A GH27 arabinopyranosidase from G. stearothermophilus (Abp) has been crystallized in the primitive orthorhombic space group P212121. Full diffraction data sets have been measured for the wild-type enzyme and its D197A catalytic mutant to maximal resolutions of 2.28 and 2.30 Å, respectively, for use in a detailed three-dimensional structural analysis of the Abp protein.
Keywords: Geobacillus stearothermophilus, arabinopyranosidase, glycoside hydrolase, GH27, catalytic mutant
Abstract
Geobacillus stearothermophilus T-6 is a thermophilic soil bacterium that possesses an extensive system for the utilization of hemicellulose. The bacterium produces a small number of endo-acting extracellular enzymes that cleave high-molecular-weight hemicellulolytic polymers into short decorated oligosaccharides, which are further hydrolysed into the respective sugar monomers by a battery of intracellular glycoside hydrolases. One of these intracellular processing enzymes is β-l-arabinopyranosidase (Abp), which is capable of removing β-l-arabinopyranose residues from naturally occurring arabino-polysaccharides. As arabino-polymers constitute a significant part of the hemicellulolytic content of plant biomass, their efficient enzymatic degradation presents an important challenge for many potential biotechnological applications. This aspect has led to an increasing interest in the biochemical characterization and structural analysis of this and related hemicellulases. Abp from G. stearothermophilus T-6 has recently been cloned, overexpressed, purified, biochemically characterized and crystallized in our laboratory, as part of its complete structure–function study. The best crystals obtained for this enzyme belonged to the primitive orthorhombic space group P212121, with average unit-cell parameters a = 107.7, b = 202.2, c = 287.3 Å. Full diffraction data sets to 2.3 Å resolution have been collected for both the wild-type enzyme and its D197A catalytic mutant from flash-cooled crystals at 100 K, using synchrotron radiation. These data are currently being used for a high-resolution three-dimensional structure determination of Abp.
1. Introduction
Arabinan is a branched, pectic polysaccharide consisting of a backbone of α-1,5-linked l-arabinofuranosyl units which are further decorated with α-1,2- and α-1,3-linked arabinofuranosides (Mohnen, 2008 ▶). In the plant cell wall, arabinan is generally linked to rhamnogalacturonan I (RG-I; Gilbert, 2010 ▶). l-Arabinose residues are relatively abundant in plants and are found mainly in arabinan polysaccharides and in other arabinose-containing polysaccharides, such as arabinoxylans and pectic arabinogalactans. The majority of the arabinose units in plants are present in the furanose form (five-membered ring configuration); however, small amounts of the arabinopyranose form (the six-membered ring configuration) are also present at the side-chain terminal ends of some hemicellulolytic polysaccharides such as arabinan and xylan (Mohnen, 2008 ▶).
Geobacillus stearothermophilus T-6 is a Gram-positive thermophilic soil bacterium that possesses an extensive system for the utilization of hemicellulose (Shulami et al., 1999 ▶, 2011 ▶; Tabachnikov & Shoham, 2013 ▶). The bacterium produces a small number of endo-acting extracellular enzymes that cleave the high-molecular-weight polymers into short decorated oligosaccharides. These short oligosaccharides enter the cell via specialized ABC transporters (Rees et al., 2009 ▶) and are further hydrolysed into the respective sugar monomers by a battery of intracellular glycoside hydrolases. For example, for the full utilization of xylan, the bacterium initially secretes an extracellular xylanase (XT6; Gat et al., 1994 ▶; Teplitsky et al., 1997 ▶, 2004 ▶; Bar et al., 2004 ▶), which partially degrades xylan to decorated xylo-oligomers that are transported into the cell via the ABC transport system (Shulami et al., 2007 ▶). Inside the cell, the decorated xylo-oligomers are hydrolysed by side-chain-cleaving enzymes, including arabinofuranosidases (Shallom, Belakhov, Solomon, Gilead-Gropper et al., 2002 ▶; Shallom, Belakhov, Solomon, Shoham et al., 2002 ▶; Hövel et al., 2003 ▶), glucuronidases (Teplitsky et al., 1999 ▶; Zaide et al., 2001 ▶; Golan et al., 2004 ▶; Shallom et al., 2004 ▶) and acetyl-esterases (Alalouf et al., 2011 ▶; Lansky et al., 2013 ▶), and finally by an intracellular xylanase (IXT6; Teplitsky et al., 2000 ▶; Solomon et al., 2007 ▶) and xylosidases (Bravman et al., 2003 ▶; Shallom et al., 2005 ▶; Brüx et al., 2006 ▶; Ben-David et al., 2007 ▶).
