The nucleocapsid protein from Tomato spotted wilt virus has been crystallized as a trimer. The crystal diffracted to resolution of 2.7 Å.
Keywords: Tomato spotted wilt virus, Tospovirus
Abstract
Tomato spotted wilt virus (TSWV), which causes severe damage to various agricultural crops such as tomato, pepper, lettuce and peanut, is a negative-stranded RNA virus belonging to the Tospovirus genus of the Bunyaviridae family. Viral genomic RNA molecules are packaged in the form of ribonucleoprotein complexes, each of which contains one viral RNA molecule that is coated with many nucleocapsid (N) proteins. Here, the expression and crystallization of TSWV N protein are reported. Native and selenomethionine-substituted crystals of N protein belonged to the same space group P21. Their unit-cell parameters were a = 66.8, b = 97.2, c = 72.0 Å, β = 112.8° and a = 66.5, b = 96.3, c = 72.1 Å, β = 113.4°, respectively.
1. Introduction
Tomato spotted wilt virus (TSWV) is classified as the type member of the genus Tospovirus, which includes Impatiens necrotic spot virus (INSV) and Watermelon silver mottle virus (WSMoV), within the family Bunyaviridae. The Bunyaviridae family has five genera, only one of which consists of plant-infecting viruses (King et al., 2011 ▶); the others (Orthobunyavirus, Hantavirus, Nairovirus and Phlebovirus) are animal viruses, several of which are the causative agents of serious human disease (Elliott, 1990 ▶). TSWV has a worldwide distribution and causes severe damage to various agricultural crops, including tomato, pepper, lettuce and peanut. With an estimated economic loss owing to TSWV in excess of US$1 billion annually in 1994, control of TSWV is urgently required (Goldbach & Peters, 1994 ▶).
The TSWV genome consists of small (S), medium (M) and large (L) RNA segments. The S RNA segment encodes the nucleocapsid (N) and NSs proteins, the M RNA segment encodes glycoproteins (Gn and Gc) and NSm proteins, and the L RNA segment encodes the RNA-dependent RNA polymerase (RdRp) (Adkins, 2000 ▶). The numerous N proteins encapsidate viral genome RNAs and, in association with a few RdRps, constitute vRNP (viral ribonucleoprotein) complexes (Uhrig et al., 1999 ▶; Richmond et al., 1998 ▶). These vRNP complexes perform genome RNA replication and viral mRNA transcription. Moreover, N protein has been shown to interact with Gn/Gc protein and it has therefore been suggested that the N protein plays a crucial role in viral encapsidation (Ribeiro et al., 2009 ▶). Overall, the N protein is an important factor in the TSWV life cycle.
Recently, the structures of N proteins from three animal virus genera of the Bunyaviridae family have been reported and several encapsidation mechanisms of viral genome RNAs have been suggested based on the formation of different oligomers of N proteins (Raymond et al., 2010 ▶, 2012 ▶; Ferron et al., 2011 ▶; Guo et al., 2012 ▶; Carter et al., 2012 ▶; Wang et al., 2012 ▶; Dong et al., 2013 ▶; Niu et al., 2013 ▶; Li et al., 2013 ▶; Reguera et al., 2013 ▶). Interestingly, the sequence and structure of N proteins are highly conserved within a genus but differ between genera. For a full understanding of the genome encapsidation mechanism of the genus Tospovirus, the structure of the N protein from TSWV is indispensable, although the domains or amino-acid residues contributing to RNA binding or oligomer formation have been suggested by biochemical analyses (Richmond et al., 1998 ▶; Uhrig et al., 1999 ▶). Here, we report the crystallization and preliminary X-ray crystallographic analysis of the TSWV N protein to gain further insight into its oligomeric state and the mechanism of RNA binding.
