The recombinant tetrathionate hydrolase from the acidophilic chemolithoautotrophic bacterium A. ferrooxidans was refolded for activation, purified and crystallized. Diffraction data were collected to 2.15 Å resolution.
Keywords: tetrathionate hydrolase, Acidithiobacillus ferrooxidans
Abstract
Tetrathionate hydrolase (4THase) from the iron- and sulfur-oxidizing bacterium Acidithiobacillus ferrooxidans catalyses the disproportionate hydrolysis of tetrathionate to elemental sulfur, thiosulfate and sulfate. The gene encoding 4THase (Af-tth) was expressed as inclusion bodies in recombinant Escherichia coli. Recombinant Af-Tth was activated by refolding under acidic conditions and was then purified to homogeneity. The recombinant protein was crystallized in 20 mM glycine buffer pH 10 containing 50 mM sodium chloride and 33%(v/v) PEG 1000 using the hanging-drop vapour-diffusion method. The crystal was a hexagonal cylinder with dimensions of 0.2 × 0.05 × 0.05 mm. X-ray crystallographic analysis showed that the crystal diffracted to 2.15 Å resolution and belongs to space group P31 or P32, with unit-cell parameters a = b = 92.1, c = 232.6 Å.
1. Introduction
Acidithiobacillus ferrooxidans is an acidophilic and a chemolithoautotrophic bacterium capable of oxidizing ferrous iron and various reduced inorganic sulfur compounds (RISCs), including elemental sulfur, thiosulfate and tetrathionate. Owing to its oxidizing abilities, this bacterium has been studied extensively and applied in industrial biomining to recover precious metals such as copper from low-grade ores (Rawlings, 2002 ▶, 2005 ▶; Rawlings & Johnson, 2007 ▶). Although the mechanism of iron oxidation in A. ferrooxidans has been deduced, the mechanism of oxidation of RISCs needs to be established (Quatrini et al., 2009 ▶; Valdes et al., 2008 ▶). In order to elucidate the RISC oxidation pathway, it is necessary to identify the enzymes and the cofactors involved in this oxidation process. A detailed investigation of the reaction mechanisms of these enzymes is also essential to understand the industrial potential of this bacterial species.
Tetrathionate hydrolase (4THase) is one of the unique enzymes in dissimilatory sulfur metabolism in acidophilic bacteria and archaea. This enzyme has been purified from the genus Acidithiobacillus (A. ferrooxidans, A. thiooxidans and A. caldus; de Jong et al., 1997b ▶; Tano et al., 1996 ▶; Bugaytsova & Lindström, 2004 ▶; Rzhepishevska et al., 2007 ▶), Acidiphilium acidophilum (de Jong et al., 1997a ▶) and the thermoacidophilic archaeon Acidianus ambivalens (Protze et al., 2011 ▶). We identified the 4THase gene for the first time in A. ferrooxidans ATCC23270 (Af-tth) by a nucleotide BLAST (Altschul et al., 1997 ▶) search using the N-terminal amino-acid sequence of purified Af-Tth (Kanao et al., 2007 ▶). Prior to this identification, the corresponding gene in this bacterium was recognized as a sulfur-regulated outer membrane protein with unknown function (Buonfiglio et al., 1999 ▶). Af-tth encoded a protein of 499 amino acids with a putative signal peptide (32 amino acids from the N-terminal). Thus, the processed mature protein of Af-Tth consisted of 467 amino acids with a molecular mass of 49 714 Da. The apparent molecular mass of the enzyme purified from A. ferrooxidans was determined to be 93 kDa by gel filtration. This suggested that the native enzyme was a homodimer. The biochemical and physiological properties have previously been reported in detail (Kanao et al., 2007 ▶). 4THase plays an important role in the thiosulfate oxidation pathway with tetrathionate as the intermediate (known as the S4-intermediate pathway) in a dissimilatory sulfur oxidation pathway in A. ferrooxidans (Kelly et al., 1997 ▶). 4THase purified from A. ferrooxidans catalyses the hydrolysis of tetrathionate to generate elemental sulfur, thiosulfate and sulfate. This hydrolysis reaction is thought to result from both enzymatic as well as abiotic reactions since one of the reaction products is highly reactive. It is speculated that sulfate and a chemically unstable compound, disulfane monosulfonic acid (HS2SO3 −), are generated during the hydrolysis of tetrathionate by 4THase. Subsequently, hydrogen sulfide, sulfite and elemental sulfur (S8) are chemically generated from HS2SO3 − (Beard et al., 2011 ▶). Thiosulfate might be generated from hydrogen sulfide and sulfite by abiotic reactions. However, the precise reaction mechanism underlying the action of this unique enzyme has not been completely understood yet.