We have recently characterized the l-arabinan-utilization system in G. stearothermophilus (Shulami et al., 2011 ▶). The system is located on a 38 kb gene segment and contains 23 genes. According to the locations of potential transcription terminators, the genes in the arabinan-utilization cluster appeared to be organized into five transcriptional units, each of which is proposed to have a different biological function. These units include, amongst others, genes for five glycoside hydrolases, extracellular and intracellular arabinanases (AbnA and AbnB, respectively; Alhassid et al., 2009 ▶), two intracellular arabinofuranosidases (AbfA and AbfB; Gilead & Shoham, 1995 ▶; Shallom, Belakhov, Solomon, Gilead-Gropper et al., 2002 ▶; Hövel et al., 2003 ▶) and an intracellular β-l-arabinopyranosidase (Abp; Salama et al., 2012 ▶).
While most genes in the l-arabinan-utilization system have been well characterized in the last few years, relatively little was known until recently about the abp gene and its protein gene product (Abp). The Abp protein shows high homology to enzymes of glycoside hydrolase family 27 (GH27), which act via a retaining mechanism (hydrolysis with net retention of the anomeric configuration). The majority of the GH27 family enzymes exhibit α-d-galactosidase activity (Henrissat, 1991 ▶), but this activity does not seem to correlate with the physical location of the abp gene in the context of the arabinan operon. Recent studies have shown that some enzymes from the GH27 family exhibit β-l-arabinopyranosidase activity (Ichinose et al., 2009 ▶; Sakamoto et al., 2010 ▶). In correlation with these observations, we have recently demonstrated that Abp is a β-l-arabinopyranosidase and that the purified protein exhibits activities on related synthetic and native substrates (Salama et al., 2012 ▶). Moreover, using 13C NMR spectroscopy, we have unequivocally demonstrated that the enzyme is capable of removing β-l-arabinopyranose residues from the natural arabino-polysaccharide larch arabinogalactan (Salama et al., 2012 ▶).
In this report, we describe the crystallization of G. stearothermophilus Abp and the preliminary crystallographic characterization of the resulting Abp crystals. Complete diffraction data sets to 2.3 Å resolution have been collected for both the wild-type enzyme (Abp-WT) and its D197A catalytic mutant (Abp-D197A). These data sets are currently being used for the full three-dimensional structure determination of the Abp protein using molecular-replacement techniques for phasing.
2. Experimental
2.1. Expression and purification of Abp-WT
The expression of the His-tagged fused abp gene was carried out using Escherichia coli BL21 (DE3) carrying pET9d-abp, essentially as described previously (Salama et al., 2012 ▶). In brief, E. coli BL21(pET9d-abp) cultures were grown (500 ml in 2 l baffled shake flasks, shaken at 230 rev min−1 and 310 K) overnight in Terrific broth medium with kanamycin (25 µg ml−1). Following overnight growth (final OD600 nm of 20–23), the cells from 1 l of culture were harvested, resuspended in about 65 ml of buffer (20 mM imidazole, 20 mM phosphate buffer, 500 mM NaCl pH 7.0), disrupted by two passages through a French press and centrifuged (14 000 rev min−1 for 30 min) to obtain a soluble extract. The His-tagged protein (at the N-terminal) was isolated using a 5 ml HisTrap column (GE Healthcare), mounted on an ÄKTAexplorer fast protein liquid chromatography system (GE Healthcare) according to the manufacturer’s instructions. The Abp protein appeared as a distinct peak, which was then collected and dialysed overnight against 2 l of 50 mM Tris–HCl buffer pH 7.0, 100 mM NaCl, 0.02% sodium azide. An average amount of 150 mg protein was typically obtained from 1 l of overnight E. coli culture, and the protein was more than 95% pure based on SDS–PAGE.
