Abstract
Background/Aims
Compounds of Cannabis sativa are known to exert anti-inflammatory properties, some of them without inducing psychotropic side effects. Cannabidiol (CBD) is such a side effect-free phytocannabinoid that improves chemically induced colitis in rodents when given intraperitoneally. Here, we tested the possibility whether rectal and oral application of CBD would also ameliorate colonic inflammation, as these routes of application may represent a more appropriate way for delivering drugs in human colitis.
Methods
Colitis was induced in CD1 mice by trinitrobenzene sulfonic acid. Individual groups were either treated with CBD intraperitoneally (10 mg/kg), orally (20 mg/kg) or intrarectally (20 mg/kg). Colitis was evaluated by macroscopic scoring, histopathology and the myeloperoxidase (MPO) assay.
Results
Intraperitoneal treatment of mice with CBD led to improvement of colonic inflammation. Intrarectal treatment with CBD also led to a significant improvement of disease parameters and to a decrease in MPO activity while oral treatment, using the same dose as per rectum, had no ameliorating effect on colitis.
Conclusion
The data of this study indicate that in addition to intraperitoneal application, intrarectal delivery of cannabinoids may represent a useful therapeutic administration route for the treatment of colonic inflammation.
Key Words: Inflammatory bowel disease, Experimental colitis, Cannabinoids
Introduction
Inflammatory bowel diseases (IBD) affect more than 3 million people in the western world [1]. Advances in the therapy of IBD have been achieved with new immunosuppressive and immunomodulatory agents; however, current pharmacological treatment still relies on nonsteroidal and steroidal anti-inflammatory drugs, treatments that may cause severe side effects [2]. With the advent of the so-called novel biologicals (e.g. tumor necrosis factor α antibodies), new hope was sparked for a more effective treatment of IBD, yet severe side effects and tolerance associated with long-term use of these drugs have dampened these outlooks [3]. Although currently used medication can keep IBD patients in relatively long states of remission, a more effective cure with fewer side effects is a desirable aim. Cannabinoids have recently moved into the center of inflammation research. But despite the fact that Cannabis sativa has traditionally been used for centuries as an analgesic and anti-inflammatory remedy, modern pharmacological therapy of inflammation with cannabinoids is still at the beginning. A recent article has highlighted that between 33 and 50% of people suffering from IBD have been using Cannabis to relieve IBD-related symptoms [4]. In line with this, animal models of IBD largely suggest that cannabinoid compounds and activation of cannabinoid (CB) receptors significantly suppress the severity of colitis [5, 6, 7]. In addition, non-CB receptor-mediated effects of cannabinoids can also cause improvement of experimental colitis [8, 9].
A major obstacle for the pharmacological exploitation of cannabinoids lies in their psychotropic side effects. This applies particularly to cannabinoids with strong cannabinoid 1 receptor (CB1) activity, such as Δ9-tetrahydrocannabinol (THC) [10] but also for CB1 antagonists, such as rimonabant [11]. However, some cannabinoids, e.g. cannabidiol (CBD) and O-1602, are known to be anti-inflammatory [9, 12, 13] while being free of adverse central side effects [12, 14]. CBD has already proven effective in decreasing the severity of experimental colitis in rodents [8]. Due to its low activity at CB receptors [15, 16] and its lack of psychoactivity [17, 18], CBD could become an important candidate for the treatment of IBD. Since orally taken cannabinoids are prone to significant metabolization in the liver [19], the route of application for cannabinoids is of major importance. For instance, Sativex®, a 1:1 mixture of THC and CBD (GW Pharma, Salisbury, UK), is given as an oromucosal spray to avoid first-pass metabolism by the liver and degradation in the intestine [20].
To address the question of the appropriate route for the application of cannabinoids in IBD treatment, we used an established mouse model of colitis (trinitrobenzene sulfonic acid [TNBS] model) and applied CBD systemically, orally and per rectum, to prevent the severity of colitis. We found that CBD not only protected from colitis when given systemically, but was also effective when given locally per rectum. Oral application of CBD, used at the same dose as per rectum, was not effective in protecting from colitis in this mouse model.
