Abstract
SUMMARY
Brewing beer involves microbial activity at every stage, from raw material production and malting to stability in the package. Most of these activities are desirable, as beer is the result of a traditional food fermentation, but others represent threats to the quality of the final product and must be controlled actively through careful management, the daily task of maltsters and brewers globally. This review collates current knowledge relevant to the biology of brewing yeast, fermentation management, and the microbial ecology of beer and brewing.
INTRODUCTION
Beer, like any fermented food, is an immutably microbial product. Microbial activity is involved in every step of its production, defining the many sensory characteristics that contribute to final quality. While fermentation of cereal extracts by Saccharomyces is the most important microbial process involved in brewing, a vast array of other microbes affect the complete process (Fig. 1). Microbial interdiction at every step of the barley-to-beer continuum greatly influences the quality of beer. For an overview of the processes of malting and brewing, see the work of Bamforth (1).
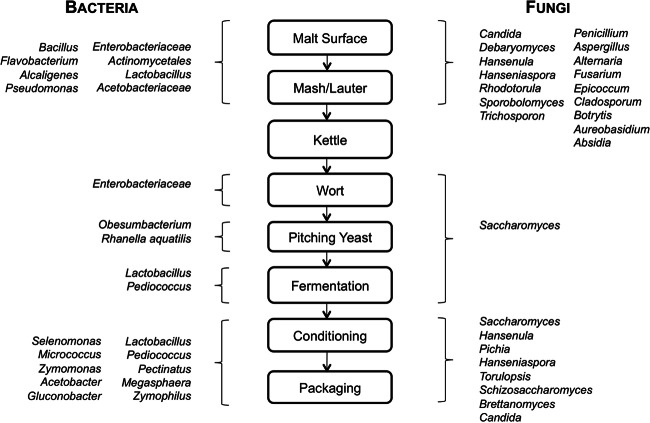
Fig 1.
Microbiota of malting and brewing. The diagram shows an overview of bacterial and fungal species previously reported at all major stages of beer production. (Adapted from reference 156 with permission of the publisher.)
BREWING YEAST
Although all strains of Saccharomyces will produce ethanol as a fermentation end product, in practice the strains employed in the production of beers worldwide are classified into the categories of ale and lager yeasts. The seminal text on brewing yeast is that of Boulton and Quain (2).
Ale yeasts, which are Saccharomyces cerevisiae strains, are the more diverse yeasts and have been isolated in innumerable locations worldwide. Such yeasts are often referred to as “top-fermenting” yeasts, insofar as in traditional open fermenters they rise to the surface of the vessel, facilitating their collection by skimming, ready for repitching into the next fermentation. The hydrostatic pressure in modern cylindroconical fermenters, many of which may contain up to 10,000 hl of fermenting beer (3), tends to overcome this tendency of ale yeast, which accordingly collects in the cone of the tank.
The nomenclature of lager yeast (“bottom-fermenting” yeast, on account of its tendency not to rise to the surface under any set of fermentation conditions) has evolved, passing through iterations of S. carlsbergensis and S. cerevisiae lager type to the currently accepted name, S. pastorianus (4–6). Irrespective of its name, lager yeast is a more complex organism than ale yeast, and it has been proposed that it arose in perhaps two separate steps involving the hybridization of S. cerevisiae with S. bayanus (7, 8).
It has generally come to be considered that lager yeast (unlike ale yeast) is not readily isolable from nature, though it was recently proposed that the cryotolerant strain of yeast that melded with S. cerevisiae in domestication circumstances to produce S. pastorianus originated in southern beech forests in Patagonia and represents Saccharomyces eubayanus sp. nov. (9).
There is far more diversity among ale strains than among lager strains (10). The latter can be divided into the Carlsberg and Tuborg types, based on chromosomal fingerprints (11), and there are comparatively minor differences between them. Casey (11) suggests that this far greater diversity of ale strains reflects their isolation in multiple locations, whereas the lager strains emerged from a very limited locality.
The genome of S. cerevisiae has been sequenced fully (12). Whereas the strains used for sequencing were haploid, brewing strains of yeast are polyploid or aneuploid, with 3 or 4 copies of each chromosome (13, 14). There is only limited information on the significance of this for yeast behavior, with one of the few studies being that of Galitski et al. (15), who found very few effects.
It is generally believed that the multiplicity of gene copies makes for a more stable yeast organism (10), and there may be a boost of enzyme production leading to more rapid metabolism of wort components, e.g., maltose (14). There appear to be some fundamental differences between the chromosomes in haploid and polyploid strains (10, 16). Despite the polyploid nature of brewing strains, there is evidence that there is chromosomal instability (11, 17). Repercussions include changes in flocculation and utilization of maltotriose (18). Yeast drift can also arise through the partial or complete loss of mitochondrial DNA, leading to the production of so-called “petites” (19–21). Although alcoholic fermentation is anaerobic, meaning there is no role for a respiratory function in mitochondria, the latter organelles do have other metabolic functions in brewery fermentations (22–24).
Typing of Yeast
The differentiation of brewing strains has been reviewed by Quain (25) and Casey et al. (26). Traditional approaches include examining colony morphology on plates (27), the ability of yeasts to metabolize melibiose (lager strains can do so due to their elaboration of an α-galactosidase, whereas ale strains cannot [28]), temperature tolerance (29), flocculation tests (2), behavior in small-scale fermenters (30, 31), and oxygen requirements (32, 33). Latterly, the emphasis has been on DNA-based techniques, including restriction fragment length polymorphism analysis (34), PCR (35, 36), karyotyping (11), and amplified fragment length polymorphism analysis (37). Additionally, pyrolysis mass spectroscopy (38), Fourier transform infrared spectroscopy (39), fatty acid methyl ester profiling (40), and protein fingerprinting (41) are other possibilities.
Yeast Resources and Handling
Several yeast culture collections and providers are available (Table 1). Larger brewing companies, however, tend to manage their own in-house strains, including the storage of master cultures (43, 44). Back-ups of these organisms are deposited with third parties. Storage of cultures in liquid nitrogen is deemed preferable in terms of survival, shelf life, and genetic stability compared to storage on agar, in broth, or by lyophilization (43).
TABLE 1.
List of culture collectionsa
| Collection | Type of organisms | Web address |
|---|---|---|
| American Type Culture Collection (ATCC) | All types | www.atcc.org |
| CABI Bioscience | Filamentous fungi | www.cabi-bioscience.org |
| Centraalbureau voor Schimmelcultures | Filamentous fungi and yeasts | www.cbs.knaw.nl/ |
| Collection Nationale de Cultures de Microorganismes | All types | http://www.pasteur.fr/recherche/unites/Cncm/index-en.html |
| Die Deutsche Sammlung von Mikroorganismen und Zellkulturen | All types | http://www.dsmz.de/ |
| Herman J. Phaff Culture Collection | Yeasts and fungi | http://www.phaffcollection.org/ |
| National Collection of Industrial and Marine Bacteria | Bacteria | www.ncimb.co.uk |
| National Collection of Yeast Cultures | Yeasts | www.ncyc.co.uk |
Derived from the work of Bamforth (42).
While there are still brewers who simply repitch yeast from one fermentation to the next ad infinitum (“backslopping”), concerns about genetic drift and selection of variants mean that most brewers pitch with yeast newly propagated from the master cultures at intervals. The frequency is typically 10 to 15 “generations” (this word in a brewing context refers to successive fermentation batches), though even this may be excessive in terms of yeast deterioration (45–47). The chronological events occurring in the life cycle of yeast in brewery fermentations and the consequences for population ageing have been addressed (48).
Yeast propagation, involving batches of successively increasing volumes, has been reviewed by Maule (49) and Quain (44). Yields of biomass can be limited at the high sugar concentrations employed (Crabtree effect), and some have advocated fed-batch systems analogous to those used in the production of baker's yeast (50). Gene transcription during propagation (51) and fermentation (52) has been investigated (also see reference 53). Newly propagated yeast does not usually “perform” as expected in the initial commercial fermentation, in part due to a lack of synchronicity in the cell population (54).
An alternative approach to handling yeast that is attracting some attention in brewing but which is already applied widely in wineries is the use of dried yeast (55–58). Concerns include an impaired ability to handle vicinal diketones (VDKs) (59; see below), impaired flocculation of yeast, and deteriorating foam and clarity in the beer (60).
Key to successful storage and handling of brewing yeast, irrespective of whether it is handled as a slurry or as a dried product, are the storage carbohydrates that it elaborates (61). Glycogen has attracted much study as an important carbon and energy reserve in brewing yeast (62), while the importance of trehalose as a stress protectant is well studied (63).
Fermentation Control
In pursuit of a constant fermentation performance, brewers seek to achieve consistent fermentations, which demands control of the key variables of yeast quantity and health, oxygen input, wort nutritional status, temperature, and yeast-wort contact (mixing).
While traditional techniques for counting yeast, such as counts with a hemocytometer, are still widely applied, there is increasing use of instrumental approaches, often inserted in-line to achieve automated pitching control. Devices include those operating on the basis of assessing capacitance/permittivity (64, 65) and according to principles of light scatter (66).
The viability of yeast has long been assessed by staining of cells with methylene blue; however, other staining approaches have been proposed (67, 68). While these techniques inform about whether cells are alive or dead, they do not gauge the healthfulness (vitality) of the cells (69). Diverse procedures have been nominated for assessing this parameter, but none has been adopted universally. Techniques include assessments of glycogen (70), sterols (71), ATP (72), oxygen uptake rate (73), and acidification power (74, 75), as well as modifications of the methylene blue viability test (76).
While it has long been recognized that a proportion of oxygen is needed by all yeast cells to support the production of the sterols and unsaturated fatty acid components of the cell membranes (77, 78), there is a less-than-clear appreciation of why different yeast strains vary considerably in the amount that they demand (32, 79). Traditionally, the oxygen is introduced to the wort, although there have been proposals to pitch unaerated wort with yeast that has been supplied directly with oxygen (80). Ensuring contact of all yeast cells with oxygen when yeast is present at a high density is important (81). On the other hand, oxygen represents one of the stress factors encountered by yeast (82), while others include ethanol, which limits the practical alcohol concentrations that can be achieved in brewery fermentations (83). Accordingly, there is interest in the development of yeast strains with greater tolerance of high-gravity conditions (84). A review of all the stresses likely to be encountered by brewing yeast has been provided by Gibson et al. (85). There is extensive use of high-gravity brewing in commercial brewing (86), with the attendant osmotic and alcohol stresses.
