Abstract
Introduction
Significant preclinical evidence exists for a synergistic interaction between the opioid and the serotonin systems in determining alcohol consumption. Naltrexone, an opiate receptor antagonist, is approved for the treatment of alcohol dependence. This double-blind placebo-controlled study examined whether the efficacy of naltrexone would be augmented by concurrent treatment with sertraline, a selective serotonin receptor uptake inhibitor (SSRI).
Methods
One hundred and thirteen participants meeting DSM IV alcohol dependence criteria, who were abstinent from alcohol between 5 and 30 days, were randomly assigned to receive one of two treatments at two sites. One group received naltrexone 12.5 mg once daily for 3 days, 25mg once daily for 4 days, and 50 mg once daily for the next 11 weeks, together with placebo sertraline. The other group received naltrexone as outlined and simultaneously received sertraline 50 mg once daily for 2 weeks, followed by 100 mg once daily for 10 weeks. Both groups received group relapse prevention psychotherapy on a weekly basis.
Results
Compliance and attendance rates were comparable and high. The groups did not differ on the two primary outcomes, time to first drink and time to relapse to heavy drinking, or on secondary treatment outcomes. With the exception of sexual side effects which were more common in the combination group, most adverse events were similar for the two conditions.
Conclusions
At the doses tested in combination with specialized behavioral therapy, this study does not provide sufficient evidence for the combined use of sertraline and naltrexone above naltrexone alone.
Keywords: Alcohol Dependence, Sertraline, Naltrexone, Clinical Trial, Alcohol Typology
1. Introduction
The FDA approval of the opioid antagonist naltrexone on the basis of two successful clinical trials (O'Malley et al., 1992; Volpicelli et al., 1992) opened a new vista for the use of pharmacotherapy in conjunction with psychotherapy to treat alcohol dependent individuals. With the publication of further positive trials of naltrexone (for reviews see Kranzler and Van Kirk, 2001; Pettinati et al., 2006) including a large NIH sponsored multi-site study (Anton et al., 2006), it has become clear that naltrexone has a useful place in the range of treatment options. Strategies to further enhance treatment response are being examined, including long acting naltrexone formulations (Garbutt et al., 2005 Kranzler et al, 2004; O’Malley et al., 2007) and combination therapies (e.g., Anton et al., 2006; Johnson et al., 2000; Keifer et al., 2003).
Several lines of research suggest that selective serotonin reuptake inhibitors (SSRIs) may be useful for augmenting naltrexone response. The serotonin system has long been found to be abnormal in preclinical and clinical studies of alcoholism (LeMarquand et al., 1994a; 1994b). Preclinical models have also shown that the combination of an SSRI and naltrexone results in greater suppression of drinking compared to either drug alone (Zink et al., 1997; Rezvani et al., 2000). Potential additive effects are also suggested by preclinical work showing that fluoxetine reduces stress induced reinstatement of drinking and naltrexone reduces alcohol reinstatement (Le et al., 1999). Although most clinical trials find that SSRIs are ineffective in treating alcohol dependence in unselected patients (Mann, 2004), some SSRIs may have therapeutic benefit in at least a typology-based subgroup (Kranzler et al., 1996; Pettinati et al., 2001; Tiihonen et al., 1996). For example, Pettinati and colleagues (2001) found increased abstinence with the use of 200 mg of sertraline in Type A (less severe) alcohol dependence. Finally, preliminary evidence from small open label studies combining naltrexone with agents targeting the serotonin system suggested benefit in nondepressed alcohol dependent patients (Farren et al., 2000; Johnson et al., 2000).
We thus examined the addition of the SSRI sertraline to naltrexone, in combination with standard cognitive behavioral psychotherapy, in a 12-week double-blind placebo-controlled trial in non-depressed alcohol dependent individuals. Exploratory analyses examined whether age of onset (early versus late) influenced the results.
2. Materials and Methods
The study was carried out at two sites, Yale University School of Medicine in New Haven, CT and Mount Sinai School of Medicine in New York City. The protocol was approved by the local IRBs and all participants provided informed consent.
