Abstract
In recent years, there have been multiple breakthroughs in our understanding of lung cancer biology. Despite significant advances in molecular targeted therapies DNA-damaging cytotoxic therapies will remain the mainstay of lung cancer management for the foreseeable future. Similar to the concept of personalized targeted therapies there is mounting evidence that perturbations in DNA repair pathways are common in lung cancers, altering the resistance of the affected tumors to many chemotherapeutics as well as radiation. Defects in DNA repair may be due to a multitude of mechanisms including gene mutations, epigenetic events, and alterations in signal transduction pathways such as EGFR and PI3K/AKT. Functional biomarkers that assess the subcellular localization of central repair proteins in response to DNA damage may prove useful for individualization of cytotoxic therapies including PARP inhibitors. A better mechanistic understanding of cellular sensitivity and resistance to DNA damaging agents should facilitate the development of novel, individualized treatment approaches. Absolute resistance to radiation therapy, however, does not exist. To some extent, radiation therapy will always have to remain unselective and indiscriminant to eradicate persistent, drug-resistant tumor stem cell pools.
Introduction
In this past decade, there have been significant breakthroughs in our knowledge of lung cancer biology. The identification of driver oncogenes in adenocarcinomas of the lung, and emerging also in squamous cell carcinomas, has led to a paradigm change in the treatment of these tumors. Biological agents targeting these genomic alterations have proven to be highly effective in subsets of lung cancer patients. In light of the success of molecular targeted therapies, there appears to be a growing conviction in the field that cytotoxic therapies will become obsolete at some point in the future. However, chemotherapeutics can achieve substantial therapeutic benefit in individual patients, but what is lacking is a better understanding of the mechanisms of chemotherapy sensitivity and resistance and associated biomarkers. There is a growing body of data suggesting the presence of perturbed DNA repair in a large number of human cancers including lung cancer. Defects in certain repair pathways, such as homologous recombination repair, can render the affected tumors highly sensitive to genotoxic agents, such as cisplatin1–3. There is also substantial radiobiological data on factors that likely influence the sensitivity of lung tumors to radiation therapy4. Here, we will review clinically relevant mechanisms affecting resistance and sensitivity of lung cancer to radiation and chemotherapeutics. We will focus on the promise of research into perturbations of DNA repair in human tumors, which may yield biomarkers of sensitivity to specific chemotherapeutics and agents targeting DNA repair pathways such as PARP inhibitors. This is a large field, and we apologize for being able to only include a fraction of pertinent research articles.
Clinically Relevant Mechanisms of Radiosensitivity and Radioresistance
Effect of radiation on clonogenic or tumor stem cells
In radiation biology, clonogenic tumor cells have been defined as cells that have the capacity to produce an expanding family of daughter cells and form colonies following irradiation in an in-vitro assay or give rise to a recurrent tumor in in-vivo models. Whether clonogenic cells fully represent tumor stem cells is unclear but more recently the terms have been used interchangeably5,6. The goal of radiation therapy in curative intent is to eradicate the last surviving tumor stem cell, as a single stem cell remaining after completion of treatment may give rise to a local tumor recurrence (reviewed in 4). Radiation-induced cell killing (or radiobiologically called cell “inactivation”) is random: lethal unrepairable DNA double-strand breaks (DSB) are generated randomly in a cell population of similar cellular radiosensitivity, at low doses eradicating some but not all stem cells. Increasing the radiation dose, for a given number of tumor stem cells, will lead to higher odds that a stem cell gets hit with at least one lethal event, thereby decreasing the number of surviving cells. Vice versa, an increasing number of stem cells for a given dose of radiation will reduce the likelihood of inflicting a lethal event per stem cell, hence increasing the likelihood of local tumor relapse. Because of this dose-response relationship, it can be inferred that absolute radiation resistance does not exist. If only an adequately high radiation dose could be delivered, any number of tumor stem cells would be eradicated. However, the radiation tolerance of normal organs and tissues surrounding the tumor typically limits the maximum amount of radiation that can be delivered to the tumor.
In addition to the number of tumor stem cells present before initiation of radiation therapy, several other factors are thought to influence the sensitivity of a tumor to radiation (Figure 1). These include the ability of tumor stem cells to increase in number during a several-week course of radiation via a process termed accelerated repopulation, effects of the tumor microenvironment such as hypoxia, and variations in the intrinsic sensitivity of cells to radiation damage to DNA, for example by up- or down-regulation of DNA repair pathways and modulation of cell survival pathways (reviewed below). Data on differences in radiosensitivity between putative stem cells and other tumor cells are sparse but generally suggest a greater intrinsic radioresistance of stem cells6.
Figure 1.

Known and putative factors influencing radiosensitivity and radioresistance of lung cancers.
Importance of tumor volume for radiation therapy
While relatively little is currently known about the nature of stem cells in lung cancer7, there is little doubt that increasing primary tumor size or volume (and hence the number of stem cells) is associated with a lower likelihood of local tumor control8–13. Radiobiological principles predict that a similar dose-response relationship should apply to the volume of nodal disease, but there exists little data on this issue10. In addition, whether increasing primary tumor size, which may also be associated with more aggressive biological behavior and poor prognosis, increases radiation resistance through factors other than a mere increase in stem cell number is not established. Locally advanced non-small cell lung cancer (NSCLC) at diagnosis (i.e., stage II/III) is typically large in volume. It is therefore not surprising that standard doses of radiation therapy, i.e., ~ 60 Gy, are associated with local failure rates of at least 30–50%, even in the presence of concurrent, radiosensitizing chemotherapy14,15. There have been numerous attempts to increase the radiation dose to >70 Gy, in order to reduce the probability of local relapse. However, recent data from the randomized phase III RTOG 0617 study suggest that the delivery of 74 Gy may exceed normal tissue tolerance, at least with the radiation techniques employed in that trial16.
If the maximum amount of radiation that can be given to the chest is restricted, then radiosensitizing agents need to be employed to increase tumor cell kill for a given physical dose of radiation, but without increasing radiation toxicity. There is currently great interest in exploring the utility of novel targeted drugs concurrently with radiation in this regard17,18. Alternatively, an emerging new paradigm involves the use of targeted agents as induction therapy in genotype-defined lung cancers. Figure 2 illustrates that the bulk of lung cancer is often too large for the achievable dose of radiation to obtain satisfactory local tumor control. Tumors expressing wild-type epidermal growth factor receptor (EGFR) may be treated with a radiosensitizing EGFR-directed monoclonal antibody or tyrosine kinase inhibitor (TKI), thereby increasing the amount of cell kill achieved with a given dose of radiation. In a tumor with mutant EGFR, the use of an induction TKI could take advantage of the high tumor response rates seen in the setting of EGFR mutant stage IV disease (~70% response) and the high degree of cell kill observed in pre-clinical models (~99% cell kill)19,20. A substantial reduction in tumor volume (and number of tumor stem cells) following 2–3 months of TKI treatment would be predicted to increase local tumor control rates for a standard dose of radiation.