2.2. Construction of the Abp-D197A nucleophile mutant
Site-directed mutagenesis was performed using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, California, USA). The mutagenic primer for the mutation was 5′-GGGAGTCGATTTTGTAAAAGTCGCGGATATTGTTGCATCAAAAC-3′ (the mutated nucleotides are shown in bold letters). The mutation was created by a double base-pair substitution in order to avoid translational mis-incorporation during protein synthesis by the host cell (Shallom et al., 2002 ▶). The mutated gene was sequenced to confirm that only the desired mutation was inserted, and the mutant protein was overexpressed and purified as described for the native enzyme.
2.3. Crystallization experiments
Crystallization experiments were set up immediately after the last purification step of the recombinant Abp protein (no His-tag removal was attempted). The purified protein was concentrated to approximately 6 mg ml−1 using Centricon centrifugal concentrators (Millipore, Massachusetts, USA) and this protein solution was directly used in the crystallization experiments. All initial crystallization experiments were performed by the hanging-drop vapour-diffusion method, using an extensive series of different factorial screens (Jancarik & Kim, 1991 ▶). In general, these initial conditions were based on commercially available sets of ‘ready-to-use’ screening solutions. Once positive results were obtained (i.e. crystals or micro-crystals), further refinement of these crystallization conditions was performed with specially prepared solutions, optimizing parameters such as pH, ionic strength and protein concentration (Almog et al., 1993 ▶, 1994 ▶; Teplitsky et al., 1997 ▶, 1999 ▶, 2000 ▶; Bar et al., 2004 ▶).
The final Abp protein crystallization drops were prepared by mixing the concentrated protein solution (4–6 mg ml−1) with an equal amount of each of the specific screen conditions to give a final drop volume of 4.0 µl. Each of these protein drops was suspended over a 1.0 ml reservoir solution in 4 × 6 VDX crystallization plates (Hampton Research, California, USA) for a period of about 1–8 d at a constant temperature of 293 K. One of these refined crystallization conditions resulted in single crystals, which were later found to be suitable for further crystallographic analysis (see below).
All diffraction data measurements were performed at the European Synchrotron Research Facility (ESRF), Grenoble, France. Processing and scaling of the diffraction data were conducted using the DENZO and SCALEPACK crystallographic programs (Otwinowski, 1993 ▶; Otwinowski & Minor, 1997 ▶).
3. Results and discussion
3.1. Crystallographic characterization of the Abp crystals
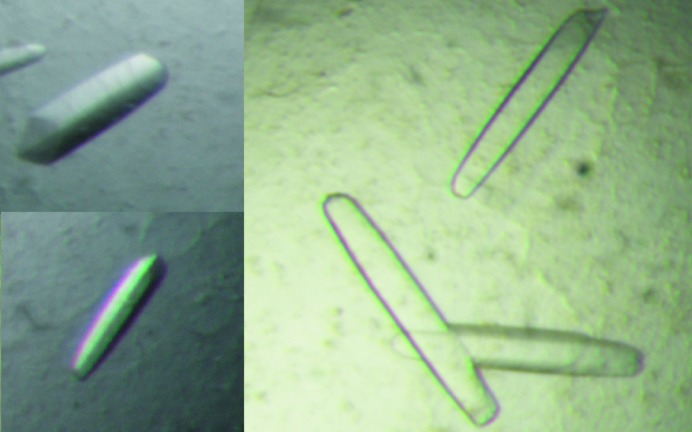
Optimal Abp crystals were obtained at a protein concentration of 4–6 µg µl−1 and in a reservoir solution of 1.8 M ammonium sulfate, 0.1 M citrate buffer pH 4.8. These crystals grew to their full size after about 6–7 d. The Abp crystals appeared usually as elongated elliptical plates, growing along their long axis. Their final shape was usually similar to an elongated boat (EB habit), with typical dimensions of about 0.6 × 0.2 × 0.1 mm (Fig. 1 ▶).