Materials and Methods
CD1 mice (males, 5–9 weeks old, 24–35 g) were purchased from Charles River (Deisenhofen, Germany) and kept in house at least for 2 weeks prior to experiments. Mice were housed in plastic sawdust floor cages at a constant temperature (22°C) and a 12-hour:12-hour light-dark cycle with free access to standard laboratory chow and tap water. Experimental procedures were approved by the ethics committee of the Austrian Federal Ministry of Science and Research (BMWF-66.010/0109-II/3b/2010) and carried out in line with the European Communities Council Directive.
Induction of TNBS Colitis
Animals were lightly anesthetized with isoflurane. TNBS (4 mg in 100 μl of 30% ethanol) was then infused into the colon through a catheter (outside diameter 1 mm) inserted 3 cm proximally to the anus. Solvent alone (100 μl of 30% ethanol) was administered in control experiments. The dose of TNBS was previously found to induce reproducible colitis with mortality rates in the published range [6].
Drugs and Pharmacological Treatments
TNBS was purchased from Sigma-Aldrich (Vienna, Austria) and CBD was provided by GW Pharma. CBD treatment was started 1 day before TNBS induction and given once daily until the end of the experiments (3 days after TNBS induction). For intraperitoneal treatment (10 mg/kg), CBD was dissolved in vehicle (ethanol, Tween 20 and sterile saline at 1:1:8). For intragastric (by gavage) and intrarectal (by use of a catheter) treatments, canola oil was used as a vehicle. Experiments were also performed with the respective vehicles.
Macroscopic Scoring and Damage Assessment
At the end of the TNBS colitis experiments, mice were killed by cervical dislocation. The colon was immediately removed, rinsed gently with saline solution, opened longitudinally along the mesenteric border and examined. Colonic damage was assessed by a semiquantitative scoring system adapted for mice in the present study [6]. Macroscopic damage was scored according to the following scale, adding individual scores for ulcer, adhesion, colonic shortening, wall thickness, and presence of hemorrhage, fecal blood or diarrhea. Ulcer: 0.5 points for each 0.5 cm; adhesion: 0 points = absent, 1 point = 1 adhesion, 2 points = 2 or more adhesions or adhesions to organs; shortening of the colon: 1 point = >115%, 2 points = >125% (based on a mean length of the untreated colon); wall thickness measured in millimeters. The presence of hemorrhage, fecal blood or diarrhea increased the score by 1 point for each additional feature.
Histology
Following macroscopic scoring, segments of the distal colon were stapled flat onto cardboard with the mucosal side up and fixed for 24 h in 10% neutral-buffered formalin. Tissue was then dehydrated, embedded in paraffin and standard hematoxylin/eosin staining was performed on 5-µm-thick sections.
Determination of Tissue Myeloperoxidase Activity
Myeloperoxidase (MPO) activity represents an index of neutrophil accumulation in the tissue and correlates with the severity of the colitis [21]. Samples of colon were weighed, immediately frozen, and stored at −80°C prior to further processing. For the determination of MPO activity, tissue was placed in 0.5% hexadecyltrimethylammonium bromide buffer (50 mg of tissue/ml; pH 6.0) and disrupted with a homogenizer (UltraTurrax®, IKA, Germany). Hexadecyltrimethylammonium bromide (Sigma-Aldrich) is a detergent that releases MPO from the primary granules of neutrophils and enhances enzyme activity by the presence of bromide. Afterwards, the homogenate was centrifuged for 15 min at maximum speed and 4°C. Before reading MPO activity, 7 μl of supernatant was added to 200 μl of 50 mmol/l potassium phosphate buffer (pH 6.0) containing 0.167 mg/ml of O-dianisidine hydrochloride and 0.5 μl of 1% H2O2/ml. The kinetics of MPO activity was measured at 460 nm (xMarkTM, Bio-Rad, Austria). A mean was calculated for the respective TNBS + vehicle-treated groups and set at 100%. Values of the CBD treatment groups are expressed as percent of the respective TNBS + vehicle-treated group.
Statistical Analysis
From every experimental group, a mean was calculated and differences of means between groups were analyzed by one-way ANOVA followed by Tukey's post hoc test using Graph Pad Prism (Graph Pad Software, San Diego, Calif., USA). p values >0.05 were considered significant.