We have developed a method to obtain the recombinant Af-Tth in an active form from Escherichia coli cells harbouring Af-tth (Kanao et al., 2010 ▶). Here, we describe a method for the crystallization and the preliminary X-ray crystallographic investigations of the recombinant Af-Tth.
2. Materials and methods
2.1. Protein expression, refolding and purification
The pET4TH expression vector was constructed and introduced into E. coli BL21 Star (DE3) cells to obtain recombinant Af-Tth, as described previously (Kanao et al., 2007 ▶). E. coli BL21 Star (DE3) cells harbouring pET4TH were cultured in modified Terrific broth (TB) medium supplemented with 50 µg ml−1 ampicillin at 310 K to an OD660 nm of 1.0. The expression of Af-tth in pET4TH was induced by adding 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) to the culture, followed by incubation at 293 K for 36 h. The recombinant protein was synthesized in inclusion bodies in an inactive form. The inclusion bodies were collected and were washed three times with 100 mM potassium phosphate buffer (KPB) pH 7.0 containing 4%(v/v) Triton X-100. The inclusion bodies were washed again with sterilized distilled water to remove the detergent. The recombinant proteins were solubilized with a 6 M guanidine hydrochloride (Gua–HCl) solution containing 10 mM dithiothreitol (DTT) and subsequently centrifuged at 10 000g for 10 min. The supernatant (1 ml) containing solubilized Af-Tth was dialysed against the refolding buffer (100 ml) at 277 K with gentle stirring for 1 h. A solution consisting of 4 M Gua–HCl, 10 mM β-alanine, 30%(v/v) glycerol, 0.4 M ammonium sulfate, 2 mM DTT was used as the initial refolding buffer. The pH was adjusted to 4.0 with sulfuric acid. After 1 h of dialysis, the concentration of Gua–HCl in the refolding buffer was gradually decreased by pumping the same buffer without Gua–HCl into the refolding buffer using a peristaltic pump (90 s ml−1) to dilute the Gua–HCl solution. When the volume of the refolding buffer reached 200 ml (the Gua–HCl concentration was 2 M at this stage), 100 ml of the refolding buffer was removed. This dilution step was performed four times in total. After the repetition, the concentration of Gua–HCl in the refolding buffer should be diluted to 0.25 M. The refolding buffer was then replaced with 0.1 M β-alanine buffer pH 4.0 containing 0.4 M ammonium sulfate. The recombinant protein solution (1 ml) was dialysed against the buffer (1000 ml) for 3 h with gentle stirring at 277 K. After dialysis, the protein solution was centrifuged at 10 000g for 10 min to remove insoluble proteins. Soluble and active Af-Tth were obtained in the supernatant. The protocol for Af-Tth refolding for its activation has been described in detail previously (Kanao et al., 2010 ▶).
After refolding, ammonium sulfate was slowly added to the enzyme solution to a final concentration of 1.3 M and the solution was gently stirred on ice for 1 h. The resultant solution was centrifuged to remove the precipitated protein. The supernatant containing refolded Af-Tth was applied onto a hydrophobic chromatography column (TOYOPEARL Butyl-600M, Tosoh, Tokyo, Japan) equilibrated with 10 mM β-alanine buffer pH 4.0 containing 1.3 M ammonium sulfate. The protein was eluted using a linear gradient of 1.3–0.0 M ammonium sulfate. The active fractions were concentrated using a Centricon YM-30 concentrator (Merck Millipore, Darmstadt, Germany) and further purified by gel-filtration column chromatography using a TSKgel G3000SW (Tosoh) column equilibrated with 10 mM β-alanine buffer pH 4.0 containing 0.4 M ammonium sulfate. All samples and buffers described above were filtered through a 0.45 µm diameter disc filter and the procedures were performed at 277 K. The 4THase activity of Af-Tth was measured by a modified cyanolysis assay as described previously (Kanao et al., 2007 ▶). The protein concentration of purified Af-Tth was determined by the Bradford method using bovine serum albumin as a standard. The purified Af-Tth was stored at 277 K for crystallization.
