Abstract
Objective
To explore the safety and efficacy of CF101, an A3 adenosine receptor agonist, in patients with moderate-to-severe dry eye syndrome
Design
Phase 2, multicenter, randomized, double-masked, placebo-controlled, parallel-group study.
Participants
68 patients completed the study, 35 patients in the placebo group and 33 patients in the CF101 group.
Intervention
Patients were orally treated with either 1 mg CF101 pills or matching vehicle-filled placebo pills, given twice daily for 12 weeks, followed by a 2-week post-treatment observation.
Main Outcome Measures
Efficacy
an improvement of >25% over baseline at week 12 in one of the following parameters: (a) tear break-up time (BUT); (b) superficial punctate keratitis assessed by fluorescein staining (FS); (c) Schirmer tear test 1 (ST1).
Safety
clinical laboratory safety tests, ophthalmic examinations, intraocular pressure (IOP) measurements, electrocardiographic evaluations, vital sign measurements and monitoring of adverse events.
Results
A statistically significant increase in the proportion of patients who achieved more than 25% improvement in the corneal staining and in the clearance of corneal staining was noted between the CF101-treated group and the placebo group. Treatment with CF101 resulted in a statistically significant improvement in the mean change from baseline at week 12 of the corneal staining, BUT, and tear meniscus (TM) height in the CF101-treated group CF101 was well tolerated and exhibited an excellent safety profile with no serious adverse events. A statistically significant decrease from baseline was observed in the IOP of the CF101-treated group in comparison with the placebo group.
Conclusions
CF101, given orally, induced a statistically significant improvement in the corneal staining and an improvement in the BUT and TM in patients with moderate-to-severe dry eye syndrome. The drug was very well tolerated. These data and the anti-inflammatory characteristic of CF101 support further study of the drug as a potential treatment for the signs and symptoms of dry eye syndrome.
Introduction
Dry eye syndrome is a multi-factorial disease involving inflammation, autoimmunity, and damage to the surface of the eye. Dry eye syndrome has mainly two types: aqueous tear-deficient dry eye and evaporative dry eye. A combination of both has also been reported.1,2 A more detailed classification system is based on: a. etiopathogenicity, in which dry eye syndrome is divided into aqueous deficiency (Sjögren’s or non-Sjögren’s related) and evaporation (due to intrinsic or extrinsic causes); b. mechanistic causes including tear hyperosmolarity and tear film instability; c. severity of the disease with regard to visual symptoms, conjuctival injection, conjunctival staining, corneal staining, corneal/tears signs, lid/meibonian glands, BUT and Schirmer tear test.3
Dry eye syndrome is typically an inflammatory condition due to high levels of pro-inflammatory cytokines such as TNF-α, interleukin-1β (IL-1β), MMP-9 and the chemokines MIP-1α which were found in tear film and ocular surface epithelia.4-7 In addition, examination of conjunctival biopsy specimens from patients with dry eye syndrome revealed massive lymphocyte infiltration and increased expression of HLA-DR, HLA-DQ, ICAM-1, CD40, CD40 ligand and apoptotic marker APO2.7.8-10
The current most widely used treatment for dry eye is artificial tears which provide some relief from eye irritation, blurred vision symptoms and improve BUT and FS. Further management may emphasize either immunosuppressive or anti-inflammatory drugs such as cordicosterotids, tetracyclines, and cyclosporine A, or agents which work via the secretagogue route, aiming at the promotion of tear production. Such agents include mucin secretion stimulants, diadenosine phosphatases, and purinergic P2Y2 receptor agonists.1,11-14 Current treatments are directed toward symptomatic therapeutic approaches. Thus, management focused on the underlying pathogenic pathways may offer better outcomes.
