Abstract
Opioid overdose morbidity and mortality is recognized to have epidemic proportions. Medical and public health agencies are adopting opioid harm reduction strategies to reduce the morbidity and mortality associated with overdose. One strategy developed by emergency medical services and public health agencies is to deliver the opioid antidote naloxone injection intranasally to reverse the effects of opioids. Paramedics have used this route to quickly administer naloxone in a needle-free system and avoiding needle-stick injuries and contracting a blood-born pathogen disease such as hepatitis or human immunodeficiency virus. Public health officials advocate broader lay person access since civilians are likely witnesses or first responders to an opioid overdose in a time-acute setting. The barrier to greater use of naloxone is that a suitable and optimized needlefree drug delivery system is unavailable. The scientific basis for design and study of an intranasal naloxone product is described. Lessons from nasal delivery of opioid analgesics are applied to the consideration of naloxone nasal spray.
Keywords: Intranasal, Naloxone, Opioid, Overdose, Antidote
Introduction
In 2008, poisoning surpassed motor vehicle accidents as the leading cause of “injury deaths” in the United States (U.S.) [1]. Nearly 90% of poisoning deaths are caused by drugs. During the past 3 decades, the number of drug poisoning deaths increased six-fold from about 6,100 in 1980 to 36,500 in 2008. Of the 36,500 drug poisoning deaths in 2008, 14,800 involved prescription opioid analgesics. Approximately 3,000 deaths also involved heroin overdose. In 2008 the overall death rate in the US was 4.8 per 100,000 for non-medical use of prescription opioids [2, 3].
The opioid overdose crisis is a world-wide phenomenon crossing sovereign, cultural and socio-economic boundaries. The U.S. Centers for Disease Control considers prescription drug overdose in epidemic proportions, in particular, the morbidity and mortality associated with use, abuse and misuse of prescription opioids [4]. Hospitalizations from prescription opioid poisoning increased by over 50% from 1999-2006, paralleling the increased prescribing of these medications for the treatment of pain [5, 6]. Although many deaths are associated with drug-abuse, there is also a growing trend of therapeutic misadventures for pain patients prescribed powerful analgesics, including opioids. Chronic cancer and non-malignant pain pharmacotherapy regimens frequently involve combinations of medications with additive or synergistic central nervous system depression adverse effects.
Injection drug use, principally heroin, is one of the most significant correlates to opiate use mortality. Eurasia, Australia, Canada, Italy and Great Britain, among others, all describe significant injection drug use populations that experience drug overdose with similar rates of mortality regardless of socioeconomic status [7-12].
Government and non-government public health agencies, the pharmaceutical industry, and others are adopting prevention and intervention strategies in an attempt to reduce opioid overdose mortality. One “harm-reduction” strategy has been to provide education and training on opioid overdose recognition and emergency treatment to addicts and their close daily contacts [13]. In addition, the addict and their loved ones are trained to rescue breathe, call emergency medical services, and to administer the opioid antidote naloxone.
Naloxone is the drug of choice to reverse respiratory and central nervous system (CNS) depression caused by opioid overdose [14]. Naloxone injection has been marketed in the U.S. for 41 years, initially under the trade name Narcan®. Naloxone hydrochloride (HCl), known chemically as 17-Allyl-4,5α,-epoxy-3, 14-dihydroxymorphinan-6-one hydrochloride, is a potent mu-receptor antagonist. It has subsequently become a multi-source prescription generic drug manufactured by International Medication Systems, Limited and Hospira, Inc. [15, 16]. Ampoules of naloxone injection are also available in many countries. The injection is available in two strengths, 0.4 mg/mL and 1.0 mg/mL. Naloxone is a standard drug carried by emergency services personnel in ambulances and medication kits for reversal of suspected opioid overdose, whether accidental or intentional. Hospital emergency departments also use this medication routinely for this purpose. The initial adult dose of naloxone in known or suspected narcotic overdose is 0.4 to 2 mg, which may be repeated to a total dose of 10 mg. The current formulations of naloxone are approved for intravenous (IV), intramuscular (IM) and subcutaneous (SC) administration, with IV being the recommended route [17, 18]. Naloxone is also indicated as a reversal agent when the effects of therapeutic use of opioids are no longer medically necessary, such as in reversal of opioid effects in general anesthesia [15, 16]. Lastly, naloxone is co-formulated with buprenorphine as an oral product providing an abuse-deterrent formulation for opioid maintenance in opioid dependent patients.
In the last several years, the emergency medical systems (EMS) community in the U.S. and elsewhere has developed an interest in administering naloxone in a needleless system via the intranasal (IN) route. Some EMS programs have now moved toward intranasal administration of naloxone since many of the patients needing naloxone are injection drug users; 80% of the injection drug user population in large metropolitan areas is Hepatitis C positive or HIV positive. For example, the Denver and San Francisco EMS uses this drug administration technique as standard of care to prevent needle-stick injuries to Emergency Medical Technicians (EMTs) [17, 19, 20].
Some EMS programs have developed a system using existing technologies of an approved drug and an existing medical device to administer this opioid antidote, albeit in a non-FDA-approved manner [19, 20]. This has been accomplished by using the injectable formulation (1 mg/mL) and administering 1 mL per nostril via a marketed nasal atomizer/nebulizer device. The system combines an FDA-approved naloxone injection product (with a Luer fitted tip, no needles) with a marketed, [510(k) exempt] medical device called the Mucosal Atomization Device (MAD™ Nasal, Wolfe Tory Medical, Inc.). This initiative is consistent with the U.S. Needlestick Safety and Prevention Act (Public Law 106-430) [21-25].
The EMS programs recognize limitations of this system, one limitation being that it is not assembled and ready-to-use. Although this administration mode appears to be effective in reversing narcosis, the formulation is not concentrated for retention in the nasal cavity. The 1 mL delivery volume per naris is larger than that generally utilized for intranasal drug administration. Therefore, there is loss of drug from the nasal cavity, due either to drainage into the nasopharynx or externally from the nasal cavity. An improvement would be to design a ready-to-use product specifically optimized, concentrated, and formulated for nasal delivery.
The drug abuse treatment and overdose prevention communities world-wide have also recognized the desire for a needle-free system for naloxone delivery [26]. Clients at needle-exchange centers provide kits containing naloxone, with either needles or nasal spray atomizers, and instructions for use. Such programs are well-described in the U.S. and Great Britain, commonly operating in conjunction with needle-exchange programs [27-33]. In May 2012 the British Advisory Council on the Misuse of Drugs published a report advocating for greater distribution of naloxone and training for administration [34]. The Council reiterates that naloxone is safe and effective, that there is evidence that take-home naloxone can be effective for reversing heroin overdoses. Cost-effectiveness of these programs is still being assessed.
An unmet medical need exists to provide greater access to the opioid antidote naloxone. A significant barrier to this goal is that naloxone is only available as an injection for IV, IM or SC administration. A needleless system that integrates a concentrated formulation and a nasal delivery device would help satisfy this unmet need.
Nasal physiology, drug and formulation considerations for nasal delivery
Nasal physiology
Intranasal sprays of medication intended for systemic drug absorption are generally designed to target the turbinates on the medial wall of the nasal cavity. The turbinates serve as a baffle in which inspired air is humidified and filtered. This region of the nasal cavity is covered with a thin mucus layer, a monolayer ciliated epithelium, with an abundant underlying blood supply. These conditions are ideal to permit passive diffusion (transcelluar) of medications with certain chemical characteristics across cell membranes and into the bloodstream. Some medications also transit to the blood stream by passing through the tight-cell junctions between cells (paracellular) [35]. To reach the turbinates the nasal spray device must be inserted fully into the nasal vestibule with the atomizer tip placed at the nasal valve, and then aimed laterally toward the turbinates. Activation of the device ejects the liquid as an atomized spray or plume. The bulk of the spray impacts the anterior and inferior portions of the nasal cavity as a function of straight-line impact of particles greater than 10 microns in size [36-38]. The smallest particles, less than 10 microns in size, may be carried by air currents more superiorly in the nasal cavity and impact on the superior turbinate and possibly reach the olfactory region and nerve. There is substantial evidence in animals, and some evidence in man, that the olfactory nerve can absorb or actively transport medications to the central nervous system via the olfactory bulb (nose to brain theory). Differences in animal and human nasal apparatus anatomy, and certain characteristics of the medication, seem to play roles as to whether medication is transported to the brain via this mechanism and if a pharmacologic effect is observed [39-41].