2.2. Crystallization and X-ray data collection
Recombinant Af-Tth protein purified to homogeneity was concentrated to 10 mg ml−1 by ultrafiltration for crystallization. Crystallization of Af-Tth was carried out using the hanging-drop vapour-diffusion technique employing a Cryschem crystallization plate and the commercially available Crystal Screen, Crystal Screen 2 (Hampton Research, Aliso Viejo, California, USA) and MemGold (Molecular Dimensions, Suffolk, England) screens. Equal volumes (1.0 µl) of the protein solution and reservoir solution were mixed and incubated at 291 K in a chamber. Small crystals were observed under several conditions in the initial screenings. After optimization of the crystallization procedure, the following protocol was selected: 1 µl Af-Tth solution (10 mg ml−1) was mixed with an equal volume of reservoir solution consisting of 50 mM sodium chloride, 20 mM glycine pH 10, 33%(v/v) PEG 1000. The mixture was suspended over 1 ml reservoir solution in a well at 291 K for 5 d.
The crystals were soaked for a few seconds in reservoir solution containing 10%(v/v) glycerol. Flash-cooled crystals were mounted in a nitrogen stream at 100 K. X-ray diffraction data were collected from the Af-Tth crystal using synchrotron radiation of wavelength 1.0 Å and were recorded on an MX-225HE system (Rayonix, USA) on the BL41XU beamline at SPring-8 (Hyogo, Japan). Data collection was performed with a total oscillation range of 0–180°, a step size of 1.0° and an exposure time of 10 s per frame. The diffraction data were processed and scaled with HKL-2000 (Otwinowski & Minor, 1997 ▶). The molecular-replacement (MR) analyses were performed in the resolution range 10–4 Å using Phaser (McCoy et al., 2007 ▶) and MOLREP (Vagin & Teplyakov, 2010 ▶) in the CCP4 program suite (Winn et al., 2011 ▶).
3. Results and discussion
The recombinant Af-Tth protein was successfully obtained as an active form by refolding and was then purified to homogeneity (Fig. 1 ▶). Crystals of the recombinant Af-Tth with dimensions of 0.2 × 0.05 × 0.05 mm appeared using the hanging-drop method at 291 K after 5 d (Fig. 2 ▶). The crystals diffracted to 2.15 Å resolution at 100 K on the BL41XU beamline at SPring-8. The Af-Tth crystals belonged to space group P31 or P32, with unit-cell parameters a = b = 92.1, c = 232.6 Å. Assuming the presence of two or three Af-Tth dimers in the asymmetric unit, the calculated Matthews coefficient (Matthews, 1968 ▶) was 2.9 or 1.9 Å3 Da−1, corresponding to a solvent content of 57 or 36%, respectively. The data-collection and processing statistics are shown in Table 1 ▶.
Figure 1.
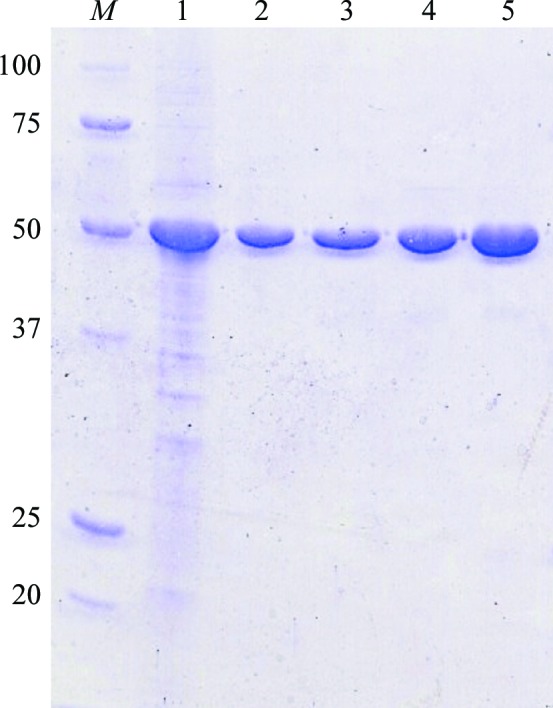
SDS–PAGE of recombinant Af-Tth. Lane 1, insoluble fraction of E. coli BL21 Star (DE3) harbouring pET4TH after induction by IPTG. Lane 2, soluble recombinant Af-Tth after refolding treatment. Lane 3, supernatant of 1.3 M ammonium sulfate treatment. Lanes 4 and 5, the active fractions of TOYOPEARL Butyl-600M hydrophobic chromatography and TSKgel G3000SW gel-filtration chromatography, respectively. Lane M, molecular-mass markers. The molecular masses are indicated on the left in kDa.