Figure 2.
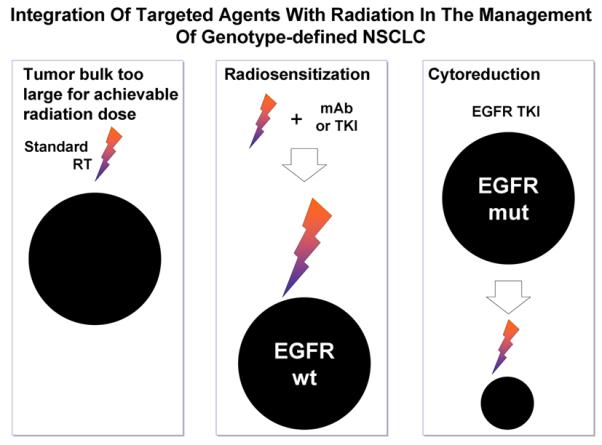
Incorporation of EGFR-directed agents with radiation in the treatment of locally advanced NSCLC. RT, radiation therapy; mAb, monoclonal antibody; TKI, tyrosine kinase inhibitor; wt, wild-type; mut, mutant
The problem of accelerated tumor cell repopulation
The time over which the total dose of radiation is delivered becomes important if there is repopulation of stem cells within the irradiated tumor during a several-week course of radiation therapy (reviewed in4). Tumor stem cell repopulation is thought to be an adaptive response to the cytotoxic effects of radiation, and is likely due to several factors including an increased cell proliferation rate, reduction in asymmetric cell division leading to a larger number of daughter stem cells, re-entry of quiescent stem cells into the growth fraction, and/or a reduction of the fraction of cells lost from the tumor. Because repopulation is able to compensate for radiation-induced cell death during fractionated treatment, it leads to an increased likelihood of surviving stem cells and local relapse.
Repopulation is accelerated when the doubling time of the proliferative cell pool is shorter than the doubling time before start of radiation therapy. During treatment, the doubling time of the tumor stem cell fraction may be as short as 4 to 5 days, compared with an average doubling time of > 2 months for NSCLC. Lacking reliable stem cell markers, it is currently not possible to detect the fraction of stem cells undergoing accelerated repopulation in a tumor during a fractionated course of radiation6. Thus, even though a tumor may initially respond to radiation, as evident by tumor shrinkage on a computed tomography scan, the stem cell subpopulation within that tumor may already be rapidly proliferating, thereby offsetting the delivered radiation dose.
Evidence for the presence of accelerated repopulation in human tumors has been reviewed elsewhere4,21,22. In lung cancer, some of the best supporting data come from randomized phase III trials involving two arms of radiation therapy with varying overall treatment time but same or similar radiation dose23–25. If there is accelerated repopulation during radiation therapy, a longer overall treatment time will yield inferior results due to the continuous increase in the number of stem cells during the course of therapy. For example, in the British Continuous Hyperfractionated Accelerated Radiotherapy (CHART) trial patients with inoperable NSCLC were randomized to conventional radiation of 60 Gy in 6 weeks or 54 Gy in 12 days23. The 12-day schedule achieved a higher overall survival rate than the 6-week schedule, with the benefit most pronounced in the squamous cell carcinoma subset. In limited-stage small cell lung cancer (SCLC), a 3-week schedule of 45 Gy yielded superior local tumor control and overall survival compared to a 5-week regimen with the same dose, both with the same concurrent chemotherapy24. These and other data also suggest that chemotherapeutic agents are not able to block accelerated tumor cell proliferation26. In fact, chemotherapy by itself may even trigger accelerated repopulation as can be inferred from experimental and clinical data27–29.
The tissue, cellular, and molecular mechanisms of accelerated repopulation are the subject of intense research5,6,30. Interestingly, in head and neck squamous cell carcinoma, shortening the overall treatment time only benefits patients whose tumors have high EGFR expression, while experimental data suggest that EGFR blockade may reduce tumor cell repopulation31,32. Whether a similar relationship between EGFR activity and tumor cell repopulation exists in lung cancer is not known.
Hypoxia and radioresistance
Cells irradiated in the absence of oxygen are 2- to 3-fold more radioresistant than well-oxygenated cells (reviewed in4). The mechanism underlying this so called “oxygen effect” may involve the generation of DNA damage by reaction of molecular oxygen with radiation-induced DNA radicals. Tumor hypoxia may be caused by multiple factors including chaotic tumor vasculature, anemia, and smoking33–36. Hypoxic cells in theory limit the sensitivity of tumors to high single doses of radiation, which preferentially kills well-oxygenated cells, leaving hypoxic cells behind. If this occurred during a several-week course of radiation therapy, the fraction of hypoxic cells would slowly increase, thereby increasing the radioresistance of the irradiated tumor. However, it is thought that a process of “reoxygenation” returns the high proportion of hypoxic cells, created immediately after each dose fraction, back toward the level that existed before the delivery of that fraction. However, direct evidence for this mechanism in human tumors is lacking. The success of stereotactic body radiation therapy in early-stage lung cancer is noteworthy as it suggests that either sufficient reoxygenation occurs even after 1–3 large fractions or hypoxia that is present at time of diagnosis may not be radiobiologically relevant. Molecular imaging studies may produce predictors of treatment-relevant hypoxia and are the subject of intense research (reviewed in37).