2.1 Medication
Participants were randomly assigned to one of two medication groups. The naltrexone only group received naltrexone 12.5 mg daily for 3 days, 25mg daily for 4 days, and 50 mg daily for the next 11 weeks, together with placebo sertraline. The combination group received the same naltrexone regimen, but simultaneously received sertraline 50 mg daily for 2 weeks, followed by 100 mg daily for 10 weeks. Groups were randomized within site according to a computerized schedule. Pill counts were performed weekly for the first 2 weeks, and biweekly for the next 10 weeks. Participants also self-reported medication compliance. Riboflavin was added to the sertraline/placebo capsules by the dispensing pharmacy and urinary fluorescence was measured at the same frequency (Del Boca et al., 1996).
2.2 Therapy
Subjects received weekly group relapse prevention psychotherapy (Project Match Research Group, 1997) for 12 weeks. Therapy sessions were conducted by masters or doctoral level psychotherapists and subjects were encouraged to attend Alcoholics Anonymous meetings.
2.3 Recruitment
Potential participants responded to local advertisements. Subjects ranged in age from 19–64 and met DSM-IV criteria for alcohol dependence (Spitzer and Williams, 1992). Between 5 and 30 days of alcohol abstinence prior to medication administration was required. Subjects were excluded if they met criteria for current abuse or dependence on any substance other than nicotine or alcohol, had a current Axis I disorder in addition to alcohol dependence (including major depression), or reported any past illicit opiate use. Other exclusions were significant liver disease (AST or ALT > 300% of upper limit of normal or bilirubin > 110% of upper limit of normal), a positive BAL during evaluation, or any major physical illness.
2.4 Assessments
The Addiction Severity Index (ASI) (McLellan et al., 1980), the Obsessive Compulsive Drinking Scale (OCDS) (Anton et al., 1996), the Beck Depression Inventory (BDI) (Beck et al., 1961), and the Time-line Follow-back Interview (TLFB) (Sobell et al., 1988) were completed at baseline. Blood tests including serum aspartate aminotransferase (AST) and gamma-glutamyltransferase (GGT) were obtained at baseline, 4 and 12 weeks. The following assessments were made weekly for the first two weeks and biweekly subsequently: the OCDS, the BDI, the TLFB, and a side-effects scale.
The primary outcomes included time to first drink and time to first relapse to heavy drinking (with relapse defined as 5 drinks in one day for men, and 4 drinks in one day for women). Percent days abstinent, number of drinks per drinking day for drinkers, change in Obsessive Compulsive Drinking Scale total scores, and ALT and GGT change from baseline were secondary outcomes.
2.5 Data Analysis
Data was analyzed using SAS version 9.1 (SAS Institute, Inc, Cary, NC). The primary analysis was conducted on the intention to treat sample (n = 111). The survival analysis with maximum likelihood method was used to analyze the time-to-event outcomes. Missing data was censored. Mixed models were used for repeated outcomes and Analysis of Variance was applied to all other continuous outcomes. Site was a covariate in the analyses.
3 Results
3.1 Subject Characteristics
The study was completed over 5 years. A total of 605 patients (299 Yale, 306 Mt Sinai) were screened to produce randomization of 113 subjects (41 Yale, 72 Mt Sinai) over the two sites. The majority of patients were ineligible for randomization because of the presence of depressive symptoms or failure to follow up. Two subjects were subsequently excluded from the intention to treat analysis at Mt. Sinai because they failed to meet entry criteria yielding a final sample of 111. Although participants at the two sites were similar in most characteristics, the Mt. Sinai group had a higher percentage of participants with high school education or greater (98.5% vs. 78.1%, p< .001), had fewer Whites (61.2% vs. 87.8%, p< .01), and reported fewer baseline drinks per drinking day (5.7 ± 2.90 vs. 10.0 ± 6.0, p<0.01).
Table 1 presents the baseline characteristics of the sample. Fifty-seven participants received Naltrexone with Sertraline and 54 subjects received Naltrexone and Placebo. Twenty-three of the combination group did not complete, with 11 lost to follow up, 6 having poor compliance, and 6 due to side effects. Fourteen of the placebo group did not complete with 10 being lost to follow up, 2 having poor compliance, and 2 due to side effects.