2. Materials and methods
2.1. Cloning, expression and purification
TSWV-infected leaves of Pericallis × hybrida (MAFF No. 260050) were obtained from the National Institute of Agrobiological Sciences Genebank, Tsukuba, Ibaraki, Japan. Total RNA was isolated from TSWV-infected leaves with RNAiso Plus (Takara Bio, Japan) according to the manufacturer’s protocol. The purified total RNA was used as a template to synthesize the first-strand of cDNA of the S RNA segment. To obtain the full cDNA template of the S RNA segment, the primers TSWV-SP (5′-AGAGCAATCGTGTCAATTTTGTGTTCATACCTTAACACTC-3′) and TSWV-SN (5′-AGAGCAATTGTGTCAGAATTTTGTTCATAATCAAACCT-3′) were designed with reference to the sequence of TSWV CPNH9 strain (GenBank D00645.1). Reverse transcription (RT) was performed using primer TSWV-SP and ReverTra Ace reverse transcriptase (Toyobo, Japan) according to the manufacturer’s protocol. The RT product was then PCR-amplified using KOD -Plus- polymerase (Toyobo, Japan) with primer pairs of TSWV-SP and TSWV-SN, and cloned into pCR-Blunt II-TOPO vector (Life Technologies, California, USA). After identifying the sequence of this cDNA template, we amplified the N-protein ORF (258 amino acids, molecular mass 29 kDa, theoretical pI of 9.5) by PCR using KOD -Plus- polymerase (Toyobo, Japan) with primer pairs FP (5′-AATTCATATGTCTAAGGTTAAGCTCACTAAGG-3′) and RP (5′-AATTCTCGAGTTAAGCAAGTTCTGCAAGTTTTGCC-3′). This PCR product was digested with NdeI/XhoI and inserted into the expression vector pET28a fused with extra amino acids (MNHKHHHHHHSSGENLYFQGH from the N-terminus to the C-terminus) including a D-box (MNHK), a His6 tag (HHHHHH) and a TEV cleavage site (ENLYFQG) to the N-terminus of the N-protein ORF. To confirm oligomeric formation of the N protein, we prepared a double mutation F242A/F246A (Phe242 and Phe246 were substituted with Ala). The mutant variant F242A/F246A was produced using the expression plasmid of the wild-type N protein as a template with specific primer pairs mutFP (5′-GCTTATGAAATGGCTGGGGTTAAAAAACAGGCAAAACTTG-3′) and mutRP (5′-CTTGTTAAGAGTTTCACTGTAATGTTCCAT-3′).
The resulting plasmid was transformed into Escherichia coli strain B834 (DE3) + pRARE2 cells by electroporation. For the preparation of native N protein, the transformed cells were cultivated in Luria broth (LB) containing 25 µg ml−1 kanamycin and 34 µg ml−1 chloramphenicol. For the preparation of selenomethionine-substituted (SeMet) N proteins, we used medium containing 25 µg ml−1 kanamycin and 34 µg ml−1 chloramphenicol, 1500 µg ml−1 adenine, 480 µg ml−1 thymine, 1980 µg ml−1 guanosine, 1500 µg ml−1 uracil, 120 µg ml−1 of each amino acid (except methionine) and 75 µg ml−1 selenomethionine. Transformed cells were cultivated at 310 K until the optical density at 600 nm (OD600) reached about 0.6 and gene expression was induced by adding isopropyl β-d-1-thiogalactopyranoside (IPTG) to a final concentration of 1 mM. The cells were cultured at 293 K for a further 18 h, harvested by centrifugation and resuspended in lysis buffer [50 mM Tris–HCl pH 7.5, 500 mM NaCl, 10 mM MgCl2, 10%(v/v) glycerol]. The resuspended cells were sonicated at 277 K and cleared by ultracentrifugation for 30 min at 40 000g at 277 K to remove cell debris.
The cleared cell lysate was loaded onto a HisTrap HP column (GE Healthcare) pre-equilibrated with His buffer A (lysis buffer containing 20 mM imidazole). The column was then washed with His buffer A and the bound protein was eluted with a linear gradient of 0–50% buffer B (lysis buffer containing 500 mM imidazole). The N-terminal His6 tag was not removed because TEV protease was not able to digest the His6 tag perfectly. Next, eluted fractions were applied onto a HiTrap Heparin HP column (GE Healthcare) to remove the endogenous nucleotides with heparin buffer A [50 mM Tris–HCl pH 7.5, 1 mM MgCl2, 10%(v/v) glycerol] and the bound protein was eluted with a linear gradient of 0–100% buffer B [50 mM Tris–HCl pH 7.5, 1 mM MgCl2, 2 M NaCl, 10%(v/v) glycerol]. After heparin column purification, the A 280:A 260 ratio of a sample fraction had increased from 0.7 to 1.6, indicating that most of the host-derived nucleic acids had been removed. Finally, the collected fractions were concentrated and subjected to size-exclusion chromatography on a Superdex 200 16/60 column (GE Healthcare) pre-equilibrated with 20 mM Na HEPES pH 7.5, 100 mM NaCl, 10%(v/v) glycerol. At each step in the purification procedure, fractions were analysed by SDS–PAGE and appropriate fractions were pooled. The final eluted protein was concentrated to 5 mg ml−1, flash-cooled and stored at 193 K for further crystallization.