Mechanisms of Chemoresistance
It is fair to summarize that almost all lung cancers are either initially, or ultimately, resistant to current anti-cancer drugs. In patients selected for drug treatment based on histopathology, the best results have been observed using cisplatin-based combinations of cytotoxic chemotherapy. The best initial response rate using a cisplatin-based, two-drug combination for stage IV NSCLC is about 30%, with less than 5% of patients living beyond 3 years.38 For extensive-stage SCLC, initial response rates to cisplatin combination are higher, 50–80%, but again fewer than 5% long term survivors.39 The toxicity of cisplatin-based chemotherapy is substantial, and includes nausea, fatigue, cytopenias, nerve damage, kidney damage, and a 1–3% chance of death. These observations resulted in early nihilism that chemotherapy was at all worthwhile for patients with stage IV NSCLC. In fact, patients with stage IV NSCLC were still being randomized to supportive care only as a standard of care as recently as the last decade.40
Fortunately, outcomes for patients have improved by small increments over the last 20 years. For all patients with lung cancer, progress has come not from the development of any one drug, but rather several new drugs with variable mechanisms of action and better toxicity profiles, but each drug only active in a minority of patients. There are now 17 drugs invoked in contemporary chemotherapy treatment guidelines for NSCLC, including platinum analogs, other DNA-damaging agents, nucleoside analogs, anti-metabolites, topoisomerase inhibitors, vinca alkaloids, taxanes, and drugs targeting specific proto-oncogenes including EGFR, ALK, and VEGF.41,42 Therefore, the challenge of chemoresistance in lung cancer has been met to date by finding drugs with variable mechanisms of action, and by trial and error figuring out which drugs to give to which patient. Based on this approach, some lung cancer patients are now identified by molecular pathology as having an activating/sensitizing mutation in EGFR (10% of patients), or rearrangement in ALK (1% of patients), and offered targeted kinase inhibitors which provide response rates over 70% and 3 year survival proportions of 20%.43, 44
Still, the low rates of cure observed even in patients treated with appropriate targeted chemotherapy requires that the challenge of chemoresistance be met one drug at a time. In this regard, chemoresistance may be defined in two general categories: de novo resistance in untreated patients, and acquired resistance which develops in patients previously-treated with a drug. Current models of lung tumor biology recognize molecular heterogeneity as a substantial problem in lung cancer such that mechanisms of de novo, and acquired chemoresistance, may be identical and observed early, or late, depending on the frequency of resistant clones in a given tumor at a given time, and natural selection of resistant clones by treatment with a given drug.45,46 From there, mechanisms of resistance fall into broad categories best defined as pre-target, on-target, post-target, and off-target resistance.47 Pre-target, post-target, and off-target mechanisms of resistance may overlap for a variety of drugs. For example, multi-drug resistance may be due to high level activity of non-selective ATP-driven drug efflux pumps in lung cancer cells, such as P-glycoprotein, MDR1 and MRP.48 The same goes for overexpression of detoxifying enzymes, and glutathione metabolism, which may provide broad resistance to cytotoxic drugs.49 Since the final common pathway of lung cancer cell death in response to drug therapy is programmed cell-death, it is widely believed that cross-resistance to most anti-cancer drugs is a result of a disconnect between cellular proliferation and apoptosis. Therefore, any observation defining chemoresistance to a specific drug as related to a post-target defect in cell death may explain resistance to a wide variety of drugs.
Since cisplatin is considered to be the single most effective drug for all patients with lung cancer, mechanisms of resistance to cisplatin are the most widely studied. In patients with earlier stages of NSCLC treated with surgery and chemotherapy, it is interesting to note that most genes implicated in cisplatin resistance are post-target, and related to the cell cycle (p27), or cell death (p53, BAX).50–52 Other prominent genes are on-target and directly inhibit the action of cisplatin by fostering DNA repair, including nucleotide-excision repair (NER) (ERCC1), or mismatch repair (MSH2).53, 54
DNA Double-Strand Breaks and Damage-Based Therapies
DNA damage induction and response
A common characteristic of ionizing radiation (IR) and many chemotherapeutics is the induction of various types of DNA damage, directly leading to tumor cell kill (reviewed in3,55). A critical feature of the eukaryotic cell is its ability to maintain genome stability across generations, attributed, in part, to the sophisticated and precisely regulated DNA lesion-specific repair mechanisms. Diminished DNA damage response and repair (DRR) have been strongly associated with lung cancers.56 The pathways by which cells remove DSBs are of particular interest, as one unrepaired DSB can trigger apoptosis,57 which underlies the efficacy of DNA damage-based therapies involving IR and platinum-based agents such as cisplatin, as well as etoposide.
In fact, DNA damage induction is the primary mechanism of action of most lung cancer therapeutics3. Taxanes are cytotoxic due to their stabilization of microtubules, which results in cell cycle arrest during mitosis.58 Pemetrexed, an antifolate, serves to inhibit nucleotide synthesis, thus depriving cells of the raw materials necessary to synthesize new copies of the genome.59 Incorporation of the nucleoside analog gemcitabine must be rectified by the NER pathway in order to prevent eventual cell death.60 The cumulative failure of the cell to repair these DNA lesions by their corresponding repair mechanisms may lead to their conversion to DSBs, eventually triggering cell death.
As such, efficient and faithful DSB repair is critical to normal cell survival, but is also a mechanism contributing to tumor cell therapeutic resistance. Therefore, we focus below on the current understanding of DSB repair regulation, as well as its role in lung cancer's response to DNA damage based therapy. It has been well established that, in mammalian cells, DSBs are repaired through at least two distinct pathways: non-homologous end-joining (NHEJ) and homologous recombination (HR). Both repair mechanisms can impact tumor response to treatment, as well as potentially serve as novel therapeutic targets in lung cancer management.
Non-Homologous End-Joining
NHEJ is a major DSB repair pathway in mammalian cells. In contrast to HR, NHEJ repairs a wide variety of DSBs with distinct break structures and sequences with high efficiency61 and functions predominantly during G1 but also during S/G2.62 However, NHEJ demonstrates decreased repair fidelity compared to HR, as it repairs DSBs using no or little homologous template to ensure that the repaired strand reflects the original sequence.63 NHEJ repair follows a basic motif of damaged base digestion, re-polymerization/repair, and ligation. Its details have been well characterized by others.63 Briefly, following identification of the DSB in the cell by the MRN complex (MRe11/Rad50/NBS1). DRR signaling is then initiated through a cascade of DNA damage response kinases, typically ATM/Chk1, which facilitate Ku70 and Ku80 bind to the exposed breakpoints as a heterodimer and serve to recruit other necessary proteins.64 DNA-dependent protein kinase (DNA-PK) is recruited to the site and exposes the DNA ends to recruited nucleases.65,66 DNA-PK also activates the G1 DNA damage checkpoint and arrest the G1-S transition.67 A wide variety of nucleases digest nucleotides from the DNA on both strands, and strand re-extension is facilitated by X family DNA polymerases,68 although in a less extensive fashion than occurs in HR.69 Finally, the DNA ligase IV complex joins the two repaired DNA ends.70
Homologous Recombination
HR is a critical pathway for accurate repair of DSBs and maintenance of genomic stability (reviewed in 1). Homology-mediated repair is characterized by deriving of the correct sequence from a homologous strand of intact DNA. This modeling process allows for high-fidelity repair of DSBs, much more so than repair via NHEJ.69 It is the primary pathway of DSB repair during the S and G2 phases of the cell cycle, in part due to the availability of sister chromatids to be used as repair templates.71
Homology-mediated DSB repair follows a general scheme of nuclease-mediated resection of damaged DNA ends, polymerization of new DNA, and ligation to restore strand integrity. Detailed biochemistry of the pathway has been well described by others.72 Rad51 and its paralogs such as XRCC3 are critical in homology searching to identify a homologous sequence on the sister chromatid.73,74 When a homologous sequence is found, there is an exchange of the damaged strands, such that each strand is now paired with a homologous template. The damaged strands are then extended and ligated, restoring the original double-strand.75,76
HR not only occurs at frank DSBs but also at DNA replication forks encountering various types of DNA damage (Figure 3A) (reviewed in1). The HR pathway is important for the stabilization and/or recovery of stalled replication forks. Defects in HR, for example due to mutation or downregulation of BRCA1, BRCA2, or RAD51, cause hypersensitivity to agents such as cisplatin. Even though the damage spectrum after cisplatin treatment is dominated by intrastrand adducts, with interstrand crosslinks (ICL) comprising only less than 10% of the total, it is the ICLs that present a particular impediment to fork progression and likely underlie the toxicity of cisplatin in HR-defective cells. ERCC1, which has been explored as a biomarker in lung cancer therapy, does not only act on intrastrand crosslinks via the NER pathway, but also is as part of the ERCC1-XPF endonuclease complex involved in ICL unhooking at stalled or collapsed replication forks. In fact, the hypersensitivity of ERCC1-deficient cells to cisplatin is likely due to an inability to excise ICLs and complete HR rather than defective NER77.