Table 1.
Baseline characteristics and drinking outcomes [mean +/− S.D.].
| Characteristic | Naltrexone + Sertraline (N=57) |
Naltrexone + Placebo (N=54) |
|---|---|---|
| Age | 41.5 (9.55) | 44.9 (9.68) |
| Male Gender (%) | 80.7 | 83.3 |
| Race or Ethnic group (%): | ||
| White | 71.4 | 71.2 |
| Black | 12.5 | 11.5 |
| Hispanic | 10.7 | 11.5 |
| Other | 5.4 | 5.8 |
| Marital Status: | ||
| % Married or living with partner | 33.9 | 46.2 |
| High school or greater | 91.1 | 90.4 |
| Age of onset of alcohol-related problems | 30.8 (10.09) | 33.3 (8.72) |
| % days abstinent | 29.3 (19.13) | 25.6 (18.66) |
| Drinks per drinking day in previous 90 days | 7.3 (4.82) | 7.4 (4.78) |
| ALT | 7.8 (5.72) | 7.3 (5.30) |
| GGT | 92.7 (122.39) | 118.0 (208.76) |
| Drinking Outcomes (%) | ||
| Drinks per drinking day for drinkers a | 5.3 (4.06) | 4.3 (4.29) |
| Drinks per drinking day for all subjects | 2.8 (3.95) | 3.0 (4.09) |
| Percent days abstinent | 79.2 (30.48) | 84.5 (17.47) |
| OCDS Total b | 7.4 (5.50) | 7.7 (6.23) |
| GGT Change from Baseline | 26.4 (44.70) | 60.5 (170.88) |
| ALT Change from Baseline | 4.0 (33.9) | 10.3 (23.8) |
No significant differences between groups in their baseline characteristics or drinking outcomes.
N = 28 for naltrexone + sertraline and N = 37 for naltrexone + placebo.
Mixed model.
3.2 Compliance
During treatment, medication compliance rates by pill count and urinary riboflavin were similar. The number of therapy sessions attended was equivalent, with 7.3 (± 0.5) for the combination group and 8.5 (± 0.3) for the placebo group. The number and percentage of each group with complete drinking data was also not significantly different between the combination group (42, 73.7%) and the placebo group (44, 81.5%).
3.3 Outcomes
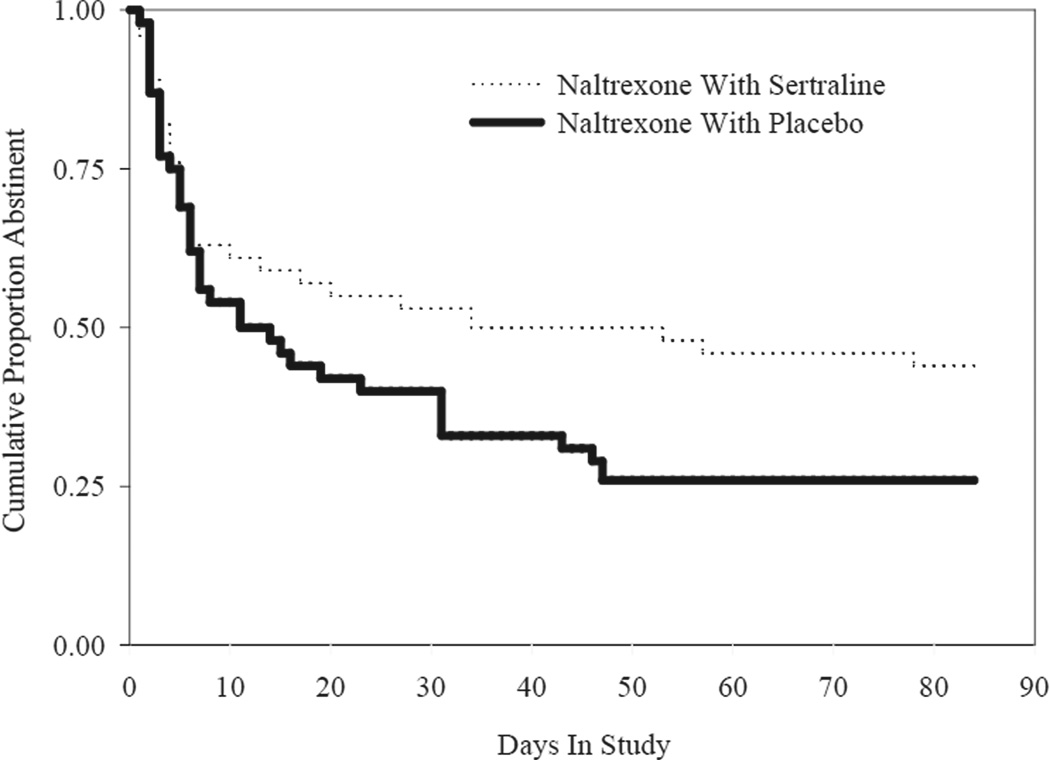
Time to the first day of drinking was longer, although not statistically different (p = 0.10; Figure 1) for the combination group (49.1% abstinent) compared to the naltrexone alone group (31.5% abstinent). There was no significant difference between groups in time to first relapse to heavy drinking (p = 0.13), time to second drink (p = 0.13), or in percent days abstinent (p=0.19). Among those subjects who drank (n = 64), the groups were similar on drinks per drinking day (p=0.19). There was also no significant difference in change in GGT (p=0.30), ALT scores (p=0.31), BDI or OCDS scores.
Figure 1. Time to first drink for naltrexone with sertraline (N=57) and naltrexone with placebo (N=54) groups.
With missing data as censored, the analysis showed a time to first drinking day for the naltrexone plus sertraline group as 29 days (50.88% censored) versus 18 (33.33% censored) for the naltrexone alone group, p=0.10.