Under ideal conditions most medication is absorbed from the nasal cavity and into the bloodstream within 15-20 minutes, thus generally avoiding the gut first-pass metabolism [35, 36]. Medication remaining in the nasal cavity beyond this time is subject to elimination via various enzyme systems present within the nasal mucus and by swallowing. A second absorption phase (oral) can be observed with nasally administered medications having incomplete nasal absorption that are not subject to high first pass gut metabolism.
Nasal physiologic changes during pathologic conditions, such as polyposis and allergic and vasomotor rhinitis, could theoretically alter the biopharmaceutics of intranasal medications intended for systemic drug administration [35, 36, 42, 43]. Physical obstruction of the nasal passage(s) due to prior trauma and subsequent deflection of the passageways is another possibility. Concurrent use of medications with vasoconstriction or vasodilation properties may also affect drug absorption. Lastly, increases in mucus production and changes in mucociliary clearance rates could affect bioavailability [35-38].
Pharmaceutical regulatory agencies have required studies of the effect of rhinitis on nasal drug delivery biopharmaceutics [43, 44]. It has been demonstrated that there is a lack of effect of nasal mucosal inflammation on the absorption of hydromorphone, butorphanol, buserelin and triamcinolone acetonide - an exception was reported for desmopressin. Inconsistent results have been reported on the biopharmaceutical disposition of these medications when pretreatment with oral or topical decongestants was administered {37, 43, 44]. Small but statistically measureable changes in rate or extent of absorption have been reported when decongestants were co-administered.
Drug and formulation considerations
Many intranasal delivery products are designed to serve certain purposes or unmet medical needs. Clearly, a nasal spray can remove the needle from drug administration, as is the case with the conversion of the protein calcitonin from a daily injection to a nasal spray. Furthermore, beyond just removing the needle from delivery, intranasal products are designed for rapid action, such as those products designed to treat seizures (benzodiazepines) migraine headache (sumatriptan, butorphannol, zolmitriptan, dihydroergotamine) or pain in general (fentanyl, hydromorphone) [45-49].
Successful intranasal products satisfied several design fundamentals necessary for intranasal delivery. The properties of the drug generally follow these characteristics:
Mass of 20 mg or less per dose
Molecular weight < 1000 Daltons
Excellent water solubility
Ionization and pH control of aqueous solutions
Osmolality - isotonic to slightly hypertonic
Stability in processing and storage
Compatibility container closure and sprayer components
Compatible with excipients (buffers, antioxidants, cosolvents, etc.)
Physical-chemical properties of the candidates must also be considered. Water-solubility is important for formulation considerations. Log P, derived from the octanol/water partition coefficient, is a surrogate for lipophilicity and potential for compounds to diffuse across biologic membranes. Successful intranasal medications tend to be water soluble and have sufficient lipophilic character to readily cross biologic membranes[35, 36].
The dose must have sufficient solubility to be administered in approximately 100 μL to 200 μL (1 spray per naris) of solution. The nasal cavity can retain 100 to 150 μL without causing immediate run-off out the front of the nose or down the naso-pharynx [35, 36]. Additional solubilization strategies may be necessary including the use of organic co-solvents, excipients such as cyclodextrins or other agents to from water-soluble inclusion complexes, or preparation of emulsions. Permeation enhancers may also be necessary to enhance drug penetration through cell membranes [50]
Design of the formulation must account for other factors as well. It is useful to design the formulation to be isotonic to slightly hypertonic to optimize absorption and tolerability. Viscosity enhancing agents such as methylcellulose and others can promote retention in the nasal cavity by slowing the ciliary movement of mucus [35, 36]. Surfactants or polymers can be employed to enhance transmembrane permeation [50]. Lastly, the drug and formulation have to be stable in the device during processing, i.e., sterilization and storage, and thus may require stabilizers.
The choice of delivery device for the medication is another critical consideration. Squeeze bottles are available but have no metering device appropriate to administer potent systemic medications. Multi-dose bottles provide a metered dose and are available for chronic drug administration. A standard syringe with Luer fitting to accept a nasal atomizer has been used to draw up and administer injection based drug solutions into the nasal cavity for opiate overdoses, acute pain and to deliver midazolam injection to the nasal cavity of a seizing patient. Unit-dose devices similar to those used for migraine treatment are also available and being used in development of benzodiazepine nasal spray products. The choice of device depends upon factors such as intended clinical use, setting, stability with the drug and formulation, among others [35, 36].
Ideally, a well designed formulation must not induce localized toxicity with acute or chronic use. For example, chronic vasoconstriction, irritation or inflammation can induce tissue damage including ulceration, epistaxis, nasal-septal defects and fistulae. Formulations should not cause damage to the cilia. Chronic, or daily use, of an irritating product could lead to more serious sequallae from nasal delivery [36].
Properties of naloxone hydrochloride dihydrate
Naloxone is supplied as Naloxone HCl dihydrate. The empirical formula of naloxone HCl dihydrate is C19H22ClNO4, 2H2O and its molecular weight is 399.9 g/mol. The structural formula for Naloxone is described in Figure 1a [15, 16].
Fig. 1.
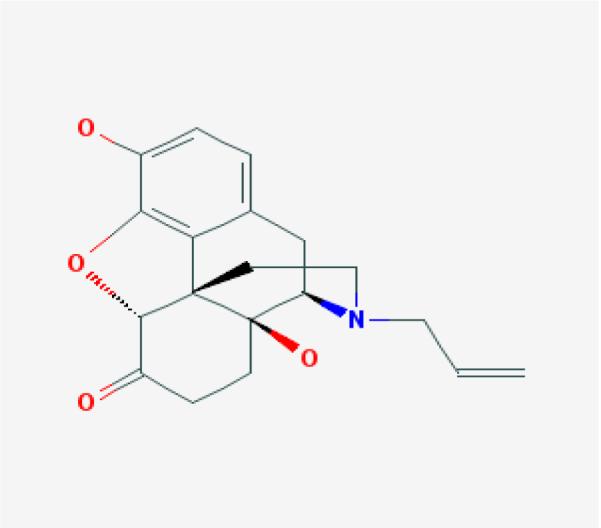
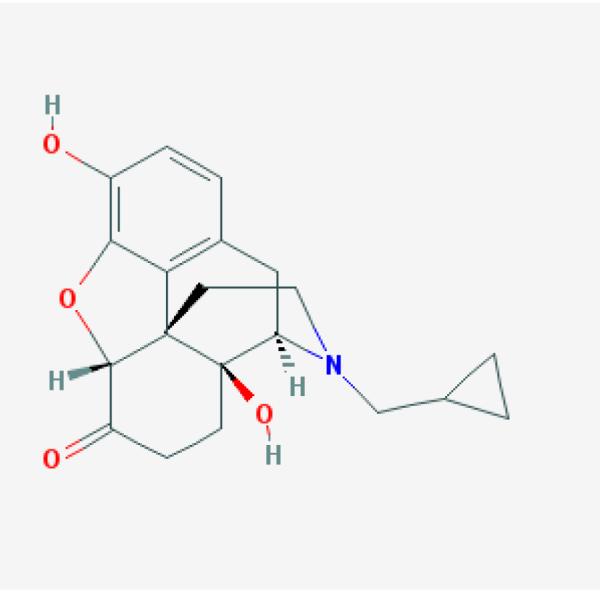
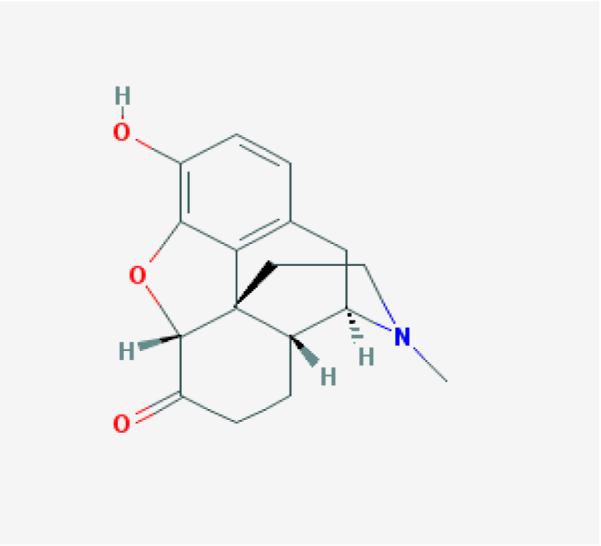

Chemical structure of naloxone (a), naltrexone (b), hydromorphone (c) and butorphanol (d) (Pub Chem pubchem.ncbi.nlm.nih.gov)
Naloxone HCl has a physical form of white, or almost white, crystalline powder, and is hygroscopic. Its melting point is 200-205 °C. Naloxone HCl is freely soluble in water and 96% alcohol, but practically insoluble in toluene. It is also slightly soluble in alcohol and practically insoluble in ether or chloroform. The dissociation constant pKa of naloxone is 7.9 and the Log P is 1.92. These physical-chemical characteristics suggest a naloxone aqueous solution is likely feasible [36].