Figure 3.
Importance of homologous recombination (HR) in the response to lung cancer therapies. (A) Cell cycle dependence of HR mechanisms, acting on stalled replication forks in S-phase and mediating sister chromatid mediated repair of two-ended DNA double-strand breaks in late S/G2. (B) Illustration of an ex-vivo foci biomarker assay for the detection of HR defects in lung cancers.
Targeting HR and Functional Biomarkers
Because of its importance during the S and G2 phase of the cell cycle (Figure 3A), the HR pathway is heavily favored by rapidly dividing tissues, including neoplasms. This reliance on HR underlies the cytotoxicity of cisplatin, which binds guanine, resulting in inter-strand DNA crosslinking.78 In addition to DNA damage-based therapeutics like cisplatin recent evidence shows that molecularly targeted agents may also act via the HR pathway. The TKI erlotinib has been previously shown to disrupt the nuclear function of BRCA1 and attenuate Rad51 and overall HR activity, therefore sensitizing cancer cells, including lung cancer cell lines, to IR.79,80 Recent studies have also demonstrated that direct inhibition of HR proteins by a variety of known proteasome inhibitors also results in cisplatin sensitization.81
Experiments conducted in our laboratory (XF) have demonstrated a similar HR attenuation in breast cancer and glioma cell models via (1) siRNA-mediated BRCA1 knockdown, (2) IR-mediated export of BRCA1 to the cytosol, and (3) erlotinib-induced BRCA1 nuclear depletion. siRNA-mediated BRCA1 knockdown results in suppression of HR repair in several cancer and non-cancer cell model systems. BRCA1 is also a nuclear-cytosolic shuttling protein, and its function is regulated by its subcellular location. When in the nucleus, BRCA1 participates in HR repair of DSBs. When in the cytosol, it enhances apoptosis. We have recently found that IR induces nuclear export of BRCA1 in response to DNA damage via the CRM1/exportin-dependent pathway. Additionally, inhibition of EGFR by erlotinib results in DNA damage and BRCA1 nuclear export.79 We believe that biology-based combined use of cisplatin and erlotinib in defined sequential fashion may synergistically enhance tumor killing and further sensitize lung tumors to other DNA damage-based therapeutic agents.
Additionally, recent preclinical and clinical studies of poly(ADP-ribose) polymerase 1 (PARP1) inhibition have been promising. PARP1 plays a key role in the repair of single-strand breaks (SSB)82. Inhibitors of this enzyme, including olaparib, target cancers that are deficient in the repair of HR-mediated DSBs, exhibit up to 1,000-fold selectivity in killing HR-deficient mutant BRCA1/2 cells, and provide an overall survival and progression-free survival benefit with minimal toxicity in breast cancers.83–87 intriguingly, we have been able to induce HR deficiency not only via BRCA1 knockdown, but also by erlotinib- or IR-induced BRCA1 nuclear export. When coupled with PARP inhibition therapy, we were able to demonstrate decreased HR-mediated DSB repair, and increased tumor cytotoxicity response.79 Therefore, cytosolic sequestration of BRCA1 and HR attenuation by IR and/or EGFR inhibitors provide a new strategy for converting HR-competent lung cancers into ones that are susceptible to PARP1 inhibition. Further studies toward development of similar therapeutic schemes that combine induced HR deficiency with known DNA damage-based agents, such as cisplatin or PARP inhibitors88, may provide potential therapeutic gain via selective cytotoxicity to proliferating lung cancer cells.
As an alternative strategy, there is increasing evidence that a significant fraction of NSCLCs harbor genetic or epigenetic defects in the HR pathway2,89–91. In addition, perturbations of the PI3K-AKT pathways, which are frequent in lung cancer, have been shown to affect HR activity92,93. HR may also be downregulated in hypoxic tumor regions94,95. Pre-existing defects in HR should not only predict an increased likelihood of hypersensitivity to cisplatin but also PARP inhibitors2. The identification of HR-deficient cancers in the clinic is a major challenge especially when taking into account the complexity of the HR pathway with many yet to be identified pathway components1. Additionally, assessing the expression of individual pathway components, such as BRCA1 or ERCC1, is unlikely to reveal the overall incidence of defects that can occur anywhere in pathway. Lastly, it is not established whether reduced gene expression translates into functional repair defects. Recent efforts have been aimed at assessing the ability of repair proteins to concentrate in subnuclear foci as a surrogate of HR activity1. Subnuclear foci are multiprotein complexes that are organized into centers surrounding damaged DNA and can be visualized as “dots” using immunofluorescence microscopy or immunohistochemistry. These complexes are highly dynamic structures involved in DNA repair and checkpoint responses. Defective formation of BRCA1, FANCD2, or RAD51 foci in cancer tissues following in-vivo or ex-vivo treatment with cytotoxic therapies may be indicative of an HR defect, while a concurrent increase in gamma-H2AX foci may signal persistent, unrepaired DNA damage2,96,97 (Figure 3B). The advantage of using foci as functional biomarkers is that they can detect repair defects due to several mechanisms such as mutations, epigenetic alterations, or perturbations in signal transduction pathways. Moreover, they provide a global measurement of HR proficiency without needing to know the identities of all the pathway components, many of which remain unknown. One can envision developing mechanism-based “HR foci signatures” that reflect nodal points in the HR pathway or network of associated DDR proteins.
