The DNA-binding domain of the response regulator SaeR was crystallized. Determination of its structure will reveal the residues that are critical for protein–DNA recognition.
Keywords: two-component systems, SaeR, Staphylococcus epidermidis
Abstract
SaeR is the response regulator of the SaeRS two-component signal transduction system, which is involved in regulating bacterial autolysis and biofilm formation. SaeR comprises an N-terminal receiver domain and a C-terminal effector domain. The effector domain possesses DNA-binding and transactivation functions. Here, the effector domain of SaeR from Staphylococcus epidermidis was purified and crystallized using the sitting-drop vapour-diffusion method. The crystals diffracted to a resolution of 2.15 Å and belonged to space group P212121, with unit-cell parameters a = 34.20, b = 53.78, c = 111.66 Å. Determining the structure will provide insights into the mechanisms underlying DNA binding.
1. Introduction
Staphylococcus epidermidis is the most frequent cause of nosocomial infections related to implanted medical devices. The formation of a biofilm on the surface of medical devices is a crucial factor for the pathogenicity of S. epidermidis (McCann et al., 2008 ▶). The resistance of bacteria in biofilms to antibiotics is often under the control of two-component regulatory systems (TCSs; Mikkelsen et al., 2011 ▶). TCSs are widely distributed among bacteria and enable them to adapt to environmental changes. A typical TCS consists of a membrane-associated sensor kinase (SK) and its cognate response regulator (RR). In response to a specific signal, SK autophosphorylates a histidine residue and the phosphate is then transferred to an aspartate residue in the N-terminal domain of the RR. Phosphorylation triggers a conformational change in RR that enables it to interact with the promoters of target genes (Groisman, 2001 ▶).
The S. epidermidis genome encodes 16 or 17 TCSs that have been identified by sequence homology. A previous study of the S. epidermidis SaeRS (SeSaeRS) TCS showed that under anaerobic conditions an SaeR deletion mutant grew more slowly but exhibited slightly higher biofilm-forming activity than the wild-type strain (Lou et al., 2011 ▶; Handke et al., 2007 ▶). SaeR significantly affects the ability of S. aureus to form biofilms and plays a particularly crucial role in the production of multiple virulence factors such as coagulase, α-haemolysin and fibronectin-binding proteins (Jeong et al., 2011 ▶).
SaeR belongs to the OmpR/PhoB subfamily of RRs. Sequence analysis has shown that SaeR contains a receiver domain and an effector domain connected by a flexible linker. The effector domain contains a winged-helix–turn–helix motif that is involved in DNA binding. SaeR recognizes the direct repeat GTTAAN6GTTAA, assuming that two molecules of SaeR bind to target DNA (Sun et al., 2010 ▶). SaeR was chosen for structural studies because of this unique DNA-binding specificity; the protein structure of SaeR has not been elucidated to date. To understand its structure–function relationships, we purified and crystallized SeSaeR and conducted preliminary X-ray analysis of its DNA-binding domain.
2. Materials and methods
2.1. Cloning, expression and purification
A DNA fragment encoding the DNA-binding domain (residues 129–229) of SeSaeR was amplified using standard polymerase chain reaction techniques with the forward primer 5′-GGGAATTCCATATGGAGCAATTAGAATTTGAT-3′ and the reverse primer 5′-CCGCTCGAGTCGGCTCCTTTCAAA-3′. The amplified product was cloned into the plasmid pET21b(+), which directs the expression of a C-terminal His6-tagged protein. The plasmid was used to transform Escherichia coli strain BL21 (DE3) and the cells were grown at 310 K in 1 l Luria–Bertani medium with 50 µg ml−1 ampicillin. Protein expression was induced by adding 1.0 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) when the cells reached an optical density at 600 nm of approximately 1.0. The cells were then grown for an additional 20 h at 293 K. After this treatment, the cultured cells were harvested by centrifugation at 1590g for 30 min at 277 K. The cell pellet was resuspended in buffer A (50 mM Tris–HCl pH 8.0, 500 mM NaCl, 5 mM imidazole) and the cells were disrupted using sonication. The crude lysate was centrifuged at 20 000g at 277 K for 90 min and the resulting supernatant was loaded onto an Ni–NTA His-bind resin column equilibrated with buffer A. Fractions containing the DNA-binding domain of SeSaeR were concentrated and purified using a HiLoad 16/60 Superdex-200 size-exclusion column (GE Healthcare), which was first equilibrated with buffer C [50 mM Tris–HCl pH 8.0, 100 mM NaCl, 5% glycerol, 2 mM tris(2-carboxyethyl)phosphine hydrochloride]. The fractions containing the DNA-binding domain of SaeR were pooled and concentrated to 30 mg ml−1 for crystallization.