Given its high water solubility, naloxone HCl is an excellent candidate to consider for intranasal delivery and satisfies many criteria necessary for this route. Naloxone is a high first-pass metabolism medication; oral bioavailability is reported to be <5%. The parenteral dose is 2 mg or less; it is highly potent when injected [14-16].
Nasal sprays of compounds chemically related to naloxone have been described. Medications studied include the opioid antagonist naltrexone, and the opioid agonists hydromorphone and butorphanol (Fig 1 b,c,d). Examination of the formulation methods and human biopharmaceutics of other chemically related compounds will be instructive for considerations of a naloxone nasal spray[36, 45, 48, 49].
Translation of intranasal opioid formulations to naloxone nasal spray
Naturally occurring and semi-synthetic opioids and antagonists share the core structure of thebaine [51]. The addition of functional groups to the core structure imparts different physical-chemical and pharmacologic properties. However, the molecular weights and pKa values are roughly similar and the acid salts tend to be freely soluble in water. Certain cogeners have relatively higher log P as compared to others and so transmembrane delivery may be more accommodative for these molecules [36]. Functional group changes also impart pharmacological properties of being a full mu-receptor agonist, partial agonist, or antagonist. Similarly, these changes may affect the degree of first pass metabolism upon oral administration. Many of these cogeners are also quite potent - doses may range from 0.5 to 10 mg.
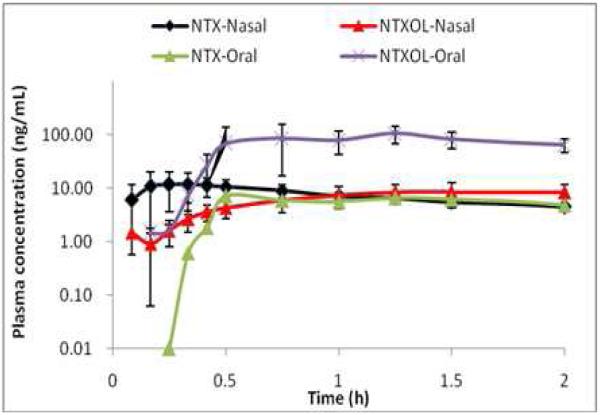
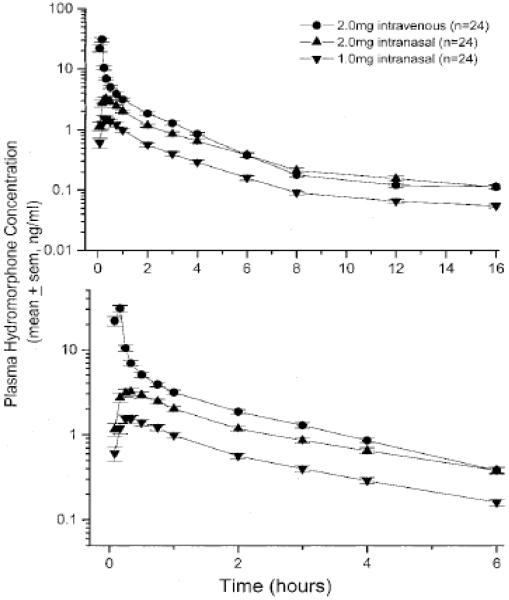
A recent manuscript has provided a comprehensive review on intranasal delivery of opioids [36]. Tables on physical-chemical properties and biopharmaceutics of various agents are provided. Data for naltrexone, hydromorphone and butorphanol are provided. These molecules may be of particular interest since the intravenous and intranasal dosing appears to be similar and the molecules share chemical characteristics to naloxone. Table 2 provides a comparison of the biopharmaceutics of nasally administered naltrexone, hydromorphone and butorphanol. Interestingly, the clinical doses of these drugs (dose normalized for naltrexone) are comparable to that of naloxone injection. Concentration-time profiles for naltrexone, hydromorphone and butorphanol are provided in Figure 2 (dose normalized). These data may suggest the likely outcome of an intranasally delivered concentrated solution of naloxone HCl. A 2 mg nasal solution dose will likely have a Cmax of 3-5 ng/mL and a tmax of approximately 20 minutes, similar to naltrexone and hydromorphone [48]. The greater absorption of butorphanol is likely related to its higher Log P and ability to diffuse across biologic membranes.
Table 2.
| Drug and Dose | Cmax (ng/mL) | Tmax (minutes) | Bioavailability (%) |
|---|---|---|---|
| Naltrexone HC l 10 mg |
14.9 | 22 | 600 (to oral) |
| Hydromorphone HCl 2 mg |
3.5 | 20 | 50-60 |
| Butorphanol tartrate 2 mg |
5.5 | 10 | 60-70 |
Fig 2.
Concentration-time profiles for naltrexone (10 mg intranasal and 50 mg oral) (a), hydromorphone (2 mg IV and 1 and 2 mg intranasal (b), and butorphanol (1 and 2 mg intranasal) (c). [48, 49]
Biopharmaceutics of intranasal naloxone
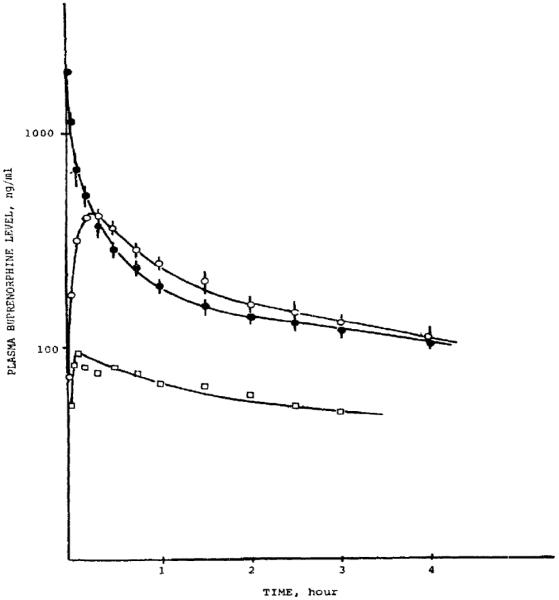
The nasal administration of 3H-naloxone to anesthetized male rats (n = 3/group) at a single dose of 30 μg (0.1 mg/kg, based on their average weight of 270 g/rat) in 0.1 mL was compared to a similar dose in 0.1 mL given by the intravenous and intraduodenal routes [52]. Nasally administered naloxone was rapidly and completely absorbed (Figure 3). The plasma elimination half-life of radioactivity was found to be 40-45 minutes. The nasal bioavailability for naloxone calculated from the ratio of the AUC0-INF (nasal/intravenous = 1517.5/1498.7 ng·h/mL) was 101%. The intraduodenal bioavailability for naloxone was only 1.5% (intraduodenal/intravenous = 22.0/1498.7 ng·h/mL). These results established the nasal route for the administration of naloxone in rats was equivalent to the parenteral route.
Fig 3.
Mean Plasma Naloxone Levels in Rats after a Single Nasal (○), Intravenous (●), or Intraduodenal (□) Dose of 30 μg of 3H-Naloxone [52]
The pharmacokinetic properties of intranasal naloxone in humans are not well described. A literature review found there are no papers describing the human pharmacokinetics of intranasal naloxone using what might be considered a highly concentrated nasal solution formulation. One paper describes pharmacokinetics of nasal administration of commercial injectable naloxone in man [53]. The intranasal formulation employed was an injection in which 0.8 mg was administered in a volume of 2 mL (1mL/naris) even though it is commonly understood the nasal cavity can retain only 100 to 200 μL per naris. The study compared intravenous and intramuscular administration to intranasal administration. The reported intranasal bioavailability of 4% is dependent upon this non-optimized delivery volume, as it can be assumed that much of the medication ran away from the site of absorption. Therefore, the report may be misleading regarding predicting nasal naloxone absorption in humans using a solution concentrated and designed to accommodate the absorptive surface of the nares.