Figure 1.
Typical crystals of Abp-WT (with the EB crystal habit). Such crystals were used for the measurement of the full diffraction data sets described here at 2.9 and 2.3 Å resolution.
Several Abp crystals of the EB habit were used for a detailed crystallographic characterization and measurement of X-ray diffraction data at cryogenic conditions (90–100 K). These experiments were performed using X-ray synchrotron radiation (λ = 0.954 Å) and a MAR 225 CCD area detector (MAR Research, USA) on the BM14 beamline of the ESRF synchrotron facility. The crystal-cooling procedure used for these experiments included a short soak of the selected crystal (for about 20–60 s) in a cryoprotecting solution consisting of the original crystallization reservoir solution (1.8 M ammonium sulfate, 0.1 M citrate buffer pH 4.8) and 20%(v/v) glycerol. Such pre-soaked crystals were then submitted to immediate flash-cooling directly within a cold nitrogen-gas stream (100 K; Oxford Cryosystems).
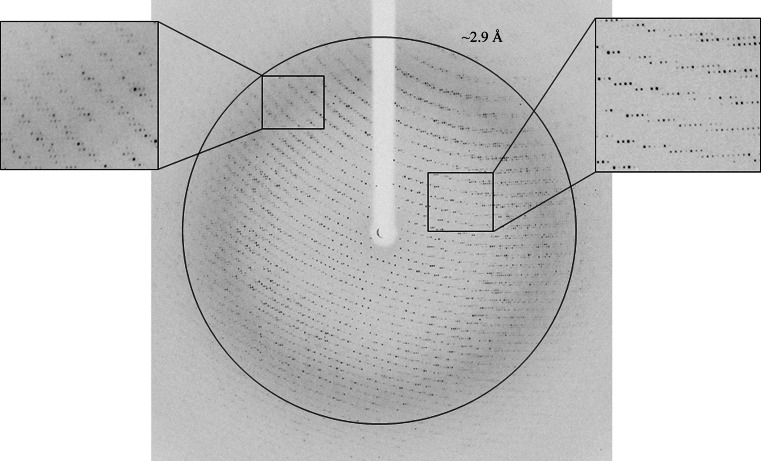
The first set of diffraction experiments indicated that the Abp crystals diffracted to well beyond 3 Å resolution, but owing to geometrical limitations of the diffraction collection and a relatively long unit-cell axis, only a medium resolution of 2.9 Å could be efficiently measured (Fig. 2 ▶). At this point, two such full data sets were collected from Abp-WT and Abp-D197A (only the 2.9 Å data for Abp-D197A are summarized in Table 1 ▶ and shown in Fig. 2 ▶) and used for the initial crystallographic characterization. These diffraction patterns indicated that the crystals belonged to a primitive orthorhombic crystal system, with average crystallographic unit-cell parameters a = 107.7, b = 202.2, c = 287.3 Å. Further analysis of the diffraction data confirmed that the space group of these crystals was P212121. Different crystals of this crystallization batch gave similar unit-cell parameters, with deviations from these average values of less than 0.3%.
Figure 2.
Medium-resolution X-ray diffraction pattern of the Abp crystals (Abp-D197A) obtained using a synchrotron source (BM14, ESRF). The outer circle corresponds to a 2.9 Å resolution limit. The insets represent magnified views of the sections indicated by the corresponding squares, demonstrating the practical resolution limit of the diffraction measurement using this experimental setup.
Table 1. Representative parameters from the crystallographic data measurement of Abp.
