10.1128/AAC.05113-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levi M, de Jonge E, van der Poll T. Sepsis and disseminated intravascular coagulation. J Thromb Thrombolysis. 2003;16:43–47. doi: 10.1023/B:THRO.0000014592.27892.11. [DOI] [PubMed] [Google Scholar]
- 41.Dixon B. The role of microvascular thrombosis in sepsis. Anaesth Intensive Care. 2004;32:619–629. doi: 10.1177/0310057X0403200502. [DOI] [PubMed] [Google Scholar]
- 42.Leifsson PS, Iburg T, Jensen HE, et al. Intravenous inoculation of Staphylococcus aureus in pigs induces severe sepsis as indicated by increased hypercoagulability and hepatic dysfunction. FEMS Microbiol Lett. 309:208–216. doi: 10.1111/j.1574-6968.2010.02042.x. [DOI] [PubMed] [Google Scholar]
- 43.ten Cate H, Timmerman JJ, Levi M. The pathophysiology of disseminated intravascular coagulation. Thromb Haemost. 1999;82:713–717. [PubMed] [Google Scholar]
- 44.Dempfle CE. Disseminated intravascular coagulation and coagulation disorders. Curr Opin Anaesthesiol. 2004;17:125–129. doi: 10.1097/00001503-200404000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Dempfle CE. Coagulopathy of sepsis. Thromb Haemost. 2004;91:213–224. doi: 10.1160/TH03-03-0182. [DOI] [PubMed] [Google Scholar]
- 46.Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586–592. doi: 10.1056/NEJM199908193410807. [DOI] [PubMed] [Google Scholar]
- 47.Miner JR, Heegaard W, Mapes A, et al. Presentation, time to antibiotics, and mortality of patients with bacterial meningitis at an urban county medical center. J Emerg Med. 2001;21:387–392. doi: 10.1016/s0736-4679(01)00407-3. [DOI] [PubMed] [Google Scholar]
- 48.Kollef MH, Rello J, Cammarata SK, et al. Clinical cure and survival in Gram-positive ventilator-associated pneumonia: retrospective analysis of two double-blind studies comparing linezolid with vancomycin. Intensive Care Med. 2004;30:388–394. doi: 10.1007/s00134-003-2088-1. [DOI] [PubMed] [Google Scholar]
- 49.Larche J, Azoulay E, Fieux F, et al. Improved survival of critically ill cancer patients with septic shock. Intensive Care Med. 2003;29:1688–1695. doi: 10.1007/s00134-003-1957-y. [DOI] [PubMed] [Google Scholar]
- 50.Nobre V, Sarasin FP, Pugin J. Prompt antibiotic administration and goal-directed hemodynamic support in patients with severe sepsis and septic shock. Curr Opin Crit Care. 2007;13:586–591. doi: 10.1097/MCC.0b013e3282e7d8e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.