2.2. Crystallization
Crystallization screening of native N protein was performed using commercial kits (The JCSG Core Suites I, II, III and IV, and The PEGs and PEGs II Suites, Qiagen) by the sitting-drop vapour-diffusion method in 96-well plates. A 0.75 µl drop of the protein sample (5 mg ml−1) was mixed with an equal volume of reservoir solution and the mixture was equilibrated against 75 µl reservoir solution at 293 K. Crystals of the N protein were obtained from condition No. 77 of The JCSG Core I Suite [0.1 M citric acid, 10%(w/v) PEG 6000, final pH 5.0], condition No. 49 of The JCSG Core Suite II [0.2 M sodium chloride, 0.1 M sodium/potassium phosphate pH 6.2, 20%(w/v) PEG 1000], condition No. 22 of The JCSG Core Suite III (0.1 M Tris pH 8.5, 2.4 M ammonium sulfate, final pH 8.0) and condition No. 15 of The PEGs II Suite [0.1 M Na HEPES pH 7.5, 25%(w/v) PEG 1000]. For optimization, the concentration of individual components and pH were adjusted and Additive Screen HT (Hampton Research) was also used under the above conditions via the hanging-drop vapour-diffusion method in 24-well plates. The best crystal was grown using 0.1 M Na HEPES pH 7.5, 25%(w/v) PEG 1000, 10 mM betaine hydrochloride. The crystals of the SeMet derivative of the N protein were obtained in 100 mM Na HEPES pH 7.5, 25%(w/v) PEG 1000, 50 mM NaCl by the hanging-drop vapour-diffusion method in 24-well plates. The largest crystal obtained under this condition was used for data collection (Fig. 1 ▶).
Figure 1.
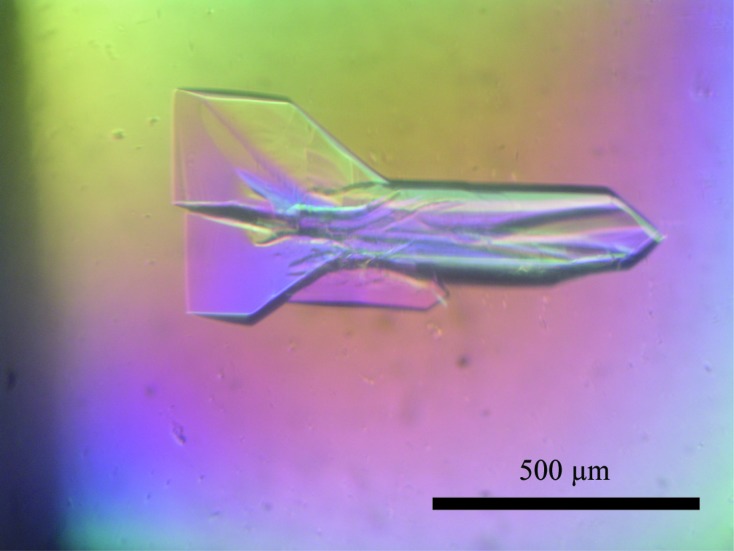
Crystals of SeMet N protein obtained by the hanging-drop vapour-diffusion method.
2.3. Data collection
For data collection at 100 K, the crystals of N protein were transferred into cryoprotectant solution containing 10% glycerol (native) or 5.6% sucrose (SeMet) and flash-cooled under a stream of liquid nitrogen. Diffraction data for the native N protein were collected using a Quantum 270 detector on BL-1A at the Photon Factory synchrotron facility, Tsukuba, Japan, while an Se-SAD data set was collected for the SeMet derivative of the N protein using a CCD detector (Rayonix MX225HE) on BL41XU at SPring-8, Harima, Japan. An X-ray wavelength of 0.9790 Å was used corresponding to the maximum f′′ for the Se-SAD data set based on the fluorescence spectrum of the Se atom. All data sets were indexed, integrated, scaled and merged using the HKL-2000 program suite (Otwinowski & Minor, 1997 ▶).