Rates of the majority of reported side effects, including disrupted sleep, decreased sex drive, low energy, nausea, headache and altered appetite were not significantly different between groups. There were more reported sexual side effects in the combination group (68% vs. 24%, p<0.001). The incidence of reported side effects peaked at week one in both groups (data not shown), with the frequency and intensity of most side effects, except sexual side effects, declining over time.
Exploratory analyses of outcomes by subtype of alcoholism were also completed with age of onset used as the criterion for classifying participants as having Type I (late onset at age 25 or older) or Type II (early onset prior to age 25) alcoholism. There were 81 Type I (75.7%) and 26 Type II (24.3%) individuals with 4 patients unassigned due to missing data on age of onset. The previous models of drinking outcomes were repeated examining the main and interactive effects of medication group and typology. All main effects and interactions were non-significant.
4. Discussion
This placebo-controlled study did not support the hypothesis that sertraline would augment the efficacy of naltrexone in combination with group relapse prevention therapy. Although the combination group had a somewhat higher rate of abstinence compared to the naltrexone only group, this difference was not statistically significant. Moreover, there was no added benefit of the combined treatment in terms of prevention of relapse to heavy drinking or on any other secondary outcomes. The negative results are in contrast to our preliminary study which compared two groups of matched patients treated open label with either the combination or naltrexone monotherapy (Farren et al., 2000), but are consistent with a recent placebo-controlled study conducted in Alaska (O’Malley et al., 2008).
The decision to recruit non-depressed individuals with alcohol dependence was made on the basis of the preclinical and clinical evidence for the general involvement of the serotonin system in alcoholism (LeMarquand et al., 1994a). In retrospect, the choice of a non-depressed group may have made a positive outcome less likely, as one prior study noted a benefit of serotonin medications in depressed alcohol dependent patients (Cornelius et al., 1997), although other studies have not (Moak et al., 2003; Kranzler et al., 2006). Type A alcohol dependent patients have been shown to benefit from SSRI’s in prior research (Pettinati et al., 2000), and we conducted exploratory analyses examining alcoholism typology (early versus late onset) as a predictor of response, but did not find an advantage for the combination of sertraline and naltrexone in Type 1 or early onset patients. Possible explanations include the small sample size and the fact that all patients received active naltrexone, an effective treatment that may have obscured any potential benefit of sertraline for this subgroup. Perhaps analyses using a typology based on severity rather than age of onset might have had different results; unfortunately we did not have the data needed to classify participants according the Babor typology (Pettinati et al., 2001; Kranzler et al., 1996).