One major variable that perhaps receives less detailed analysis and control than others in fermenter control is actually the wort composition (87, 88). Most brewers simply regulate the strength of the wort (degrees Plato) and pitch on that basis, assuming that the relative balance of the diverse nutrients within the feedstock is consistent and modulated by the malt selection and how that malt is processed in the brewhouse. To a first approximation, this seems to be a reasonable situation on an experiential basis, although there are two variables that many brewers do seek to regulate more closely, i.e., the clarity of the wort and the concentration of zinc ions (89, 90), although other additions to promote fermentations, particularly those with higher-strength wort, may be employed (91, 92). The presence of insoluble particles in wort (which are derived in the brewhouse and are present at a level in inverse proportion to the extent that they are removed in clarification stages prior to fermentation) promotes yeast action by their ability to nucleate carbon dioxide, thereby releasing bubbles (93). Two effects may be at play, namely, the increased resulting tendency of yeast to be moved through the fermenter and the impact that this has on lowering dissolved CO2 levels in the wort from inhibitory concentrations (94).
The contact of yeast and wort in fermentation is not inconsequential. Often, huge fermenters are filled with several batches of wort, leading to quandaries over precisely when the yeast should be added to the fermenter and how to ensure homogeneity of yeast-wort contact throughout the vessel (95). Mechanical mixing is uncommon but advocated (96).
Fermentations may be monitored in various ways, including measuring the decrease in specific gravity of the wort (including in-process measurements) (97–99), CO2 evolution (100, 101), the pH decrease (102), and ethanol formation (103), as well as camera-based observation of events in the fermenter (104).
At the completion of fermentation, yeast is recovered either for disposal (commonly to animal feed or production of yeast extracts [105]) or for repitching. For open fermenters, ale yeast is skimmed from the surface of the vessel, but for closed cylindroconical vessels the yeast is harvested from the cone. The population of yeast cells differs in the cone, with stratification such that older cells are located beneath the younger, more vital ones (64, 106, 107).
Harvested yeast may either be pumped to the next fermenter filling with fresh wort (cone-to-cone pitching) or stored in either a pressed or slurry form (2). It may receive acid washing to kill any bacteria that may have developed in the slurry (108). Its collection from fermenters is often through the use of centrifuges, creating damage that has implications for subsequent performance (109). The impact of serial repitching was addressed by Jenkins et al. (110), who showed that extents of deterioration vary between yeast cells.
Flocculation
A key influence on harvesting of yeast is its flocculation behavior. The flocculation of brewer's yeast was recently reviewed by Soares (111), Vidgren and Londesborough (112), and Verstrepen et al. (113). The clumping of yeast cells involves the binding of lectin-like proteins to mannoprotein receptors, promoted by calcium ions to overcome the negative zeta potential. The surface hydrophobicity of the cell is also important, and this may relate to the tendency of cell aggregates to migrate to the surface of a fermenter (top-fermenting yeast) (114). There are factors present in certain malts that lead to the premature flocculation of yeast (115, 116; see below), and meanwhile, there may be additional antiyeast materials in malt (117).
Products of Yeast Metabolism in Brewery Fermentations
During fermentation, yeast excretes a range of molecules, in addition to ethanol and CO2, that can affect flavor (Fig. 2). While there are diverse brewing yeast strains, it has been argued that the vast majority do not differ very widely in their gene complement such that they produce unique flavor components. Strain-to-strain variation exists in the levels of some products, but there are extremely limited instances of brewing yeasts procuring flavor-active species that are not produced to at least some extent by other brewery strains.
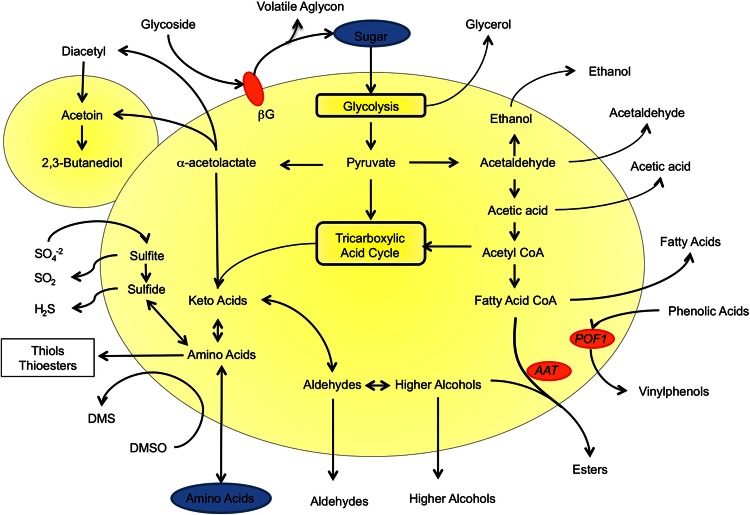
Fig 2.
Overview of Saccharomyces metabolic activities influencing beer quality. This simplified schematic summarizes the main metabolic pathways linked to beer flavor modulation by Saccharomyces. βG, β-glycosidase; DMS, dimethyl sulfide; DMSO, dimethyl sulfoxide.
The exception is the ale strains used for the production of traditional hefeweizen products in Germany. They have a gene coding for ferulic acid decarboxylase, which converts ferulate derived from cereal cell walls to 4-vinylguaiacol (118–121), imparting a spicy, clove-like character.
All brewing strains produce glycerol (120–122), vicinal diketones (VDKs) (123), alcohols (124, 125), esters (126, 127), short-chain fatty acids (33), organic acids (120), and diverse sulfur-containing substances (128, 129). The levels of each category that are found in beer are dependent in part upon the yeast strain, but at least as important are the precise fermentation conditions that exist, including pitching rate (130), temperature, extent of oxygen addition, C:N ratio, and duration of fermentation and maturation (2).
Of especial significance are the VDKs, diacetyl and pentanedione, which afford a buttery or honey-like character that is undesirable for most beers (123). They are produced during fermentation by the nonenzymatic degradation of acetolactate and acetohydroxybutyrate, which are metabolic intermediates in pathways of amino acid synthesis that leak out into fermenting wort. Yeast, however, will scavenge the diacetyl and pentanedione, reducing them to butanediol and pentanediol, respectively, using a range of enzymes (131–133), provided there is sufficient healthy yeast to do so. This can, however, be a relatively prolonged event, depending on the level to which the brewer seeks to lower the VDKs. Recent developments targeted toward accelerating the handling of VDKs include the addition of the enzyme acetolactate decarboxylase (e.g., derived from Klebsiella aerogenes), which leads to the conversion of acetolactate directly to acetoin (134). An alternative approach has been to thermally degrade newly fermented beer (denuded of yeast) to break down the precursor molecules before diverting the stream through a column of immobilized yeast (135). This represents the largest extant commercial use of immobilized yeast, although there is much interest in the potential for such yeast in continuous beer production systems (136).
A range of esters are produced by brewing yeast, with perhaps the most important being isoamyl acetate, owing to its very low flavor threshold. Such esters are produced by the action of the enzyme alcohol acetyltransferase (AAT) on higher alcohols and acetyl-coenzyme A (acetyl-CoA) (137, 138). A major factor affecting the extent of lipid production—and, by extension, ester formation—is the amount of oxygen and unsaturated fatty acids in wort (139). AAT is also responsible for the production of thioesters (140). The mechanisms and physiological roles of ester formation in Saccharomyces fermentation were recently reviewed elsewhere (137, 141).
There is some interest in selecting yeast strains with elevated β-glycosidase (β-G) activity for enhancing the aroma of specialty beers. β-Glycosidases in Saccharomyces cleave nonvolatile glycosides derived from hops, fruit, and other plants used in brewing, cleaving a sugar moiety from the aglycon. The free aglycon may exhibit aromatic activity in this state and represents a largely untapped source of aroma in beer (142). However, there are doubts about whether the cellular location of β-G is commensurate with an ability to release such aroma compounds (143).
Production of SO2 by yeast is significant not only with respect to a direct contribution of this material to aroma but also on account of its role in protecting against flavor deterioration, notably by scavenging the carbonyl substances that afford staling (144). Mutant yeasts capable of increased production of SO2 have been reported (145, 146). Wort composition, for example, the level of lipid material in the wort, also has a profound effect on SO2 production (147).
The production of SO2 and hydrogen sulfide is linked a priori, through reduction of the former by sulfite reductase (148). However, it is possible to regulate their levels independently (149).
Dimethyl sulfide (DMS) is often a significant contributor to the character of lager beers, though sometimes it is reviled (150). While the bulk of the DMS originates from thermal degradation of a malt-derived precursor (3), some yeast strains are capable of reducing dimethyl sulfoxide that also originates in malt (151). Mutant forms of yeast lacking the necessary enzyme have been isolated (146), and it appears that mitochondrial function is required for the activity (24).
Genetic Modification
Saerens et al. (152) and Nevoigt (153) have reviewed genetic strategies for improving brewing yeast. As regards the application of genetic modification (154), there is sensibly no such modified strain in commercial use globally (155).
MICROBIAL ECOLOGY OF MALTING AND BREWING
While beer fermentation itself is a monocultural microbial phenomenon—with few exceptions—the complete process of beer production involves a succession of microbial constituents that dramatically influence the final product (Fig. 3). The knowledge and management of these constituents at all stages have led to dramatic increases in beer quality. Methods for interrogating the microbial consortia of beer and brewing ingredients have been reviewed elsewhere (156, 157).
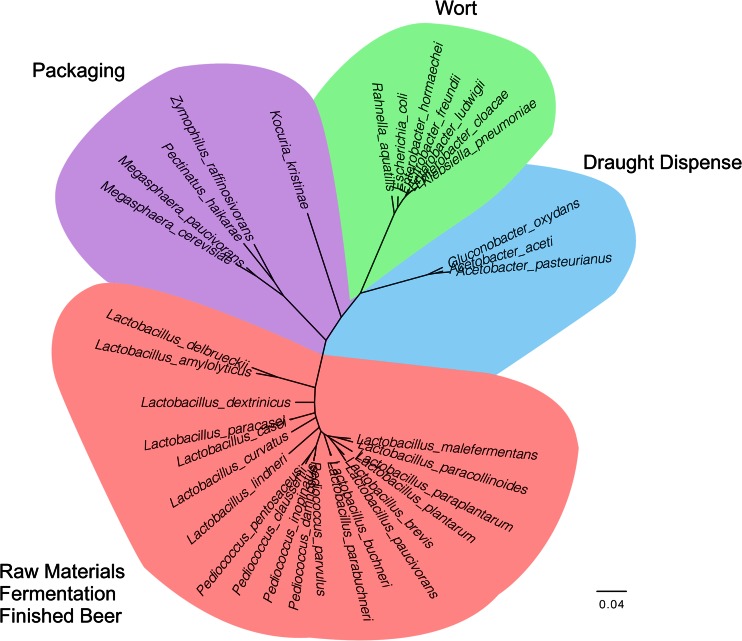
Fig 3.
Phylogeny of primary beer spoilage bacteria. The maximum-likelihood tree shows the most common beer spoilage bacteria, colored by ecological niche and taxonomic group. Red, Lactobacillales, isolated primarily from raw materials, fermenting beer, and packaged beer; blue, acetic acid bacteria (Rhodospirillales), limited primarily to spoilage of draft dispensers; green, Enterobacteriaceae, occasional wort contaminants; purple, Veillonellaceae, which cause spoilage in packaged beer.