2.2. Crystallization
Initial screening for the crystallization conditions using the sitting-drop vapour-diffusion method on 24-well VDX plates (Hampton Research) was performed with commercially available kits (Hampton Research, Emerald BioStructures and Molecular Dimensions). Drops consisting of 1 µl protein solution (30 mg ml−1 in buffer C) and 1 µl reservoir solution were equilibrated against 400 µl reservoir solution at 277 K. Of the 480 conditions screened, the DNA-binding domain of SaeR could be crystallized using Index condition No. 82 [0.1 M bis-tris pH 5.5, 25%(w/v) PEG 3350, 0.2 M MgCl2] and Natrix condition No. 42 (0.05 M Tris–HCl pH 7.5, 0.01 M MgCl2, 5% isopropanol). The conditions were then optimized at 277 K by varying the PEG concentration (15–30%) and the buffer pH (5.0–6.0) using PEG 3350, PEG 4000 and PEG 8000 as precipitants. The effects of adding salt (0.0–400 mM MgCl2) and organic solvent (0.0–15% isopropanol) were also examined. The best crystals were grown using a reservoir solution consisting of 0.1 M bis-tris pH 5.3, 23%(w/v) PEG 3350, 0.2 M MgCl2. The crystals grew as prisms, reaching dimensions of 0.05 × 0.05 × 0.1 mm (Fig. 1 ▶).
Figure 1.
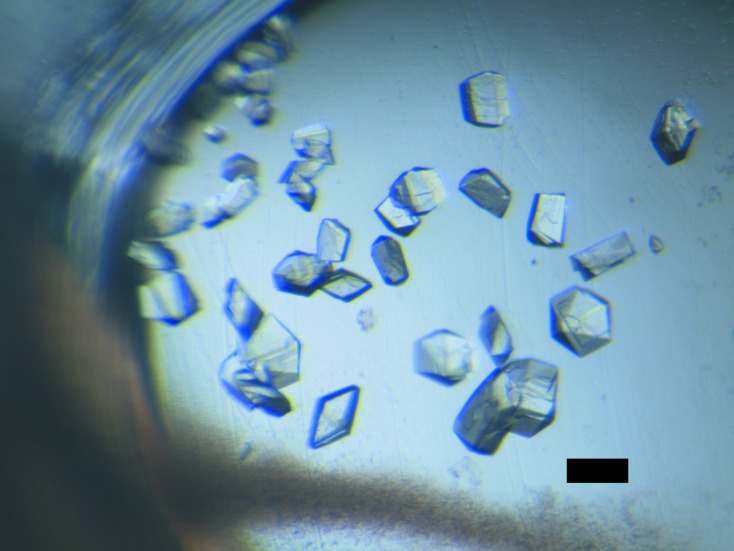
Crystals of the DNA-binding domain of SaeR. The dimensions of the crystal are approximately 0.05 × 0.05 × 0.1 mm. The bar represents a length of 100 µm.