3. Results and discussion
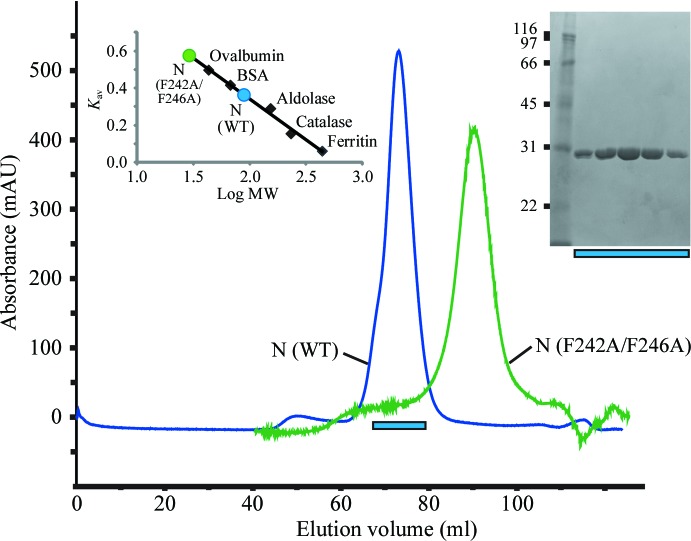
In the final step of purification by size-exclusion chromatography using a Superdex 200 16/60 column (GE Healthcare), we estimated that the N protein formed trimers in solution, which was confirmed by analysis relative to molecular-weight standards (Fig. 2 ▶).
Figure 2.
Elution profile of the TSWV N protein on HiLoad 16/60 Superdex 200. Left inset, the standard curve was obtained using the proteins ferritin (440 kDa), catalase (232 kDa), aldolase (153 kDa), bovine serum albumin (67 kDa) and ovalbumin (43 kDa). From its elution volume, purified native N protein (wild type; WT) was estimated at 87 kDa, which is nearly equal to the size of a trimer. We also analysed the double mutant F242A/F246A, which lost the oligomeric interaction (Uhrig et al., 1999 ▶). The estimated size of this N protein mutant is about 30 kDa, which is nearly equal to the size of a monomer. K av = (V e − V o)/(V t − V o), where V e is the elution volume of the protein, V o is the void volume and V t is the bed volume. The elution pattern of the SeMet N protein was similar to that of the WT. Right inset, SDS–PAGE of the eluted fractions of WT. The left lane contains molecular-mass markers (labelled in kDa).
Both native and SeMet N protein crystals belonged to space group P21, with unit-cell parameters a = 66.8, b = 97.2, c = 72.0 Å, β = 112.8° and a = 66.5, b = 96.3, c = 72.1 Å, β = 113.4°, respectively (Table 1 ▶). For tag-fused N protein with molecular weight 31 452 Da, the Matthews equation (Matthews, 1968 ▶) indicated that there are three molecules with a V M of 2.25 Å3 Da−1 (solvent content = 45%) per asymmetric unit. The result of a self-rotation search with POLARRFN (Winn et al., 2011 ▶) did not show obvious local threefold symmetry. The initial phases were obtained by the Se-SAD method using SHELXC/D/E (Sheldrick, 2008 ▶). The value of d′′/sig was estimated to be larger than 0.61 from 3.1 Å, and 24 of 30 Se sites were determined with CC(all) = 50.8 and CC(weak) = 31.6. After phase improvement the pseudo-free CC volume showed that the inverted Se sites were correct. Initial model building was carried out with the program ARP/wARP (Langer et al., 2008 ▶) after phase improvement. Refinement of this model is currently in progress in our laboratory.
Table 1. Data for native and SeMet N protein crystals.
Values in parentheses are for the outer resolution shell.
| Native N protein | SeMet N protein | |
|---|---|---|
| Beamline | BL-1A, Photon Factory | BL41XU, SPring-8 |
| Wavelength (Å) | 1.0000 | 0.9790 |
| Detector | Quantum 270 | Rayonix MX225HE |
| Crystal-to-detector distance (mm) | 360 | 280 |
| Rotation range per image (°) | 1 | 1.4 |
| Total rotation range (°) | 180 | 360 |
| Exposure time per image (s) | 2 | 1 |
| Space group | P21 | P21 |
| Unit-cell parameters (Å,°) | a = 66.8, b = 97.2, c = 72.0, β = 112.8 | a = 66.5, b = 96.3, c = 72.1, β = 113.4 |
| Mosaicity (°) | 0.97–1.46 | 0.56–1.77 |
| Resolution range (Å) | 50.0–3.25 (3.37–3.25) | 50.0–2.70 (2.80–2.70) |
| Total No. of reflections | 46995 | 169351 |
| No. of unique reflections | 13251 (1330) | 22739 (2268) |
| Completeness (%) | 99.4 (99.7) | 99.9 (99.9) |
| Multiplicity | 3.5 (3.6) | 7.4 (7.4) |
| R merge † (%) | 8.1 (58.9) | 12.9 (68.6) |
| 〈I〉/〈σ (I)〉 | 14.2 (1.5) | 15.3 (2.2) |
R
merge = 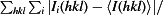
 .