Results
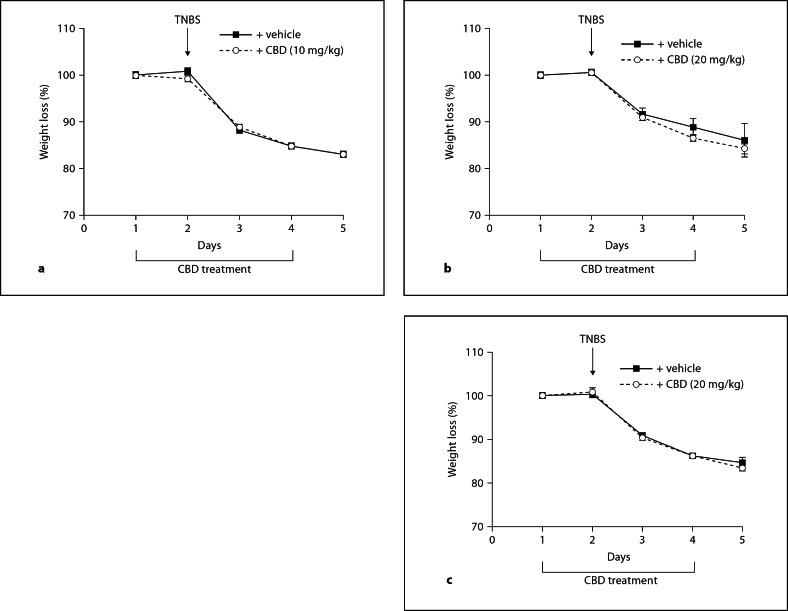
In all treatment groups, mice lost around 10–15% of their body weights after induction of TNBS colitis; however, no differences were observed in body weights between CBD-treated (intraperitoneal, intragastric and intrarectal) and the respective vehicle-treated mice (fig. 1).
Fig. 1.
Graphs showing weight changes (%) in mice during TNBS colitis. No significant differences were seen between vehicle-treated TNBS mice and TNBS mice that received CBD intraperitoneally (a), intragastrically (b) or intrarectally (c). Treatment with CBD (or with the respective vehicle) was started 1 day before application of TNBS and continued once daily for 3 more days. Until the end of the experiment, mice lost about 15% of their body weight. n = 8; significances were tested by one-way ANOVA and Tukey's post hoc test.
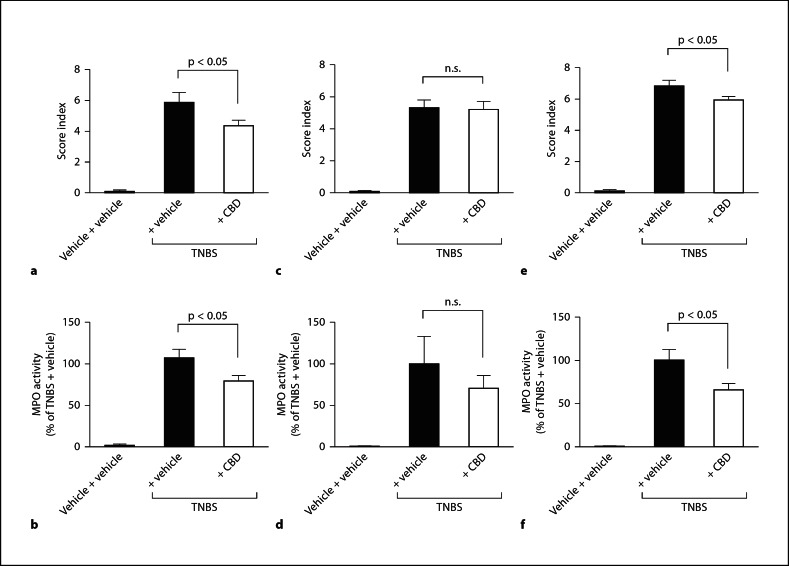
In accordance with Borrelli et al. [8], intraperitoneal injection of 10 mg/kg CBD (once daily) caused a significant improvement of the colitis score index and a decrease in MPO activity (fig. 2). In mice treated with intraperitoneal CBD, histological sections from lesioned areas revealed less destruction of the epithelial lining, a reduction in colon thickness and less infiltration of immunocytes, as compared to intraperitoneally vehicle-treated mice (fig. 3). Intragastric treatment with CBD (20 mg/kg; once daily) did not lead to an improvement of the colitis score (fig. 2). The 20 mg/kg dose for the intragastric treatment was chosen because it proved effective in an inflammatory and neuropathic pain model of the rat [22]. By applying the same dose for intrarectal treatment (20 mg/kg; once daily), a small but significant improvement of the colitis score index was observed (fig. 2). MPO activity was significantly decreased indicating a reduction in the severity of the inflammation (fig. 2). A representative histological section from a lesioned area in the colon of intrarectally CBD-treated mice shows reduced leukocyte infiltration and partially preserved crypt architecture in comparison to intrarectally vehicle-treated mice (fig. 3).