The A3 adenosine receptor (AR) is a Gi protein-coupled cell surface receptor which belongs to the adenosine receptor family that includes also the A1, A2A and A2BARs.15 CF101, generically known as IB-MECA, is an A3AR agonist shown in pre-clinical and clinical studies to mediate a marked anti-inflammatory effect. The binding of CF101 to the A3AR initiates downstream signal transduction pathways, which entail down-regulation of PKB/Akt and NF-κB, resulting in the inhibition of TNF-α and MIP-1α. In addition, CF101 inhibits the proliferation of auto-reactive T cells and the production of chemokines.16-19 The therapeutic potential for CF101 as an anti-inflammatory agent was established in experimental animal models of arthritis, inflammatory bowel disease, osteoarthritis, and septic peritonitis, in which CF101 treatment suppressed inflammatory manifestations and prevented tissue damage.16-22
Human Phase 1 clinical studies, including single- and repeated-dose trials in healthy volunteers, established CF101 as apparently safe and well-tolerated. Pharmacokinetic parameters, which were linearly proportional to dose, demonstrated that a maximal plasma concentration of CF101 was achieved at 1-2 hours, with an elimination half-life of approximately 9 hours.23 In a Phase 2a clinical study in patients with rheumatoid arthritis, CF101 administered twice daily (BID) orally for 12 weeks resulted in an improvement of disease signs and symptoms and appeared to act as a disease modifying anti-rheumatic drug.24 In the rheumatoid arthritis population, CF101 was safe and very well tolerated.
The marked anti-inflammatory effect of CF101, together with its good safety profile and oral bioavailability, led us to explore its effect on moderate-to-severe dry eye syndrome. Based on prior experience in rheumatoid arthritis trials, a CF101 dose of 1 mg BID was selected.
Methods
Study design
This report describes a randomized, multi-center, doubled-masked, placebo-controlled, parallel-group, Phase 2 clinical study to explore the safety and efficacy of daily CF101 administered orally in patients with moderate-to-severe dry eye syndrome. The study was composed of a screening period of up to 4 weeks, which included a 2-week run-in period, followed by a 12-week treatment period and a 2-week follow-up period. The study was conducted in 5 investigative sites in Israel, in compliance with Good Clinical Practices, investigational site Institutional Review Board Regulations, Informed Consent Regulations, and the Declaration of Helsinki.
Patients
Patients were required to be ≥18 years of age, with a diagnosis of moderate-to-severe dry eye syndrome as defined by: (1) at least one of the following ocular symptoms scored at ≥2, where 0 = none and 4 = very severe/interferes with normal activities: photophobia, blurred vision, foreign body sensation, soreness or pain, itching, burning, dryness; and (2) ST1, without anesthesia and <7mm/5 min in either eye; and (3) positive fluorescein staining (FS), defined as a corneal punctate fluorescein staining score of ≥1 in either eye, where 0 = none and 3 = severe. Patients were not allowed to use any topical ocular treatments except unpreserved artificial tears. In addition, particular cosmetic application was not allowed for the duration of the study.
Patients were excluded from the study if they had a history of Sjögren’s syndrome with significant systemic non-exocrine gland involvement, Stevens-Johnson syndrome, post-burn ocular injury, or chronic ocular disease other than dry eye syndrome requiring topical treatment. Also excluded were patients being administered topical cyclosporine eye drops or systemic cyclosporine within 3 months prior to the screening visit; disease-modifying drugs, including methotrexate and biological agents, whose dose had been changed within 3 months prior to the screening visit or was expected to change during the trial; oral corticosteroids consisting of >10 mg prednisone, or equivalent, per day; or topical steroids within 2 weeks prior to the screening visit and for the duration of the study. Additional exclusion criteria included ocular herpes simplex virus infection; use of contact lenses concomitantly or within 3 months; persistent intraocular inflammation or infection; active blepharitis of greater than mild degree; recent surgical occlusion of the lacrimal puncta; subepithelial corneal scarring; anesthetic or neurotrophic corneas; presence or history of uncontrolled asthma; any evidence of clinically significant heart disease; pregnancy, planned pregnancy, lactation, or inadequate contraception as judged by the investigator; participation in another investigational drug or vaccine trial concurrently or within 30 days; or other conditions which would confound the study evaluations or endanger the safety of the patient.
Study Protocol
This was a Phase 2, randomized, double-masked, placebo-controlled, parallel-group study. Patients were randomly assigned to treatment with either 1 mg CF101 (methyl 1-[N6-(3-iodobenzyl)-adenin-9-yl]-β-D-ribofuronamide) pills or matching vehicle-filled placebo pills, given twice daily for 12 weeks. Patients were also provided with individually packaged preservative-free artificial tears (REFRESH® Lubricant Eye Drops, Allergan, Inc.) which served as adjuvant treatment to be used up to 8 times/day for the duration of the trial.