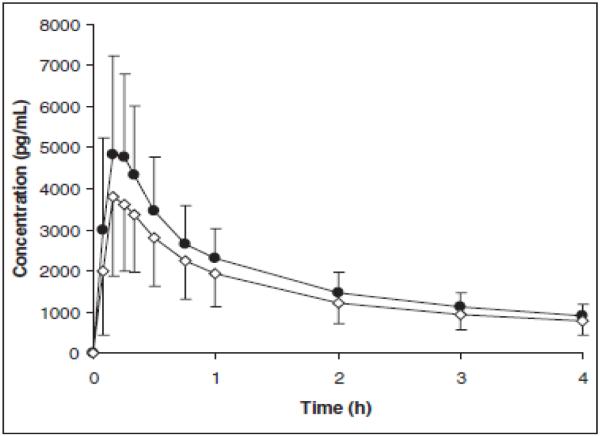
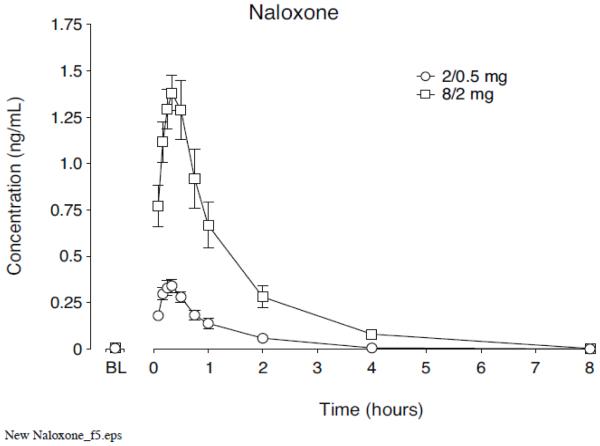
A recent publication provides a more relevant examination of the possible pharmacokinetic profile of a formulated naloxone nasal spray. The study provides information regarding the nasal absorption of naloxone in humans from a powder obtained from crushed Suboxone® (buprenorphine and naloxone sublingual tablets) [54]. After administration of 2 mg (naloxone) nasal powder, the absolute bioavailability was 30%, with a tmax of 20 minutes and a Cmax of 1.6 ng/mL. A powder will behave somewhat differently than a solution administered intranasally because dissolution must occur during the time that the naloxone powder is present in the nasal cavity and before the ciliated epithelia sweep the product posterior to be swallowed. A solution of naloxone may have a slightly higher bioavailability and Cmax. Figure 4 presents a concentration-time profile for 0.5 and 2.0 mg of intranasal naloxone powder from a Suboxone® tablet.
Fig 4.
Mean±SEM for plasma concentrations of naloxone in volunteers, analyzed for up to 8 hours after 0.5mg or 2 mg dose of naloxone from Suboxone® tablets [54]
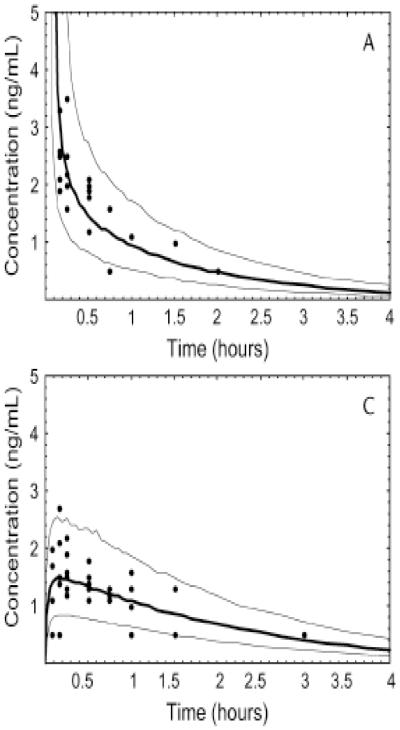
The nasal delivery of naloxone powder, and subsequent exposure, can be used as a surrogate marker as to the potential for naloxone nasal spray to reverse narcosis. The essential question remains as to whether naloxone nasal spray will produce an exposure that is clinically relevant. The Dowling paper suggests that naloxone nasal spray will produce a systemic exposure that is clinically relevant if compared to the profiles of intravenous and intramuscular administration. Figure 5 presents the concentration-time profile for 0.8 mg naloxone, a clinically relevant dose within the naloxone prescribing label, for intravenous and intramuscular administration. The intramuscular administration is particularly relevant given the absorption phase. A Cmax of about 1.5 ng/mL and a tmax 12 minutes were derived from this route of administration [53]. These data are not greatly different from the profile demonstrated from intranasal naloxone powder administration.
Fig 5.
Concentration-time profile for 0.8 mg naloxone give IV and IM [53]
Clinical experience with intranasal naloxone in reversing narcosis
Emergency medicine practitioners and drug abuse treatment and prevention clinicians have considerable experience with administering naloxone injection for treatment of suspected opioid overdose. It appears the practice of nasal naloxone administration has outpaced the biopharmaceutic and clinical pharmacologic aspects typical of understanding the properties of a medication and delivery system. Regardless, the system appears to work and is further described.
Nasal administration of naloxone was first reported by Loimer in 1992 [55]. Naloxone was studied using a 1 mg intranasal dose to identify potentially physically-dependent opioid users. Withdrawal distress, pupillary response, pulse rate and blood pressure were recorded. Withdrawal symptoms highly correlated with subject history and the presence of opioid metabolites in urine. The authors conclude that nasal delivery of naloxone is as effective as intravenous injection to identify physically-dependent opioid users and could be useful in emergency medicine treatment.
A second study by Loimer was conducted in 17 opioid-dependent patients to compare the efficacy of 1 mg of intranasal naloxone to intramuscular and intravenous naloxone administration [56]. Withdrawal symptoms and vital sign changes were again used as endpoints at 1, 5, 15, 45, 90 and 180 minutes after administration. The data demonstrated that intranasal administration had a more rapid onset and intensity of withdrawal as compared to intramuscular administration, but was not as rapid or as intense as intravenous administration. All endpoints returned to baseline by 180 minutes in all groups.
Naloxone is approved for use in the United States by IV, IM, or SC routes [14-16]. It is suggested that the onset of action of the IV will be faster, so is preferred in emergency situations. However, obtaining IV access in the pre-hospital setting, especially among injection drug abusers can be time consuming and difficult. Wanger conducted a study to determine the actual onset of effect (defined as a respiratory rate of ≥10 breaths/minute) calculated from the time of arrival at the patient’s side using two approved injection routes [57]. That is, this approach takes into account the time to set up and deliver the drug by the intended route.
The study utilized naloxone injection, as used in non-hospital settings for presumed heroin overdoses. Patients were enrolled in series, rather than randomized. The first series treated patients with naloxone 0.4 mg IV followed by an additional 0.4 mg IV in the case of unsatisfactory response by 5 minutes (satisfactory response was ≥10 breaths/minute). The second phase of the protocol treated patients with 0.8 mg naloxone SC. If a satisfactory response was not observed by 5 minutes, the IV protocol was followed.
While the time to effect after IV administration was faster, it was offset by the time needed to obtain IV access and administer the drug, as compared to SC administration, such that there was no difference in time to response. The SC response data may be quite relevant to assessing possible doses and response profile of naloxone nasal spray. A second dose was needed in 35% of the patients treated with IV naloxone and a second dose (given IV) was needed in 15% of the patients treated with SC naloxone. Subjectively, the ambulance attendants administering the naloxone indicated that emergence was more gradual, resulting in less violence and aggression at the scene after SC administration as compared to IV administration. A similar effect may be seen after intranasal route of administration (See Table 3).
Table 3.