Fig. 2.
Macroscopic scoring and MPO activity assays of mice with TNBS colitis. Intraperitoneal injection of 10 mg/kg CBD (once daily) caused a significant improvement of the colitis score index (a; n = 11–13) and a decrease in MPO activity (b; n = 9–10). Intrarectal application of 20 mg/kg CBD also significantly improved colitis parameters (e; n = 11) and decreased MPO activity (f; n = 11). c Intragastric treatment with CBD (20 mg/kg) did not lead to improvement of the colitis score (n = 11–12). d Differences in MPO activity were not significant (n = 8). Significances were tested by one-way ANOVA and Tukey's post hoc test.
Fig. 3.
The pictures depict representative histological sections of lesioned areas in the colon of TNBS mice. Moderate destruction of the epithelial lining and a reduction in leukocyte infiltration is seen in intraperitoneally (IP) CBD-treated mice, as compared to intraperitoneally vehicle-treated mice. An image of a lesioned area in the colon shows that the crypt architecture of the epithelium was more preserved in intrarectally (IR) CBD-treated mice than in intrarectally vehicle-treated mice. Calibration bar = 500 μm.
Discussion
Over the last decade, cannabinoids and the endocannabinoid system (ECS) have become a hot topic in inflammation research, and evidence is multifold that cannabinoids can protect against different forms of inflammation by targeting CB receptors and other structures within the ECS. Regarding the gastrointestinal tract, cannabinoid-induced protection against experimental gastrointestinal inflammation was first shown by Massa et al. [5] in 2004 and later confirmed and further characterized by others [6, 8, 9, 23, 24]. We now know that these effects involve CB1 and CB2 receptors. Additional targeting of structures within the ECS, like the anandamide-degrading enzyme fatty acid amide hydrolase and the endocannabinoid membrane transporter, results in protection against intestinal inflammation [25]. More importantly, translational studies indicate that the ECS is activated in human IBD [26, 27], suggesting that the ECS not only plays a crucial role in animal models of IBD, but also in human gastrointestinal inflammation. Concepts need to be developed therefore, as to how the knowledge generated from basic research can be translated into humans and how the discovered mechanisms may be applied to result in future treatments of human IBD. Our present study confirms previously published studies [8] for it shows that intraperitoneal CBD protects against intestinal inflammation and we can furthermore show that not only systemically, but also topically applied CBD is protective. This represents a significant extension of our knowledge on how to apply cannabinoids since previous studies in animal models of IBD focussed on pathophysiological mechanisms and, for the ease of use, cannabinoids were applied by intraperitoneal injections. Whereas this way of application is feasible in an experimental setup, there is little doubt that such a mode of action is unlikely to be translated into future therapeutic use for humans [28]. The intraperitoneal route has advantages over the oral route as it bypasses the hepatic metabolism of the employed cannabinoids. It is known that hepatic metabolization is a limiting factor of systemic cannabinoid use [19]. Because promotion of inhaled (smoked) cannabinoids is not advisable from a health perspective, our study is of major interest as it shows for the first time that intrarectal application protects against intestinal inflammation. Intrarectal application is an easy mode of drug application, especially for patients with distal colitis, rectosigmoiditis, proctitis and pouchitis. In human use, it is also one of the preferred modes of application for other compounds like steroids and aminosalicylates [29]. Thus, our results promote the notion that a clinical investigation using CBD in patients with the above-mentioned diseases should be considered. Because CBD, as compared to other cannabinoids, has a favorable side effect profile [18], such a study seems feasible. Hepatic first-pass effects were also demonstrated for cannabinoids of other composition, such as for THC [19], but in contrast to CBD, systemic (e.g. psychotropic) effects were still prevalent [30].