Patients qualified for the trial after a screening period of up to 4 weeks, which included a 2-week run-in period during which time they were instructed to discontinue use of all topical ophthalmic medications except for REFRESH. Patients who successfully completed the run-in period were randomized with respect to their assigned medication. The patients returned for clinical assessments and a new supply of study medication at weeks 2, 4, 8 and 12, and at week 14 for a final follow-up assessment after 2 weeks off treatment.
Outcome measures
Efficacy
The primary efficacy endpoint was the proportion of successes, where a success was defined to be an improvement of ≥25% over baseline at week 12 in BUT, superficial punctate keratitis as assessed by FS or ST1. The assessment of superficial punctate erosions using FS was the sums of scores from nasal, temporal, pupil, and inferior segments (graded on a scale from 0 = none to 3 = severe). The primary efficacy analysis was performed for one eye (target eye), defined as the eye with the worse Schirmer value at baseline. If both eyes had the same ST1 value at baseline, the worse eye was considered the eye with the worse superficial punctate keratitis value at baseline.
The analyses of the components of the success criterion (change from baseline at week 12 for BUT, superficial punctate keratitis as assessed by FS, and ST1) for the target eye were performed as secondary analyses, as were the same analyses for the non-target eye, and also using the average assessment for both eyes. Other secondary analyses were performed for change from baseline at week 12 in TM for the target eye, and the Dry Eye Symptom Score (DESS).25 The DESS is a questionnaire consisting of 12 questions designed to assess the symptoms of ocular irritation, covering 3 areas: ocular symptoms, environmental triggers and vision-related function.
Safety
The safety of CF101 was assessed by recording the nature, severity, and duration of all adverse events and their relationship to the study medication, as judged by the investigator. Additional safety endpoints included clinical laboratory safety testing (clinical chemistry, hematology, and urinalysis), physical examinations, slit lamp and ophthalmic examinations, IOP measurements, electrocardiographic evaluations, and vital sign measurements.
Safety was evaluated at all visits, starting from baseline throughout the study and on week 14.
Statistical considerations
The between-treatment comparison with the success rate was performed using Fisher’s Exact Test. The analyses of the secondary variables, other than change from baseline in TM, were performed using analysis of covariance (ANCOVA) with the baseline assessment of the variable as the covariate. Change from baseline in TM was performed using the Wilcoxon rank sum test. All tests were performed at the 0.05 level. Safety endpoints were summarized by treatment group using descriptive statistics.
Results
Participant Flow and Follow-up
A total of 80 patients were enrolled, 38 in the placebo group and 42 in the CF101-treated group, and 85% (68/80) completed the study (Table 1). The first patient was enrolled in November, 2008, and the last patient completed the 12-week treatment and 2-week follow up in May, 2009. Patient disposition is presented in Table 1.
Table 1.
Patient Disposition
| Placebo | CF101 | |
|---|---|---|
| Enrolled | 38 | 42 |
| Completed | 35 (92%) | 33 (79%) |
| Discontinued | ||
| - Withdrawal of consent by the patient | 0 | 4 (9%) |
| - Occurrence of adverse events | 0 | 2 (5%) |
| - Noncompliance | 3 (8%) | 2 (5%) |
| - The patient condition required a change in a concomitant medication | 0 | 1 (2%) |
Patient Demographics Patients’ Characteristics at Baseline
All the patients were white. Most of the patients were women (49/76, 65%). There was no statistically significant difference in the patients’ age between CF101-treated and the placebo-treated groups (Table 2). At baseline, no statistical differences in FS, BUT, TM and ST1 were recorded between the placebo and the CF101-treated groups (Table 3).
Table 2.
Patient Demographics
| Placebo | CF101 | |
|---|---|---|
| Age | 61.73±1.94 | 52.56±2.19 |
| Sex | ||
| - Male | 10 (27%) | 17 (44%) |
| - Female | 27 (73%) | 22 (56%) |
Table 3.