Barley
Brewing microbiology begins in the barley field, where plant-microbial interactions and the microbiological status of the grain both pre- and postharvest can have serious implications for brewhouse processing and beer quality. Although these microbes do not survive the malting and brewing processes, secretory factors may persist, affecting downstream quality.
In the field, a vast range of bacteria and fungi are present on the barley, originating from the surrounding environment, insects, and animals. Weather and other conditions will naturally affect the microbial community growing on barley, and unusually wet years in particular can encourage microbial growth and pathogenesis (158). Following harvest, barley may be stored for a time prior to malting to overcome dormancy. During this time, microbes continue to grow on and interact with the living grain, and conditions must be monitored carefully to ensure that the grain is stored in a low-moisture, low-temperature environment to minimize microbial growth (159), which can be extremely detrimental to beer quality.
A diverse set of microbes has been detected on barley (for a thorough list, see reference 158), but only a few plant-pathogenic fungi have notable relevance to beer quality. Fusarium spp. and several other fungal pathogens of barley and other cereals are capable of producing mycotoxins that survive the brewing process and can be detected in finished beer (160–162). A number of mycotoxins have been detected in barley, including deoxynivalenol (DON; also known as “vomitoxin”), nivalenol, T-2 toxin, HT-2 toxin, and diacetoxyscirpenol (163). DON has been implicated as the most abundantly and commonly produced mycotoxin in Fusarium-infected grain (164). The toxicogenic effects of DON and related mycotoxins are well established for animals and humans (for a review, see reference 165), leading to the adoption of strict quality standards for DON in malt. In addition to potentially threatening human health, high concentrations of these mycotoxins have been shown to inhibit yeast growth during beer fermentation (166, 167).
Fungal infection of barley also causes a problem with more immediate consequences to the consumer: gushing. This phenomenon is caused by hydrophobic fungal peptides (hydrophobins), which serve as nucleation sites for CO2 bubbles in beer, resulting in the spontaneous release of gas and overfoaming once the container is opened (168). Hydrophobins are surface-active, amphipathic proteins produced by most filamentous fungi to shield the growing hyphal tip, facilitating growth across liquid-air interfaces (169). As a result of ubiquitous expression, dangerous levels are introduced into the beer process stream when excess fungal growth occurs on the grain preharvest or during storage. The link between fungal growth and gushing is well established, and suppression of Fusarium growth on barley by in-field application of lactic acid bacterium (LAB) starters has been used successfully to diminish gushing (170).
Fungal infection of barley may also promote gushing by eliciting a stress response in barley leading to the production of foam-active compounds, principally the plant pathogenesis-related nonspecific lipid transfer proteins (nsLTPs) (171, 172). These are normally expressed in healthy barley corns and are important foam-promoting factors when expressed at normal levels (171–173). In response to microbial infection, expression levels are increased (174), a phenomenon that has been suggested to explain gushing in beers brewed from infected grain (171, 175). In addition, nsLTPs and other pathogenesis-related proteins are toxic to yeast cells and inhibit respiration at high concentrations (176–178). However, other authors dispute the gushing potential of these proteins and instead have found that only high-molecular-weight barley proteins are positively correlated with beer gushing (179).
Different plant pathogenesis factors can also precipitate premature yeast flocculation (PYF) during fermentation. Yeast flocculation occurs when cell wall mannoproteins bind lectin-like glycoproteins on other cells, resulting in aggregation and settling (180). This normally occurs at the end of fermentation, as sugars present during early fermentation associate with the lectin surface, preventing interaction (180). In PYF, yeast aggregation and settling occur prior to full attenuation of sugar, resulting in incomplete fermentation, off-flavors, and significantly decreased beer quality (181–183). PYF can be initiated by a range of polysaccharides naturally occurring in the barley husk (183–185), released either in response to microbial infection or by degradation of the husk by microbial enzymatic activity (186).
Malt
The process of malting comprises three primary steps—steeping, germination, and kilning. The successive steeping and aeration cycles promote more than plant growth, and although kilning diminishes viable counts of microbes (159, 187), microbial activity during germination can influence beer quality downstream. After kilning, low-moisture conditions must be maintained carefully to avoid microbial spoilage, especially as malt is somewhat hygroscopic and rich in soluble nutrients at this stage.
Upon steeping, microbial cells multiply rapidly on the grain and in the steep water, stimulated by dissolved nutrients, moisture, warmth, and aeration (159, 187, 188). In general, the growth of microbes during germination is deleterious to malt quality, and microbes residing on the surfaces of barley corns can compete for oxygen with the embryo, inhibiting germination (188, 189) and decreasing rootlet growth and alpha-amylase activity (190). In addition, several bacteria and fungi isolated from barley could produce significant quantities of the plant hormone indole-3-acetate in vitro, as well as low quantities of gibberellic acid and abscisic acid, potentially affecting germination and enzyme production (191).
The inhibitory effects of microbial growth on malt quality may be diminished by changing the steep liquor between air rests, reducing dissolved nutrients and reintroduction of suspended biomass (188), and by controlling the steep temperature, as microbial growth is limited at lower temperatures (159). Several authors have also recommended microbial inoculation of steep liquor to control the growth of detrimental microbiota during germination. Wickerhamomyces anomalus has been shown to inhibit Fusarium growth on malt when added during steeping, thereby preventing hydrophobin production and beer gushing (192). Geotrichum candidum (193) and Lactobacillus plantarum (194) have also been shown to diminish Fusarium growth on malt. The addition of LAB has also been shown to decrease rootlet growth, diminishing malting loss (195).
Wort
During the mashing of malt, the microbial load diminishes, but thermotolerant microbes, especially homofermentative LAB, remain active in the nutrient-rich, high-moisture environment (187). Bacterial growth during mashing can have beneficial consequences, and mash acidification by lactic acid bacteria can improve the extraction, fermentability, and nitrogen yield of wort and the foam stability, color, and flavor of beer (196). The beneficial effects of mash acidification are achieved in most breweries by direct acid addition, but microbial acidification remains the only acceptable means for mash acidification in breweries adhering to the Reinheitsgebot German beer purity law (196). Bacterial growth can also cause serious problems during extended mashing. For example, Bacillus spp. can cause excessive acidification and nitrosamine formation by reduction of nitrate to nitrite (197, 198). Growth of Clostridium in the mash or in wort can produce high levels of butyric acid, giving the beer a cheese-like aroma (199). Excessive bacterial growth on malt can also retard mash filtration, probably due to production of dextrans (200), and suppression of bacterial growth has been shown to improve the filterability, extraction efficiency, and nitrogen yield during mashing (192). Fungi growing on malt can produce beta-glucanases and xylanases, lowering wort viscosity and improving mash filtration (192), though this lower wort viscosity has been negatively correlated with beer foam quality (201).
Following the mash, wort is boiled for an extended period, effectively sterilizing the wort. However, wort is a nutrient-rich, high-pH (∼5.5) medium, so once it leaves the kettle it is vulnerable to opportunistic spoilage agents if appropriate precautions are not taken to ensure rapid fermentation, which serves to stabilize the wort against most contaminants. The most prevalent wort spoilers are Gram-negative enterobacteria, especially species of Klebsiella, Citrobacter, Enterobacter, Obesumbacterium, and Escherichia (202). In wort, these bacteria produce DMS, organic acids, and 2,3-butanediol in abundance, giving beer an unpleasant fruity or vegetal aroma (202, 203). Growth of enterobacteria also inhibits the growth of Saccharomyces (202). Enterobacteria are aerobic and are not sensitive to hop-derived antimicrobials, so they can thrive in the oxygenated, high-sugar, high-pH environment of wort, but they are inhibited by ethanol and low pH, so they are not found in finished beer (202). However, some enterobacteria, especially Obesumbacterium and Enterobacter, are contaminants of pitching yeast, leading to serial inoculation into successive batches (202). By modern brewing convention, these bacteria are categorically considered contaminants, but it has been suggested (202) that limited activity of enterobacteria was once characteristic of certain English ales. Increased hygienic standards and updated equipment have dramatically changed this perspective in the past 40 years, and enterobacteria are now considered unwelcome (and uncommon) guests in most worts.
Beer
The cooled, oxygenated wort is pumped to fermenters, where strains of Saccharomyces are added to rapidly convert the wort to beer through the fermentation of maltose and other sugars to ethanol and carbon dioxide. The resulting conditions are hostile to the growth of most microorganisms: beer is high in ethanol and carbon dioxide, contains hop-derived antimicrobial compounds, and is low in pH, oxygen, and residual nutrients, though ∼20% of all reducing sugars in all-malt wort consist of oligosaccharides that are not utilized by Saccharomyces and are felt by many brewers to contribute to the mouthfeel and flavor of the beer, as well as supporting potential microbial spoilage. The stringent conditions of beer fermentation have selected for unique groups of yeast and bacteria specialized for growth in beer—and usually not much else. All species described in this section are the most prevalent contaminants of beer from the start of fermentation through to the packaged product.
Gram-positive bacteria.
LAB are prevalent in nature, associated with plant matter (including barley and malt) and humans, among other environments. Thus, their entry into the brewery is both frequent and inevitable, and their widespread dispersion in malt dust, aerosols, and equipment is unquestionable. Fortunately, most LAB are prevented from growing in beer due to the antibacterial activity of hop-derived compounds (see below). However, those that have adapted to the stringent conditions of beer (namely, developed hop tolerance) are the most prevalent beer spoilage microorganisms of the present day. These include Pediococcus damnosus, Pediococcus inopinatus, Pediococcus dextrinicus, Pediococcus pentosaceous (204), Pediococcus parvulus (205), Pediococcus claussenii (206), Lactobacillus brevis, Lactobacillus casei, Lactobacillus paracasei, Lactobacillus plantarum, Lactobacillus buchneri, Lactobacillus curvatus, Lactobacillus coryneformis, Lactobacillus parabuchneri, Lactobacillus delbrueckii, Lactobacillus fermentum, Lactobacillus fructivorans, (207), Lactobacillus paucivorans (208), Lactobacillus paracollinoides (209), Lactobacillus amylolyticus (210), Lactobacillus lindneri (211), Lactobacillus paraplantarum (212), Lactobacillus brevisimilis (213), and Lactobacillus malefermentans (214). Note that the above list includes all recognized species of LAB previously detected in beer, though not all exhibit high spoilage potential. Among these, L. brevis and P. damnosus probably represent the greatest threat to beer, being the most commonly reported contaminants of finished beers. Most species of LAB show high degrees of ethanol tolerance, but ethanol tolerance is conserved within species, and hop resistance plays a more prevalent role in conferring beer spoilage capability (215). Thus, LAB involved in other food and beverage fermentations, such as Leuconostoc, Oenococcus, Lactococcus, Streptococcus, and Enterococcus, have not been isolated from beer.