A different dose of sertraline may have been more effective. For example, Pettinati et al (2000) reported positive results using 200mg daily of sertraline. Our study was initiated prior to that report, and the 100 mg dose was selected based on the results of a pilot study (Farren et al., 2000), preclinical studies (Gill, et al, 1988; Higgins et al, 1992; Rezvani et al., 1998), and the possible escalation of side effects with higher doses of sertraline, particularly in combination with naltrexone. In the current study, although only sexual side effects were higher in the combination group, 40.3% (23/57) of participants in the combination group failed to complete treatment compared to 25.9% (14/54) of participants in the naltrexone only group. In addition, discontinuation due to adverse events was more common in the combination group (6 vs. 2), suggesting that the potential for increased efficacy with a higher sertraline dose might be compromised by poorer tolerability, at least in combination with naltrexone. There is no evidence in the extant literature for a pharmacokinetic interaction between the two compounds that might alter either’s blood levels or side effect profiles.
In conclusion, the study’s findings do not support the use of 100 mg of sertraline to augment the efficacy of naltrexone for the treatment of nondepressed alcohol dependent patients. Other doses, other induction schedules or durations of treatment may have been more effective. Future trials of combination drug therapies should consider incorporating a less intensive behavioral platform, as there may be a ceiling effect when pharmacotherapies are tested with intensive psychotherapy. In the present study, all participants received naltrexone and CBT therapy, both effective treatments (Anton et al., 2006), and both groups showed substantial improvements.
Acknowledgements
This study was supported by National Institutes of Health grants RO1AA11122, K05AA014715, by the Mount Sinai GCRC, and by the State of Connecticut, Department of Mental Health and Addiction Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism or the National Institute of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anton R, Moak DH, Latham P. The obsessive-compulsive drinking scale: A new method of assessing outcome in alcoholism treatment studies. Arch Gen Psychiatry. 1996;53:225–231. doi: 10.1001/archpsyc.1996.01830030047008. [DOI] [PubMed] [Google Scholar]
- Anton RF, Pettinati H, Zweben A, Kranzler HR, Johnson B, Bohn M, McCaul ME, Anthenelli R, Salloum I, Galloway G, Garbutt J, Swift R, Gastfriend D, Kallio A, Karhuvarra S. A multi-site dose ranging study of nalmefene in the treatment of alcohol dependence. J Clin Psychopharmacol. 2004;24:421–428. doi: 10.1097/01.jcp.0000130555.63254.73. [DOI] [PubMed] [Google Scholar]
- Anton R, O’Malley S, Ciraulo D, Cisler R, Couper D, Donovan D, Hosking J, Johnson B, LoCastro J, Longabaugh R, Mason B, Mattson M, Miller W, Pettinati H, Randall C, Swift R, Weiss R, Williams L, Zweben A. Combination pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized clinical trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Beck A, Ward C, Mendelson M. An inventory for measuring depression. Arch Gen Psychiatry. 1961;3:461–471. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Cornelius J, Salloum I, Ehler J. Fluoxetine in depressed alcoholics: a double-blind, placebocontrolled trial. Arch Gen Psychiatry. 1997;54:700–770. doi: 10.1001/archpsyc.1997.01830200024004. [DOI] [PubMed] [Google Scholar]