Baseline Dry Eye Syndrome Parameter Values
| Placebo | CF101 | |
|---|---|---|
| FS (score) | 3.16±0.33 | 2.8±0.42 |
| BUT (sec) | 4.34±0.5 | 5.06±0.45 |
| ST1 (mm) | 2.97±0.33 | 3.0±0.33 |
| IOP (mmHg) | 14.23±0.44 | 13.31±0.43 |
BUT = tear break-up time; FS = fluorescein staining; IOP = intraocular pressure; ST1 = Schirmer tear test 1.
Efficacy analysis
In the CF101-treated group, 84.6% of the patients achieved more than 25% improvement in the corneal staining (success as defined by the primary efficacy end point of the trial) in comparison with 52.2% in the placebo group (P = 0.06 on week 12). Discontinuation of the treatment led to a reduction in the proportion of successes in the CF101-treated group, suggesting that the improvement in the corneal staining was attributed to CF101 (Fig 1). Furthermore, analysis of mean change from baseline of the corneal staining (measured by FS) revealed a progressive improvement in the CF101-treated group versus placebo throughout the treatment duration, with a statistically significant difference on week 12 (P = 0.004; Fig 2).
Figure 1.

Graph showing the percent of patients who achieved more than 25% improvement from baseline in the corneal staining score. *Statistically significant difference between CF101-treated group and placebo (P = 0.006). Placebo group, n = 37; CF101 group, n = 39.
Figure 2.
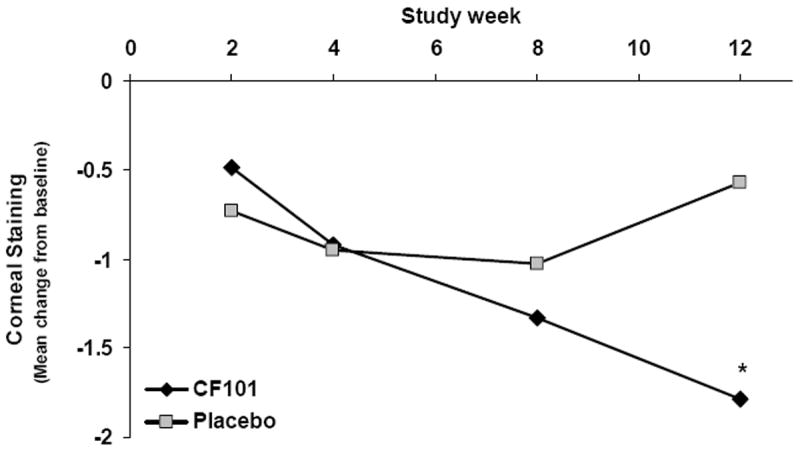
Graph showing the mean change from baseline in the corneal staining. *Statistically significant difference between CF101-treated group and placebo (P = 0.004). Placebo group, n = 37; CF101 group, n = 39.
Additionally, there was a statistically significant difference in the clearing of corneal staining between the CF101 and the placebo-treated groups in the nasal, temporal, pupil and inferior parts of the cornea, indicating consistency of effect across the ocular surface (Figure 3).
Figure 3.
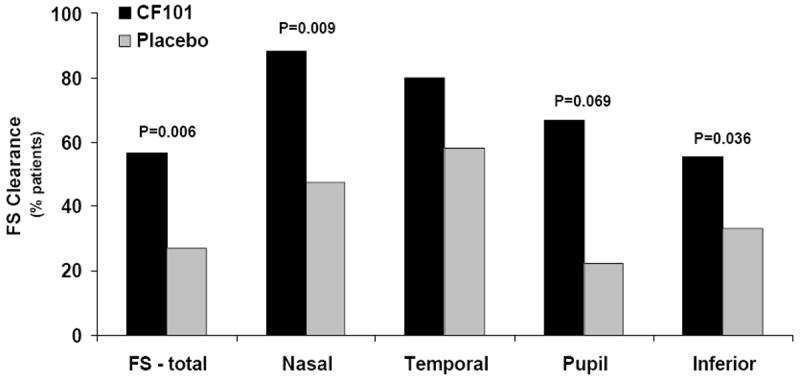
Bar graph showing the percentage of patients that achieved clearing of fluorescein staining (FS) in the various regions of the cornea at week 12.