Time intervals for patients in the out-of-hospital setting receiving naloxone 0.4 mg IV vs naloxone 0.8 mg SC [57]
| IV (n=74) |
SC (n=122) |
p-value | |
|---|---|---|---|
| Time interval from arrival at patient’s side to drug administration (min) |
5.7±3.8 | 4.0±3.0 | 0.002 |
| Time interval from drug administration to resp. rate ≥10 breaths/min (min) |
3.8±3.1 | 5.5±3.9 | 0.001 |
| Time interval from arrival at patient’s side to resp. rate ≥10 breaths/min (min) |
9.3±4.2 | 9.6±4.6 | 0.67 |
| Duration of bag-valve-mask ventilation (min) |
8.1±6.0 | 9.1±4.8 | - |
Barton was the first to demonstrate intranasal delivery of naloxone by paramedics. Thirty patients in Denver, CO encountered by paramedics received 2 mg of naloxone (injection formulation, 1mg/mL) administered intranasally with the disposable nasal spray atomizer called the Mucosal Atomization Device [58]. One mL was administered into each naris upon initial patient contact. Paramedics then assessed/provided airway management and IV line placement unless the patient initially responded to the naloxone and no additional treatment was needed. Nasal abnormalities were also noted. Eighty-three percent of the patients with an opioid overdose responded to intranasal naloxone, with an average response time of 3.4 minutes. One patient responded to IV naloxone and not to IN naloxone alone. Sixty-four percent of the naloxone responders did not require an IV placement. This was the first paper demonstrating use of this delivery route in clinical practice.
Barton published a final report of the aforementioned study. In this analysis of 95 patients, response rates to treatment remained similar [59]. That is, 83% of naloxone responders responded to intranasal naloxone. Seven (16%) of the intranasal responders required additional doses of IV naloxone. None of the “naloxone responders” were reported to have severe withdrawal reactions from either IV or IN naloxone. Nasal abnormalities (epistaxis, mucus, trauma, or septal abnormalities) were noted in 5 patients (of 9) who did not respond to IN naloxone. None of the IN naloxone responders had any nasal abnormality noted by paramedics.
The mean time from drug administration to clinical response was slightly longer with intranasal (4.2 minutes) as compared to intravenous administration (3.7 minutes); the medians were not different (3.0 minutes for each route). However, because of the time needed to obtain IV access in these patients, the time from first patient contact to time of clinical response is not different between the routes. The median time from arrival to the patient’s side to clinical response was 8 minutes for IN and 10 minutes for IV (means reported as 9.9 and 12.8 minutes, respectively). Thus, the nasal route can be a quick first response while additional intervention and monitoring are conducted by rescue personnel.
A randomized trial comparing 2 mg intranasal to 2 mg intramuscular naloxone was reported by Kelly in 2005 [22]. One hundred fifty-five patients (71 IM and 84 IN) requiring pre-hospital treatment for suspected overdose received 2 mg/5 mL naloxone by one of the routes of administration. Sixty-three percent of the IN patients had 10 or more spontaneous respirations within 8 minutes of treatment as compared to 82% of the intramuscular naloxone treated patients. Additional rescue naloxone was needed in 13% of IM patients and 26% of IN patients (p=NS). The time to achieve a Glasgow Coma Scale (GCS) greater than 11 was not significantly different between groups. There were no major adverse events in either group, although the intramuscular group more commonly experienced withdrawal symptoms, especially agitation and/or irritation. The authors conclude that IN naloxone was sufficient to reverse opioid toxicity. Although the IN response rate was not as great as after IM administration, the data and study design demonstrate the importance of dose and endpoint selection. Moreover, the authors state that the IN response rate will likely improve if IN naloxone exposure were to be increased through optimization of intranasal delivery product design. The IN solution used in this experiment was 10 fold more dilute than what would be typically formulated for an IN product.
In a follow-up study designed to test a higher concentration of naloxone, Kerr enrolled 172 suspected heroin overdose patients in a randomized, controlled, open-label trial [21]. Patients received 2 mg/mL naloxone either IM or IN (0.5 mL/naris) in the pre-hospital setting. The primary outcome measure was the proportion of patients with an adequate response within 10 minutes of naloxone administration. (Response was defined as respiration rate (RR) ≥10/min and/or GCS ≥13.) Patients receiving a supplementary dose were automatically classified as not achieving adequate response within 10 minutes. Outcomes are shown in Table 4. In general the responses are quite parallel comparing IM to IN naloxone for clinical outcomes with one exception. More patients receiving IN naloxone required a second dose. However, this result could also be a function of using a dilute naloxone solution for nasal administration - the functional dose administered was less than provided due to solution run away.
Table 4.
Comparison of outcomes for patients treated by intranasal (IN) or intramuscular (IM) naloxone [21]
| Outcome | IN (83) n (%) |
IM (89) n (%) |
|---|---|---|
| Adequate response in ≤10 min | 60 (72.3) | 69 (77.5) |
| Rescue naloxone for inadequate response |
15 (18.1) | 4 (4.5) |
| Mean time to response (min)* | 8.0 | 7.9 |
| Hospitalization | 24 (28.9) | 23 (25.8) |
In another study, Robertson reported a retrospective review comparing intravenous naloxone to intranasal naloxone in the pre-hospital setting in the San Francisco EMS service area [23, 24]. The study was done to assess the implementation of the IN protocol as it was being employed. The IN protocol was to give 1 mg/mL/nostril (2 mg total); if no adequate response was observed in 10 minutes, then 1 mg naloxone was to be administered by slow IV push. The principal variables reported were time from medication to patient response and time from patient contact to clinical response in patients with suspected opioid overdose. Charts of 154 patients were reviewed; 104 received IV and 50 received IN treatment. Clinical response to naloxone was reported to be the same between groups: “positive” clinical response was seen in 56% of the patients treated with IV naloxone and 66% in patients treated with IN naloxone. The mean time from drug administration to clinical response was longer for IN (12.9 minutes) versus IV (8.1 minutes) administration. However, the time from patient contact to clinical response was no different (20.3 versus 20.7 minutes). More of the IN patients received a second dose of naloxone (34% of IN patients versus 18% of IV patients). The authors conclude that IN naloxone is a viable alternative rescue treatment given the hazards associated with obtaining IV access in this patient population.
Safety considerations for intranasal naloxone
Patients with severe central nervous system and respiratory depression related to opioid overdose are critically ill and need emergency resuscitation. Adverse consequences of hypoxia and hypercarbia become relevant in a matter of minutes. Administration of the antidote naloxone provides a dose-dependent reversal of the opioid effects. Rapid intravenous administration, while effective in reversal of narcosis, can result in a rapid emergence with possible agitation and violence. Rare cases of seizures, hypertension and cardiac arrest have been reported. It is difficult to separate out opioid overdose effects, concurrent co-intoxicant effects (benzodiazepines, ethanol, etc.) from naloxone effects, from the underlying hypoxia/hypercarbia and subsequent reversal. There is a suggestion that lower initial doses and or administration routes with a slower onset might minimize emergence reactions [60, 61].
Of the reports describing the response to naloxone delivered nasally, only two studies described adverse events seen in detail. Kelly conducted a prospective, randomized trial comparing 2 mg IM naloxone to 2 mg/5mL IN naloxone given by a mucosal atomizer [22]. One hundred eighty-two patients were enrolled, of whom 155 were evaluable. The patients averaged 28-30 years in age (range 13-57) and 72% were male. Patients who received IM naloxone responded faster than the IN group with respect to time until respirations >10/minute (6 minutes to response for IM versus 8 minutes to response for IN, p=0.006). Time to GCS greater than 11 was not significantly different. In the IM group, 13% of patients needed “rescue” naloxone, versus 26% in the IN group. Note the high volume (5mL) used to deliver naloxone intranasally. There were no major adverse events in either group. Adverse events listed as mild include nausea and vomiting and agitation/irritation.
In a follow-up to the Kelly study, Kerr compared safety and effectiveness of a specially-prepared concentrated naloxone formulation (2 mg/mL) given via the IN versus IM routes in a randomized, controlled, open-label trial [21]. A total of 172 patients suspected of heroin overdose were treated by emergency medical personnel and enrolled into the study: 83 received 1 mg/0.5 mL into each nostril (2 mg total) and 89 patients received 2 mg/mL IM. Seventy-four percent of the patients were male, and the average age was 31. The adverse events seen were similar between the two groups and were generally mild in nature and included agitation and nausea and vomiting.
In general patients receiving naloxone will experience some degree of withdrawal symptoms [14-16, 53, 54, 60, 61]. Unlike withdrawal symptoms precipitated by withdrawal of other agents, opioid withdrawal is not life-threatening. Withdrawal symptoms induced by naloxone administration tend to dissipate in a period of 30-60 minutes due to the relatively short half-life of naloxone. Due to naloxone’s high metabolic clearance and the fact that most opioids have a longer persistence in the blood stream, the symptoms of withdrawal dissipate, and in about 15-20% of cases, administration of a repeat dose of naloxone may become necessary if overt toxicity such as central nervous system and respiratory depression recur. Repeat dosing may also be more likely for patients receiving methadone and buprenorphine due to their long half-life and extended-release opioid preparations and transdermal patches due to the large depot of medication in the dosage form.