In the present study, no effects of CBD were seen following oral application of 20 mg/kg, a dose that was shown to improve pain in rats caused by chronic sciatic nerve constriction and intraplantar injection of complete Freund's adjuvant [22]. Whether higher doses of CBD or application twice a day and 3 times a day would result in observable effects remains unresolved at the moment. Also, whether rectal CBD protects against colitis by activation of local mechanisms or by systemic effects is unknown and needs to be addressed in a concomitant study. This is of additional interest as a true local effect would allow the development of e.g. slow-release formulations that may be ingested orally and then act throughout the colon, thus being helpful in patients with proximal colitis and pancolitis.
To summarize, CBD was given via 3 different routes of delivery to mice and its effect on the severity of TNBS colitis was compared. We confirm that CBD given intraperitoneally is protective, and we add that CBD given per rectum also offers protective effects, suggesting that rectal application of cannabinoids for the therapy of intestinal inflammation may be a feasible option.
Acknowledgements
We would like to thank Veronika Pommer for excellent technical assistance. R.S. is supported by grants from the Austrian Science Fund (FWF P 22771), Austrian National Bank (OeNB 14429) and Franz Lanyar Foundation (351). M.S. is supported by the Crohn's and Colitis Foundation of Canada and the Deutsche Forschungsgemeinschaft.
References
- 1.Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Engel MA, Neurath MF. New pathophysiological insights and modern treatment of IBD. J Gastroenterol. 2010;45:571–583. doi: 10.1007/s00535-010-0219-3. [DOI] [PubMed] [Google Scholar]
- 3.Danese S, Fiorino G, Reinisch W. Review article: causative factors and the clinical management of patients with Crohn's disease who lose response to anti-TNF-α therapy. Aliment Pharmacol Ther. 2011;34:1–10. doi: 10.1111/j.1365-2036.2011.04679.x. [DOI] [PubMed] [Google Scholar]
- 4.Lal S, Prasad N, Ryan M, Tangri S, Silverberg MS, Gordon A, Steinhart H. Cannabis use amongst patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2011;23:891–896. doi: 10.1097/MEG.0b013e328349bb4c. [DOI] [PubMed] [Google Scholar]
- 5.Massa F, Marsicano G, Hermann H, Cannich A, Monory K, Cravatt BF, Ferri GL, Sibaev A, Storr M, Lutz B. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest. 2004;113:1202–1209. doi: 10.1172/JCI19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Storr MA, Keenan CM, Zhang H, Patel KD, Makriyannis A, Sharkey KA. Activation of the cannabinoid 2 receptor (CB2) protects against experimental colitis. Inflamm Bowel Dis. 2009;15:1678–1685. doi: 10.1002/ibd.20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izzo AA, Camilleri M. Cannabinoids in intestinal inflammation and cancer. Pharmacol Res. 2009;60:117–125. doi: 10.1016/j.phrs.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Borrelli F, Aviello G, Romano B, Orlando P, Capasso R, Maiello F, Guadagno F, Petrosino S, Capasso F, Di Marzo V, Izzo AA. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J Mol Med (Berl) 2009;87:1111–1121. doi: 10.1007/s00109-009-0512-x. [DOI] [PubMed] [Google Scholar]
- 9.Schicho R, Bashashati M, Bawa M, McHugh D, Saur D, Hu HM, Zimmer A, Lutz B, Mackie K, Bradshaw HB, McCafferty DM, Sharkey KA, Storr M. The atypical cannabinoid O-1602 protects against experimental colitis and inhibits neutrophil recruitment. Inflamm Bowel Dis. 2011;17:1651–1664. doi: 10.1002/ibd.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pertwee RG. Pharmacological actions of cannabinoids. Handb Exp Pharmacol. 2005;168:1–51. doi: 10.1007/3-540-26573-2_1. [DOI] [PubMed] [Google Scholar]