An improvement in the mean change from baseline of the BUT was observed in the CF101- and placebo-treated groups (P=0.0025 and P=0.014, respectively); however, a higher rate of improvement was noted in the CF101-treated group (Figure 4). Analysis of the TM data revealed a statistically significant difference in the mean change from baseline at week 12 between the CF101-treated and the placebo groups (P=0.02) (Figure 5). No effect of the drug on ST1 or DESS was observed.
Figure 4.

Bar graph showing the mean change from baseline at week 12 in the tear film break-up time (BUT). *Statistically significant on week 12 versus baseline. Placebo group, n = 37 (P = 0.014); CF101 group, n = 39 (P = 0.0025).
Figure 5.

Bar graph showing the mean change from baseline at week 12 in the tear meniscus (TM) height. *Statistically significant on week 12 versus baseline. Placebo group, n = 37; CF101 group, n = 39 (P = 0.014).
Safety Analysis
No serious adverse events were noted through out the study. Adverse events resulting in discontinuation of the study were described in 2 patients: myalgias and recurrent corneal erosion. The frequency of adverse events was comparable in both treated groups (Table 4). The most frequently reported adverse events included: constipation, headache, palpitations, itching, abdominal pain, athralgia, myalgia, fatigure, and dry mouth (Table 5).
Table 4.
Treatment-Related Adverse Events: Safety Population
| Placebo (n=38) | CF101 (n=43) | |
|---|---|---|
| Any adverse event | 26 (68%) | 25 (58%) |
| Serious adverse events | 0 | 0 |
| Adverse events resulting in discontinuation | 0 | 2 (8%) |
| Death | 0 | 0 |
Table 5.
Most Frequently Reported Adverse Events
| Placebo (n=38) | CF101 (n=43) | |
|---|---|---|
| Constipation | 2 (5%) | 3 (7%) |
| Headace | 4 (10%) | 3 (7%) |
| Palpitations | 0 | 3 (7%) |
| Itching | 1 (3%) | 3 (7%) |
| Abdominal pain | 0 | 2 (5%) |
| Athralgia | 1 (3%) | 2 (5%) |
| Myalgia | 0 | 2 (5%) |
| Fatigue | 0 | 2 (5%) |
| Dry mouth | 1 (3%) | 0 |
Orally-administered CF101 1 mg twice daily was well tolerated and exhibited an excellent safety profile. No clinically significant changes in vital sings, electrocardiograms, blood chemistry, and hematology values were observed. Although the trial was not designed to assess the effects of treatment on IOP, it was noted that, at week 12, the CF101-treated group showed a 1.1 mmHg decrease from baseline, which was statistically significant at a P=0.048 level when compared with placebo.
Discussion
This study presents data showing that CF101, given orally, induced a statistically significant short term improvement in the corneal staining in patients with moderate-to-severe dry eye syndrome. An improvement in the BUT and TM was also observed. CF101 was well tolerated with no severe adverse events and a safety profile consistent with that reported in previous trials.23
In patients treated with CF101, the corneal staining scores were significantly lower at endpoint compared with placebo and decreased gradually over the study period. Notably, two weeks after cessation of therapy, the beneficial effects of CF101 with respect to corneal staining were diminished. The corneal staining reflects defects on the ocular surface that led to infection, inflammation, scarring and visual acuity,26,27 thus suggesting that a longer treatment period may impact on additional manifestations of dry eye syndrome and could improve DESS, as well. The time-dependent improvement of this objective measure of corneal integrity, coupled with the rebound of the disease within 2 weeks after the discontinuation of treatment, strongly suggest that CF101 is exerting an anti-inflammatory effect at the ocular surface.