Values in parentheses are for the outer diffraction shell.
| Abp-WT (high resolution) | Abp-D197A (high resolution) | Abp-D197A (medium resolution) | |
|---|---|---|---|
| Beamline | BM14, ESRF | BM14, ESRF | BM14, ESRF |
| Wavelength (Å) | 0.954 | 0.954 | 0.954 |
| Space group | P212121 | P212121 | P212121 |
| Unit-cell parameters | |||
| a (Å) | 107.71 | 107.70 | 107.54 |
| b (Å) | 202.16 | 203.53 | 201.94 |
| c (Å) | 287.29 | 286.95 | 286.43 |
| Total No. of reflections | 1665005 | 1491619 | 798708 |
| No. of unique reflections | 283329 | 270391 | 137052 |
| Multiplicity | 5.9 (3.4) | 5.5 (3.7) | 5.8 (4.1) |
| 〈I〉/〈σ(I)〉 | 9.6 (4.4) | 8.3 (4.0) | 7.0 (2.8) |
| Mosaicity (°) | 0.181 | 0.274 | 0.366 |
| Resolution range (Å) | 30.0–2.28 (2.32–2.28) | 25.00–2.30 (2.34–2.30) | 50.00–2.90 (2.95–2.90) |
| Completeness (%) | 99.8 (97.6) | 97.1 (79.1) | 98.8 (95.0) |
| R merge † (%) | 7.3 (25.2) | 8.6 (32.5) | 11.9 (37.3) |
R
merge = 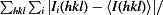
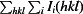 .
.
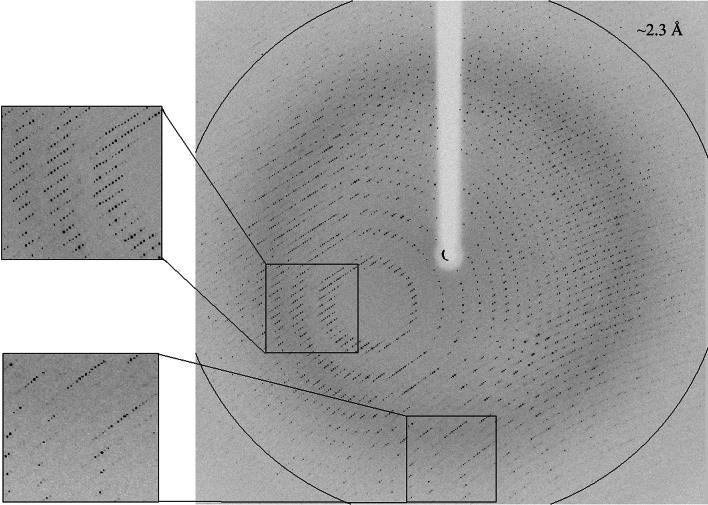
In a second set of diffraction experiments we examined different data-collection geometries in order to improve the resolution limits of the measured diffraction. These experiments indicated that in a certain crystal-mounting orientation (in which the long physical axis of the crystal was mounted along the Z axis of the goniometer), the best data measurement geometry is at a κ angle of 55°. This optimal geometry allowed us to collect full diffraction data sets (for both Abp-WT and Abp-D197A) to a resolution limit of around 2.3 Å (see below) using the same diffraction setup as described above (Fig. 3 ▶).
Figure 3.
High-resolution X-ray diffraction pattern of the Abp crystals (WT) obtained using a synchrotron source (BM14, ESRF). The outer circle corresponds to a 2.3 Å resolution limit. The insets represent magnified views of the sections indicated by the corresponding squares (top, medium resolution; bottom, high resolution).
3.2. X-ray diffraction data for Abp-WT
One of the Abp-WT crystals (of the EB crystal habit) was used for a full high-resolution X-ray diffraction data measurement. An oscillation data set (Δϕ = 0.5°, κ = 55°, 15 s exposure, 300 frames) was measured on the BM14 beamline at the ESRF (λ = 0.954 Å, 100 K). The raw CCD diffraction images were processed with DENZO and SCALEPACK (Otwinowski, 1993 ▶). A total of 1 665 005 accepted reflections [F > 0σ(F)] were measured in the 30.00–2.28 Å resolution range and resulted in 283 329 independent reflections, with 99.8% completeness to 2.28 Å resolution and 97.6% completeness for the highest resolution shell (2.32–2.28 Å). The overall multiplicity (or redundancy) in the combined data set was 5.9, the overall mosaicity was 0.181°, the average 〈I/σ(I)〉 was 9.6 and the final R merge (or R sym) for the whole data set was 7.3% (25.2% for the highest resolution shell). These parameters confirm that this data set represents a full diffraction data set of relatively high quality (Table 1 ▶).
