Abstract
The axon repulsion factor Semaphorin3A (SEMA3A) and its receptor Neuropilin-1 (NP-1) are expressed in breast tumor cells, and function as suppressors of tumor cell migration. Based on the knowledge that both SEMA3A and the α2β1 integrin suppress breast tumor cell migration, we studied the impact of SEMA3A signaling on α2β1 integrin expression/function. The incubation of breast tumor cells with SEMA3A increased α2 and β1 integrin levels, and stimulated tumor cell adhesion to the α2β1-binding matrix protein collagen I. Conversely, reducing SEMA3A expression in breast tumor cells decreased α2β1 levels and collagen adhesion. The ability of SEMA3A to increase tumor cell adhesion to collagen was dependent on both the SEMA3A receptor NP-1 and the Glycogen Synthase Kinase -3. The incubation of breast tumor cells with SEMA3A disrupted the actin cytoskeleton, and reduced both tumor cell migratory and invasive behavior. Importantly, using an α2β1-neutralizing antibody, we demonstrated that SEMA3A suppression of tumor cell migration is dependent on α2β1. Our studies indicate that expression of the α2β1 integrin, a suppressor of metastatic breast tumor growth, is stimulated in breast tumor cells by an autocrine SEMA3A pathway.
Keywords: Semaphorin3A, Neuropilin-1, integrin, migration, breast tumor
Introduction
The axon repulsion factor Semaphorin3A (SEMA3A) promotes growth cone collapse by binding to its receptor, Neuropilin-1 (NP-1) [1, 2]. Interestingly, SEMA3A and NP-1 are also expressed in endothelial cells, and serve as endogenous suppressors of integrin activity [3, 4]. Previously, our laboratory made the exciting observation that breast carcinoma cells support an autocrine signaling pathway defined by SEMA3A and NP-1 that represses cell migration [5]. However, the targets of SEMA3A signaling that impede breast tumor cell migration, and the impact of SEMA3A signaling on integrin activity in tumor cells remains unclear.
Significant evidence exists that α2β1, an integrin that binds to collagen and in some cells to laminin-1[6], is a differentiation marker for breast epithelial and tumor cells. α2 integrin knockout mice exhibit impaired mammary gland branching morphogenesis [7]. Transgenic mice expressing a β1 integrin mutant protein lacking the extracellular domain have under-developed mammary glands with defects in epithelial differentiation [8, 9]. Finally, the degree of differentiation of primary ductal breast tumors correlates positively with α2β1 integrin expression levels [10–12].
During their metastatic progression, tumor cells acquire the ability to migrate and invade tissue. Although a baseline level of the α2β1 integin is important for breast tumor cell migration and tumor growth [13, 14], excessive α2β1 integrin expression is inhibitory for tumor cell migration [15, 16]. It has been demonstrated that breast tumor cell motility is increased upon reducing α2β1 integrin expression levels in breast tumor cells [15]. Furthermore, the forced expression of α2β1 in an α2β1 integrin-deficient breast tumor cell line impairs cell motility and tumorigenesis [16]. Finally, α2β1 integrin expression is significantly reduced in metastatic relative to non-metastatic ductal breast tumors [11, 12]. Collectively, these findings suggest that the α2β1 integrin can impede breast tumor metastatic progression by suppressing cell motility. These studies stress the importance of identifying endogenous proteins in breast tumor cells that regulate α2β1 integrin expression. In the current work, we define an ability of autocrine SEMA3A to stimulate α2β1 integrin expression in breast tumor cells, resulting in impaired tumor cell migration.
Materials and Methods
Cell Culture and Reagents
Recombinant human Semaphorin-3A/Fc, recombinant human IgG1 Fc and Neuropilin-1-neutralizing antibody (MAB566) were obtained from R&D Systems, Inc. (Minneapolis, MN). Bovine collagen type I and Fibronectin were purchased from BD Biosciences (Bedford, MA). Bovine serum albumin was purchased from Sigma (Milwaukee, WI). HB1.1 (mouse anti-β1 integrin), HUTS-4 (mouse anti-β1 integrin, active conformation), BHA2.1 (mouse anti-human α2β1 integrin, neutralizing), rabbit anti-human integrin α2 (CD49b) and rabbit anti-GSK-3 were purchased from Chemicon International (Temecula, CA). Donkey anti-rabbit (Fab)2 and anti-mouse (Fab)2 antibodies conjugated with horseradish peroxidase were from Jackson ImmunoResearch Labs (West Grove, PA). The sources of other antibodies were as follows: rabbit anti-phospho-GSK-3β(Ser9) (Cell Signaling Technology; Danvers, MA), mouse anti-β-actin (Sigma; Milwaukee, WI), rabbit anti-human SEMA3A (ECM Biosciences; Versailles, KY), isotype control antibody (mouse IgG1, Jackson ImmunoResearch Labs, West Grove, PA). Western Lightning Chemiluminescence Reagent was from PerkinElmer (Boston, MA). GSK-3 inhibitor SB415286 was obtained from Biomol (Plymouth Meeting, PA).
Adhesion Assay
Serum-starved cells (10,000) were detached for 5 min with 0.25% Trypsin and resuspended in serum-free medium containing the indicated stimuli and/or antibodies. These cells were immediately plated in triplicate on 96-well microtiter plates (Fisher Scientific) pre-coated with bovine collagen type I (20μg/mL) or bovine serum albumin (BSA) (20 μg/mL), and blocked with 0.5% BSA/DMEM. After 40 min. at 37°C, these wells were washed with PBS, fixed with methanol for 10 min., and stained with 0.2% crystal violet containing 2% ethanol for 15 min. Cell adhesion was quantified in an ELISA reader by measuring absorbance (OD=595nm). Specific adhesion to collagen was determined by subtracting the mean OD595 (from triplicate wells) obtained on BSA from the mean OD595 (from triplicate wells) obtained on collagen +/− standard deviation (SD). Statistical significance was determined using a Student’s t-test, with a p<0.05 being considered statistically significant.