2.3. Data collection
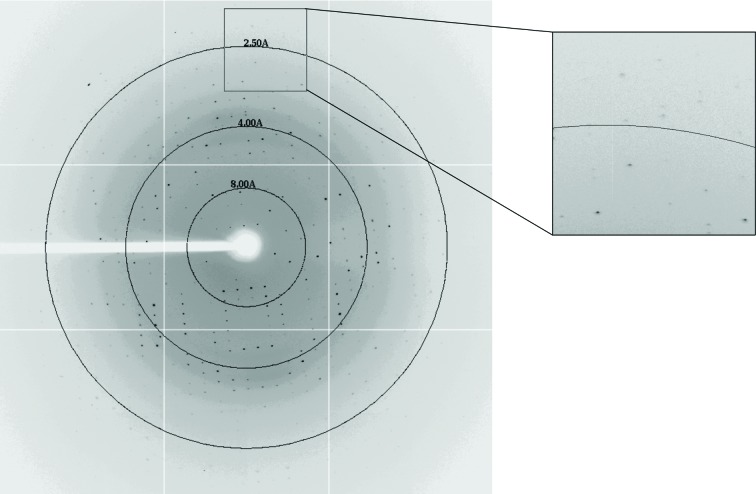
The crystal selected was immersed under optimum crystallization conditions with 15%(v/v) glycerol solution as a cryoprotectant and then flash-cooled in a stream of nitrogen gas cooled to 100 K. X-ray diffraction data were collected at the National Synchrotron Radiation Research Center (NSRRC) BL13B1 in Taiwan. The crystal-to-detector distance was 300 mm, the oscillation range was 1.0° and 180 frames of data were collected. The data were indexed and integrated using the HKL-2000 processing software (Otwinowski & Minor, 1997 ▶). An X-ray diffraction data image is shown in Fig. 2 ▶.
Figure 2.
Diffraction pattern of the DNA-binding domain of SaeR collected on the NSRRC 13B1 beamline.
3. Results and discussion
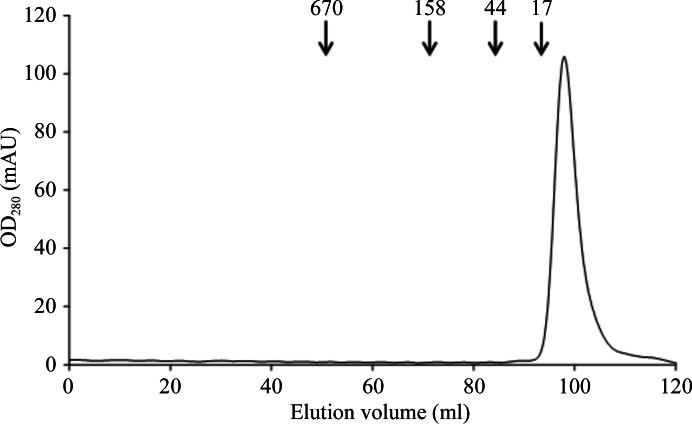
The DNA-binding domain of SeSaeR was overexpressed in E. coli with a yield of ∼30 mg protein per litre of culture after purification. The purified protein contained an extra octapeptide, LEH6, at its C-terminus. Based on the results of gel filtration, the molecular mass of the protein was found to be approximately 13 kDa, corresponding to the predicted molecular mass of the monomer (12 kDa; Fig. 3 ▶).
Figure 3.
Gel-filtration chromatography of the DNA-binding domain of SaeR. The elution volumes of the standard proteins are indicated by arrows and their molecular masses are given in kDa.