A rough calculation of the specific ratio of volume/protein (V M) was performed in order to estimate the number of protein monomers per crystallographic asymmetric unit. The volume of the WT-Abp crystallographic unit cell, as determined from the mean value of the unit-cell parameters at 100 K, is 6.26 × 106 Å3. Assuming that the V M and the overall water content values here are within the normal range observed for soluble proteins (Matthews, 1968 ▶; Kantardjieff & Rupp, 2003 ▶), there should be between eight and 18 Abp monomers (448 amino-acid residues; MW 51 190 Da; Salama et al., 2012 ▶) in the crystallographic asymmetric unit. With eight molecules in the P212121 asymmetric unit (32 in the unit cell), the calculated V M is 3.82 Å3 Da−1 and the corresponding solvent content in the crystals is 67.81%. With 18 molecules in the asymmetric unit (72 in the unit cell), the calculated V M is 1.70 Å3 Da−1 and the corresponding solvent content in the crystals is 27.57%. Both the high-limit and the low-limit V M values have been observed for soluble proteins, but it is expected that the true content of the asymmetric unit will be somewhere between these two extreme possibilities. Self-rotation calculations confirmed that there is no additional (noncrystallographic) symmetry within the crystallographic asymmetric unit and indicated that the number of monomers per asymmetric unit is closer to eight. The actual content of the Abp asymmetric unit (and the unit cell) will be unequivocally resolved only when the crystallographic protein structure is fully determined.
3.3. X-ray diffraction data for Abp-D197A
The catalytic mutant of Abp presented above, Abp-D197A, was crystallized using the same conditions as described for Abp-WT and the resulting EB habit crystals were confirmed to be completely isomorphous with the corresponding Abp-WT crystals. One of these Abp-D197A crystals was used for the measurement of a complete oscillation data set (Δϕ = 0.5°, κ = 55°, 15 s exposure, 300 frames) using the same crystallographic setup as described above for the Abp-WT crystal (beamline BM14 at ESRF, λ = 0.954 Å, 100 K). A total of 1 491 619 accepted reflections [F > 0σ(F)] were measured in the 25.00–2.30 Å resolution range, resulting in 270 391 independent reflections with 97.1% completeness to 2.30 Å resolution. The overall multiplicity in the combined data set was 5.5, the overall mosaicity was 0.274°, the average 〈I/σ(I)〉 was 8.3 and the final R merge was 8.6%. These parameters, although not as good as those of the Abp-WT crystal, still confirm that the mutant data are also of relatively high quality (Table 1 ▶).
The two data sets described above will now be used for the full three-dimensional structural analysis of both Abp-WT and Abp-D197A. A search of the Protein Data Bank (PDB) showed that a full three-dimensional structure is available for a highly homologous (68% amino-acid identity) protein, the GH27 enzyme BH1870 from Bacillus halodurans (PDB entry 3cc1; Joint Center for Structural Genomics, unpublished work). This structure should provide an excellent reference model for molecular-replacement calculations, which are expected to solve the phase problem and to provide a good starting model for the structural analysis of the Abp proteins. Such analyses are currently in progress in our laboratory.
Acknowledgments
This work was supported by Israel Science Foundation grants 500/10 and 152/11, the I-CORE Program of the Planning and Budgeting Committee, the Ministry of Environmental Protection and the Grand Technion Energy Program (GTEP), and comprises part of The Leona M. and Harry B. Helmsley Charitable Trust Reports on Alternative Energy series of the Technion, Israel Institute of Technology and the Weizmann Institute of Science. We thank the staff at the European Synchrotron Research Facility (ESRF; beamline BM14), for their helpful support in the X-ray synchrotron data measurement and analysis. The synchrotron experiments at the ESRF were also supported by the ESRF internal funding program. YS holds the Erwin and Rosl Pollak Chair in Biotechnology at the Technion.