Actin Staining
MDA-MB-231 cells were grown on chamber slides (BD Bioscience Discovery Labware, Two Oak Park, Bedford, MA) to reach 70% confluence. After serum starving these cells overnight, they were incubated with rSEMA3A for 30 minutes. After three washes with PBS, cells were fixed in 4% paraformaldehyde/PBS for 15 min, permeabilized with 0.20% Triton X-100/PBS for 20 min, and blocked in 5% Bovine Serum Albumin/PBS (BSA) for 1 hour. The slides were then incubated with Texas red-phalloidin (Becton Dickinson; 1:40) for 1 hour, washed three times with PBS, incubated with DAPI for 3 min, and mounted in glycerol containing 0.1% propylgallate. Cells were examined by confocal microscopy (Light Microscopy Core Facility, Duke University) using a 40X objective.
Migration Assay
Cell migration assays were performed as previously described [5]. In brief, serum-starved cells were seeded into the top chambers of transwell plates (Corning Inc., Acton, MA) in the presence of the indicated stimuli and/or antibodies. Culture medium containing 10% FBS was added to the bottom chambers. After 4 hours, the cells on the bottom side of the membrane were fixed and stained with 0.2% crystal violet. The number of cells on the bottom side of the membrane were counted on an inverted microscope using a 20X objective. The mean number of migrated cells from triplicate wells +/− standard deviation (SD) was determined.
Invasion Assay
MDA-MB-231 cells (80% confluence) were serum starved overnight. These cells (1 x 105) were placed in the upper chamber of transwells that had been coated with Matrigel (Becton-Dickinson; 5 μg/well) and blocked with BSA (250 μg/mL)/DMEM. The ability of these cells to invade Matrigel and migrate toward 5% FBS-containing medium in the bottom chamber was measured. After 5 hours, cells adhering to the bottom side of the membrane were fixed, stained with crystal violet, and counted as described for the migration assay.
Imaging Migratory Cells in Transwells
Cells were imaged using a Leica DMI4000 B (Leica Microsystems inc. Bannockburn IL) inverted microscope using the brightfield setting. The images were captured with a Retiga EXi camera and a Sony progressive scan interline CCD sensor (Q imaging, Burvinab BC Canada). Images were displayed using Simplepci software, version 6.1.2 (Compix Inc, Sewickley PA) on a Dell Optiplex GX620 computer (Dell Roundrock TX).
Immunoblotting and Immunoprecipitation
Proteins were extracted on ice from cells (80–90% confluence) in lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 0.5% Nonidet P-40, pH 7.8) containing proteinase inhibitor cocktail (Roche, Nutley, N.J.). After clarification at 14000 rpm/min. at 4°C, protein concentrations were determined using Bio-Rad Protein Assay (Bio-Rad Laboratories, Inc, Hercules, CA). Equivalent amounts of protein were subjected to SDS-PAGE, transferred to nitrocellulose, and probed with primary and secondary antibodies according to the manufacturer’s recommended conditions. For immunoprecipitations, antibody (2 μg) was added to protein lysate (50 μg) and incubated at 4°C with shaking for 2 hours. Protein A/G plus agarose was added to each sample, incubated for 1 hour at 4°C, and washed with protein lysis buffer.
Small hairpin RNAs (shRNA)
The complementary oligonucleotides to generate the shRNA against human SEMA3A were designed using BLOCK-iT™ RNAi Designer from Invitrogen (Carlsbad, CA). Two oligo sequences were generated: shSema1=GGGAAGAACAATGTGCCAAGG; shSema2=GCTAGAATAGGTCAGATATGC. The double stranded (ds) oligos were subcloned into pLKOpuro1 (Yang et al., 2004). pLKOpuro1-shLuc was used as a control. pLKOpuro1-shLuc and pLKOpuro1 plasmids were a generous gift from Drs. Yang and Weinberg (Whitehead Institute for Biomedical Research, Cambridge, Massachusetts). pLKOpuro1-shLUC and pLKOpuro1-shSema were co-transfected with pCMV-VSVG and pHR’8.2ΔR (Dr. Weinberg) into 293T cells. After 48 hours, the medium was collected and centrifuged for 10 min. at 2000 rpm. The supernatants were transferred into targeted cell lines, and these cell lines were selected with 1ug/ml puromycin for one week. All analyses were performed using cells that had been passaged fewer than four times.
Flow cytometry
Cells were harvested with 5mM EDTA/PBS, and incubated on ice for 30 min. with an α2β1-specific or isotype control IgG. After washing with PBS, these cells were incubated on ice for 30 min. with FITC-conjugated goat anti- mouse IgG. Bound antibodies were detected using a Facscalibur flow cytometer (Becton Dickinson).
Reverse transcription PCR
Total RNA was isolated using the TRIzol reagent (Invitrogen, Carlsbad, CA). Reverse transcription and PCR amplification were performed using the Superscript One Step RT-PCR Kit (Invitrogen, Carlsbad, CA). The following primers were used: β1 integrin(209bp):5′-catctgcgagtgtggtgtct-3′(forward),5′-ggggtaatttgtcccgactt -3′(reverse); α2 integrin( 183 bp): 5′-gggcattgaaaacactcgat -3”(forward), 5′-tcggatcccaagattttctg -3′ (reverse); β-actin (234bp): 5′-ggacttcgagcaagagatgg-3′ (forward), 5′-agcactgtgttggcgtacag-3′ (reverse). The RT-PCR conditions were as follows: 50°C for 50 min., 95°C for 2 min., followed by 29 cycles of 95°C for 30 sec., 60°C for 30 sec., 72°C for 1 min. A final 72°C extension for 3 min was performed. PCR products were size-fractionated on a 1.5% agarose gel, and DNA bands were visualized using ethidium bromide.