Furthermore, FS clearance was observed consistently across most portions of the cornea, being statistically significant in favor of CF101 in all but the temporal sector. This result appears to have both precedence and clinical relevance, since the FS clearance parameter was recently incorporated as an efficacy endpoint for a Phase 3 dry eye syndrome trial of Diquafosol tetrasodium (Inspire Pharmaceuticals, Inc., Durham, NC), which targets the Gq protein-coupled P2Y2 receptor, a member of the ATP receptor family. Diquafosol promotes non-glandular secretion of fluid (water transport via chloride channel activation), mucin secretion, and possibly lipid production in the meibomian glands.14,28 Since common mechanistic pathways are shared between the different purine receptor family members, it may be that, in addition to the anti-inflammatory effect mediated via the A3AR, CF101 may also induce its beneficial effect via fluid and mucin secretion. Indeed, previous studies showed that the A3AR is the only AR that activates chloride channels in pigmented and nonpigmented epithelial cells. This was shown both in vitro and in vivo utilizing A3AR agonists such as IB-MECA and Cl-IB-MECA.29-31
The core mechanisms of dry eye are driven by tear hyperosmolarity and tear film instability. Tear hyperosmolarity causes damage to the surface epithelium by activating a cascade of inflammatory events at the ocular surface and a release of inflammatory mediators into the tears. Epithelial damage involves cell death by apoptosis, a loss of goblet cells, and disturbance of mucin expression, leading to tear film instability.32,33 The capability of CF101 to act as an anti-inflammatory agent, together with its effects on ion transport, may suggest its beneficial effect in dry eye syndrome.
ST1 was conducted in the present study without anesthesia and is known to be cumbersome, rough, primitive, and inaccurate, which might be the reason for the negative outcome. Similar data were found in moderate-to-severe patients with dry eye syndrome, treated with Restasis. However, significant improvement in ST conducted with anesthesia were observed in the Restasis trial after 4 months,34 suggesting that the design for the ST analysis in the Restasis study needs to be adopted for future studies with CF101.
In addition, this study demonstrated that CF101 treatment induced a statistically significant reduction in the IOP. During the last decade Civan and Avila have published that A3AR agonists activate Cl- channels in the non-pigmental ciliary epithelial cells, leading to an increase of the water inflow to the anterior chamber, thereby elevating the IOP both in vitro and in vivo.30 A point to note is that under these lab conditions, the animals were treated once, and IOP measurement was examined immediately after the treatment. In our clinical study, the patients were chronically treated, and the drug reached steady state plasma levels to induce either a direct or indirect effects.
The concept of treating dry eye syndrome with an oral drug is based on much better patient compliance than a topical treatment. The utilization of CF101 for the treatment of dry eye syndrome was enabled based on its apparently safety profile and continued anti-inflammatory effect for a long period of time, up to 18 month (unpublished data). Long term studies to treat dry eye syndrome patients with CF101 are underway to establish this drug as an efficacious treatment for this condition. The anti-inflammatory effect of CF101 which may affect the long-range disease pathogenesis can be suggested as a novel approach to treat the cause of the disease rather than its symptoms only. This approach distinguishes CF101 from the current standard care of treatment today. Thus, in future clinical development, CF101 will most probably be compared to placebo.
To conclude, the clinical results from this proof-of-concept study support further evaluation of CF101 as a potential treatment for the signs and symptoms of dry eye disease. The safety profile of CF101, as initially established in this and other trials,23 supports the long-term investigation that is necessitated by the chronic inflammatory nature of dry eye disease.
Acknowledgments
Sponsored by Can-Fite BioPharma Ltd, Petach Tikva, Israel. Supported in part by the Intramural Research Program of the National Institute of Diabetes & Digestive & Kidney Diseases, National Institutes of Health, Bethesda, Maryland.