Genomic Biomarkers of Radiosensitivity
There has been a plethora of studies trying to establish predictive biomarkers of radioresistance, and a discussion of these is beyond the scope of this review. As radiation causes complex damages in cells and tissues, which can differ between individual tumors, it is perhaps doubtful whether universal, single biomarkers can ever be identified. However, our increasing knowledge of lung cancer genotypes, coupled with preclinical data on gene function, may ultimately produce genomic biomarkers that correlate with tumor radiosensitivity or radioresistance. In this regard, it is worth emphasizing that DSB produced by IR are particularly genotoxic and are repaired mostly by NHEJ (and less so by HR) (reviewed in 55). It follows that genomic alterations that disturb this repair process would be likely to alter the radiosensitivity of an affected tumor. For example, experimental data indicate that mutations in EGFR impair NHEJ and increase cellular radiosensitivity, and clinical observations are consistent with improved local tumor control for EGFR mutant NSCLC treated with radiation44,98. Conversely, it has been suggested that mutant KRAS promotes cellular radioresistance by promoting NHEJ, though whether KRAS mutant NSCLC are more radioresistant than KRAS wild-type cancers remains to be established99,100. Mutations in the ATM kinase, which controls aspects of NHEJ, occurs in ~7% of lung adenocarcinoma51,101. Thus, one would predict that the affected tumors may display increased radiosensitivity. However, there are no recurrent ATM mutations reported, thus limiting the ability of PCR-based clinical tests to capture these alterations45. Furthermore, it needs to be kept in mind that a particular mutation in ATM may not necessarily be functionally relevant, let alone influence the protein's role in NHEJ.
Conclusion
In the future, therapeutic gain will be maximized by individualized therapies that are based on phenotypic and genotypic profiling of lung cancers patients. For example, predicting which tumors respond to radiation with accelerated tumor cell repopulation will allow us to add drugs that can inhibit repopulation such as EGFR-directed agents. The combination of radiation therapy with molecularly targeted agents will be a highly individualized treatment approach. In addition, the identification of HR defects in lung cancers, or the conversion of HR-proficient to - deficient cancers, should facilitate rational combinations with radiosensitizing chemotherapeutics and targeted agents. However, to some degree, radiation therapy will always have to remain unselective and indiscriminant to eradicate the last surviving dormant and probably drug-resistant tumor stem cell4.
Acknowledgments
Source of Funding: H.W. is currently receiving partial support from the Dana-Farber/Harvard Cancer Center SPORE in Lung Cancer, NCI P50 CA090578 and the Federal Share of program income earned by Massachusetts General Hospital on C06 CA059267, Proton Therapy Research and Treatment Center; FX is currently receiving R01 NIH NCI CA163838.
Footnotes
Conflicts of Interest For the remaining authors none were declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Willers H, Pfäffle HN, Zou L. Targeting Homologous Recombination Repair in Cancer. In: Kelley MR, editor. DNA Repair in Cancer Therapy: Molecular Targets and Clinical Applications. Academic Press; Elsevier: 2012. pp. 119–60. [Google Scholar]
- 2.Birkelbach M, Ferraiolo N, Gheorghiu L, et al. Detection of Impaired Homologous Recombination Repair in NSCLC Cells and Tissues. J Thorac Oncol. 2013;8(3):279–86. doi: 10.1097/JTO.0b013e31827ecf83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8(3):193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- 4.Willers H, Held KD. Introduction to clinical radiation biology. Hematol Oncol Clin North Am. 2006;20(1):1–24. doi: 10.1016/j.hoc.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Baumann M, Krause M, Dikomey E, et al. EGFR-targeted anti-cancer drugs in radiotherapy: preclinical evaluation of mechanisms. Radiother Oncol. 2007;83(3):238–48. doi: 10.1016/j.radonc.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Krause M, Yaromina A, Eicheler W, Koch U, Baumann M. Cancer stem cells: targets and potential biomarkers for radiotherapy. Clin Cancer Res. 2011;17(23):7224–9. doi: 10.1158/1078-0432.CCR-10-2639. [DOI] [PubMed] [Google Scholar]
- 7.O'Flaherty JD, Barr M, Fennell D, et al. The cancer stem-cell hypothesis: its emerging role in lung cancer biology and its relevance for future therapy. J Thorac Oncol. 2012;7(12):1880–90. doi: 10.1097/JTO.0b013e31826bfbc6. [DOI] [PubMed] [Google Scholar]
- 8.Dubben HH, Thames HD, Beck-Bornholdt HP. Tumor volume: a basic and specific response predictor in radiotherapy. Radiother Oncol. 1998;47(2):167–74. doi: 10.1016/s0167-8140(97)00215-6. [DOI] [PubMed] [Google Scholar]
- 9.Yaromina A, Krause M, Thames H, et al. Pre-treatment number of clonogenic cells and their radiosensitivity are major determinants of local tumour control after fractionated irradiation. Radiother Oncol. 2007;83(3):304–10. doi: 10.1016/j.radonc.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Alexander BM, Othus M, Caglar HB, Allen AM. Tumor volume is a prognostic factor in non-small-cell lung cancer treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2011;79(5):1381–7. doi: 10.1016/j.ijrobp.2009.12.060. [DOI] [PubMed] [Google Scholar]
- 11.Soliman M, Yaromina A, Appold S, et al. GTV differentially impacts locoregional control of non-small cell lung cancer (NSCLC) after different fractionation schedules: Subgroup analysis of the prospective randomized CHARTWEL trial. Radiother Oncol. 2013 doi: 10.1016/j.radonc.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Werner-Wasik M, Swann RS, Bradley J, et al. Increasing tumor volume is predictive of poor overall and progression-free survival: secondary analysis of the Radiation Therapy Oncology Group 93–11 phase I–II radiation dose-escalation study in patients with inoperable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;70(2):385–90. doi: 10.1016/j.ijrobp.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 13.Zhao L, West BT, Hayman JA, Lyons S, Cease K, Kong FM. High radiation dose may reduce the negative effect of large gross tumor volume in patients with medically inoperable early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2007;68(1):103–10. doi: 10.1016/j.ijrobp.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 14.Le Chevalier T, Arriagada R, Quoix E, et al. Radiotherapy alone versus combined chemotherapy and radiotherapy in nonresectable non-small-cell lung cancer: first analysis of a randomized trial in 353 patients. J Natl Cancer Inst. 1991;83(6):417–23. doi: 10.1093/jnci/83.6.417. [DOI] [PubMed] [Google Scholar]