LAB spoil beer through acidification, haze formation, and/or diacetyl production, which gives the beer an intense aroma of artificial butter. Many strains can also produce exopolysaccharides (EPS) in beer, lending an oily consistency or, in extreme cases, the formation of slime (215, 216). Pediococcus spp., in particular, are known for diacetyl and EPS production, and because they exhibit strong growth at low temperatures, they are common contaminants of both lager and ale breweries (204).
Aside from LAB, very few Gram-positive organisms have been reported in beer. Kocuria kristinae (previously Micrococcus kristinae) has been reported as a beer spoiler, but with low potential due to its sensitivity to hops, ethanol, and pH (217). The Bacillaceae have not traditionally been considered capable of beer spoilage, but four species containing the hop resistance horA gene (see below)—Bacillus cereus, Bacillus licheniformis, Staphylococcus epidermidis, and Paenibacillus humicus—have been isolated from spoiled, home-brewed beer and exhibited growth when reinoculated into beer (218).
A major factor limiting which organisms can spoil beer (particularly ethanol- and pH-tolerant Gram-positive bacteria) is the presence of hop-derived bittering compounds. Hops contain a range of compounds that inhibit the growth of Gram-positive bacteria. Principal among these are the iso-alpha-acids, which are produced from the hop alpha-acids during wort boiling (1). The iso-alpha-acids function as proton ionophores, dissipating the transmembrane proton gradient, decreasing cytoplasmic pH, and squelching proton motive force (219). This impairs enzymatic activity and nutrient transport, halting growth and ultimately killing the cell (220, 221). In addition, iso-alpha-acids participate in transmembrane redox reactions in association with manganese, causing oxidative stress to the bacterial cell (222), which explains the manganese-dependent enhancement of transmembrane potential observed previously (219, 221) (Fig. 4).
Fig 4.
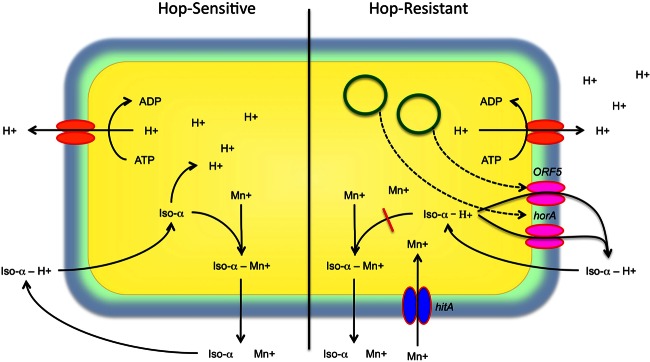
Schematic overview of main mechanisms of hop toxicity and resistance in Gram-positive bacteria. Iso-α, iso-alpha-acids. Green loops indicate plasmids carrying the hop resistance genes horA and ORF5.
Hop challenge involves multiple mechanisms for bacteriostasis, and thus hop resistance involves a complex cellular response. A key factor in hop resistance is the plasmid-encoded, ATP-dependent transporter protein HorA, which purges hop compounds from the cell (223). Another plasmid-encoded multidrug transporter, ORF5, has been shown to confer hop resistance across multiple species of LAB (209). In addition, resistant cells upregulate expression of the hop-inducible cation transporter HitA, which may facilitate manganese transport into hop-stressed cells despite proton gradient dissipation (224). Hop stress in L. brevis also induces expression of a broad range of proteins involved in redox homeostasis, DNA repair, and protein repair, facilitating a shift toward energy balance and metabolic regulation to cope with low-pH conditions and oxidative stress (225). The multiple modes of inhibition exerted by hop challenge and the complex response elicited in resistant bacteria indicate that hop-resistant LAB are specialized for growth in beer through resistance to oxidative and acid stress (219, 222). Early work demonstrated that iso-alpha-acids have no impact on the growth of Gram-negative bacteria (226), but no further work has been done to determine the mechanism for resistance. Iso-alpha-acids also display little or no inhibition of yeasts. In Saccharomyces cerevisiae, this is due to relegation of iso-alpha-acids within the vacuole, their active expulsion across the cell membrane, and modification of the cell wall structure in response to hop stress (227), but the mechanism has not been studied in other yeasts.
Gram-negative bacteria.
The aerobic, Gram-negative acetic acid bacteria (AAB) were once a serious threat to beer production, but their activity in modern beer production is negligible, as oxygen exposure can be avoided (204). In a bygone age, when beer was aged in barrels without the luxuries at the disposal of the modern brewer (e.g., conical steel fermenters and controlled headspace), AAB were a more prevalent threat, and they are still commonly found in barrel-aged beers (228). These AAB include Acetobacter aceti, Acetobacter pasteurianus, and Gluconobacter oxydans. These bacteria spoil beer through the oxidation of ethanol to acetate, effectively transforming beer into vinegar.
As dissolved oxygen concentrations declined in beers with the introduction of modern techniques, a new threat replaced the enemies of old. These new contaminants were the obligate anaerobic Veillonellaceae organisms, including Pectinatus, Megasphaera, Selenomonas, and Zymophilus. Members of this family belong to the Gram-positive phylum Firmicutes but stain Gram negative and possess a lipid bilayer. Most Veillonellaceae organisms are found in aquatic sediment or mammalian intestines, but those mentioned above have been reported only for beer, where they cause spoilage through haze formation, overwhelming production of propionic acid, acetic acid, hydrogen sulfide, and mercaptans, and inhibition of yeast growth and alcohol production (229). Veillonellaceae organisms have been reported to grow in beer at a pH of ≥4.3 and with ≤5% (wt/vol) ethanol (204). Similar to enterobacteria, some of these bacteria can be introduced to beer through their association with pitching yeast (230), causing product spoilage before ethanol and pH reach inhibitory levels and contaminating future batches through repitching. Spoilage cases from these organisms have surfaced only in recent years, concurrent with the growth of nonpasteurized beers and with improved bottling equipment leading to lower dissolved oxygen in the packaged beer (204).
Zymomonas mobilis is a problem in beers containing adjunct sugars. This bacterium can grow under conditions of extreme pH (>3.4) and ethanol content (<10% [wt/vol]), is iso-alpha-acid resistant, and spoils beer through production of acetaldehyde and hydrogen sulfide, giving the beer an aroma of rotten eggs (204). However, this bacterium cannot ferment maltose or maltotriose, the primary carbohydrates in wort and beer, so it is not a common contaminant of beer (204). Spoilage is limited to beers supplemented with other sugars, e.g., sucrose added to carbonated English cask ales (231).
Wild yeasts.
Any organism that has not intentionally been introduced to a beer by the brewer is considered a spoilage organism. Thus, the principal form of wild yeast contamination in beer is from rogue strains of Saccharomyces cerevisiae (232). These spoil beer through ester or phenolic off-flavor production (POF), formation of haze or sediment, or superattenuation, leading to overcarbonation and diminished body. In Saccharomyces and other yeasts, POF is caused by decarboxylation of p-coumaric acid and ferulic acid to 4-vinylphenol and 4-vinylguaiacol, respectively, a property engendered by the POF1 gene (233). These compounds give beer an unusual medicinal or spicy clove aroma and are atypical for most beers, though they are considered a marker trait of German wheat beers and some Belgian ales, as the yeasts used in these beers are POF positive.
Brettanomyces yeasts (teleomorph Dekkera), including Brettanomyces bruxellensis, Brettanomyces custersii, and Brettanomyces anomalus, are nefarious contaminants of most beers and other alcoholic beverages, though their presence is often encouraged in other types of beer (see Deviant Fermentations). These yeasts spoil beer through the production of the highly volatile phenolic compounds 4-ethylguaiacol and 4-ethylphenol, lending the aroma of bandages, sweat, and smoke. A number of other metabolites, including copious acetate production in the presence of oxygen (234), result in a wide range of off-flavors produced by these yeasts. In spite of its reputation, Brettanomyces is a desired component of certain beers, particularly Belgian lambic (see below) and fruit beers, in which its beta-glycosidase activity enhances fruit aroma (235). In a bygone age, Brettanomyces character was even considered an indispensable element of proper English stock beers, and it was first described for English beer, giving this yeast its name (236).
A large number of other non-Saccharomyces yeasts are capable of growth in beer, but their spoilage potential is limited under optimal storage conditions, due to the combined factors of oxygen limitation, ethanol toxicity, and competition with Saccharomyces. These include Pichia anomala, Pichia fermentans, Pichia membranifaciens, Pichia guilliermondii, Candida tropicalis, Candida boidinii, Candida sake, and Candida parapsilosis (all reported in reference 232); Candida guilliermondii, Candida glabrata, Candida valida, Saccharomyces unisporus, Torulaspora delbrueckii, and Issatchenkia orientalis (all reported in reference 237); and Kluyveromyces marxianus, Debaryomyces hansenii, Zygosaccharomyces bailii, Zygosaccharomyces bisporus, Schizosaccharomyces pombe, and Kloeckera apiculata (all reported in reference 207). Most of these yeasts spoil beer through the production of off-flavors (especially organic acids and POF), haze, sediment, or surface films. Like AAB, these yeasts are common throughout breweries, especially in unwashed sampling ports and on other surfaces contacting beer. They are opportunistic contaminants, causing spoilage when conditions are favorable, but are generally not an issue in modern brewing practices, due to improved oxygen control. These yeasts are more of an issue in barrel-fermented beers, where oxygen ingress stimulates their growth, hence the need to limit the headspace during barrel maturation.
Biogenic Amines
Biogenic amines (BAs) and polyamines present another serious consequence of microbial contamination of beer (238–243; for a review of BAs in beer, see reference 244). These compounds are found in a wide range of foods and beverages, including fish, meat, cheese, and wine, and are formed by microbial decarboxylation of amino acids (245, 246). BAs pose a health hazard to sensitive individuals, resulting in allergy-like reactions (247), migraine (248), and/or toxic reactions with monoamine oxidase inhibitor drugs (249, 250). BAs in beer are formed primarily during fermentation but can also be produced by microbes in barley, malt, wort, and hops (251, 252). LAB are most commonly implicated in biogenic amine formation (251), but enterobacteria and some strains of Saccharomyces may also play a role (251). Therefore, limitation of microbial activity during malting, wort production, and fermentation is the best strategy for minimizing BA formation (239). In mixed-culture and “spontaneous” fermentations (see Deviant Fermentations), however, many of these organisms are crucial components of the fermentation, and these beers often contain higher levels of BAs than other beers (238, 251).
Packaging and Distribution
Packaging and distributing beer represent the two greatest challenges to the microbial stability of beer. During all previous brewing processes, from wort boiling to cold conditioning, wort and beer are contained within eminently cleanable, seamless stainless steel vessels (assuming that state-of-the-art equipment and hygienic practices are employed). Upon packaging, however, the virgin product travels across complex surfaces in the filling equipment, is briefly exposed to the atmosphere, and is parsed into small vessels. Biofilms may form on the surfaces of filler heads and in filling areas, increasing the risk of microbial contamination (253). Kegs represent a particular risk, as these are reused constantly, often circulated among different breweries, and contain enclosed, complex surfaces. Kegs may see questionable conditions during return to the brewery—including prolonged exposure to warm temperatures and air—making them a potential breeding ground for colonization and biofilm formation by the microbial panoply described above.