Results and Discussion
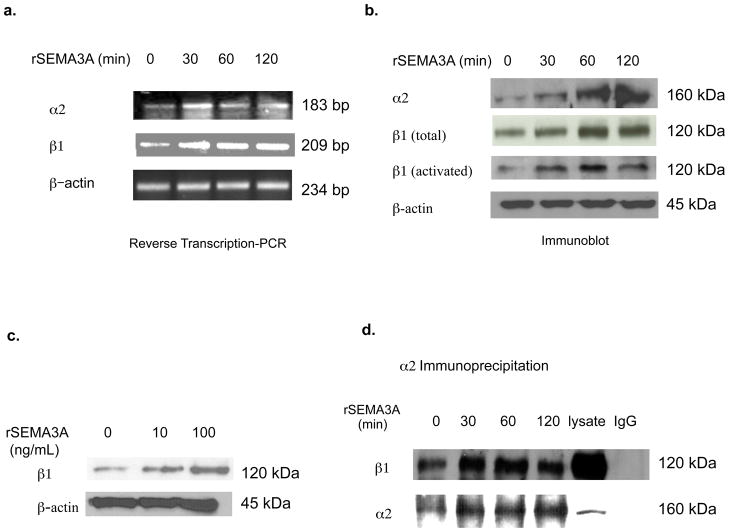
Both SEMA3A [5] and the α2β1 integrin [15, 16] have been independently implicated in the suppression of breast tumor cell migration, and SEMA3A reduces integrin activity in endothelial cells [4]. In the current study, we investigated SEMA3A regulation of the α2β1 integrin in breast tumor cells. The incubation of MDA-MB-231 cells with a recombinant human SEMA3A-Fc fusion protein (rSEMA3A) increased α2 and β1 subunit mRNA levels, but did not impact β-actin mRNA levels (Fig. 1a). In addition, rSEMA3A increased the levels in MDA-MB-231 cells of α2, β1, and activated β1 protein (Fig. 1b). As evidence that this activity was not an artifact of the Fc fusion domain of this recombinant protein, the incubation of these cells with a human Fc protein did not impact expression of these integrin subunits (data not shown). α2 and β1 integrin expression levels were increased as early as 30 minutes after the addition of rSEMA3A (Fig. 1b), and the ability of rSEMA3A to increase β1 integrin expression was concentration-dependent (Fig. 1c). Finally, α2β1 integrin heterodimer expression was increased in rSEMA3A-pulsed as compared to control cells, as assessed by co-immunoprecipitation of the integrin subunits (Fig. 1d). In contrast, rSEMA3A did not increase the expression of α5β1 integrin, as assessed by co-immunoprecipitation of the integrin subunits (data not shown). Confirming our results in MDA-MB-231 cells, we observed an ability of rSEMA3A to increase α2β1 integrin expression levels in SUM159 breast tumor cells (data not shown). These studies indicate that SEMA3A increases α2β1 integrin expression levels in breast tumor cells.
Figure 1. SEMA3A increases α2β1 integrin mRNA and protein expression levels in breast carcinoma cells.
(a and b) MDA-MB-231 cells were grown to 80–90% confluence and serum-starved overnight. These cells were incubated with recombinant SEMA3A (rSEMA3A, 100 ng/mL) for the indicated times. (a) Total RNA was isolated, and α2, β1 integrin and β-actin-specific reverse transcription PCR reactions were performed. PCR products were analyzed on a 1.5% agarose gel. (b) Total cellular proteins were extracted from these cells, and equivalent amounts were subjected to SDS-PAGE, transferred to nitrocellulose, and immunoblotted with antibodies specific for α2, β1, activated β1, or β-actin, followed by the appropriate species secondary antibody. (c) MDA-MB-231 cells were grown to 90% confluence and serum-starved overnight. These cells were detached with trypsin, washed with serum-free medium, and plated on collagen-coated dishes in the absence or presence of rSEMA3A at the indicated concentration. After 40 minutes, total cellular proteins were extracted from these cells, and equivalent amounts of protein were subjected to SDS-PAGE. These proteins were transferred to nitrocellulose and probed with antibodies specific for β1 integrin or β-actin, followed by the appropriate secondary antibody. (d) MDA-MB-231 cells were incubated with rSEMA3A for the indicated times as described in (a). α2 integrin (or isotype control IgG) immunoprecipitations were performed on equivalent amounts of total cellular protein extracted from the cells. These immunoprecipitates were subjected to SDS-PAGE, transferred to nitrocellulose, and immunoblotted with a β1 or α2 integrin antibody, followed by the appropriate secondary antibody.
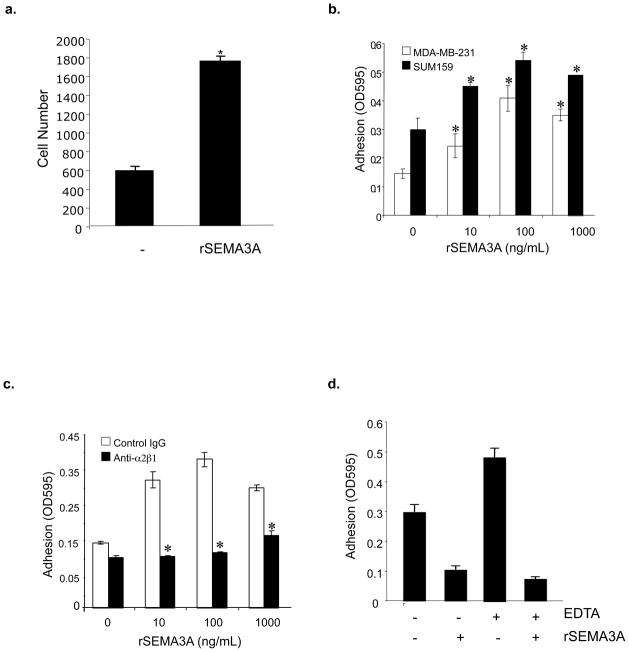
We next examined the effect of incubating breast tumor cells with recombinant SEMA3A on their ability to adhere to the α2β1-binding matrix protein collagen I. As shown in Figs. 2a and b, the incubation of either MDA-MB-231 or SUM159 breast tumor cells with a recombinant human SEMA3A-Fc fusion protein (rSEMA3A) significantly increased their adhesion to collagen I, with peak activity observed at 100 ng/mL. As a control, we showed that rSEMA3A did not influence the adhesion of these tumor cells to bovine serum albumin (BSA). We next examined if rSEMA3A impacts collagen adhesion in an α2β1 integrin-dependent manner. As shown in Fig. 2c, the incubation of MDA-MB-231 cells with an α2β1 integrin-neutralizing antibody suppressed the ability of rSEMA3A to increase cell adhesion to collagen. The incubation of these tumor cells with rSEMA3A in medium containing EDTA did not increase their adhesion to collagen, indicating the cation-dependence of this SEMA3A activity (Fig. 2d).