- 15.Curran WJ, Jr., Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103(19):1452–60. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox JD. Are the results of RTOG 0617 mysterious? Int J Radiat Oncol Biol Phys. 2012;82(3):1042–4. doi: 10.1016/j.ijrobp.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 17.Ausborn NL, Le QT, Bradley JD, et al. Molecular profiling to optimize treatment in non-small cell lung cancer: a review of potential molecular targets for radiation therapy by the translational research program of the radiation therapy oncology group. Int J Radiat Oncol Biol Phys. 2012;83(4):e453–64. doi: 10.1016/j.ijrobp.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 18.Provencio M, Sanchez A, Garrido P, Valcarcel F. New molecular targeted therapies integrated with radiation therapy in lung cancer. Clin Lung Cancer. 2010;11(2):91–7. doi: 10.3816/CLC.2010.n.012. [DOI] [PubMed] [Google Scholar]
- 19.Neal JW, Sequist LV. First-line use of EGFR tyrosine kinase inhibitors in patients with NSCLC containing EGFR mutations. Clin Adv Hematol Oncol. 2010;8(2):119–26. [PubMed] [Google Scholar]
- 20.Sharma SV, Lee DY, Li B, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141(1):69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JJ, Tannock IF. Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat Rev Cancer. 2005;5(7):516–25. doi: 10.1038/nrc1650. [DOI] [PubMed] [Google Scholar]
- 22.Krause M, Zips D, Thames HD, Kummermehr J, Baumann M. Preclinical evaluation of molecular-targeted anticancer agents for radiotherapy. Radiother Oncol. 2006;80(2):112–22. doi: 10.1016/j.radonc.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Saunders M, Dische S, Barrett A, Harvey A, Gibson D, Parmar M. Continuous hyperfractionated accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small-cell lung cancer: a randomised multicentre trial. CHART Steering Committee. Lancet. 1997;350(9072):161–5. doi: 10.1016/s0140-6736(97)06305-8. [DOI] [PubMed] [Google Scholar]
- 24.Turrisi AT, 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340(4):265–71. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 25.Baumann M, Herrmann T, Koch R, et al. Final results of the randomized phase III CHARTWEL-trial (ARO 97–1) comparing hyperfractionated-accelerated versus conventionally fractionated radiotherapy in non-small cell lung cancer (NSCLC) Radiother Oncol. 2011;100(1):76–85. doi: 10.1016/j.radonc.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 26.Machtay M, Hsu C, Komaki R, et al. Effect of overall treatment time on outcomes after concurrent chemoradiation for locally advanced non-small-cell lung carcinoma: analysis of the Radiation Therapy Oncology Group (RTOG) experience. Int J Radiat Oncol Biol Phys. 2005;63(3):667–71. doi: 10.1016/j.ijrobp.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 27.Milas L, Nakayama T, Hunter N, et al. Dynamics of tumor cell clonogen repopulation in a murine sarcoma treated with cyclophosphamide. Radiother Oncol. 1994;30(3):247–53. doi: 10.1016/0167-8140(94)90465-0. [DOI] [PubMed] [Google Scholar]
- 28.Chen CP, Weinberg VK, Jahan TM, Jablons DM, Yom SS. Implications of delayed initiation of radiotherapy: accelerated repopulation after induction chemotherapy for stage III non-small cell lung cancer. J Thorac Oncol. 2011;6(11):1857–64. doi: 10.1097/JTO.0b013e318229a41e. [DOI] [PubMed] [Google Scholar]
- 29.El Sharouni SY, Kal HB, Battermann JJ. Accelerated regrowth of non-small-cell lung tumours after induction chemotherapy. Br J Cancer. 2003;89(12):2184–9. doi: 10.1038/sj.bjc.6601418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Q, Li F, Liu X, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011;17(7):860–6. doi: 10.1038/nm.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bentzen SM, Atasoy BM, Daley FM, et al. Epidermal growth factor receptor expression in pretreatment biopsies from head and neck squamous cell carcinoma as a predictive factor for a benefit from accelerated radiation therapy in a randomized controlled trial. J Clin Oncol. 2005;23(24):5560–7. doi: 10.1200/JCO.2005.06.411. [DOI] [PubMed] [Google Scholar]
- 32.Krause M, Ostermann G, Petersen C, et al. Decreased repopulation as well as increased reoxygenation contribute to the improvement in local control after targeting of the EGFR by C225 during fractionated irradiation. Radiother Oncol. 2005;76(2):162–7. doi: 10.1016/j.radonc.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 33.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 34.Nieder C, Bremnes RM. Effects of smoking cessation on hypoxia and its potential impact on radiation treatment effects in lung cancer patients. Strahlenther Onkol. 2008;184(11):605–9. doi: 10.1007/s00066-008-1877-4. [DOI] [PubMed] [Google Scholar]
- 35.Hoff CM, Grau C, Overgaard J. Effect of smoking on oxygen delivery and outcome in patients treated with radiotherapy for head and neck squamous cell carcinoma--a prospective study. Radiother Oncol. 2012;103(1):38–44. doi: 10.1016/j.radonc.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Langendijk H, de Jong J, Wanders R, Lambin P, Slotman B. The importance of pre-treatment haemoglobin level in inoperable non-small cell lung carcinoma treated with radical radiotherapy. Radiother Oncol. 2003;67(3):321–5. doi: 10.1016/s0167-8140(03)00057-4. [DOI] [PubMed] [Google Scholar]
- 37.Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol. 9(12):674–87. doi: 10.1038/nrclinonc.2012.171. [DOI] [PubMed] [Google Scholar]
- 38.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 39.Socinski MA, Smit EF, Lorigan P, et al. Phase III study of pemetrexed plus carboplatin compared with etoposide plus carboplatin in chemotherapy-naive patients with extensive-stage small-cell lung cancer. J Clin Oncol. 2009;27(28):4787–92. doi: 10.1200/JCO.2009.23.1548. [DOI] [PubMed] [Google Scholar]