The best crystals were obtained using the sitting-drop vapour-diffusion method in a buffer composed of 0.1 M bis-tris pH 5.3, 23%(w/v) PEG 3350, 0.2 M MgCl2. Statistical analysis of the data collected is summarized in Table 1 ▶. The average dimensions of the crystal after 3 weeks were approximately 0.05 × 0.05 × 0.1 mm. Diffraction data were collected to a resolution limit of 2.15 Å (Fig. 2 ▶). The preliminary crystallographic analysis indicated that the crystal belonged to the space group P212121, with unit-cell parameters a = 34.20, b = 53.78, c = 111.66 Å. The initial model was obtained by the molecular-replacement method using AutoMR in PHENIX (Adams et al., 2010 ▶). The coordinate of the structure of the response regulator VicR DNA-binding domain (PDB entry 2hwv; Blanco et al., 2011 ▶) was used as the search model. The rotation and translation functions yielded two clear positions for the model, indicating the presence of two molecules in the asymmetric unit with a V M value of 2.14 Å3 Da−1 (Matthews, 1968 ▶). The Z scores from AutoMR for the rotation and translation functions were 3.8 and 6.0, respectively. Subsequent rigid-body refinement of the model gave an R factor of 50%. The model was automatically rebuilt and refined using AutoBuild in PHENIX (Adams et al., 2010 ▶). After autobuilding, the R factor and R free quickly converged to 29 and 36%, respectively. Studies involving electron-density interpretation and structure refinement are in progress.
Table 1. Data-collection statistics for SaeR crystal.
Values in parentheses are for the outermost shell.
| X-ray wavelength (Å) | 1.0000 |
| Space group | P212121 |
| Unit-cell parameters (Å) | a = 34.20, b = 53.78, c = 111.66 |
| Resolution (Å) | 30.0–2.15 (2.23–2.15) |
| No. of measured reflections | 79346 (7578) |
| No. of unique reflections | 11686 (1131) |
| Multiplicity | 6.8 (6.7) |
| R merge † (%) | 3.1 (15.2) |
| Data completeness (%) | 99.1 (99.8) |
| 〈I/σ(I)〉 | 55.88 (12.68) |
R
merge = 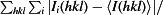
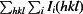 .
.
Acknowledgments
We thank the National Synchrotron Radiation Research Center (NSRRC, Taiwan) for assistance during data collection. We are grateful to the staff of TCX-D900, Technology Commons, College of Life Science and the Center for System Biology, NTU for help with the Art Robbins Instruments Phoenix protein crystallization robot. This work was supported by grants from the National Science Council (NSC99-2313-B-241-001 and NSC100-2313-B-241-006 to YC).
References
- Adams, P. D. et al. (2010). Acta Cryst. D66, 213–221.
- Blanco, A. G., Canals, A., Bernués, J., Solà, M. & Coll, M. (2011). EMBO J. 30, 3776–3785. [DOI] [PMC free article] [PubMed]
- Groisman, E. A. (2001). J. Bacteriol. 183, 1835–1842. [DOI] [PMC free article] [PubMed]
- Handke, L. D., Rogers, K. L., Olson, M. E., Somerville, G. A., Jerrells, T. J., Rupp, M. E., Dunman, P. M. & Fey, P. D. (2007). Infect. Immun. 76, 141–152. [DOI] [PMC free article] [PubMed]
- Jeong, D.-W., Cho, H., Lee, H., Li, C., Garza, J., Fried, M. & Bae, T. (2011). J. Bacteriol. 193, 4672–4684. [DOI] [PMC free article] [PubMed]
- Lou, Q., Zhu, T., Hu, J., Ben, H., Yang, J., Yu, F., Liu, J., Wu, Y., Fischer, A., Francois, P., Schrenzel, J. & Qu, D. (2011). BMC Microbiol. 11, 146. [DOI] [PMC free article] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- McCann, M. T., Gilmore, B. F. & Gorman, S. P. (2008). J. Pharm. Pharmacol. 60, 1551–1571. [DOI] [PubMed]
- Mikkelsen, H., Sivaneson, M. & Filloux, A. (2011). Environ. Microbiol. 13, 1666–1681. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Sun, F., Li, C., Jeong, D., Sohn, C., He, C. & Bae, T. (2010). J. Bacteriol. 192, 2111–2127. [DOI] [PMC free article] [PubMed]