Take-home prescription naloxone
Large metropolitan cities that have significant injection-based drug abuse, primarily heroin, adopted pilot studies in the 1990s to determine if addicts could be trained to rescue other individuals that may be experiencing an overdose. A 2001 review by Baca provides a summary of evidence to that point [26]. The basic premise was the needle-exchange programs commonly have repeated access to addicts. Also, it was generally understood that abuse occurs in small groups of individuals and that a fellow addict is likely to be the observer and potential first-responder to a person overcome by the effects of heroin and in need of resuscitation. Initial programs developed a medical protocol in which naloxone injection was prescribed to an addict and was trained on overdose recognition and treatment. A simple kit was provided to the addict that contained naloxone injection along with a needle or nasal atomizer adaptor, a rescue breathing mask and a message to call 911. The concept was simple, give naloxone, rescue breathe if apneic, and call for EMS [62-64] .
A pilot study in which pairs of San Francisco based heroin users were trained to recognize overdose and complete the three step process of naloxone administration, rescue breathe and call 911 for help [65]. Twenty-four pairs were enrolled and followed for one year after being provided training and a naloxone rescue kit. Twenty witnessed overdoses were reported and the rescue methods employed. All subjects survived the overdose incident.
In 2006 Maxwell and the Chicago Recovery Alliance report their multi-year experience designed similarly to the San Francisco pilot study. Over several years the Alliance distributed over 3500 vials of naloxone injection to addicts. The study reports 319 incidents of peer reversals. During the study period the Cook County Medical Examiner reports a 20 % decrease in opioid deaths in the first year and an additional 10 % reduction for each of the second and third years of implementation.
Similar programs have been described for Boston, New York City, Baltimore and the state of New Mexico. Sporer goes further to explain how to establish medical programs for heroin overdose prevention in other locales [65]. Green in 2008 has published an evaluation of six different naloxone training and distribution programs in the U.S. [66]. Strang, in 2008, and subsequently Sherman, presented an additional critical consideration for training on intervention and preventing overdoses [67]. It was a simple notion that family caregivers, meaning a spouse, loved one, girl or boy friend, or any family member in close contact with an addict should be trained on recognition of suspected overdose and what to do. A family member of an addict is likely the first responder and can provide rescue until arrival of emergency medical services [68].
Doe-Simkins described the Boston experience with intranasal delivery of naloxone injection and training of addicts on drug overdose prevention [13]. The article goes further to outline the regulatory and legal barriers that must be overcome to establish a harm reduction program. The most recent summary of naloxone expanded access is provided by Kim [69]. The Kim article provides a global overview of the opioid overdose epidemic, the nature of programs in existence, and how additional considerations of FDA regulatory status, including developing and approving a naloxone product designed for nasal delivery, or over-the-counter status for naloxone, could help expand access.
Project Lazarus in the U.S. state of North Carolina attempts to address the prescription opioid overdose in a rural region of the state [70]. The organizers address the need for overdose prevention education and training for both prescribers and patients - in particular patients who a prescribed high potency, high-dose opioids. Moreover the program has identified many high-risk clinical scenarios that warrant education, training, and most of all, a prescription for take-home naloxone. A new notion of “co-prescribing” has been introduced such that any high risk patient and their close relations should receive training on overdose recognition and access to naloxone through a prescription. Thus, when attending to their pharmacy, the patient will receive the analgesic medication along with the antidote and instructions for use.
The principles espoused by overdose prevention programs are very similar to those for emergency administration of epinephrine or glucagon for anaphylaxis or hypoglycemia from insulin or antihyperglycemic overdose [70]. It is generally recognized in public health and law that a citizen may administer these antidotes without criminal or civil liability in a legitimate attempt to save another person’s life. Many locales are considering or have passed legislation permitting naloxone administration from one layperson to another [13, 68, 70].
Data from the practice of peer to peer administration are somewhat sparse, at least in terms of well-controlled research. However, the centers providing such services report hundreds of thousands of naloxone doses dispensed and thousands of reversals without apparent serious consequences. Leaders of these programs now assert that broader distribution is so safe that over-the-counter regulatory status should be considered or that a new product be developed and researched with an over-the-counter marketing in mind [69].
Gaps in knowledge for naloxone nasal spray
As stated previously, there are no data describing a highly concentrated naloxone nasal spray formulation integrated with an appropriate nasal spray device. The formulation would have to satisfy general formulationrequirements, be physically, chemically and microbiologically stable, and compatible with the delivery system. The delivery system must contemplate a wide array of clinical circumstances including standard medical use and use under austere conditions by a lay person. The biopharmaceutics of such a formulation must be determined and applied to select a dose that is clinically relevant and comparable to routes and doses used in clinical medicine today. Clinical studies under various conditions of use may be necessary for premarketing and or post-marketing studies. The ultimate goal of reducing opioid overdose death rates must be demonstrated for a cost-effectiveness determination.
Conclusion
Opioid overdose is a world-wide public health crisis regardless of whether heroin or prescription medications are involved. Naloxone is a well-known antidote with decades of clinical experience as an injection-based pharmaceutical. Alternative routes of delivery, removing the needle from administration, appear to be an unmet medical need. Paramedics and public health workers have demonstrated that intranasal administration of naloxone injection reverses narcosis - however no naloxone nasal spray product is currently available. While no pharmacokinetic data has been generated with a naloxone solution prepared/formulated specifically for intranasal administration, the chemistry profile and animal studies suggest it should have significant bioavailability as a nasal solution. Administration of a naloxone powder intranasally to humans demonstrated significant absorption in blood level ranges known to be pharmacologically active. Administration of naloxone injection intranasally by EMS workers appears to be a successful practice in reversing suspected opioid overdose in the field. Chemical relatives to naloxone, including naltrexone, hydromorphone and butorphanol, have all been administered to humans intranasally at similar doses and produce systemically-active blood concentrations without producing clinically significant acute local effects in the upper airway. Studies of naloxone injection in normal volunteers show it is well tolerated at doses used clinically. Studies of injectable naloxone given intranasally also show it to be well tolerated in normal volunteers at clinical doses. The available data suggest the development and marketing of a naloxone nasal spray is highly feasible and may satisfy an emergency medicine and public health unmet medical need.
Table 1.
Chemical properties of naloxone, naltrexone, hydromorphone and butorhpanol [36]
| Drug Name | Molecular Weight | pKa | Log P |
|---|---|---|---|
| Naloxone | 327 | 7.9 | 2 |
| Naltrexone | 341 | 8.1 | 1.9 |
| Hydromorphone | 285 | 8.5 | 1.8 |
| Butorphanol Tartrate |
327 | 8.6 | 3.77 |
Acknowledgements
This work was supported in part by an STTR grant (NIDA DA 4R42DA030001-02) from the National Institute on Drug Abuse.