Footnotes
- Avni I; Stock options owner; Can-Fite BioPharma
- Fishman S; Employee; Can-Fite BioPharma
- Harpaz Z; Employee; Can-Fite BioPharma
- Farbstein M; Employee; Can-Fite BioPharma
- Bar Yehuda S; Employee; Can-Fite BioPharma
- Silverman MH; Employee; Can-Fite BioPharma
- Kerns WD; Employee; Can-Fite BioPharma
- Cohn I; Employee; Can-Fite BioPharma
- Fishman P; Employee; Can-Fite BioPharma
All the rest of the authors have no financial interests to disclose
References
- 1.Latkany R. Dry eyes: etiology and management. Curr Opin Ophthalmol. 2008;19:287–91. doi: 10.1097/ICU.0b013e3283023d4c. [DOI] [PubMed] [Google Scholar]
- 2.Fox RI, Stern M, Michelson P. Update in Sjögren’s syndrome. Curr Opin Rheumatol. 2000;12:391–8. doi: 10.1097/00002281-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 3.DEW. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop. Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 4.Luo L, Li DQ, Doshi A, et al. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;4:4293–301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 5.Acera A, Rocha G, Vecino E, et al. Inflammatory markers in the tears of patients with ocular surface disease. Ophthalmic Res. 2008;40:315–21. doi: 10.1159/000150445. [DOI] [PubMed] [Google Scholar]
- 6.Pflugfelder SC, Farley W, Luo L, et al. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol. 2005;166:61–71. doi: 10.1016/S0002-9440(10)62232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon KC, De Paiva CS, Qi H, et al. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest Ophthalmol Vis Sci. 2007;48:2561–9. doi: 10.1167/iovs.07-0002. [DOI] [PubMed] [Google Scholar]
- 8.Tsubota K, Fukagawa K, Fujihara T, et al. Regulation of human leukocyte antigen expression in human conjunctival epithelium. Invest Ophthalmol Vis Sci. 1999;40:28–34. [PubMed] [Google Scholar]
- 9.Pisella PJ, Brignole F, Debbasch C, et al. Flow cytometric analysis of conjunctival epithelium in ocular rosacea and keratoconjunctivitis sicca. Ophthalmology. 2000;107:1841–9. doi: 10.1016/s0161-6420(00)00347-x. [DOI] [PubMed] [Google Scholar]
- 10.de Paiva CS, Pflugfelder SC. Rationale for anti-inflammatory therapy in dry eye syndrome. Arq Bras Oftalmol. 2008;71:89–95. doi: 10.1590/s0004-27492008000700017. [DOI] [PubMed] [Google Scholar]
- 11.Peral A, Domínguez-Godínez CO, Carracedo G, Pintor J. Therapeutic targets in dry eye syndrome. Drug News Perspect. 2008;21:166–76. [PubMed] [Google Scholar]
- 12.Donnenfeld E, Pflugfelder SC. Topical ophthalmic cyclosporine: pharmacology and clinical uses. Surv Ophthalmol. 2009;54:321–38. doi: 10.1016/j.survophthal.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Pintor J, Peral A, Hoyle CH, et al. Effects of diadenosine polyphosphates on tear secretion in New Zealand white rabbits. J Pharmacol Exp Ther. 2002;300:291–7. doi: 10.1124/jpet.300.1.291. [DOI] [PubMed] [Google Scholar]
- 14.Tauber J, Davitt WF, Bokosky JE, et al. Double-masked, placebo-controlled safety and efficacy trial of diquafosol tetrasodium (INS365) ophthalmic solution for the treatment of dry eye. Cornea. 2004;23:784–92. doi: 10.1097/01.ico.0000133993.14768.a9. [DOI] [PubMed] [Google Scholar]
- 15.Stiles GL. Adenosine receptors and beyond: molecular mechanisms of physiological regulation. Clin Res. 1990;38:10–8. [PubMed] [Google Scholar]
- 16.Baharav E, Bar-Yehuda S, Madi L, et al. Antiinflammatory effect of A3 adenosine receptor agonists in murine autoimmune arthritis models. J Rheumatol. 2005;32:469–76. [PubMed] [Google Scholar]
- 17.Fishman P, Bar-Yehuda S, Madi L, et al. The PI3K–NF-κ B signal transduction pathway is involved in mediating the anti-inflammatory effect of IB-MECA in adjuvant-induced arthritis. Arthritis Research &Therapy. 2006;8:1–9. doi: 10.1186/ar1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rath-Wolfson L, Bar Yehuda S, Madi L, et al. IB-MECA, an A3 adenosine receptor agonist prevents bone resorption in rats with adjuvant induced arthritis. Clin & Exp Rheum. 2006;24:400–6. [PubMed] [Google Scholar]