.
Acknowledgments
This work was supported by a Grant-in-Aid for Young Scientists (Start-up; No. 22880001) from the Japan Society for the Promotion of Science (JSPS), Japan. The X-ray experiments were performed on BL41XU at SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI; proposal No. 2011A1272) and on BL-1A at the Photon Factory. We thank Dr Yoshikazu Tanaka for assistance with data collection and Dr Masayuki Ishikawa and the National Institute of Agrobiological Sciences for allowing the use of the TSWV cDNA clones.
References
- Adkins, S. (2000). Mol. Plant Pathol. 1, 151–157. [DOI] [PubMed]
- Carter, S. D., Surtees, R., Walter, C. T., Ariza, A., Bergeron, É, Nichol, S. T., Hiscox, J. A., Edwards, T. A. & Barr, J. N. (2012). J. Virol. 86, 10914–10923. [DOI] [PMC free article] [PubMed]
- Dong, H., Li, P., Elliott, R. M. & Dong, C. (2013). J. Virol. 87, 5593–5601. [DOI] [PMC free article] [PubMed]
- Elliott, R. M. (1990). J. Gen. Virol. 71, 501–522. [DOI] [PubMed]
- Ferron, F., Li, Z., Danek, E. I., Luo, D., Wong, Y., Coutard, B., Lantez, V., Charrel, R., Canard, B., Walz, T. & Lescar, J. (2011). PLoS Pathog. 7, e1002030. [DOI] [PMC free article] [PubMed]
- Goldbach, R. & Peters, D. (1994). Semin. Virol. 5, 113–120.
- Guo, Y., Wang, W., Ji, W., Deng, M., Sun, Y., Zhou, H., Yang, C., Deng, F., Wang, H., Hu, Z., Lou, Z. & Rao, Z. (2012). Proc. Natl Acad. Sci. USA, 109, 5046–5051. [DOI] [PMC free article] [PubMed]
- King, A. M. Q., Adams, M. J., Carstens, E. B. & Lefkowitz, E. J. (2011). Editors. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. London, Waltham, San Diego: Academic Press.
- Langer, G., Cohen, S. X., Lamzin, V. S. & Perrakis, A. (2008). Nature Protoc. 3, 1171–1179. [DOI] [PMC free article] [PubMed]
- Li, B., Wang, Q., Pan, X., Fernández de Castro, I., Sun, Y., Guo, Y., Tao, X., Risco, C., Sui, S.-F. & Lou, Z. (2013). Proc. Natl Acad. Sci. USA, doi:10.1073/pnas.1222552110. [DOI] [PMC free article] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Niu, F., Shaw, N., Wang, Y. E., Jiao, L., Ding, W., Li, X., Zhu, P., Upur, H., Ouyang, S., Cheng, G. & Liu, Z.-J. (2013). Proc. Natl Acad. Sci. USA, doi:10.1073/pnas.1300035110. [DOI] [PMC free article] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Raymond, D. D., Piper, M. E., Gerrard, S. R., Skiniotis, G. & Smith, J. L. (2012). Proc. Natl Acad. Sci. USA, 109, 19208–19213. [DOI] [PMC free article] [PubMed]
- Raymond, D. D., Piper, M. E., Gerrard, S. R. & Smith, J. L. (2010). Proc. Natl Acad. Sci. USA, 107, 11769–11774. [DOI] [PMC free article] [PubMed]
- Reguera, J., Malet, H., Weber, F. & Cusack, S. (2013). Proc. Natl Acad. Sci. USA, 110, 7246–7251. [DOI] [PMC free article] [PubMed]
- Ribeiro, D., Borst, J. W., Goldbach, R. & Kormelink, R. (2009). Virology, 383, 121–130. [DOI] [PubMed]
- Richmond, K. E., Chenault, K., Sherwood, J. L. & German, T. L. (1998). Virology, 248, 6–11. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Uhrig, J. F., Soellick, T. R., Minke, C. J., Philipp, C., Kellmann, J. W. & Schreier, P. H. (1999). Proc. Natl Acad. Sci. USA, 96, 55–60. [DOI] [PMC free article] [PubMed]
- Wang, Y., Dutta, S., Karlberg, H., Devignot, S., Weber, F., Hao, Q., Tan, Y. J., Mirazimi, A. & Kotaka, M. (2012). J. Virol. 86, 12294–12303. [DOI] [PMC free article] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.