Figure 2.
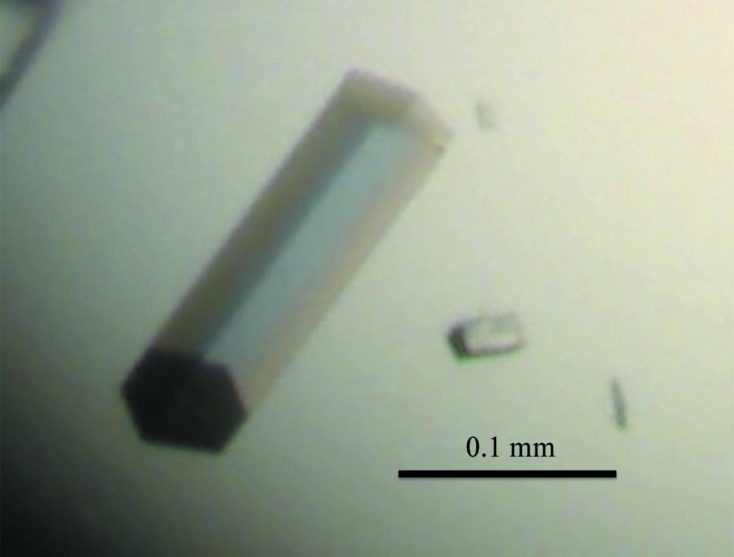
Crystals of recombinant Af-Tth. The Af-Tth crystal was a hexagonal cylinder with dimensions of 0.2 × 0.05 × 0.05 mm.
Table 1. Data-collection statistics.
Values in parentheses are for the outermost resolution shell.
| Space group | P31 or P32 |
| Unit-cell parameters (Å) | a = b = 92.1, c = 232.6 |
| Wavelength (Å) | 1.000 |
| Resolution range (Å) | 46.7–2.15 (2.23–2.15) |
| Completeness (%) | 100 (100) |
| No. of measured reflections | 646775 |
| No. of unique reflections | 116955 (11711) |
| R merge † | 0.095 (0.329) |
| Multiplicity | 5.5 (5.5) |
| 〈I/σ(I)〉 | 17.7 (6.1) |
| Mosaicity (°) | 0.66 |
R
merge = 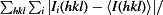
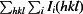 , where Ii(hkl) is the value of the ith intensity measurement and 〈I(hkl)〉 is the mean value of all measurements of I(hkl).
, where Ii(hkl) is the value of the ith intensity measurement and 〈I(hkl)〉 is the mean value of all measurements of I(hkl).
The amino-acid sequence of Af-Tth without signal peptide showed similarity to the pyrroloquinoline quinone (PQQ) domain of a quinoprotein alcohol dehydrogenase (QgdA; PDB entry 1yiq; Toyama et al., 2005 ▶) and a lipoprotein (BamB) in the β-barrel assembly machinery complex (PDB entry 3q7m; Noinaj et al., 2011 ▶) by a protein BLAST search. The similarity and identity of the primary structures between Af-Tth and QgdA were 34.0 and 18.8%, respectively. Af-Tth showed 31.5% sequence similarity and 16.7% sequence identity to BamB. Although the primary structure of Af-Tth showed a relatively low identity (∼20%) to QgdA and BamB, MR analyses using these two search models and two programs (Phaser and MOLREP) gave no significant solution in both possible space groups (P31 and P32) for initial phase determination. The failure of the trials may indicate significant differences in the structures between Af-Tth and PQQ-dependent dehydrogenases. Further analyses such as multiple/single anomalous dispersion (MAD/SAD) methods are required to determine the Af-Tth structure. Because 11 methionine codons not including the start codon were observed in the Af-tth gene, MAD/SAD using selenomethionine-derivatized protein may be possible. Preparation of selenomethionyl Af-Tth protein for MAD/SAD analyses is in progress.