The moment beer leaves the brewery it is out of the brewers' control and is subject to whatever conditions distributors, retailers, and consumers may impose. The package may be exposed to fluctuations in temperature, light, and/or turbulence, all of which degrade the quality of the inner product and (with the exception of light exposure) promote microbial growth. Even under optimal storage conditions, a significant volume of beer may spend several months in shipment and storage prior to consumption, increasing the probability of microbial spoilage, given the scale and distance of contemporary global beer distribution. The industry long ago addressed this issue through product stabilization via filtration, pasteurization, or some combination thereof. However, increasing demand for unpasteurized beers in recent years has increased the incidence of microbial contamination in packaged beer by microbes such as Pectinatus (204).
Draft systems present a particular threat to the stability of beer, as the serving mechanism itself involves introducing foreign objects into the package in situ, after which its stability is governed by the storage, serving, and hygienic conditions of the serving site (pub, restaurant, or private residence). The container is penetrated by the coupler, which allows gas to flow into the container and beer to flow out. Compressed carbon dioxide enters the container, maintaining the appropriate level of carbonation and driving the beer through the draft lines, through the tap faucet, and into the drinking vessel. While the compressed gas itself should be sterile, microbes may be introduced directly into the keg by the gas lines and coupler if they have not been cleaned and sterilized properly. Dispensing equipment (coupler, lines, and tap faucet) comprises a large area of surface contact with the beer and contains a number of complex surfaces that are resistant to cleaning. Biofilms may hypothetically form along these surfaces, especially in microfissures in the draft line and crevices in the dispensing equipment. These biofilms may support the survival of microbes not typically found in beer, but the composition of draft beer biofilms has yet to be elucidated. Beer experiences a certain residence time in this unrefrigerated environment before it is dispensed to the next customer. During this time, cells may multiply in the beer trapped in the lines, causing spoilage through haze, off-flavors, and even BA production (250). A study of draft and bottled beers in Canada found that of all beers tested (n = 98), only the draft beers (4 of 49 beers) contained dangerous concentrations of BAs (>10 mg/liter)—implying postpackaging microbial growth—and these investigators suggested that draft beers should be avoided by BA-sensitive individuals (250). The best means of controlling draft contamination is through observation of proper hygienic practices, including cleaning and sanitization of all equipment prior to connection to a keg, replacement of all lines at regular intervals, and proper storage conditions.
DEVIANT FERMENTATIONS
For 99% of the beers on this planet, Saccharomyces is the sole microbial component, and any deviation is considered a flaw. However, other beers, which are gaining increased popularity worldwide, incorporate secondary, non-Saccharomyces starter cultures, uncharacterized “natural” starter cultures, or autochthonous, nonstarter microbiota during fermentation or maturation, leading to distinctive, unusual products.
Autochthonous Fermentations
The best-known mixed-fermentation beers are the lambics of Belgium and (to a lesser extent) their offspring, the “coolship ales” of the United States. The uniting feature of both of these beers is the lack of any inoculation whatsoever. Instead, these beers are fermented by a mixture of brewery-resident yeasts and bacteria introduced to the cooling wort during overnight exposure in a shallow, open vessel known as a coolship. The following morning, the beer is pumped into oak barrels and allowed to ferment—without racking off the lees—for up to 3 years before packaging. The brewhouse environment appears to select for similar microbiotas, as lambic and coolship ale exhibit similar successions of microbial communities. The first month is dominated by enterobacteria, including Klebsiella, Enterobacter, Escherichia, Citrobacter, Serratia, and Pectobacterium (228, 254), and non-Saccharomyces yeasts, primarily Kluyveromyces in lambic (216) and Rhodotorula in coolship ale (228). Enterobacteria present during this stage produce several compounds responsible for the aroma of 1- to 2-month-old lambic, including 2,3-butanediol, ethyl acetate, higher alcohols, and acetic, lactic, and succinic acids (255). After 1 month, LAB (primarily Pediococcus) and Saccharomyces spp. dominate the main, alcoholic fermentation, which lasts 3 to 4 months. Brettanomyces bruxellensis dominates the remainder of the fermentation and maturation (216), producing a range of characteristic aroma compounds in lambic, including caprylic and capric fatty acids and their ethyl esters (256, 257). Brettanomyces also hydrolyzes EPS produced by Pediococcus during the main fermentation, reducing the viscosity of lambic (216). Since these beers are fermented and matured in the same vessel sur lies, the unique flavor profile is likely influenced by microbial autolysis, contributing both substrates (lipids, proteins, and carbohydrates) and intracellular enzymes to participate in unbridled reactions. One- and 3-year-old lambic is often blended and allowed to re-ferment in the bottle to produce gueuze, which exhibits a markedly different aroma due to regrowth of Brettanomyces in the bottle (258).
Less familiar to Western palates are the many traditional beers enjoyed throughout Africa. These include such popular libations as ikigage of Rwanda (259), bili bili of Chad (260), tchoukoutou of Benin (261), tchapalo of Côte d'Ivoire (262), pito in Ghana, Togo, and Nigeria, and dolo in Burkina Faso (263). These beers are made from malted sorghum, and often malted millet, and otherwise involve roughly similar brewing processes consisting of a sour mash followed by alcoholic fermentation. The beers are consumed fresh in an actively fermenting state. They are opaque, sour, and mildly alcoholic and contain large amounts of suspended solids but are highly nutritious and comprise a large proportion of the local diet (261). All of these beers are fermented by backslopping flocculent yeast slurry from a previous batch, an identical process to that traditionally used for European beer inoculation. Thus, Saccharomyces cerevisiae dominates the fermentation of these beers, similar to other spontaneous beer fermentations. A range of other yeasts are involved, including Meyerozyma caribbica, Candida tropicalis, Pichia kudriavzevii, Pichia kluyveri, Kodamaea ohmeri (262), Kluyveromyces marxianus, Candida melibiosica, Cryptococcus albidius var. albidius, Dekkera bruxellensis, Rhodotorula mucilaginosa, Debaryomyces hansenii, Torulaspora delbrueckii (260), Candida inconspicua, Issatchenkia orientalis, Candida magnolia, Candida humilis (259), Candida albicans, Dekkera anomala, Candida etchellsii, Candida kuwiensis, and Saccharomyces pastorianus (261). LAB are the second most prominent category of microorganisms in most of these beers, and they carry out mash acidification, which is an important processing step. The most commonly observed LAB are Lactobacillus fermentum, Lactobacillus buchneri (259), Lactobacillus delbrueckii, Pediococcus acidilacti, Leuconostoc lactis, and Lactococcus lactis (263). During early fermentation, Enterobacteriaceae and Staphylococcus aureus can also be isolated, but they do not survive the fermentation and are likely killed by the low pH (259).
Mixed-Culture Fermentations
Many mixed-inoculum beer fermentations have traditionally been brewed in Belgium, with the most renowned group being the acid beers of Flanders. These beers are inoculated with a mixture of S. cerevisiae, Lactobacillus spp., and Pediococcus spp. and fermented in steel tanks for 7 to 8 weeks to create a fruity, refreshingly tart beer (205). Some breweries package and sell this young beer as is, while others mature the beer for 1 to 2 years in large oak casks, where Brettanomyces spp. and wild yeasts resident in the wood re-ferment the beer (205). The fully matured beer is then packaged straight, blended with some proportion of young, steel-fermented beer, or filtered and blended with non-sour ale prior to distribution, depending upon the preference of the brewery.
A number of other mixed-inoculum beers are produced globally and enjoy increasing popularity among niche markets. Many Belgian ales—most notably certain Trappist beers—are re-fermented in-bottle by Brettanomyces and occasionally other yeasts or bacteria, providing the unique sensory character of these beers. German Berliner weisse is a low-gravity wheat beer fermented with S. cerevisiae and Lactobacillus spp. in mixed culture. Finally, there is a growing trend of American craft brews incorporating Brettanomyces spp. and lactic acid bacteria in the fermentation, maturation, or bottle re-fermentation process, and even a rare few purportedly conduct a fermentation entirely by Brettanomyces.
CONCLUSION
An enormously diverse group of microbes can contribute to the production and quality of beer. For most of these organisms, it is not possible to categorically define them as making negative contributions: it really does depend on the beer or on the role that the organism is expected to play. Thus, while lactic acid bacteria are frequently undesirable as spoilage agents, they can perform necessary functions, such as the acidification of mashes according to traditional Germanic brewing practices or as key elements in the production of sour beers. A brewing company's prized brewing strain represents a wild yeast for a competing brewer. The achievement of mastery over the diverse microbial players will differ from circumstance to circumstance and is dependent on an understanding of the organisms likely to be at play from grain to glass.
Biographies

Nicholas A. Bokulich is a graduate student in the Department of Food Science and Technology at the University of California, Davis (UC Davis). He is a member of the Mills research group, studying the microbial ecology of foods as delivery systems for microbes in the human diet. He received a B.A. in microbiology, molecular biology, and brewing from Hampshire College and a master's degree in viticulture and enology from UC Davis.

Charles W. Bamforth is Professor of Malting & Brewing Sciences at UC Davis and Honorary Professor in the School of Biosciences at the University of Nottingham, England. He is a fellow of the Institute of Brewing & Distilling, the Society of Biology, and the International Academy of Food Science & Technology. He is Editor in Chief of the Journal of the American Society of Brewing Chemists and Vice President of the Institute of Brewing & Distilling.