- 40.Spiro SG, Rudd RM, Souhami RL, et al. Chemotherapy versus supportive care in advanced non-small cell lung cancer: improved survival without detriment to quality of life. Thorax. 2004;59(10):828–36. doi: 10.1136/thx.2003.020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azzoli CG, Baker S, Jr., Temin S, et al. American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol. 2009;27(36):6251–66. doi: 10.1200/JCO.2009.23.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.NCCN Guidelines . Non-Small Cell Lung Cancer. 2013. [Google Scholar]
- 43.Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J Clin Oncol. 2011;29(21):2866–74. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 44.Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12(11):1004–12. doi: 10.1016/S1470-2045(11)70232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turke AB, Zejnullahu K, Wu YL, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17(1):77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galluzzi L, Senovilla L, Vitale I, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31(15):1869–83. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 48.Chen KG, Sikic BI. Molecular pathways: regulation and therapeutic implications of multidrug resistance. Clin Cancer Res. 2012;18(7):1863–9. doi: 10.1158/1078-0432.CCR-11-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sau A, Pellizzari Tregno F, Valentino F, Federici G, Caccuri AM. Glutathione transferases and development of new principles to overcome drug resistance. Arch Biochem Biophys. 2010;500(2):116–22. doi: 10.1016/j.abb.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 50.Filipits M, Pirker R, Dunant A, et al. Cell cycle regulators and outcome of adjuvant cisplatin-based chemotherapy in completely resected non-small-cell lung cancer: the International Adjuvant Lung Cancer Trial Biologic Program. J Clin Oncol. 2007;25(19):2735–40. doi: 10.1200/JCO.2006.08.2867. [DOI] [PubMed] [Google Scholar]
- 51.Tsao MS, Aviel-Ronen S, Ding K, et al. Prognostic and predictive importance of p53 and RAS for adjuvant chemotherapy in non small-cell lung cancer. J Clin Oncol. 2007;25(33):5240–7. doi: 10.1200/JCO.2007.12.6953. [DOI] [PubMed] [Google Scholar]
- 52.Brambilla E, Bourredjem A, Lantuejoul S, et al. Bax Expression as a Predictive Marker of Survival Benefit in Non-small Cell Lung Carcinoma Treated by Adjuvant Cisplatin-based Chemotherapy. J Thorac Oncol. 2010;5(12, suppl 7):S503. [Google Scholar]
- 53.Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355(10):983–91. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 54.Kamal NS, Soria JC, Mendiboure J, et al. MutS homologue 2 and the long-term benefit of adjuvant chemotherapy in lung cancer. Clin Cancer Res. 2010;16(4):1206–15. doi: 10.1158/1078-0432.CCR-09-2204. [DOI] [PubMed] [Google Scholar]
- 55.Willers H, Dahm-Daphi J, Powell SN. Repair of radiation damage to DNA. Br J Cancer. 2004;90(7):1297–301. doi: 10.1038/sj.bjc.6601729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei Q, Cheng L, Hong WK, Spitz MR. Reduced DNA repair capacity in lung cancer patients. Cancer Res. 1996;56(18):4103–7. [PubMed] [Google Scholar]
- 57.Frosina G. DNA repair and resistance of gliomas to chemotherapy and radiotherapy. Mol Cancer Res. 2009;7(7):989–99. doi: 10.1158/1541-7786.MCR-09-0030. [DOI] [PubMed] [Google Scholar]
- 58.Hagiwara H, Sunada Y. Mechanism of taxane neurotoxicity. Breast Cancer. 2004;11(1):82–5. doi: 10.1007/BF02968008. [DOI] [PubMed] [Google Scholar]
- 59.Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther. 2007;6(2):404–17. doi: 10.1158/1535-7163.MCT-06-0343. [DOI] [PubMed] [Google Scholar]
- 60.Pauwels B, Korst AE, Pattyn GG, et al. Cell cycle effect of gemcitabine and its role in the radiosensitizing mechanism in vitro. Int J Radiat Oncol Biol Phys. 2003;57(4):1075–83. doi: 10.1016/s0360-3016(03)01443-3. [DOI] [PubMed] [Google Scholar]
- 61.Lieber MR, Ma Y, Pannicke U, Schwarz K. Mechanism and regulation of human nonhomologous DNA end-joining. Nat Rev Mol Cell Biol. 2003;4(9):712–20. doi: 10.1038/nrm1202. [DOI] [PubMed] [Google Scholar]
- 62.Shibata A, Conrad S, Birraux J, et al. Factors determining DNA double-strand break repair pathway choice in G2 phase. Embo J. 2011;30(6):1079–92. doi: 10.1038/emboj.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weterings E, Chen DJ. The endless tale of non-homologous end-joining. Cell Res. 2008;18(1):114–24. doi: 10.1038/cr.2008.3. [DOI] [PubMed] [Google Scholar]
- 64.Spagnolo L, Rivera-Calzada A, Pearl LH, Llorca O. Three-dimensional structure of the human DNA-PKcs/Ku70/Ku80 complex assembled on DNA and its implications for DNA DSB repair. Mol Cell. 2006;22(4):511–9. doi: 10.1016/j.molcel.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 65.Moll U, Lau R, Sypes MA, Gupta MM, Anderson CW. DNA-PK, the DNA-activated protein kinase, is differentially expressed in normal and malignant human tissues. Oncogene. 1999;18(20):3114–26. doi: 10.1038/sj.onc.1202640. [DOI] [PubMed] [Google Scholar]
- 66.Ma Y, Pannicke U, Schwarz K, Lieber MR. Hairpin opening and overhang processing by an Artemis/DNA-dependent protein kinase complex in nonhomologous end joining and V(D)J recombination. Cell. 2002;108(6):781–94. doi: 10.1016/s0092-8674(02)00671-2. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki K, Kodama S, Watanabe M. Recruitment of ATM protein to double strand DNA irradiated with ionizing radiation. J Biol Chem. 1999;274(36):25571–5. doi: 10.1074/jbc.274.36.25571. [DOI] [PubMed] [Google Scholar]
- 68.Yamtich J, Sweasy JB. DNA polymerase family X: function, structure, and cellular roles. Biochim Biophys Acta. 2010;1804(5):1136–50. doi: 10.1016/j.bbapap.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mao Z, Bozzella M, Seluanov A, Gorbunova V. Comparison of nonhomologous end joining and homologous recombination in human cells. DNA Repair (Amst) 2008;7(10):1765–71. doi: 10.1016/j.dnarep.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson TE, Grawunder U, Lieber MR. Yeast DNA ligase IV mediates non-homologous DNA end joining. Nature. 1997;388(6641):495–8. doi: 10.1038/41365. [DOI] [PubMed] [Google Scholar]
- 71.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 5th Edition. Garland Science; Oxford: 2008. 5 ed. [Google Scholar]