- 19.Bar-Yehuda S, Silverman MH, Kerns WD, et al. The anti-inflammatory effect of A3 adenosine receptor agonists: a novel targeted therapy for rheumatoid arthritis. Expert Opin Inves Drugs. 2007;16:1601–13. doi: 10.1517/13543784.16.10.1601. [DOI] [PubMed] [Google Scholar]
- 20.Mabley J, Soriano F, Pacher P, et al. The adenosine A3 receptor agonist, N6-(3-iodobenzyl)-adenosine-5’-N-methyluronamide, is protective in two murine models of colitis. Eur J Pharmacol. 2003;466:323–9. doi: 10.1016/s0014-2999(03)01570-x. [DOI] [PubMed] [Google Scholar]
- 21.Bar Yehuda S, Rat-Wolfson L, Del Valle L, et al. CF101 induces anti-inflammatory effect and prevents cartilage damage in rat knee osteoarthritis. Arthritis & Rheumatol accepted. 2009 doi: 10.1002/art.24817. [DOI] [PubMed] [Google Scholar]
- 22.Lee HT, Kim M, Joo JD, et al. A3 adenosine receptor activation decreases mortality and renal and hepatic injury in murine septic peritonitis. Am J Physiol Regul Integr Comp Physiol. 2006;291:R959–69. doi: 10.1152/ajpregu.00034.2006. [DOI] [PubMed] [Google Scholar]
- 23.van Troostenburg AR, Clark EV, Carey WDH, et al. Tolerability, pharmacokinetics, and concentration-dependent hemodynamic effects of oral CF101, an A3 adenosine receptor agonist, in healthy young men. Int J Clin Pharmacol Ther. 2004;42:534–42. doi: 10.5414/cpp42534. [DOI] [PubMed] [Google Scholar]
- 24.Silverman MH, Strand V, Markovits D, et al. Clinical evidence for utilization of the A3 adenosine receptor as a target to treat rheumatoid arthritis: data from a phase II clinical trial. Rheumatol. 2008;35:41–8. [PubMed] [Google Scholar]
- 25.Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–21. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 26.Cohen IJ. Use of fluorescein in the eye. J Occup Med. 1963;5:540–1. [PubMed] [Google Scholar]
- 27.Holland MC. Fluorescein staining of the cornea. JAMA. 1964;188:81. doi: 10.1001/jama.1964.03060270087025. [DOI] [PubMed] [Google Scholar]
- 28.Kellerman D, Rossi Mospan A, Engels J, et al. Denufosol: a review of studies with inhaled P2Y2 agonists that led to Phase 3. Pulm Pharmacol Ther. 2008;21:600–7. doi: 10.1016/j.pupt.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Carré DA, Mitchell CH, Peterson-Yantorno K, et al. Similarity of A3-adenosine and swelling-activated Cl(-) channels in nonpigmented ciliary epithelial cells. Am J Physiol Cell Physiol. 2000;279:C440–51. doi: 10.1152/ajpcell.2000.279.2.C440. [DOI] [PubMed] [Google Scholar]
- 30.Yang H, Avila MY, Peterson-Yantorno K, et al. The cross-species A3 adenosine-receptor antagonist MRS 1292 inhibits adenosine-triggered human nonpigmented ciliary epithelial cell fluid release and reduces mouse intraocular pressure. Curr Eye Res. 2005;30:747–54. doi: 10.1080/02713680590953147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Do CW, Civan MM. Swelling-activated chloride channels in aqueous humour formation: on the one side and the other. Acta Physiol (Oxf) 2006;187:345–52. doi: 10.1111/j.1748-1716.2006.01548.x. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Begley CG, Chen M, et al. A link between tear instability and hyperosmolarity in dry eye. Invest Ophthalmol Vis Sci. 2009;50:3671–9. doi: 10.1167/iovs.08-2689. [DOI] [PubMed] [Google Scholar]
- 33.Yeo AC, Carkeet A, Carney LG, Yap MK. Relationship between goblet cell density and tear function tests. Ophthalmic Physiol Opt. 2003;23:87–94. doi: 10.1046/j.1475-1313.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 34.Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107:631–9. doi: 10.1016/s0161-6420(99)00176-1. [DOI] [PubMed] [Google Scholar]