Figure 2. Incubation of breast tumor cell lines with recombinant SEMA3A increases their adhesion to collagen I in an α2β1 integrin-dependent manner.
(a) MDA-MB-231 cells were grown to 80–90% confluence and serum-starved overnight. Cells were resuspended in serum-free medium in the absence or presence of rSEMA3A (100 ng/mL), and plated on 96-well microtiter plates pre-coated with bovine collagen type I (20 μg/mL). After 40 minutes, non-adherent cells were removed, bound cells were fixed with methanol, and adherent cells were stained with crystal violet. Adherent cells were counted by phase contrast microscopy using a 10X objective. Data is presented as the mean number of cells per well from each of three wells (+/− SD). (b) MDA-MB-231 (white bar) and SUM159 (black bar) cells were grown to 80–90% confluence and serum-starved overnight. Cells were resuspended in serum-free medium in the absence or presence of rSEMA3A (0, 10, 100, 1000ng/mL), and plated on 96-well microtiter plates pre-coated with bovine collagen type I (20ug/mL) or bovine serum albumin (20 μg/mL). After 40 minutes, non-adherent cells were removed, adherent cells were fixed with methanol and stained with crystal violet, and cells were permealized with 1% SDS. Adhesion was quantified in an ELISA reader by measuring absorbance at 595nm (OD595). Data are presented as the difference between the mean OD595 on BSA (from triplicate wells) and the mean OD595 on collagen (from triplicate wells) ± standard deviation (SD). *p < 0.05 in a student’s t-test. Similar results were obtained in two independent experiments. (c) The adhesion of MDA-MB-231 cells to collagen was measured as described in (b) except that cells were resuspended in serum-free medium in the presence or absence of rSEMA3A in addition to an isotype control IgG or α2β1 integrin antibody (Anti-α2β1) (1ug/mL). *p < 0.05 in a student’s t-test. (d) Serum-starved MDA-MB-231 cells were pre-incubated in EDTA (5 mM)-containing or control medium for 30 minutes. These cells were then incubated in the presence or absence of rSEMA3A (100 ng/mL) in 96 well plates coated with collagen or BSA, as described for (b). Data are reported as indicated for (b). *p < 0.05 in a student’s t-test.
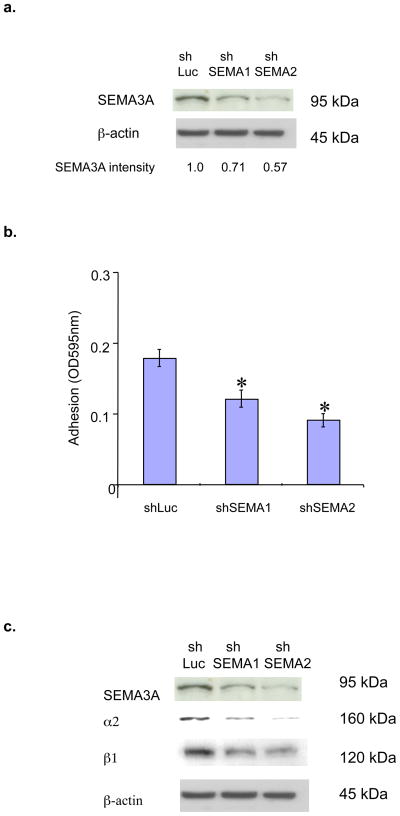
Our previous studies demonstrated that SEMA3A is expressed in breast tumor cell lines and primary tumors [5]. Accordingly, we next investigated if endogenous SEMA3A impacts the adhesion of breast tumor cells to collagen. MDA-MB-231 cells were infected with lentiviruses expressing either of two SEMA3A-specific small hairpin RNAs (shRNAs) or a control Luciferase shRNA (shLuc RNA). SEMA3A expression was reduced in cells infected with these SEMA3A shRNA-expressing vectors as compared to that observed in shLuc RNA-expressing cells (Fig. 3a). Importantly, SEMA3A shRNA-expressing cells exhibited a significantly reduced ability to bind to collagen I as compared to control infectants (Fig. 3b). Moreover, reducing SEMA3A expression in these cells decreased the levels of α2 and β1 integrin subunits (Fig. 3c). Collectively, these results indicate that autocrine SEMA3A stimulates breast tumor cell adhesion to collagen by increasing α2β1 expression.
Figure 3. Endogenous SEMA3A in breast tumor cells promotes α2β1 expression and collagen adhesion.