- 72.Moynahan ME, Jasin M. Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis. Nat Rev Mol Cell Biol. 2010;11(3):196–207. doi: 10.1038/nrm2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brenneman MA, Wagener BM, Miller CA, Allen C, Nickoloff JA. XRCC3 controls the fidelity of homologous recombination: roles for XRCC3 in late stages of recombination. Mol Cell. 2002;10(2):387–95. doi: 10.1016/s1097-2765(02)00595-6. [DOI] [PubMed] [Google Scholar]
- 74.Zou Y, Liu Y, Wu X, Shell SM. Functions of human replication protein A (RPA): from DNA replication to DNA damage and stress responses. J Cell Physiol. 2006;208(2):267–73. doi: 10.1002/jcp.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maloisel L, Fabre F, Gangloff S. DNA polymerase delta is preferentially recruited during homologous recombination to promote heteroduplex DNA extension. Mol Cell Biol. 2008;28(4):1373–82. doi: 10.1128/MCB.01651-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goetz JD, Motycka TA, Han M, Jasin M, Tomkinson AE. Reduced repair of DNA double-strand breaks by homologous recombination in a DNA ligase I-deficient human cell line. DNA Repair (Amst) 2005;4(6):649–54. doi: 10.1016/j.dnarep.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 77.Al-Minawi AZ, Lee YF, Hakansson D, et al. The ERCC1/XPF endonuclease is required for completion of homologous recombination at DNA replication forks stalled by inter-strand cross-links. Nucleic Acids Res. 2009;37(19):6400–13. doi: 10.1093/nar/gkp705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McHugh PJ, Spanswick VJ, Hartley JA. Repair of DNA interstrand crosslinks: molecular mechanisms and clinical relevance. Lancet Oncol. 2001;2(8):483–90. doi: 10.1016/S1470-2045(01)00454-5. [DOI] [PubMed] [Google Scholar]
- 79.Li L, Wang H, Yang ES, Arteaga CL, Xia F. Erlotinib attenuates homologous recombinational repair of chromosomal breaks in human breast cancer cells. Cancer Res. 2008;68(22):9141–6. doi: 10.1158/0008-5472.CAN-08-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chinnaiyan P, Huang S, Vallabhaneni G, et al. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva) Cancer Res. 2005;65(8):3328–35. doi: 10.1158/0008-5472.CAN-04-3547. [DOI] [PubMed] [Google Scholar]
- 81.Jacquemont C, Simon JA, D'Andrea AD, Taniguchi T. Non-specific chemical inhibition of the Fanconi anemia pathway sensitizes cancer cells to cisplatin. Mol Cancer. 2012;11:26. doi: 10.1186/1476-4598-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26(8):882–93. doi: 10.1002/bies.20085. [DOI] [PubMed] [Google Scholar]
- 83.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–7. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 84.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 85.Turner NC, Lord CJ, Iorns E, et al. A synthetic lethal siRNA screen identifying genes mediating sensitivity to a PARP inhibitor. Embo J. 2008;27(9):1368–77. doi: 10.1038/emboj.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–34. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 87.Underhill C, Toulmonde M, Bonnefoi H. A review of PARP inhibitors: from bench to bedside. Ann Oncol. 2011;22(2):268–79. doi: 10.1093/annonc/mdq322. [DOI] [PubMed] [Google Scholar]
- 88.Johnson N, Li YC, Walton ZE, et al. Compromised CDK1 activity sensitizes BRCA-proficient cancers to PARP inhibition. Nat Med. 2010;17(7):875–82. doi: 10.1038/nm.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marsit CJ, Liu M, Nelson HH, Posner M, Suzuki M, Kelsey KT. Inactivation of the Fanconi anemia/BRCA pathway in lung and oral cancers: implications for treatment and survival. Oncogene. 2004;23(4):1000–4. doi: 10.1038/sj.onc.1207256. [DOI] [PubMed] [Google Scholar]
- 90.Rosell R, Skrzypski M, Jassem E, et al. BRCA1: A Novel Prognostic Factor in Resected Non-Small-Cell Lung Cancer. PLoS ONE. 2007;2(11):e1129. doi: 10.1371/journal.pone.0001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 92.Plo I, Laulier C, Gauthier L, Lebrun F, Calvo F, Lopez BS. AKT1 inhibits homologous recombination by inducing cytoplasmic retention of BRCA1 and RAD51. Cancer Res. 2008;68(22):9404–12. doi: 10.1158/0008-5472.CAN-08-0861. [DOI] [PubMed] [Google Scholar]
- 93.McEllin B, Camacho CV, Mukherjee B, et al. PTEN loss compromises homologous recombination repair in astrocytes: implications for glioblastoma therapy with temozolomide or poly(ADP-ribose) polymerase inhibitors. Cancer Res. 2010;70(13):5457–64. doi: 10.1158/0008-5472.CAN-09-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bindra RS, Schaffer PJ, Meng A, et al. Down-regulation of Rad51 and decreased homologous recombination in hypoxic cancer cells. Mol Cell Biol. 2004;24(19):8504–18. doi: 10.1128/MCB.24.19.8504-8518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chan N, Koritzinsky M, Zhao H, et al. Chronic hypoxia decreases synthesis of homologous recombination proteins to offset chemoresistance and radioresistance. Cancer Res. 2008;68(2):605–14. doi: 10.1158/0008-5472.CAN-07-5472. [DOI] [PubMed] [Google Scholar]
- 96.Graeser M, McCarthy A, Lord CJ, et al. A marker of homologous recombination predicts pathologic complete response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res. 2010;16(24):6159–68. doi: 10.1158/1078-0432.CCR-10-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Willers H, Taghian AG, Luo C-M, Treszezamsky AD, Sgroi D, Powell SN. Utility of DNA repair protein foci for the detection of putative BRCA1-pathway defects in breast cancer biopsies. Mol Cancer Res. 2009;7:1304–9. doi: 10.1158/1541-7786.MCR-09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Das AK, Sato M, Story MD, et al. Non-small-cell lung cancers with kinase domain mutations in the epidermal growth factor receptor are sensitive to ionizing radiation. Cancer Res. 2006;66(19):9601–8. doi: 10.1158/0008-5472.CAN-06-2627. [DOI] [PubMed] [Google Scholar]
- 99.Mak RH, Doran E, Muzikansky A, et al. KRAS Mutation Is Associated With Decreased Overall Survival After Thoracic Radiation Therapy In Patients With Locally Advanced Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2010;78(3 (Suppl. 1)) abstract #76. [Google Scholar]
- 100.Bernhard EJ, Stanbridge EJ, Gupta S, et al. Direct evidence for the contribution of activated N-ras and K-ras oncogenes to increased intrinsic radiation resistance in human tumor cell lines. Cancer Res. 2000;60(23):6597–600. [PubMed] [Google Scholar]
- 101.Riballo E, Kuhne M, Rief N, et al. A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to gamma-H2AX foci. Mol Cell. 2004;16(5):715–24. doi: 10.1016/j.molcel.2004.10.029. [DOI] [PubMed] [Google Scholar]