References
- Alalouf, O., Balazs, Y., Volkinshtein, M., Grimple, Y., Shoham, G. & Shoham, Y. (2011). J. Biol. Chem. 286, 41933–42001. [DOI] [PMC free article] [PubMed]
- Alhassid, A., Ben-David, A., Tabachnikov, O., Libster, D., Naveh, E., Zolotnitsky, G., Shoham, Y. & Shoham, G. (2009). Biochem. J. 422, 73–82. [DOI] [PubMed]
- Almog, O., Greenblatt, H. M., Spungin, A., Ben-Meir, D., Blumberg, S. & Shoham, G. (1993). J. Mol. Biol. 230, 342–344. [DOI] [PubMed]
- Almog, O., Klein, D., Braun, S. & Shoham, G. (1994). J. Mol. Biol. 235, 760–762. [DOI] [PubMed]
- Bar, M., Golan, G., Nechama, M., Zolotnitsky, G., Shoham, Y. & Shoham, G. (2004). Acta Cryst. D60, 545–549. [DOI] [PubMed]
- Ben-David, A., Bravman, T., Balazs, Y. S., Czjzek, M., Schomburg, D., Shoham, G. & Shoham, Y. (2007). Chembiochem, 8, 2145–2151. [DOI] [PubMed]
- Bravman, T., Zolotnitsky, G., Belakhov, V., Shoham, G., Henrissat, B., Baasov, T. & Shoham, Y. (2003). Biochemistry, 42, 10528–10536. [DOI] [PubMed]
- Brüx, C., Ben-David, A., Shallom-Shezifi, D., Leon, M., Niefind, K., Shoham, G., Shoham, Y. & Schomburg, D. (2006). J. Mol. Biol. 359, 97–109. [DOI] [PubMed]
- Gat, O., Lapidot, A., Alchanati, I., Regueros, C. & Shoham, Y. (1994). Appl. Environ. Microbiol. 60, 1889–1896. [DOI] [PMC free article] [PubMed]
- Gilbert, H. J. (2010). Plant Physiol. 153, 444–455. [DOI] [PMC free article] [PubMed]
- Gilead, S. & Shoham, Y. (1995). Appl. Environ. Microbiol. 61, 170–174. [DOI] [PMC free article] [PubMed]
- Golan, G., Shallom, D., Teplitsky, A., Zaide, G., Shulami, S., Baasov, T., Stojanoff, V., Thompson, A., Shoham, Y. & Shoham, G. (2004). J. Biol. Chem. 279, 3014–3024. [DOI] [PubMed]
- Henrissat, B. (1991). Biochem. J. 280, 309–316. [DOI] [PMC free article] [PubMed]
- Hövel, K., Shallom, D., Niefind, K., Belakhov, V., Shoham, G., Baasov, T., Shoham, Y. & Schomburg, D. (2003). EMBO J. 22, 4922–4932. [DOI] [PMC free article] [PubMed]
- Ichinose, H., Fujimoto, Z., Honda, M., Harazono, K., Nishimoto, Y., Uzura, A. & Kaneko, S. (2009). J. Biol. Chem. 284, 25097–25106. [DOI] [PMC free article] [PubMed]
- Jancarik, J. & Kim, S.-H. (1991). J. Appl. Cryst. 24, 409–411.
- Kantardjieff, K. A. & Rupp, B. (2003). Protein Sci. 12, 1865–1871. [DOI] [PMC free article] [PubMed]
- Lansky, S., Alalouf, O., Solomon, V., Alhassid, A., Govada, L., Chayan, N. E., Belrhali, H., Shoham, Y. & Shoham, G. (2013). Acta Cryst. F69, 430–434. [DOI] [PMC free article] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Mohnen, D. (2008). Curr. Opin. Plant Biol. 11, 266–277. [DOI] [PubMed]
- Otwinowski, Z. (1993). Proceedings of the CCP4 Study Weekend. Data Collection and Processing, edited by L. Sawyer, N. Isaacs & S. Bailey, pp. 56–62. Warrington: Daresbury Laboratory.