References
- 1.Warner M, Chen LH, Makuc DM, Anderson RN, Miniño AM. NCHS data brief. 81. National Center for Health Statistics; Hyattsville, MD: 2011. Drug poisoning deaths in the United States; pp. 1980–2008. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention, National Center for Health Statistics Deaths: preliminary data for 2009. National Vital Statistics Reports. 2011;59:17–20. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention, Morbidity and Mortality Weekly Report Vital Signs: Overdoses of prescription opioid pain relievers - United States, 199-2008. 2011 Nov 1;60:1–6. [PubMed] [Google Scholar]
- 4.Centers of Disease Control and Prevention, Morbidity and Mortality Weekly Report CDC Grand Rounds: Prescription drug overdoses - a U.S. epidemic. 2012 Jan 13;61(1):10–14. [PubMed] [Google Scholar]
- 5.Coben JH, Davis SM, Furbee PM, et al. Hospitalizations for poisoning by prescription opioids, sedatives, and tranquilizers. Am J Prev Med. 2010 May;38(5):517–524. doi: 10.1016/j.amepre.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 6. [Accessed October, 2009];Dawn Report - Emergency department visits involving nonmedical use of selected pharmaceuticals. 2006 R 23 http://DAWNinfo.samsha.gov. [Google Scholar]
- 7.Milloy MJ, Wood E, Reading C, Kane D, Montaner J, Kerr T. Elevated overdose mortality rates among first nations individuals in a Canadian setting: a population-based analysis. Addiction. 2010;105:1962–70. doi: 10.1111/j.1360-0443.2010.03077.x. [DOI] [PubMed] [Google Scholar]
- 8.Strang J, Manning V, Mayet S, et al. Overdose training and take-home naloxone for opiate users: prospective cohort study of impact on knowledge and attitudes and subsequent management of overdoses. Addiction. 2008;103:1648–57. doi: 10.1111/j.1360-0443.2008.02314.x. [DOI] [PubMed] [Google Scholar]
- 9.Preti A, Miotto P, De Coppi M. Deaths by unintentional illicit drug overdose in Italy, 1984-2000. Drug Alcohol Depend. 2002;66:275–82. doi: 10.1016/s0376-8716(01)00207-1. [DOI] [PubMed] [Google Scholar]
- 10.Coffin P. Overdose: A major cause of preventable death in central and eastern Europe and central Asia: Recommendations and overview of the situation in Latvia, Kyrgyzstan, Romania, Russia and Tajikistan. Eurasian Harm Reduction Network (EHRN); Vilnius: Aug, 2008. [Google Scholar]
- 11.Quan VM, Minh NL, Ha TV, et al. Mortality and HIV transmission among male Vietnamese injection drug users. Addiction. 2011;106:583–9. doi: 10.1111/j.1360-0443.2010.03175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall WD, Degenhardt LJ, Lynskey MT. Opioid overdose mortality in Australia, 1964-1997: birth-cohort trends. Med J Aust. 1999;171:34–7. doi: 10.5694/j.1326-5377.1999.tb123495.x. [DOI] [PubMed] [Google Scholar]
- 13.Doe-Simkins M, Walley AY, Epstein A, Moyer P. Saved by the nose: bystander-administered intranasal naloxone hydrochloride for opioid overdose. Am J Public Health. 2009;99:788–91. doi: 10.2105/AJPH.2008.146647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.AHFS Drug Information Naloxone Hydrochloride monograph. American Society of Health System Pharmacists. 2009:2251–54. [Google Scholar]
- 15.International Medication Systems, Limited Naloxone hydrochloride injection prescribing information. 2001 [Google Scholar]
- 16.Hospira, Inc. Naloxone hydrochloride injection prescribing information. 2006 Available from: http://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=2668.
- 17.Sporer KA, Kral AH. Out of hospital treatment of opioid overdoses in an urban setting. Acad Emerg Med July. 1996;3(7):660–667. doi: 10.1111/j.1553-2712.1996.tb03487.x. [DOI] [PubMed] [Google Scholar]
- 18.Belz D, Lieb J, Rea T, Eisenberg MS. Naloxone use in a tiered response emergency medical services system. 2006;10(4):468–471. doi: 10.1080/10903120600885134. [DOI] [PubMed] [Google Scholar]
- 19.Barton ED, Ramos J, Colwell C, Benson J, Baily J, Dunn W. Intranasal administration of naloxone by paramedics. 2002 Jan-Mar;:54. doi: 10.1080/10903120290938797. [DOI] [PubMed] [Google Scholar]
- 20.Barton ED, Colwell CB, Wolfe T, et al. Efficacy of intranasal naloxone as a needleless alternative for treatment of opioid overdose in the pre-hospital setting. J of Emerg Med. 2005;29(3):265–271. doi: 10.1016/j.jemermed.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Kerr D, Dietze P, Kelly A. Intranasal naloxone for the treatment of suspected heroin overdose. Addiction. 2008;103:379–386. doi: 10.1111/j.1360-0443.2007.02097.x. [DOI] [PubMed] [Google Scholar]
- 22.Kelly AM, Kerr D, Dietze P, Patrick I, Walker T, Koutsogiannis Z. Randomized trial of intranasal versus intramuscular naloxone in prehospital treatment of suspected opioid overdose. MJA. 2005;182(1):24–27. doi: 10.5694/j.1326-5377.2005.tb06550.x. [DOI] [PubMed] [Google Scholar]
- 23.Robertson GW, Stroh G, Shalit M. Intranasal vs intravenous naloxone for prehospital narcotic overdose. 2005: Acad Emerg Med. 2005;12(5):166. doi: 10.1080/10903120903144866. Suppl 1. [DOI] [PubMed] [Google Scholar]
- 24.Robertson TM, Hendey GW, Stroh G, Shalit M. Intranasal naloxone is a viable alternative to intravenous naloxone for prehospital narcotic overdose. PreHospital Emergency Care. 2009;13(4):512–515. doi: 10.1080/10903120903144866. [DOI] [PubMed] [Google Scholar]
- 25.Merlin MA, Saybolt M, Kapitanyan R, Alter SM, Jeges J, Lui J, et al. Intranasal naloxone delivery is an alternative to intravenous naloxone for opioid overdoses. Am J Emerg Med. 2010;28:296–303. doi: 10.1016/j.ajem.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Baca CT, Grant KJ. Take-home naloxone to reduce heroin death. Addiction. 2001;100:1823–1831. doi: 10.1111/j.1360-0443.2005.01259.x. [DOI] [PubMed] [Google Scholar]
- 27.Seal KH, Thawley R, Gee L, et al. Naloxone distribution and cardiopulmonary resuscitation training for injection drug users to prevent heroin overdose death: A pilot intervention study. Journal of Urban Health, Bulletin of the New York Academic of Medicine. 2005;82(02):303–311. doi: 10.1093/jurban/jti053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maxwell S, Bigg D, Stanczykiewicz K, Carlberg S. Prescribing naloxone to actively injection heroin users: a program to reduce heroin overdose death. J Addictive Diseases. 2006;25(3):89–96. doi: 10.1300/J069v25n03_11. [DOI] [PubMed] [Google Scholar]
- 29.Piper TM, Rudenstine S, Stancliff S, et al. Overdose prevention for injection drug users: Lessons learned from naloxone training and distribution programs in New York City. Harm Reduction Journal. 2007;4(3):1–8. doi: 10.1186/1477-7517-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sporer KA, Kral AH. Prescription naloxone: A novel approach to heroin overdose prevention. Ann Emerg Med. 2007;49(2):172–177. doi: 10.1016/j.annemergmed.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 31.Green TC, Heimer R, Grau LE. Distinguishing signs of opioid overdose and indication for naloxone: an evaluation of six overdose training and distribution programs in the US. Addiction. 2008:1–11. doi: 10.1111/j.1360-0443.2008.02182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strang J, Manning V, Mayet S, et al. Family carers and the prevention of heroin overdose deaths: Unmet training need and overlooked intervention opportunity of resuscitation training and supply of naloxone. Drug Education, prevention and Policy. 2008;15(2):211–218. [Google Scholar]
- 33.Sherman SG, Gann DS, Tobin KE, et al. “The life they same may be mine”: Diffusion of overdose prevention information from a city sponsored programme. Int J Drug Policy. 2009;20:137–142. doi: 10.1016/j.drugpo.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Advisory Council on Misuse of Drugs [Accessed June 1, 2012];Consideration of naloxone. 2012 May; http://www.homeoffice.gov.uk/publications/agencies-public-bodies/acmd1/consideration-of-naloxone?view=Binary.