(a) MDA-MB-231 cells were infected with lentiviruses expressing shSEMA3A RNA#1 (shSema1), shSEMA3A RNA#2 (shSema2) or shLuciferase RNA (shLuc). Equivalent amounts of protein extracted from these cells (90% confluence) were subjected to SDS-PAGE, transferred to nitrocellulose and immunoblotted with a SEMA3A or β-actin antibody, followed by the appropriate secondary antibody. The intensity of SEMA3A bands was quantified by densitometry using ImageJ software (NIH). (b) MDA-MB-231 subclones expressing shSEMA3A RNAs (shSema1 and shSema2) or shLuc RNA were grown to confluence, resuspended in serum-free medium, and plated on 96-well microtitre plates coated with bovine collagen type I (20ug/mL). After 40 min, collagen adhesion was measured as described in (A). Data are presented as the difference between the mean OD595 on BSA (from triplicate wells) and the mean OD595 on collagen (from triplicate wells) (± SD). *p < 0.05 in a student’s t-test. Similar results were obtained in two independent experiments. (c) Total cellular proteins were extracted from MDA-MB-231 subclones expressing shSEMA3A RNAs (shSema1 and shSema2) or shLuciferase (shLuc). Equivalent amounts of protein were subjected to SDS-PAGE and immunoblotted with a SEMA3A, α2, β1, or β-actin antibody, followed by the appropriate species secondary antibody.
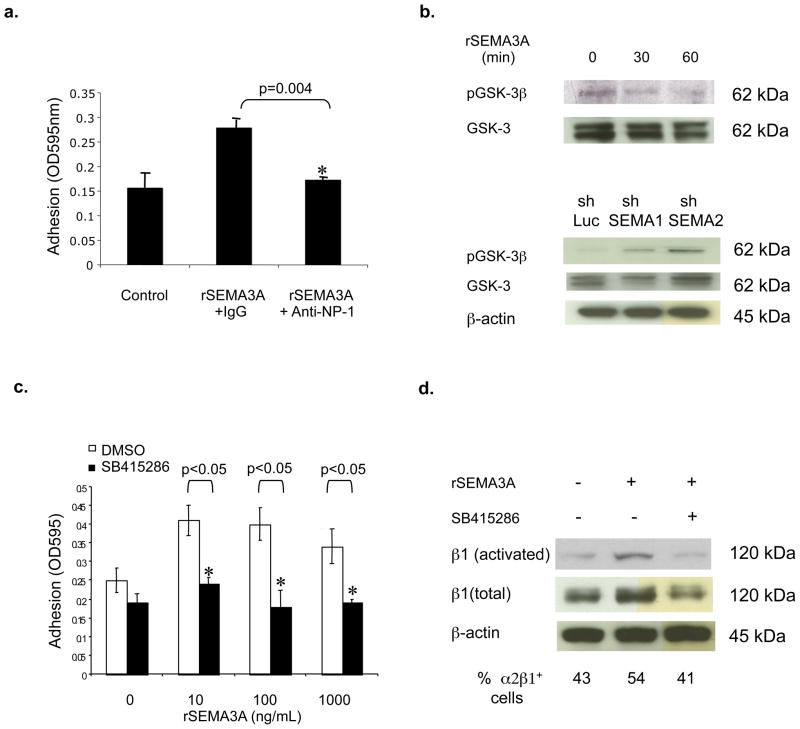
Based on our previous demonstration that the SEMA3A receptor NP-1 is expressed in breast tumor cells [5], we examined a role for NP-1 in regulating tumor cell adhesion to collagen. As shown in Fig. 4a, the ability of rSEMA3A to stimulate MDA-MB-231 cell adhesion to collagen was inhibited by incubating these cells with a NP-1-neutralizing antibody. Because SEMA3A stimulates the serine/threonine kinase GSK-3 by binding to NP-1 in neurons [17], we next examined a role for this kinase in regulating α2β1 integrin expression in breast tumor cells. The incubation of MDA-MB-231 cells with rSEMA3A stimulated GSK-3 activity, as evidenced by reduced levels of phosphorylated GSK-3 (the inactive form of GSK-3) in rSEMA3A-treated compared to control cells (Fig. 4b). Conversely, we observed increased levels of phosphorylated GSK-3 in SEMA3A shRNA-expressing as compared to control MDA-MB-231 cells (Fig. 4b). As evidence that GSK-3 is important for SEMA3A regulation of cell adhesion, the ability of rSEMA3A to increase MDA-MB-231 cell adhesion to collagen was inhibited in cells that had been pre-incubated with SB415286, a highly-specific small molecule GSK-3 inhibitor [18](Fig. 4c). Furthermore, rSEMA3A did not increase β1, activated β1, or α2β1 expression levels in MDA-MB-231 cells incubated with SB415286 (Fig. 4d). These data indicate that SEMA3A induction of α2β1 integrin expression and adhesive function is dependent on its activation of GSK-3. Although previous studies defined a role for GSK-3 in integrin recycling [19], our work indicates that the SEMA3A/GSK-3 signaling axis influences α2β1 integrin expression levels in tumor cells. Further work is needed to determine the level at which GSK-3 regulates α2β1 integrin expression, and to identify the target of GSK-3 kinase activity that regulates expression of this integrin.
Figure 4. The ability of SEMA3A to increase breast carcinoma adhesion to collagen is dependent on NP-1 and GSK-3.