- 11.Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- 12.Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30:515–527. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Schuelert N, McDougall JJ. The abnormal cannabidiol analogue O-1602 reduces nociception in a rat model of acute arthritis via the putative cannabinoid receptor GPR55. Neurosci Lett. 2011;500:72–76. doi: 10.1016/j.neulet.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, Razdan RK, Zimmer A, Kunos G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunha JM, Carlini EA, Pereira AE, Ramos OL, Pimentel C, Gagliardi R, Sanvito WL, Lander N, Mechoulam R. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 1980;21:175–185. doi: 10.1159/000137430. [DOI] [PubMed] [Google Scholar]
- 18.Bergamaschi MM, Queiroz RH, Zuardi AW, Crippa JA. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf. 2011;6:237–249. doi: 10.2174/157488611798280924. [DOI] [PubMed] [Google Scholar]
- 19.Huestis MA. Pharmacokinetics and metabolism of the plant cannabinoids, delta9-tetrahydrocannabinol, cannabidiol and cannabinol. Handb Exp Pharmacol. 2005;168:657–690. doi: 10.1007/3-540-26573-2_23. [DOI] [PubMed] [Google Scholar]
- 20.Karschner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA. Plasma cannabinoid pharmacokinetics following controlled oral delta9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin Chem. 2011;57:66–75. doi: 10.1373/clinchem.2010.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- 22.Costa B, Trovato AE, Comelli F, Giagnoni G, Colleoni M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur J Pharmacol. 2007;556:75–83. doi: 10.1016/j.ejphar.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Sibaev A, Massa F, Yüce B, Marsicano G, Lehr HA, Lutz B, Göke B, Allescher HD, Storr M. CB1 and TRPV1 receptors mediate protective effects on colonic electrophysiological properties in mice. J Mol Med (Berl) 2006;84:513–520. doi: 10.1007/s00109-006-0040-x. [DOI] [PubMed] [Google Scholar]
- 24.Kimball ES, Schneider CR, Wallace NH, Hornby PJ. Agonists of cannabinoid receptor 1 and 2 inhibit experimental colitis induced by oil of mustard and by dextran sulfate sodium. Am J Physiol Gastrointest Liver Physiol. 2006;291:G364–G371. doi: 10.1152/ajpgi.00407.2005. [DOI] [PubMed] [Google Scholar]
- 25.Storr MA, Keenan CM, Emmerdinger D, Zhang H, Yüce B, Sibaev A, Massa F, Buckley NE, Lutz B, Göke B, Brand S, Patel KD, Sharkey KA. Targeting endocannabinoid degradation protects against experimental colitis in mice: involvement of CB1 and CB2 receptors. J Mol Med. 2008;86:925–936. doi: 10.1007/s00109-008-0359-6. [DOI] [PubMed] [Google Scholar]
- 26.Stintzing S, Wissniowski TT, Lohwasser C, Alinger B, Neureiter D, Ocker M. Role of cannabinoid receptors and RAGE in inflammatory bowel disease. Histol Histopathol. 2011;26:735–745. doi: 10.14670/HH-26.735. [DOI] [PubMed] [Google Scholar]
- 27.Marquéz L, Suárez J, Iglesias M, Bermudez-Silva FJ, Rodríguez de FF, Andreu M. Ulcerative colitis induces changes on the expression of the endocannabinoid system in the human colonic tissue. PLoS One. 2009;4:e6893. doi: 10.1371/journal.pone.0006893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schicho R, Storr M. Targeting the endocannabinoid system for gastrointestinal diseases: future therapeutic strategies. Expert Rev Clin Pharmacol. 2010;3:193–207. doi: 10.1586/ecp.09.62. [DOI] [PubMed] [Google Scholar]
- 29.Schölmerich J. Review article: systemic and topical steroids in inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20(suppl 4):66–74. doi: 10.1111/j.1365-2036.2004.02059.x. [DOI] [PubMed] [Google Scholar]
- 30.Ashton CH. Adverse effects of cannabis and cannabinoids. Br J Anaesth. 1999;83:637–649. doi: 10.1093/bja/83.4.637. [DOI] [PubMed] [Google Scholar]