- 35.Constantino HR, Illum L, Brandt G, et al. Intl J Pharmaceutics. 2007;337:1–24. doi: 10.1016/j.ijpharm.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 36.Grassin-Delyle S, Buenestado A, Naline E, et al. Intranasal drug delivery: An efficient and non-invasive route for systemic administration. Pharmacol THer. 2012;134:366–379. doi: 10.1016/j.pharmthera.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Illum L. Nasal clearance in health and disease. J Aerosol Med. 2006;19:92–99. doi: 10.1089/jam.2006.19.92. [DOI] [PubMed] [Google Scholar]
- 38.Van den Donk HJ, van den Huervel AG, Zuidema J, Merkus FW. The effects of nasal drops and their additives on human nasal mucociliary clearance. Rhinology. 1982;20:127–137. [PubMed] [Google Scholar]
- 39.Merkus P, Ebbens FA, Muller B, Fokkens WJ. Influence of anatomy and head position on intranasal drug deposition. Eur Arch Otorhinolaryngol. 2006;263:827–832. doi: 10.1007/s00405-006-0071-5. [DOI] [PubMed] [Google Scholar]
- 40.Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci. 2000;11:1–18. doi: 10.1016/s0928-0987(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 41.Illum L. Is nose to brain transport of drugs a reality? JPP. 2004;56:3–17. doi: 10.1211/0022357022539. [DOI] [PubMed] [Google Scholar]
- 42.Merkus F, van den Berg MP. Can nasal drug delivery bypass the blood-brain barrier? Questioning the direct transport theory. Drugs. 2007;8:133–144. doi: 10.2165/00126839-200708030-00001. [DOI] [PubMed] [Google Scholar]
- 43.Davis GA, Rudy AC, Archer SA, Wermeling DP, McNamara PJ. Effect of fluticasone propionate nasal spray on bioavailability of hydromorphone hydrochloride in patients with allergic rhinitis. Pharmacotherapy. 2004;24:26–32. doi: 10.1592/phco.24.1.26.34810. [DOI] [PubMed] [Google Scholar]
- 44.Davis GA, Rudy AC, Archer SM, Wermeling DP, McNamara PJ. Bioavailability and pharmacokinetics of intranasal hydromorphone in patients experiencing vasomotor rhinitis. Clin Drug Invest. 2004;24:1–7. doi: 10.2165/00044011-200424110-00002. [DOI] [PubMed] [Google Scholar]
- 45.Veldhorst-Janssen NML, Fiddelers AAA, van der Kuy PHM, et al. A review of the clinical pharmacokinetics of opioids, benzodiazepines and antimigraine drugs delivered intranasally. Clin Ther. 2009 Nov 12;31:2954–2987. doi: 10.1016/j.clinthera.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 46.Holsti M, Sill B, Firth S, et al. Prehospital intranasal midazolam for the treatment of pediatric seizures. Ped Emerg Care. 2007;23:148–153. doi: 10.1097/PEC.0b013e3180328c92. [DOI] [PubMed] [Google Scholar]
- 47.Wermeling DP, Clinch T, Rudy AC, et al. A multicenter, open label, exploratory dose-ranging trial of hydromorphone for managing acute pain from trauma. The J of Pain. 2010;11(1):24–31. doi: 10.1016/j.jpain.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Rudy AC, Coda BA, Wermeling DP. A multiple dose phase 1 study of intranasal hydromorphone hydrochloride in healthy volunteers. Anesth Analg. 2004;99:1379–86. doi: 10.1213/01.ANE.0000132927.47528.8B. [DOI] [PubMed] [Google Scholar]
- 49.Davis GA, Rudy AC, Archer SM, Wermeling DP. Pharmacokinetics of butorphanol tartrate administered from single-dose intranasal sprayer. AM J Health-Syst Pharm. 2004;61:261–266. doi: 10.1093/ajhp/61.3.261. [DOI] [PubMed] [Google Scholar]
- 50.Illum L. Nasal drug delivery - recent developments and future prospects. J Cont Rel. 2012 Jan 24; doi: 10.1016/j.jconrel.2012.01.024. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 51.Goodman and Gilman’s . The Pharmacologic Basis of Therapeutics. 10th edition McGraw Hill; New York, NY: pp. 569–620. [Google Scholar]
- 52.Hussain A, Kimura R, Huang C-H, Kashihara T. Nasal absorption of naloxone and buprenorphine in rats. Int J Pharm. 1984;21:233–7. [Google Scholar]
- 53.Dowling J, Isbister GK, Kirkpatrick CM, Naidoo D, Graudins A. Population pharmacokinetics of intravenous, intramuscular, and intranasal naloxone in human volunteers. Ther Drug Monit. 2008;30:490–6. doi: 10.1097/FTD.0b013e3181816214. [DOI] [PubMed] [Google Scholar]
- 54.Middleton LS, Nuzzo PA, Lofwall MR, Moody DE, Walsh SL. The pharmacodynamic and pharmacokinetic profile of intranasal crushed buprenorphine and buprenorphine/naloxone tablets in opioid abusers. Addiction. 2011;106:1460–73. doi: 10.1111/j.1360-0443.2011.03424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loimer N, Hofmann P, Chaudhry HR. Nasal administration of naloxone for detection of opiate dependence. J Psychiat Res. 1992;26:39–43. doi: 10.1016/0022-3956(92)90014-f. [DOI] [PubMed] [Google Scholar]
- 56.Loimer N, Hofmann P, Chaudhry HR. Nasal administration of naloxone is as effective as the intravenous route in opiate addicts. Int J Addict. 1994;29:819–27. doi: 10.3109/10826089409047912. [DOI] [PubMed] [Google Scholar]
- 57.Wanger K, Brough L, Macmillan I, Goulding J, MacPhail I, Christenson JM. Intravenous vs subcutaneous naloxone for out-of-hospital management of presumed opioid overdose. Acad Emerg Med. 1998;5:293–9. doi: 10.1111/j.1553-2712.1998.tb02707.x. [DOI] [PubMed] [Google Scholar]
- 58.Barton ED, Colwell CB, Wolfe T, Fosnocht D, Gravitz C, Bryan T, et al. Efficacy of intranasal naloxone as a needleless alternative for treatment of opioid overdose in the prehospital setting. J Emerg Med. 2005;29:265–71. doi: 10.1016/j.jemermed.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 59.Barton ED, Ramos J, Colwell C, Benson J, Baily J, Dunn W. Intranasal administration of naloxone by paramedics. Prehosp Emerg Care. 2002;6:54–8. doi: 10.1080/10903120290938797. [DOI] [PubMed] [Google Scholar]
- 60.Buajordet I, Næss A-C, Jacobsen D, Brørs O. Adverse events after naloxone treatment of episodes of suspected acute opioid overdose. Eur J Emerg Med. 2004;11:19–23. doi: 10.1097/00063110-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 61.Osterwalder JJ. Naloxone - for intoxications with intravenous heroin and heroin mixtures - harmless of hazardous? A prospective clinical study. Clin Toxicol. 1996;34:409–16. doi: 10.3109/15563659609013811. [DOI] [PubMed] [Google Scholar]
- 62.Seal KH, Thawley R, Gee L, et al. Naloxone distribution and cardiopulmonary resuscitation training for injection drug users to prevent heroin overdose death: A pilot intervention study. Journal of Urban Health, Bulletin of the New York Academic of Medicine. 2005;82(02):303–311. doi: 10.1093/jurban/jti053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maxwell S, Bigg D, Stanczykiewicz K, Carlberg S. Prescribing naloxone to actively injection heroin users: a program to reduce heroin overdose death. J Addictive Diseases. 2006;25(3):89–96. doi: 10.1300/J069v25n03_11. [DOI] [PubMed] [Google Scholar]
- 64.Piper TM, Rudenstine S, Stancliff S, et al. Overdose prevention for injection drug users: Lessons learned from naloxone training and distribution programs in New York City. Harm Reduction Journal. 2007;4(3):1–8. doi: 10.1186/1477-7517-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sporer KA, Kral AH. Prescription naloxone: A novel approach to heroin overdose prevention. Ann Emerg Med. 2007;49(2):172–177. doi: 10.1016/j.annemergmed.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 66.Green TC, Heimer R, Grau LE. Distinguishing signs of opioid overdose and indication for naloxone: an evaluation of six overdose training and distribution programs in the US. Addiction. 2008:1–11. doi: 10.1111/j.1360-0443.2008.02182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Strang J, Manning V, Mayet S, et al. Family carers and the prevention of heroin overdose deaths: Unmet training need and overlooked intervention opportunity of resuscitation training and supply of naloxone. Drug Education, prevention and Policy. 2008;15(2):211–218. [Google Scholar]
- 68.Sherman SG, Gann DS, Tobin KE, et al. “The life they same may be mine”: Diffusion of overdose prevention information from a city sponsored programme. Int J Drug Policy. 2009;20:137–142. doi: 10.1016/j.drugpo.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 69.Kim D, Irwin KS, Khoshknod K. Expanded Access to Naloxone:Options for Critical Response to the Epidemic of Opioid Overdose and Mortality. Am J Public Health March. 2009;99:402–407. doi: 10.2105/AJPH.2008.136937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. [Accessed June 1, 2012];Project Lazarus. www.projectlazarus.org.
- 71.Leavitt SB. Intranasal naloxone for at-home opioid rescue. 2010 Practical Pain Management. 2010 Oct; [Google Scholar]