(a) MDA-MB-231 cells were incubated with a NP-1-neutralizing (Anti-NP-1) or isotype control (Control Ig) antibody (1 μg/mL) in the presence or absence of the indicated concentration of rSEMA3A. The ability of these cells to adhere to collagen was assessed as described for Fig. 2b. (b) (Top panel) MDA-MB-231, cells were grown to 80–90% confluence and serum-starved overnight. These cells were incubated with rSEMA3A at a concentration of 100ng/mL and harvested at 0, 30 or 60 min. Equivalent amounts of total cellular protein extracted from these cells were subjected to SDS-polyacrylamide gel electrophoresis, and immunoblotted with antibodies specific for phosphorylated or total GSK-3, followed by the appropriate species HRP-conjugated secondary antibody. (Bottom panel) MDA-MB-231 cells expressing shSEMA3A RNAs (shSema1 and shSema2) or shLuc RNA were serum-starved overnight, and immunoblotted with antibodies specific for phospho-GSK-3β (Ser9), GSK-3 or β-actin, followed by the appropriate species secondary antibody. (c) MDA-MB-231 cells were pre-incubated with the GSK-3 inhibitor SB415286 (25 μM) or DMSO for 1 hour, and then incubated with or without rSEMA3A at the indicated concentration. Relative cell adhesion to collagen was determined as described in Fig. 1. *p < 0.05 in a student’s t-test. (d) MDA-MB-231 cells were grown to 80–90% confluence and serum-starved overnight. The cells were pre-treated with the GSK-3 inhibitor SB415286 (25 μM) or DMSO for 1 hour, then incubated with rSEMA3A (100ng/ml) for 40 min. For immunoblots, total cellular protein was extracted from these cells, and equivalent amounts run on an SDS polyacrylamide gel. These proteins were transferred to nitrocellulose and immunobloted with an antibody specific for activated or total β1, followed by the appropriate species secondary antibody. As a loading control, a β-actin immunoblot was performed. Alternatively, to assess cell surface integrin expression, MDA-MB-231 cells were serum-starved overnight, detached with trypsin, and resuspended in serum-free medium containing: 1) DMSO, 2) rSEMA3A + DMSO, or 3) rSEMA3A + GSK-3 inhibitor (SB415286, 25 μM). These cells were immediately plated onto collagen-coated dishes. After 40 min., the cells were collected and incubated with an α2β1 integrin or isotype control antibody, followed by a FITC conjugated secondary antibody. These cells were analyzed by flow cytometry. Data are reported as the percent α2β1 integrin-positive cells, and are representative of three different trials.
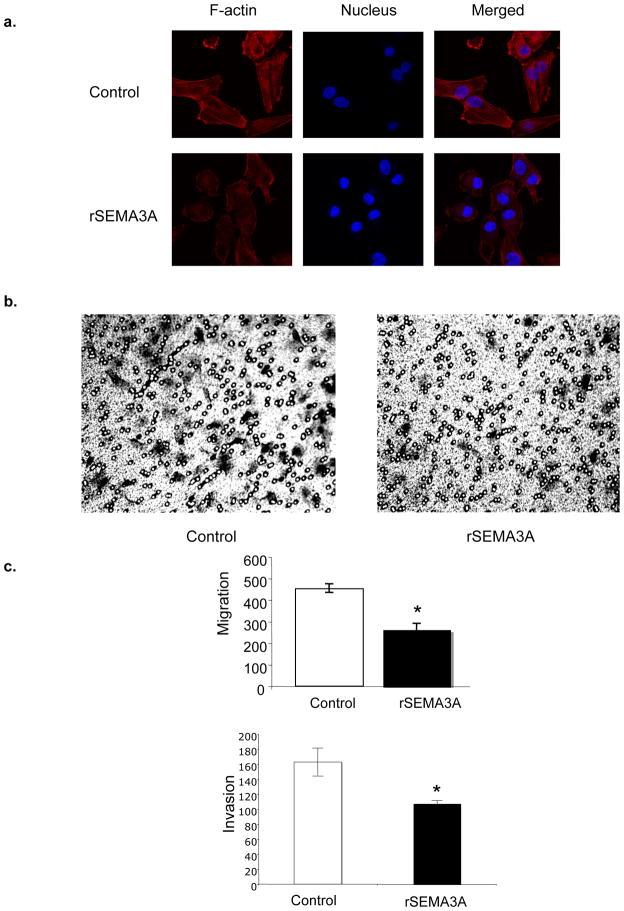
During their metastatic progression, tumor cells acquire the ability to migrate. Several studies indicate that the acquisition of an intermediate cell adhesive strength promotes optimal cell migration [20, 21]. Accordingly, while a baseline level of α2β1 is essential for cell migration, cell migration is suppressed by α2β1 integrin expression levels beyond this baseline [15, 16]. Likewise, reducing α2β1 integrin expression levels below this baseline inhibits cell migration [15]. Considering that SEMA3A is an endogenous suppressor of breast tumor cell migration [5], we sought to determine if SEMA3A suppresses breast tumor cell migration by stimulating the α2β1 integrin. Based on the knowledge that MDA-MB-231 cells are highly migratory [5], we predicted that these cells express an intermediate concentration of α2β1 optimal for migration. We hypothesized that significant increases or decreases in α2β1 expression or activity in these cells would impair cell migration. First, we assessed the effect of rSEMA3A on the actin cytoskeleton. MDA-MB-231 cells were incubated for 30 minutes with or without rSEMA3A in serum-free medium, and F-actin levels were assessed by phalloidin staining. As shown in Fig. 5a, actin filaments were abundant in control MDA-MB-231 cells, and lamellipodia were observed at the leading migration edge. In contrast, MDA-MB-231 cells incubated with rSEMA3A showed reduced phalloidin staining, exhibited a less organized actin filament network, and lacked visible lamellipodia. This ability of rSEMA3A to disrupt the actin cytoskeleton suggested that rSEMA3A may reduce tumor cell migration. To examine this question, we assessed the ability of control and rSEMA3A-incubated MDA-MB-231 cells to migrate toward serum-containing medium in a transwell assay. As shown in Fig. 5b, rSEMA3A significantly reduced MDA-MB-231 cell migration. Importantly, rSEMA3A also decreased the invasive potential of MDA-MB-231 cells, as assessed using Matrigel-coated transwells (Fig. 5c).
Figure 5. rSEMA3A decreases breast tumor cell migration and invasion.