REFERENCES
- 1. Bamforth CW. (ed). 2006. Scientific principles of malting and brewing. American Society of Brewing Chemists, St. Paul, MN [Google Scholar]
- 2. Boulton C, Quain DE. (ed). 2001. Brewing yeast and fermentation. Blackwell, Oxford, United Kingdom [Google Scholar]
- 3. Boulton C. 2006. Fermentation of beer, p 228–253 In Bamforth CW. (ed), Brewing: new technologies. Woodhead Publishing, Cambridge, United Kingdom [Google Scholar]
- 4. Barnett JA. 1992. The taxonomy of the genus Saccharomyces Meyen ex Reess—a short review for non-taxonomists. Yeast 8:1–231496857 [Google Scholar]
- 5. Vaughan-Martini A, Martini A. 1998. Saccharomyces Meyen ex Rees, p 358–371 In Kurtzman C, Fell J. (ed), The yeasts: a taxonomic study. Elsevier, Amsterdam, Netherlands [Google Scholar]
- 6. Rodrigues De Sousa H, Madeira-Lopes A, Spencer-Martins I. 1995. The significance of active fructose transport and maximum temperature for growth in the taxonomy of Saccharomyces sensu stricto. Syst. Appl. Microbiol. 18:44–51 [Google Scholar]
- 7. Turakainen H, Kristo P, Korhola M. 1994. Consideration of the evolution of the Saccharomyces-Cerevisiae Mel gene family on the basis of the nucleotide-sequences of the genes and their flanking regions. Yeast 10:1559–1568 [DOI] [PubMed] [Google Scholar]
- 8. Vaughan-Martini A, Kurtzman CP. 1985. Deoxyribonucleic acid relatedness among species of the genus Saccharomyces sensu stricto. Int. J. Syst. Bacteriol. 35:508–511 [Google Scholar]
- 9. Libkind D, Hittinger CT, Valerio E, Goncalves C, Dover J, Johnston M, Goncalves P, Sampaio JP. 2011. Microbe domestication and the identification of the wild genetic stock of lager-brewing yeast. Proc. Natl. Acad. Sci. U. S. A. 108:14539–14544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Casey GP. 1990. Yeast selection in brewing, p 65–112 In Panchal CJ. (ed), Yeast strain selection. Marcel Dekker, New York, NY [Google Scholar]
- 11. Casey GP. 1996. Practical applications of pulsed field electrophoresis and yeast chromosome fingerprinting in brewing QA and R&D. Tech. Q. Master Brew. Assoc. Am. 33:1–10 [Google Scholar]
- 12. Mewes HW, Albermann K, Bahr M, Frishman D, Gleissner A, Hani J, Heumann K, Kleine K, Maierl A, Oliver SG, Pfeiffer F, Zollner A. 1997. Overview of the yeast genome. Nature 387:7–8 [DOI] [PubMed] [Google Scholar]
- 13. Codon AC, Benitez T, Korhola M. 1998. Chromosomal polymorphism and adaptation to specific industrial environments of Saccharomyces strains. Appl. Microbiol. Biotechnol. 49:154–163 [DOI] [PubMed] [Google Scholar]
- 14. Hammond J. 1996. Yeast genetics, p 45–82 In Priest FG, Campbell I. (ed), Brewing microbiology. Chapman and Hall, London, United Kingdom [Google Scholar]
- 15. Galitski T, Saldanha AJ, Styles CA, Lander ES, Fink GR. 1999. Ploidy regulation of gene expression. Science 285:251–254 [DOI] [PubMed] [Google Scholar]
- 16. Wicksteed BL, Collins I, Dershowitz A, Stateva LI, Green RP, Oliver SG, Brown AJ, Newlon CS. 1994. A physical comparison of chromosome III in six strains of Saccharomyces cerevisiae. Yeast 10:39–57 [DOI] [PubMed] [Google Scholar]
- 17. Wightman P, Quain DE, Meaden PG. 1996. Analysis of production brewing strains of yeast by DNA fingerprinting. Lett. Appl. Microbiol. 22:90–94 [DOI] [PubMed] [Google Scholar]
- 18. Donnelly D, Hurley J. 1996. Yeast monitoring: the Guinness experience. Ferment 9:283–286 [Google Scholar]
- 19. Good L, Dowhanick T, Ernandes J, Russell I, Stewart G. 1993. Rho- mitochondrial genomes and their influence on adaptation to nutrient stress in lager yeast fermentations. J. Am. Soc. Brew. Chem. 51:36–39 [Google Scholar]
- 20. Jenkins CL, Lawrence SJ, Kennedy AI, Thurston P, Hodgson JA, Smart KA. 2009. Incidence and formation of petite mutants in lager brewing yeast Saccharomyces cerevisiae (syn. S. pastorianus) populations. J. Am. Soc. Brew. Chem. 67:72–80 [Google Scholar]
- 21. Silhankova L, Mostek JSJ. 1970. Respiratory deficient mutants of bottom brewer's yeast. I. Frequencies and types of mutant in various strains. J. Inst. Brew. 76:280–288 [Google Scholar]
- 22. O'Connor-Cox ESC, Lodolo EJ, Axcell BC. 1996. Mitochondrial relevance to yeast fermentative performance: a review. J. Inst. Brew. 102:19–25 [Google Scholar]
- 23. Samp E. 2012. Possible roles of the mitochondria in sulfur dioxide production by lager yeast. J. Am. Soc. Brew. Chem. 70:219–229 [Google Scholar]
- 24. Samp EJ, Foster RT, Edelen C. 2010. Influence of cardiolipin on lager beer dimethyl sulfide levels: a possible role involving mitochondria? J. Am. Soc. Brew. Chem. 68:204–209 [Google Scholar]
- 25. Quain DE. 1986. Differentiation of brewing yeast. J. Inst. Brew. 92:435–438 [Google Scholar]
- 26. Casey GP, Pringle AT, Erdmann PA. 1990. Evaluation of recent techniques used to identify individual strains of Saccharomyces yeasts. J. Am. Soc. Brew. Chem. 48:100–106 [Google Scholar]
- 27. Palkova Z, Janderova B, Gabriel J, Zikanova B, Pospisek M, Forstova J. 1997. Ammonia mediates communication between yeast colonies. Nature 390:532–536 [DOI] [PubMed] [Google Scholar]
- 28. Tubb RS, Liljestrom PL. 1986. A colony color method which differentiates alpha-galactosidase-positive strains of yeast. J. Inst. Brew. 92:588–590 [Google Scholar]
- 29. Lawrence DR. 1983. Yeast differentiation and identification, p 449–456 In Proceedings of the 19th Congress of the European Brewery Convention, London European Brewery Convention, Brussels, Belgium [Google Scholar]
- 30. Sigsgaard P. 1996. Strain selection and characterisation. Ferment 9:43–45 [Google Scholar]
- 31. Skands B. 1997. Studies of yeast behaviour in fully automated test plant, p 413–421 In Proceedings of the 26th Congress of the European Brewery Convention, Maastricht European Brewery Convention, Brussels, Belgium [Google Scholar]
- 32. Jakobsen M, Thorne RSW. 1980. Oxygen requirements of brewing strains of Saccharomyces-Uvarum (Carlsbergensis)—bottom fermentation yeast. J. Inst. Brew. 86:284–287 [Google Scholar]
- 33. Kirsop BH. 1977. Yeast lipids and beer flavor. Tech. Q. Master Brew. Assoc. Am. 14:227–230 [Google Scholar]
- 34. Meaden P. 1996. DNA fingerprinting of brewer's yeast. Ferment 9:267–272 [Google Scholar]
- 35. Hutzler M, Geiger E, Jacob F. 2010. Use of PCR-DHPLC (polymerase chain reaction-denaturing high performance liquid chromatography) for the rapid differentiation of industrial Saccharomyces pastorianus and Saccharomyces cerevisiae strains. J. Inst. Brew. 116:464–474 [Google Scholar]
- 36. Russell I, Dowhanick T. 1996. Rapid detection of microbial spoilage, p 209–235 In Priest FG, Campbell I. (ed), Brewing microbiology. Chapman and Hall, London, United Kingdom [Google Scholar]
- 37. De Barros Lopes M, Rainieri S, Henschke PA, Langridge P. 1999. AFLP fingerprinting for analysis of yeast genetic variation. Int. J. Syst. Bacteriol. 49:915–924 [DOI] [PubMed] [Google Scholar]
- 38. Timmins E, Quain D, Goodacre R. 1998. Differentiation of brewery yeast strains by pyrolysis mass spectrometry and Fourier transform infrared spectroscopy. Yeast 14:885–893 [DOI] [PubMed] [Google Scholar]
- 39. Hopkinson JH, Newbery JE, Spencer DM, Spencer JF. 1988. Differentiation between some industrially significant yeasts through the use of Fourier-transform infrared-spectroscopy. Biotechnol. Appl. Biochem. 10:118–123 [Google Scholar]
- 40. Augustyn OPH, Kock JLF. 1989. Differentiation of yeast species, and strains within a species, by cellular fatty-acid analysis. 1. Application of an adapted technique to differentiate between strains of Saccharomyces-cerevisiae. J. Microbiol. Methods 10:9–23 [Google Scholar]
- 41. Van Vuuren HJJ, Van der Meer L. 1987. Fingerprinting of yeasts by protein electrophoresis. Am. J. Enol. Vitic. 38:49–53 [Google Scholar]
- 42. Bamforth CW. 2010. Genetic resources of yeast and other micro-organisms, p 515–525 In Gepts P, Famula TR, Bettinger RL, Brush SB, Damania AB, McGuire PE, Qualset CO. (ed), Biodiversity in agriculture: domestication, evolution and sustainability. Cambridge University Press, New York, NY [Google Scholar]
- 43. Quain DE. 1995. Yeast supply—the challenge of zero defects, p 309–318 In Proceedings of the 25th Congress of the European Brewery Convention, Brussels European Brewery Convention, Brussels, Belgium [Google Scholar]
- 44. Quain DE. 2006. Yeast supply and propagation in brewing, p 167–182 In Bamforth CW. (ed), Brewing: new technologies. Woodhead Publishing, Cambridge, United Kingdom [Google Scholar]
- 45. Powell CD, Diacetis AN. 2007. Long term serial repitching and the genetic and phenotypic stability of brewer's yeast. J. Inst. Brew. 113:67–74 [Google Scholar]
- 46. Verbelen PJ, Dekoninck TML, Van Mulders SE, Saerens SMG, Delvaux F, Delvaux FR. 2009. Stability of high cell density brewery fermentations during serial repitching. Biotechnol. Lett. 31:1729–1737 [DOI] [PubMed] [Google Scholar]
- 47. Powell CD, Quain DE, Smart KA. 2003. The impact of brewing yeast cell age on fermentation performance, attenuation and flocculation. FEMS Yeast Res. 3:149–157 [DOI] [PubMed] [Google Scholar]
- 48. Maskell DL, Kennedy AI, Hodgson JA, Smart KA. 2003. Chronological and replicative lifespan of polyploid Saccharomyces cerevisiae (syn. S. pastorianus). FEMS Yeast Res. 3:201–209 [DOI] [PubMed] [Google Scholar]
- 49. Maule D. 1979. Propagation and handling of pitching yeast. Brew. Guard. 109:76–80 [Google Scholar]