Acknowledgments
The synchrotron-radiation experiments were performed on the BL41XU beamline at SPring-8, Hyogo, Japan. This work was financially supported by the Japan Society for the Promotion of Science (JSPS) and the Japan Oil, Gas and Metals National Corporation (JOGMEC).
References
- Altschul, S. F., Madden, T. L., Schäffer, A. A., Zhang, J., Zhang, Z., Miller, W. & Lipman, D. J. (1997). Nucleic Acids Res. 25, 3389–3402. [DOI] [PMC free article] [PubMed]
- Beard, S., Paradela, A., Albar, J. P. & Jerez, C. A. (2011). Front. Microbiol. 2, 79. [DOI] [PMC free article] [PubMed]
- Bugaytsova, Z. & Lindström, E. B. (2004). Eur. J. Biochem. 271, 272–280. [DOI] [PubMed]
- Buonfiglio, V., Polidoro, M., Soyer, F., Valenti, P. & Shively, J. (1999). J. Biotechnol. 72, 85–93. [DOI] [PubMed]
- de Jong, G. A. H., Hazeu, W., Bos, P. & Kuenen, J. G. (1997a). Eur. J. Biochem. 243, 678–683. [DOI] [PubMed]
- de Jong, G. A. H., Hazeu, W., Bos, P. & Kuenen, J. G. (1997b). Microbiology, 143, 499–504. [DOI] [PubMed]
- Kanao, T., Kamimura, K. & Sugio, T. (2007). J. Biotechnol. 132, 16–22. [DOI] [PubMed]
- Kanao, T., Matsumoto, C., Shiraga, K., Yoshida, K., Takada, J. & Kamimura, K. (2010). FEMS Microbiol. Lett. 309, 43–47. [DOI] [PubMed]
- Kelly, D. P., Shergill, J. K., Lu, W.-P. & Wood, A. P. (1997). Antonie Van Leeuwenhoek, 71, 95–107. [DOI] [PubMed]
- McCoy, A. J., Grosse-Kunstleve, R. W., Adams, P. D., Winn, M. D., Storoni, L. C. & Read, R. J. (2007). J. Appl. Cryst. 40, 658–674. [DOI] [PMC free article] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Noinaj, N., Fairman, J. W. & Buchanan, S. K. (2011). J. Mol. Biol. 407, 248–260. [DOI] [PMC free article] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Protze, J., Müller, F., Lauber, K., Nass, B., Mentele, R., Lottspeich, F. & Kletzin, A. (2011). Front. Microbiol. 2, 68. [DOI] [PMC free article] [PubMed]
- Quatrini, R., Appia-Ayme, C., Denis, Y., Jedlicki, E., Holmes, D. S. & Bonnefoy, V. (2009). BMC Genomics, 10, 394. [DOI] [PMC free article] [PubMed]
- Rawlings, D. E. (2002). Annu. Rev. Microbiol. 56, 65–91. [DOI] [PubMed]
- Rawlings, D. E. (2005). Microb. Cell Fact. 4, 13. [DOI] [PMC free article] [PubMed]
- Rawlings, D. E. & Johnson, D. B. (2007). Microbiology, 153, 315–324. [DOI] [PubMed]
- Rzhepishevska, O. I., Valdés, J., Marcinkeviciene, L., Gallardo, C. A., Meskys, R., Bonnefoy, V., Holmes, D. S. & Dopson, M. (2007). Appl. Environ. Microbiol. 73, 7367–7372. [DOI] [PMC free article] [PubMed]
- Tano, T., Kitaguchi, H., Harada, M., Nagasawa, T. & Sugio, T. (1996). Biosci. Biotechnol. Biochem. 60, 224–227. [DOI] [PubMed]
- Toyama, H., Chen, Z.-W., Fukumoto, M., Adachi, O., Matsushita, K. & Mathews, F. S. (2005). J. Mol. Biol. 352, 91–104. [DOI] [PubMed]
- Vagin, A. & Teplyakov, A. (2010). Acta Cryst. D66, 22–25. [DOI] [PubMed]
- Valdes, J., Pedroso, I., Quatrini, R., Dodson, R. J., Tettelin, H., Blake, R., Eisen, J. A. & Holmes, D. S. (2008). BMC Genomics, 9, 597. [DOI] [PMC free article] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.