(a) MDA-MB-231 cells were grown on chamber slides and serum-starved overnight. These cells were then incubated +/− rSEMA3A in serum-free medium for 30 min. Cells were fixed, permeabilized, and incubated with Texas red-phalloidin and DAPI. These samples were visualized by confocal microscopy. (b) Serum-starved MDA-MB-231 cells were added to the top chambers of a collagen-coated transwell in serum-free medium +/− rSEMA3A (100ng/ml). Culture medium containing 10% FBS was added to the bottom chambers. After 4 hours, the cells that had migrated to the bottom side of the membrane were fixed and stained with 0.2% crystal violet. Cells were visualized and counted by microscopy using a 20X objective. The data are presented as the mean number of migrated cells +/− standard deviation (SD) from three wells. *p < 0.05 in a student’s t-test. (c) Serum-starved MDA-MB-231 cells were added to the top chambers of a Matrigel-coated transwell in serum-free medium +/− rSEMA3A (100 ng/mL). Culture medium containing 10% FBS was added to the bottom chambers. After 5 hours, the cells that had invaded the Matrigel and migrated to the bottom side of the membrane were stained with crystal violet and counted as described in (b).
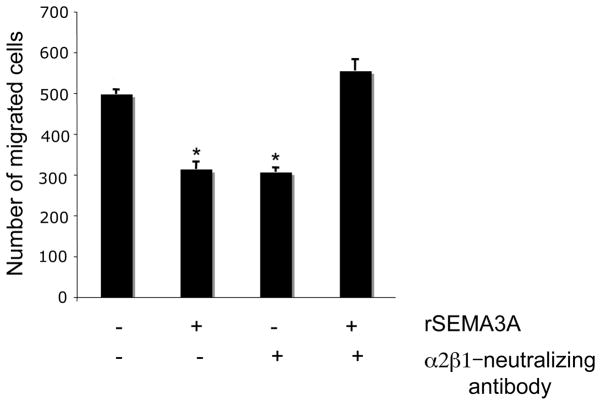
Based on our demonstration that rSEMA3A increases the expression in breast tumor cells of α2β1, an integrin that can reduce tumor cell migration[15, 16], we next investigated the importance of α2β1 for SEMA3A regulation of breast tumor cell migration. Serum-starved MDA-MB-231 cells were incubated with control medium or rSEMA3A-containing medium in the presence of a control or an α2β1 integrin-neutralizing antibody. The ability of these cells to migrate in a transwell assay was then investigated. The incubation of MDA-MB-231 cells with an α2β1-neutralizing antibody reduced cell migration, consistent with the idea that these cells are reliant on a baseline level of α2β1 integrin to support optimal migration (Fig. 6). We also observed that the incubation of MDA-MB-231 cells with rSEMA3A significantly reduced their migration (Fig. 6). However, SEMA3A did not repress the migration of MDA-MB-231 cells incubated with an α2β1 integrin-neutralizing antibody (Fig. 6). This data indicates that SEMA3A suppresses breast tumor cell migration in an α2β1 integrin-dependent manner.
Figure 6. rSEMA3A decreases breast tumor cell migration, but not in the presence of an α2β1-integrin neutralizing antibody.
MDA-MB-231 cells (80–90% confluent) were serum-starved overnight. These cells were then added to the top chambers of a collagen-coated transwell in serum-free medium +/− rSEMA3A (100ng/ml) and either an α2β1 integrin-neutralizing antibody (anti-α2β1) or isotype control antibody (control Ig) at a concentration of 1μg/ml. Culture medium containing 10% FBS was added to the bottom chambers. After 4 hours, the cells that had migrated to the bottom side of the membrane were fixed and stained with 0.2% crystal violet. Cells were visualized and counted by microscopy using a 20X objective. The data are presented as the mean number of migrated cells +/− standard deviation (SD) from three wells). *p < 0.05 in a student’s t-test.
Collectively, our work establishes that an autocrine pathway defined by SEMA3A and NP-1 drives α2β1 integrin expression and adhesive function in breast tumor cells. This finding is in agreement with previous work defining NP-1 as an adhesion-promoting molecule in endothelial cells [22]. However, our results contrast with other studies indicating that SEMA3A can repress the adhesion of other cell types to matrix proteins [4, 23–25]. These conflicting results may reflect an ability of SEMA3A and NP-1 to differentially impact the adhesion of divergent cell types based on their expression of different SEMA3A co-receptors. For example, a soluble form of the NP-1 co-receptor L1 can convert SEMA3A growth cone collapsing activity into growth cone attraction [26].
Our previous studies established that beyond its angiogenic activities, the Vascular Endothelial Growth Factor (VEGF-A) exhibits autocrine functions in breast tumor cells[5]. We also showed that VEGF-A competes with Sema3A for NP-1 binding in breast tumor cells, thus inhibiting autocrine Sema3A signaling[5]. Based on these findings, we predict that tumor cells characterized by a high VEGF-A: Sema3A ratio will exhibit reduced α2β1 expression levels and increased invasive behavior compared to tumor cells associated with a high Sema3A:VEGF-A ratio, a topic of current investigation.
Several studies indicate that the α2β1 integrin is a differentiation marker for breast tumor cells and an inhibitor of tumor growth [7–12]. Our studies are important in defining an autocrine regulator of α2β1 integrin expression in breast tumor cells. Importantly, we also show that autocrine SEMA3A reduces cell migration by stimulating the α2β1 integrin. Based on the knowledge that tumor metastasis is dependent on tumor cell migratory and invasive behavior, we predict that breast tumor cells that support autocrine Sema3A signaling are likely suppressed in their metastatic potential, a topic of current investigation. Our studies also suggest that the delivery of SEMA3A to tumors may impede their metastasis by increasing α2β1 integrin expression levels, a topic for future studies.