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Rees, D. C., Johnson, E. & Lewinson, O. (2009). Nature Rev. Mol. Cell Biol. 10, 218–227. [DOI] [PMC free article] [PubMed]
- Sakamoto, T., Tsujitani, Y., Fukamachi, K., Taniguchi, Y. & Ihara, H. (2010). Appl. Microbiol. Biot. 86, 1115–1124. [DOI] [PubMed]
- Salama, R., Alalouf, O., Tabachnikov, O., Zolotnitsky, G., Shoham, G. & Shoham, Y. (2012). FEBS Lett. 586, 2436–2442. [DOI] [PubMed]
- Shallom, D., Belakhov, V., Solomon, D., Gilead-Gropper, S., Baasov, T., Shoham, G. & Shoham, Y. (2002). FEBS Lett. 514, 163–167. [DOI] [PubMed]
- Shallom, D., Belakhov, V., Solomon, D., Shoham, G., Baasov, T. & Shoham, Y. (2002). J. Biol. Chem. 277, 43667–43673. [DOI] [PubMed]
- Shallom, D., Golan, G., Shoham, G. & Shoham, Y. (2004). J. Bacteriol. 186, 6928–6937. [DOI] [PMC free article] [PubMed]
- Shallom, D., Leon, M., Bravman, T., Ben-David, A., Zaide, G., Belakhov, V., Shoham, G., Schomburg, D., Baasov, T. & Shoham, Y. (2005). Biochemistry, 44, 387–397. [DOI] [PubMed]
- Shulami, S., Gat, O., Sonenshein, A. L. & Shoham, Y. (1999). J. Bacteriol. 181, 3695–3704. [DOI] [PMC free article] [PubMed]
- Shulami, S., Raz-Pasteur, A., Tabachnikov, O., Gilead-Gropper, S., Shner, I. & Shoham, Y. (2011). J. Bacteriol. 193, 2838–2850. [DOI] [PMC free article] [PubMed]
- Shulami, S., Zaide, G., Zolotnitsky, G., Langut, Y., Feld, G., Sonenshein, A. L. & Shoham, Y. (2007). Appl. Enviorn. Microbiol. 73, 874–884. [DOI] [PMC free article] [PubMed]
- Solomon, V., Teplitsky, A., Shulami, S., Zolotnitsky, G., Shoham, Y. & Shoham, G. (2007). Acta Cryst. D63, 845–859. [DOI] [PubMed]
- Tabachnikov, O. & Shoham, Y. (2013). FEBS J. 280, 950–964. [DOI] [PubMed]
- Teplitsky, A., Feinberg, H., Gilboa, R., Lapidot, A., Mechaly, A., Stojanoff, V., Capel, M., Shoham, Y. & Shoham, G. (1997). Acta Cryst. D53, 608–611. [DOI] [PubMed]
- Teplitsky, A., Mechaly, A., Stojanoff, V., Sainz, G., Golan, G., Feinberg, H., Gilboa, R., Reiland, V., Zolotnitsky, G., Shallom, D., Thompson, A., Shoham, Y. & Shoham, G. (2004). Acta Cryst. D60, 836–848. [DOI] [PubMed]
- Teplitsky, A., Shulami, S., Moryles, S., Shoham, Y. & Shoham, G. (2000). Acta Cryst. D56, 181–184. [DOI] [PubMed]
- Teplitsky, A., Shulami, S., Moryles, S., Zaide, G., Shoham, Y. & Shoham, G. (1999). Acta Cryst. D55, 869–872. [DOI] [PubMed]
- Zaide, G., Shallom, D., Shulami, S., Zolotnitsky, G., Golan, G., Baasov, T., Shoham, G. & Shoham, Y. (2001). Eur. J. Biochem. 268, 3006–3016. [DOI] [PubMed]