- 50. Jorgensen H, Olsson L, Ronnow B, Palmqvist EA. 2002. Fed-batch cultivation of baker's yeast followed by nitrogen or carbon starvation: effects on fermentative capacity and content of trehalose and glycogen. Appl. Microbiol. Biotechnol. 59:310–317 [DOI] [PubMed] [Google Scholar]
- 51. Gibson BR, Graham NS, Boulton CA, Box WG, Lawrence SJ, Linforth RST, May ST, Smart KA. 2010. Differential yeast gene transcription during brewery propagation. J. Am. Soc. Brew. Chem. 68:21–29 [Google Scholar]
- 52. Gibson BR, Boulton CA, Box WG, Graham NS, Lawrence SJ, Linforth RST, Smart KA. 2008. Carbohydrate utilization and the lager yeast transcriptome during brewery fermentation. Yeast 25:549–562 [DOI] [PubMed] [Google Scholar]
- 53. Smart KA. 2007. Brewing yeast genomes and genome-wide expression and proteome profiling during fermentation. Yeast 24:993–1013 [DOI] [PubMed] [Google Scholar]
- 54. Miller KJ, Box WG, Boulton CA, Smart KA. 2012. Cell cycle synchrony of propagated and recycled lager yeast and its impact on lag phase in fermenter. J. Am. Soc. Brew. Chem. 70:1–9 [Google Scholar]
- 55. Fels S, Reckelbus B, Gosselin Y. 1999. Dried yeast as an alternative to fresh yeast propagation, p 147–151 In Proceedings of the 7th Institute of Brewing Convention (Africa Section), Nairobi Institute of Brewing and Distilling, London, United Kingdom [Google Scholar]
- 56. Jenkins DM, Powell CD, Fischborn T, Smart KA. 2011. Rehydration of active dry brewing yeast and its effect on cell viability. J. Inst. Brew. 117:377–382 [Google Scholar]
- 57. Muller R, Fels S, Gosselin Y. 1997. Brewery fermentations with dried lager yeast, p 431–438 In Proceedings of the 26th Congress of the European Brewery Convention, Maastricht European Brewery Convention, Brussels, Belgium [Google Scholar]
- 58. Powell C, Fischborn T. 2010. Serial repitching of dried lager yeast. J. Am. Soc. Brew. Chem. 68:48–56 [Google Scholar]
- 59. Cyr N, Blanchette M, Price SP, Sheppard JD. 2007. Vicinal diketone production and amino acid uptake by two active dry lager yeasts during beer fermentation. J. Am. Soc. Brew. Chem. 65:138–144 [Google Scholar]
- 60. Finn DA, Stewart GG. 2002. Fermentation characteristics of dried brewers yeast: effect of drying on flocculation and fermentation. J. Am. Soc. Brew. Chem. 60:135–139 [Google Scholar]
- 61. Somani A, Bealin-Kelly F, Axcell B, Smart KA. 2012. Impact of storage temperature on lager brewing yeast viability, glycogen, trehalose, and fatty acid content. J. Am. Soc. Brew. Chem. 70:123–130 [Google Scholar]
- 62. Pham TH, Mauvais G, Vergoignan C, De Coninck J, Cachon R, Feron G. 2008. Gaseous environments modify reserve carbohydrate contents and cell survival in the brewing yeast Saccharomyces cerevisiae. Biotechnol. Lett. 30:287–294 [DOI] [PubMed] [Google Scholar]
- 63. D'Amore T, Crumplen R, Stewart GG. 1991. The involvement of trehalose in yeast stress tolerance. J. Ind. Microbiol. 7:191–195 [Google Scholar]
- 64. Boulton C, Clutterbuck VJ. 1993. Application of a radiofrequency permittivity biomass probe to the control of yeast cone cropping, p 509–516 In Proceedings of the 24th Congress of the European Brewery Convention, Oslo European Brewery Convention, Brussels, Belgium [Google Scholar]
- 65. Harris CM, Kell DB. 1985. The estimation of microbial biomass. Biosensors 1:17–84 [DOI] [PubMed] [Google Scholar]
- 66. Riess S. 1986. Automatic control of the addition of pitching yeast. Tech. Q. Master Brew. Assoc. Am. 23:32–35 [Google Scholar]
- 67. Smart KA, Chambers KM, Lambert I, Jenkins C, Smart CA. 1999. Use of methylene violet staining procedures to determine yeast viability and vitality. J. Am. Soc. Brew. Chem. 57:18–23 [Google Scholar]
- 68. Van Zandycke SM, Simal O, Gualdoni S, Smart KA. 2003. Determination of yeast viability using fluorophores. J. Am. Soc. Brew. Chem. 61:15–22 [Google Scholar]
- 69. Lodolo EJ, Cantrell IC. 2007. Yeast vitality—a holistic approach toward an integrated solution to predict yeast performance. J. Am. Soc. Brew. Chem. 65:202–207 [Google Scholar]
- 70. Quain DE, Tubb RS. 1983. A rapid and simple method for the determination of glycogen in yeast. J. Inst. Brew. 89:38–40 [Google Scholar]
- 71. Rowe SM, Simpson WJ, Hammond JRM. 1991. Spectrophotometric assay of yeast sterols using a polyene antibiotic. Lett. Appl. Microbiol. 13:182–185 [Google Scholar]
- 72. Manson D, Slaughter J. 1986. Methods for predicting yeast fermentation activity, p 295–297 In Proceedings of the Aviemore Conference on Malting, Brewing and Distilling Institute of Brewing, London, United Kingdom [Google Scholar]
- 73. Kennedy R. 1989. Measuring vitality. Brew. Guard. 119:57–58 [Google Scholar]
- 74. Gabriel P, Dienstbier M, Matoulkova D, Kosar K, Sigler K. 2008. Optimised acidification power test of yeast vitality and its use in brewing practice. J. Inst. Brew. 114:270–276 [Google Scholar]
- 75. Kara BV, Simpson WJ, Hammond JRM. 1988. Prediction of the fermentation performance of brewing yeast with the acidification power test. J. Inst. Brew. 94:153–158 [Google Scholar]
- 76. Li WL, Guo ZP, Zhang LA, Ding ZY, Wang ZX, Shi GY. 2011. A novel and rapid method for yeast vitality evaluation based on the methylene blue dye reduction test. J. Am. Soc. Brew. Chem. 69:44–49 [Google Scholar]
- 77. Brenner R. 1984. Effect of unsaturated fatty acids on membrane structure and enzyme kinetics. Prog. Lipid Res. 23:69–96 [DOI] [PubMed] [Google Scholar]
- 78. Parks LW. 1978. Metabolism of sterols in yeast. Crit. Rev. Microbiol. 6:301–341 [DOI] [PubMed] [Google Scholar]
- 79. Kirsop BH. 1974. Oxygen in brewery fermentation. J. Inst. Brew. 80:252–259 [Google Scholar]
- 80. Boulton C, Quain DE. 1987. Yeast, oxygen and the control of brewery fermentation, p 401–408 In Proceedings of the 21st Congress of the European Brewery Convention, Madrid European Brewery Convention, Brussels, Belgium [Google Scholar]
- 81. Verbelen PJ, Saerens SMG, Van Mulders SE, Delvaux F, Delvaux FR. 2009. The role of oxygen in yeast metabolism during high cell density brewery fermentations. Appl. Microbiol. Biotechnol. 82:1143–1156 [DOI] [PubMed] [Google Scholar]
- 82. Gibson BR, Lawrence SJ, Boulton CA, Box WG, Graham NS, Linforth RST, Smart KA. 2008. The oxidative stress response of a lager brewing yeast strain during industrial propagation and fermentation. FEMS Yeast Res. 8:574–585 [DOI] [PubMed] [Google Scholar]
- 83. Hu XH, Wang MH, Tan T, Li JR, Yang H, Leach L, Zhang RM, Luo ZW. 2007. Genetic dissection of ethanol tolerance in the budding yeast Saccharomyces cerevisiae. Genetics 175:1479–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Blieck L, Toye G, Dumortier F, Verstrepen KJ, Delvaux FR, Thevelein JM, Van Dijck P. 2007. Isolation and characterization of brewer's yeast variants with improved fermentation performance under high-gravity conditions. Appl. Environ. Microbiol. 73:815–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gibson BR, Lawrence SJ, Leclaire JPR, Powell CD, Smart KA. 2007. Yeast responses to stresses associated with industrial brewery handling. FEMS Microbiol. Rev. 31:535–569 [DOI] [PubMed] [Google Scholar]
- 86. Stewart GG. 2010. High-gravity brewing and distilling—past experiences and future prospects. J. Am. Soc. Brew. Chem. 68:1–9 [Google Scholar]
- 87. Bamforth CW. 2003. Wort composition and beer quality, p 77–85 In Smart K. (ed), Brewing yeast fermentation performance. Blackwell, Oxford, United Kingdom [Google Scholar]
- 88. Van Zandycke S, Fischborn T. 2008. The impact of yeast nutrients on fermentation performance and beer quality. Tech. Q. Master Brew. Assoc. Am. 45:290–293 [Google Scholar]
- 89. De Nicola R, Walker GM. 2009. Accumulation and cellular distribution of zinc by brewing yeast. Enzyme Microb. Technol. 44:210–216 [Google Scholar]
- 90. De Nicola R, Walker GM. 2011. Zinc interactions with brewing yeast: impact on fermentation performance. J. Am. Soc. Brew. Chem. 69:214–219 [Google Scholar]
- 91. Dekoninck TML, Verbelen PJ, Delvaux F, Van Mulders SE, Delvaux FR. 2012. The importance of wort composition for yeast metabolism during accelerated brewery fermentations. J. Am. Soc. Brew. Chem. 70:195–204 [Google Scholar]
- 92. Gibson BR. 2011. Improvement of higher gravity brewery fermentation via wort enrichment and supplementation. J. Inst. Brew. 117:268–284 [Google Scholar]
- 93. Siebert K, Blum P, Wisk T, Stentoos L, Anklam W. 1986. The effect of trub on fermentation. Tech. Q. Master Brew. Assoc. Am. 23:37–43 [Google Scholar]
- 94. Kruger L, Pickerell ATW, Axcell B. 1992. The sensitivity of different brewing yeast strains to carbon-dioxide inhibition-fermentation and production of flavor-active volatile compounds. J. Inst. Brew. 98:133–138 [Google Scholar]
- 95. Kapral D. 2008. Stratified fermentation—causes and corrective actions. Tech. Q. Master Brew. Assoc. Am. 45:115–120 [Google Scholar]
- 96. Nienow AW, McLeod G, Hewitt CJ. 2010. Studies supporting the use of mechanical mixing in large scale beer fermentations. Biotechnol. Lett. 32:623–633 [DOI] [PubMed] [Google Scholar]
- 97. Dutton J. 1990. FV control with real time SG monitoring. Brew. Dist. Int. 21:20–21 [Google Scholar]
- 98. Forrest I, Cuthbertson R, Dickson J, Gilchrist F, Skrgatic D. 1989. In-line measurement of original gravity by sound velocity and refractive index, p 725–732 In Proceedings of the 22nd Congress of the European Brewery Convention, Zurich European Brewery Convention, Brussels, Belgium [Google Scholar]
- 99. Moller N. 1975. Continuous measurement of wort/beer extract in a fermenter. Tech. Q. Master Brew. Assoc. Am. 12:41–45 [Google Scholar]