References
- 1.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90(4):739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 2.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90(4):753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 3.Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146(1):233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424(6947):391–397. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- 5.Bachelder RE, Lipscomb EA, Lin X, Wendt MA, Chadborn NH, Eickholt BJ, Mercurio AM. Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer Res. 2003;63(17):5230–5233. [PubMed] [Google Scholar]
- 6.Elices MJ, Hemler ME. The human integrin VLA-2 is a collagen receptor on some cells and a collagen/laminin receptor on others. Proc Natl Acad Sci U S A. 1989;86(24):9906–9910. doi: 10.1073/pnas.86.24.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Diacovo TG, Grenache DG, Santoro SA, Zutter MM. The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. The American journal of pathology. 2002;161(1):337–344. doi: 10.1016/s0002-9440(10)64185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faraldo MM, Deugnier MA, Lukashev M, Thiery JP, Glukhova MA. Perturbation of beta1-integrin function alters the development of murine mammary gland. Embo J. 1998;17(8):2139–2147. doi: 10.1093/emboj/17.8.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, Wang P, Schatzmann F, Wintermantel T, Schuetz G, et al. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol. 2005;171(4):717–728. doi: 10.1083/jcb.200503144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maschler S, Wirl G, Spring H, Bredow DV, Sordat I, Beug H, Reichmann E. Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene. 2005;24(12):2032–2041. doi: 10.1038/sj.onc.1208423. [DOI] [PubMed] [Google Scholar]
- 11.Zutter MM, Krigman HR, Santoro SA. Altered integrin expression in adenocarcinoma of the breast. Analysis by in situ hybridization. The American journal of pathology. 1993;142(5):1439–1448. [PMC free article] [PubMed] [Google Scholar]
- 12.Zutter MM, Mazoujian G, Santoro SA. Decreased expression of integrin adhesive protein receptors in adenocarcinoma of the breast. The American journal of pathology. 1990;137(4):863–870. [PMC free article] [PubMed] [Google Scholar]
- 13.Gui GP, Puddefoot JR, Vinson GP, Wells CA, Carpenter R. In vitro regulation of human breast cancer cell adhesion and invasion via integrin receptors to the extracellular matrix. Br J Surg. 1995;82(9):1192–1196. doi: 10.1002/bjs.1800820914. [DOI] [PubMed] [Google Scholar]
- 14.Lochter A, Navre M, Werb Z, Bissell MJ. alpha1 and alpha2 integrins mediate invasive activity of mouse mammary carcinoma cells through regulation of stromelysin-1 expression. Mol Biol Cell. 1999;10(2):271–282. doi: 10.1091/mbc.10.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keely PJ, Fong AM, Zutter MM, Santoro SA. Alteration of collagen-dependent adhesion, motility, and morphogenesis by the expression of antisense alpha 2 integrin mRNA in mammary cells. J Cell Sci. 1995;108 ( Pt 2):595–607. doi: 10.1242/jcs.108.2.595. [DOI] [PubMed] [Google Scholar]
- 16.Zutter MM, Santoro SA, Staatz WD, Tsung YL. Re-expression of the alpha 2 beta 1 integrin abrogates the malignant phenotype of breast carcinoma cells. Proc Natl Acad Sci U S A. 1995;92(16):7411–7415. doi: 10.1073/pnas.92.16.7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eickholt BJ, Walsh FS, Doherty P. An inactive pool of GSK-3 at the leading edge of growth cones is implicated in Semaphorin 3A signaling. J Cell Biol. 2002;157(2):211–217. doi: 10.1083/jcb.200201098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chemistry & biology. 2000;7(10):793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 19.Roberts MS, Woods AJ, Dale TC, Van Der Sluijs P, Norman JC. Protein kinase B/Akt acts via glycogen synthase kinase 3 to regulate recycling of alpha v beta 3 and alpha 5 beta 1 integrins. Mol Cell Biol. 2004;24(4):1505–1515. doi: 10.1128/MCB.24.4.1505-1515.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiMilla PA, Stone JA, Quinn JA, Albelda SM, Lauffenburger DA. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J Cell Biol. 1993;122(3):729–737. doi: 10.1083/jcb.122.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynch L, Vodyanik PI, Boettiger D, Guvakova MA. Insulin-like growth factor I controls adhesion strength mediated by alpha5beta1 integrins in motile carcinoma cells. Mol Biol Cell. 2005;16(1):51–63. doi: 10.1091/mbc.E04-05-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murga M, Fernandez-Capetillo O, Tosato G. Neuropilin-1 regulates attachment in human endothelial cells independently of vascular endothelial growth factor receptor-2. Blood. 2005;105(5):1992–1999. doi: 10.1182/blood-2004-07-2598. [DOI] [PubMed] [Google Scholar]
- 23.Kashiwagi H, Shiraga M, Kato H, Kamae T, Yamamoto N, Tadokoro S, Kurata Y, Tomiyama Y, Kanakura Y. Negative regulation of platelet function by a secreted cell repulsive protein, semaphorin 3A. Blood. 2005;106(3):913–921. doi: 10.1182/blood-2004-10-4092. [DOI] [PubMed] [Google Scholar]
- 24.Lepelletier Y, Smaniotto S, Hadj-Slimane R, Villa-Verde DM, Nogueira AC, Dardenne M, Hermine O, Savino W. Control of human thymocyte migration by Neuropilin-1/Semaphorin-3A-mediated interactions. Proc Natl Acad Sci U S A. 2007;104(13):5545–5550. doi: 10.1073/pnas.0700705104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herman JG, Meadows GG. Increased class 3 semaphorin expression modulates the invasive and adhesive properties of prostate cancer cells. International journal of oncology. 2007;30(5):1231–1238. [PubMed] [Google Scholar]
- 26.Castellani V, Chedotal A, Schachner M, Faivre-Sarrailh C, Rougon G. Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron. 2000;27(2):237–249. doi: 10.1016/s0896-6273(00)00033-7. [DOI] [PubMed] [Google Scholar]