- Del Boca F, Kranzler H, Brown J, Korner P. Assessment of medication compliance through UV light detection of a riboflavin tracer. Alcohol Clin Exp Res. 1996;20:1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Farren CK, Rezvani AH, Overstreet D, O’Malley S. Combination pharmacotherapy in alcoholism: a novel treatment approach. CNS Spectrums. 2000;5:70–76. doi: 10.1017/s1092852900012839. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O’Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, Loewy JW, Ehrich EW, Vivitrex Study G. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293(13):1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- Gill K, Amit Z, Koe BK. Treatment with sertraline, a new serotonin uptake inhibitor, reduces voluntary ethanol consumption in rats. Alcohol. 1988;5:349–354. doi: 10.1016/0741-8329(88)90019-5. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Tomkins DM, Fletcher PJ, Sellers EM. Effect of drugs influencing 5-HT function on ethanol drinking and feeding behavior in rats: studies using a drinkometer system. Neurosci Biobehav Rev. 1992;16:535–552. doi: 10.1016/s0149-7634(05)80195-2. [DOI] [PubMed] [Google Scholar]
- Johnson B, Ait-Daoud N, Prihoda T. Combining ondansetron and naltrexone effectively treats biologically predisposed alcoholics: from hypothesis to preliminary clinical evidence. Alcohol Clin Exp Res. 2000;24:737–742. [PubMed] [Google Scholar]
- Keifer F, Jahn H, Tarnaske T, Helwig H, Briken P, Holzbach R, Kahn P, Stracke R, Baehr M, Naber D, Wiedermann K. Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2003;60:92–99. doi: 10.1001/archpsyc.60.1.92. [DOI] [PubMed] [Google Scholar]
- Kranzler H, Burleson J, DelBoca F, Babor T, Korner P, Brown J, Bohn M. Buspirone treatment of anxious alcoholics. A placebo-controlled trial. Arch Gen Psychiatry. 1994;51:720–731. doi: 10.1001/archpsyc.1994.03950090052008. [DOI] [PubMed] [Google Scholar]
- Kranzler H, Burleson J, Brown J, Babor T. Fluoxetine treatment seems to reduce the beneficial effects of cognitive-behavioral therapy in type B alcoholics. Alcohol Clin Exp Res. 1996;20:1534–1541. doi: 10.1111/j.1530-0277.1996.tb01696.x. [DOI] [PubMed] [Google Scholar]
- Kranzler H, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: A meta-analysis. Alcohol Clin Exp Res. 2001;25:1334–1341. [PubMed] [Google Scholar]
- Kranzler HR, Wesson DR, Billot L Drug Abuse Sciences Naltrexone Depot Study Group. Naltrexone depot for treatment of alcohol dependence: a multicenter, randomized, placebocontrolled clinical trial. Alcohol Clin Exp Res. 2004;28:1051–1059. doi: 10.1097/01.alc.0000130804.08397.29. [DOI] [PubMed] [Google Scholar]
- Kranzler H, Mueller T, Cornelius J, Pettinati HM, Moak D, Martin PR, Anthenelli R, Brower K, O’Malley S, Mason BJ, Hasin D, Keller M. Sertraline treatment of co-occurring alcohol dependence and major depression. J Clin Psychopharmacol. 2006;26:13–20. doi: 10.1097/01.jcp.0000194620.61868.35. [DOI] [PubMed] [Google Scholar]
- Krystal J, Cramer J, Krol W, Kirk G, Rosenheck R. Naltrexone in the treatment of alcohol dependence. New Eng J Medicine. 2001;345:1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Le A, Sellers E. Interaction between opiate and 5-HT3 receptor antagonists in the regulation of alcohol intake. Alcohol Alcohol. 1996;(Suppl. 2):545–549. [PubMed] [Google Scholar]
- Le A, Poulos C, Harding S, Watchus J, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- LeMarquand D, Pihl R, Benkelfat C. Serotonin and alcohol intake, abuse and dependence: clinical evidence. Biol Psychiatry. 1994a;36:326–337. doi: 10.1016/0006-3223(94)90630-0. [DOI] [PubMed] [Google Scholar]
- LeMarquand D, Pihl R, Benkelfat C. Serotonin and alcohol intake, abuse and dependence: findings of animal studies. Biol Psychiatry. 1994b;36:395–421. doi: 10.1016/0006-3223(94)91215-7. [DOI] [PubMed] [Google Scholar]
- Mann K. Pharmacotherapy of alcohol dependence: a review of the clinical data. CNS Drugs. 2004;18:485–504. doi: 10.2165/00023210-200418080-00002. [DOI] [PubMed] [Google Scholar]
- McLellan A, Luborsky L, Woody G, O’Brien C. An improved diagnostic instrument for substance abuse subjects: the addiction severity index. J Nerv Ment Disorders. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Moak DH, Anton RF, Latham PK, Voronin KE, Waid RL, Durazo-Arvizu R. Sertraline and cognitive behavioral therapy for depressed alcoholics: results of a placebo-controlled trial. J Clin Psychopharmacology. 2003;23:553–562. doi: 10.1097/01.jcp.0000095346.32154.41. [DOI] [PubMed] [Google Scholar]
- O’Malley S, Jaffe A, Chang G, Schottenfeld R, Meyer R, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. Arch Gen Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Garbutt JC, Gastfriend DR, Dong Q, Kranzler HR. Efficacy of extendedrelease naltrexone in alcohol-dependent patients who are abstinent before treatment. J Clin Psychopharmacol. 2007;27:507–12. doi: 10.1097/jcp.0b013e31814ce50d. [DOI] [PubMed] [Google Scholar]
- OMalley SS, Robin RW, Levenson AL, GreyWolf I, Chance LE, Hodgkinson CA, Romano D, Robinson J, Meandzija B, Stillner V, Wu R, Goldman D. Naltrexone alone and with sertraline for the treatment of alcohol dependence in Alaska Natives and non-Natives residing in rural settings: a randomized controlled trial. Alc: Clin Exp Res. 2008 doi: 10.1111/j.1530-0277.2008.00682.x. published article online: 14-May-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinati HM, O’Brien CP, Rabinowitz AR, Wortman SP, Oslin DW, Kampman KM, Dackis CA. The status of naltrexone in the treatment of alcohol dependence: Specific effects on heavy drinking. J Clin Psychiatry. 2006;26:610–625. doi: 10.1097/01.jcp.0000245566.52401.20. [DOI] [PubMed] [Google Scholar]
- Pettinati H, Volpicelli J, Kranzler H, Luck G, Rukstalis M, Cnaan A. Sertraline treatment for alcohol dependence: interactive effects of medication and alcoholic subtype. Alcohol Clin Exp Res. 2000;24:1041–1049. [PubMed] [Google Scholar]
- Pettinati H, Volpicelli J, Luck G, Kranzler H, Rukstalis M, Cnaan A. Double-blind clinical trial of sertraline treatment for alcohol dependence. J Clin Psychopharmacology. 2001;21:143–153. doi: 10.1097/00004714-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Project-Match RG. Matching alcoholism treatment to client heterogeneity: Project MATCH posttreatment drinking outcomes. J Stud Alcohol. 1997;58:7–29. [PubMed] [Google Scholar]
- Rezvani A, Overstreet D, Mason G, Janowsky D, Hamedi M, Clarke EJ, Yang Y. Combination pharmacotherapy: a mixture of small doses of naltrexone, fluoxetine, and a thyrotropin-releasing hormone analogue reduces alcohol intake in three strains of alcoholpreferring rats. Alcohol Alcohol. 2000;35:76–83. doi: 10.1093/alcalc/35.1.76. [DOI] [PubMed] [Google Scholar]
- Sobell L, Sobell M, Leo G, Cancill A. Reliability of a timeline method: assessing normal drinker reports of recent drinking and a comparative evaluation across several populations. Brit J Addiction. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Spitzer R, Williams J. Structured Clinical Interview for DSM IV. New York: Biometric Research Department, New York State Psychiatric Institute; 1992. [Google Scholar]
- Tiihonen J, Ryynanen O, Kauhanen J, Hakola H, Salaspuro M. Citalopram in the treatment of alcoholism: a double-blind placebo-controlled study. Pharmacopsychiatry. 1996;29:27–29. doi: 10.1055/s-2007-979538. [DOI] [PubMed] [Google Scholar]
- Tonigan J, Miller W. Research Society on Alcoholism. San Antonio, Texas: 1993. Assessment and validation of the drinker inventory of consequences (DRINC): a multi-site outpatient aftercare clinical sample of problem drinkers. [Google Scholar]
- Volpicelli J, Alterman A, Hayashida M, O’Brien C. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Zink RW, Rohrbach K, Froehlich JC. Naltrexone and fluoxetine act synergistically to decrease alcohol intake. Alcohol Clin Exp Res. 1997;21(suppl):104A. [Google Scholar]