- 100. Stassi P, Goetzke G, Fehring J. 1991. Evaluation of an insertion thermal mass flowmeter to monitor carbon dioxide evolution rate in large scale fermentations. Tech. Q. Master Brew. Assoc. Am. 28:84–88 [Google Scholar]
- 101. Stassi P, Rice J, Munroe J, Chicoye E. 1987. Use of carbon dioxide evolution rate for the study and control of fermentation. Tech. Q. Master Brew. Assoc. Am. 24:44–50 [Google Scholar]
- 102. Leedham P. 1983. Control of brewery fermentation via yeast growth, p 153–160 In Proceedings of the 19th Congress of the European Brewery Convention, London European Brewery Convention, Brussels, Belgium [Google Scholar]
- 103. Pfisterer E, Krynicki C, Steer J, Hagg W. 1988. On-line control of ethanol and carbon dioxide in high gravity brewing. Tech. Q. Master Brew. Assoc. Am. 25:1–5 [Google Scholar]
- 104. Wasmuht K, Weinzart M. 1999. Observing fermentation with the help of a new control system referred to as ‘Topscan.’ Brau. Int. 17:512–513 [Google Scholar]
- 105. Ferreira IMPLVO, Pinho O, Vieira E, Tavarela JG. 2010. Brewer's Saccharomyces yeast biomass: characteristics and potential applications. Trends Food Sci. Technol. 21:77–84 [Google Scholar]
- 106. Hodgson J, Pinder A, Catley B, Deans K. 1999. Effect of cone cropping and serial re-pitch on the distribution of cell ages in brewery. Tech. Q. Master Brew. Assoc. Am. 36:175–177 [Google Scholar]
- 107. Powell CD, Quain DE, Smart KA. 2004. The impact of sedimentation on cone yeast heterogeneity. J. Am. Soc. Brew. Chem. 62:8–17 [Google Scholar]
- 108. Simpson WJ, Hammond JRM. 1989. The response of brewing yeasts to acid washing. J. Inst. Brew. 95:347–354 [Google Scholar]
- 109. Chlup PH, Bernard D, Stewart GG. 2008. Disc stack centrifuge operating parameters and their impact on yeast physiology. J. Inst. Brew. 114:45–61 [Google Scholar]
- 110. Jenkins CL, Kennedy AI, Hodgson JA, Thurston P, Smart KA. 2003. Impact of serial repitching on lager brewing yeast quality. J. Am. Soc. Brew. Chem. 61:1–9 [Google Scholar]
- 111. Soares EV. 2011. Flocculation in Saccharomyces cerevisiae: a review. J. Appl. Microbiol. 110:1–18 [DOI] [PubMed] [Google Scholar]
- 112. Vidgren V, Londesborough J. 2011. Yeast flocculation and sedimentation in brewing. J. Inst. Brew. 117:475–487 [Google Scholar]
- 113. Verstrepen KJ, Derdelinckx G, Verachtert H, Delvaux FR. 2003. Yeast flocculation: what brewers should know. Appl. Microbiol. Biotechnol. 61:197–205 [DOI] [PubMed] [Google Scholar]
- 114. Hinchliffe E, Box W, Walton E, Appleby M. 1985. The influences of cell wall hydrophobicity on the top fermenting properties of brewing yeast, p 323–340 In Proceedings of the 20th Congress of the European Brewery Convention, Helsinki European Brewery Convention, Brussels, Belgium [Google Scholar]
- 115. Patel JK, Speers RA, Lake HC. 2011. Colloidal examination of worts associated with premature yeast flocculation. J. Am. Soc. Brew. Chem. 69:81–90 [Google Scholar]
- 116. Lake HC, Speers RA. 2008. A discussion of malt-induced premature yeast flocculation. Tech. Q. Master Brew. Assoc. Am. 45:253–262 [Google Scholar]
- 117. van Nierop SNE, Axcell BC, Cantrell IC, Rautenbach M. 2009. Quality assessment of lager brewery yeast samples and strains using barley malt extracts with anti-yeast activity. Food Microbiol. 26:192–196 [DOI] [PubMed] [Google Scholar]
- 118. Coghe S, Benoot K, Delvaux F, Vanderhaegen B, Delvaux FR. 2004. Ferulic acid release and 4-vinylguaiacol formation during brewing and fermentation: indications for feruloyl esterase activity in Saccharomyces cerevisiae. J. Agric. Food Chem. 52:602–608 [DOI] [PubMed] [Google Scholar]
- 119. Cui YQ, Cao XH, Li SS, Thamm L, Zhou GT. 2010. Enhancing the concentration of 4-vinylguaiacol in top-fermented beers—a review. J. Am. Soc. Brew. Chem. 68:77–82 [Google Scholar]
- 120. Klopper WJ, Angelino S, Tuning B, Vermeire HA. 1986. Organic-acids and glycerol in beer. J. Inst. Brew. 92:225–228 [Google Scholar]
- 121. Omori T, Umemoto Y, Ogawa K, Kajiwara Y, Shimoda M, Wada H. 1997. A novel method for screening high glycerol- and ester-producing brewing yeasts (Saccharomyces cerevisiae) by heat shock treatment. J. Ferment. Bioeng. 83:64–69 [Google Scholar]
- 122. Nevoigt E, Stahl U. 1996. Reduced pyruvate decarboxylase and increased glycerol-3-phosphate dehydrogenase [NAD(+)] levels enhance glycerol production in Saccharomyces cerevisiae. Yeast 12:1331–1337 [DOI] [PubMed] [Google Scholar]
- 123. Inoue T. (ed). 2008. Diacetyl in fermented foods and beverages. American Society of Brewing Chemists, St. Paul, MN [Google Scholar]
- 124. Landaud S, Latrille E, Corrieu G. 2001. Top pressure and temperature control the fusel alcohol/ester ratio through yeast growth in beer fermentation. J. Inst. Brew. 107:107–117 [Google Scholar]
- 125. Quain DE, Duffield ML. 1985. A metabolic function for higher alcohol production by yeast, p 307–314 In Proceedings of the 20th Congress of the European Brewery Convention, Helsinki European Brewery Convention, Brussels, Belgium [Google Scholar]
- 126. Lyness CA, Steele GM, Stewart GG. 1997. Investigating ester metabolism: characterization of the ATF1 gene in Saccharomyces cerevisiae. J. Am. Soc. Brew. Chem. 55:141–146 [Google Scholar]
- 127. Quain DE. 1988. Studies on yeast physiology—impact on fermentation performance and product quality. J. Inst. Brew. 94:315–323 [Google Scholar]
- 128. Miracle RE, Ebeler SE, Bamforth CW. 2005. The measurement of sulfur-containing aroma compounds in samples from production-scale brewery operations. J. Am. Soc. Brew. Chem. 63:129–134 [Google Scholar]
- 129. Walker MD, Simpson WJ. 1993. Production of volatile sulfur-compounds by ale and lager brewing strains of Saccharomyces cerevisiae. Lett. Appl. Microbiol. 16:40–43 [Google Scholar]
- 130. Verbelen PJ, Dekoninck TML, Saerens SMG, Van Mulders SE, Thevelein JM, Delvaux FR. 2009. Impact of pitching rate on yeast fermentation performance and beer flavour. Appl. Microbiol. Biotechnol. 82:155–167 [DOI] [PubMed] [Google Scholar]
- 131. Bamforth CW, Kanauchi M. 2004. Enzymology of vicinal diketone reduction in brewer's yeast. J. Inst. Brew. 110:83–93 [Google Scholar]
- 132. Gonzalez E, Fernandez MR, Larroy C, Sola L, Pericas MA, Pares X, Biosca JA. 2000. Characterization of a (2R,3R)-2,3-butanediol dehydrogenase as the Saccharomyces cerevisiae YAL060W gene product—disruption and induction of the gene. J. Biol. Chem. 275:35876–35885 [DOI] [PubMed] [Google Scholar]
- 133. van Bergen B, Strasser R, Cyr N, Sheppard JD, Jardim A. 2006. Alpha,beta-dicarbonyl reduction by Saccharomyces d-arabinose dehydrogenase. Biochim. Biophys. Acta 1760:1636–1645 [DOI] [PubMed] [Google Scholar]
- 134. Godtfredsen SE, Rasmussen AM, Ottesen M, Rafn P, Peitersen N. 1984. Occurrence of alpha-acetolactate decarboxylases among lactic-acid bacteria and their utilization for maturation of beer. Appl. Microbiol. Biotechnol. 20:23–28 [Google Scholar]
- 135. Pajunen E, Grongvist A, Lommi H. 1989. Continuous secondary fermentation and maturation of beer in an immobilized yeast reactor. Tech. Q. Master Brew. Assoc. Am. 26:147–151 [Google Scholar]
- 136. Willaert R, Nedovic VA. 2006. Primary beer fermentation by immobilised yeast—a review on flavour formation and control strategies. J. Chem. Technol. Biotechnol. 81:1353–1367 [Google Scholar]
- 137. Procopio S, Qian F, Becker T. 2011. Function and regulation of yeast genes involved in higher alcohol and ester metabolism during beverage fermentation. Eur. Food Res. Technol. 233:721–729 [Google Scholar]
- 138. Verstrepen KJ, Derdelinckx G, Dufour JP, Winderickx J, Pretorius IS, Thevelein JM, Delvaux FR. 2003. The Saccharomyces cerevisiae alcohol acetyl transferase gene ATF1 is a target of the cAMP/PKA and FGM nutrient-signalling pathways. FEMS Yeast Res. 4:285–296 [DOI] [PubMed] [Google Scholar]
- 139. Moonjai N, Verstrepen KJ, Delvaux FR, Derdelinckx G, Verachtert H. 2002. The effects of linoleic acid supplementation of cropped yeast on its subsequent fermentation performance and acetate ester synthesis. J. Inst. Brew. 108:227–235 [Google Scholar]
- 140. Bamforth CW, Kanauchi M. 2003. Use of novel assays to indicate that O-esters and S-esters are produced by the same enzyme in brewing yeast. FEMS Microbiol. Lett. 228:111–113 [DOI] [PubMed] [Google Scholar]
- 141. Saerens SM, Delvaux FR, Verstrepen KJ, Thevelein JM. 2010. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 3:165–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Daenen L, Saison D, Sterckx F, Delvaux FR, Verachtert H, Derdelinckx G. 2008. Screening and evaluation of the glucoside hydrolase activity in Saccharomyces and Brettanomyces brewing yeasts. J. Appl. Microbiol. 104:478–488 [DOI] [PubMed] [Google Scholar]
- 143. Kanauchi M, Bamforth CW. 2012. β-Glucoside hydrolyzing enzymes from ale and lager strains of brewing yeast. J. Am. Soc. Brew. Chem. 70:303–307 http://dx.doi.org/10.1094/ASBCJ-2012-1012-02 [Google Scholar]
- 144. Ilett DR. 1995. Aspects of the analysis, role and fate of sulphur dioxide in beer—a review. Tech. Q. Master Brew. Assoc. Am. 32:213–221 [Google Scholar]
- 145. Chen YF, Yang X, Zhang SJ, Wang XQ, Guo CH, Guo XW, Xiao DG. 2012. Development of Saccharomyces cerevisiae producing higher levels of sulfur dioxide and glutathione to improve beer flavor stability. Appl. Biochem. Biotechnol. 166:402–413 [DOI] [PubMed] [Google Scholar]
- 146. Hansen J, Bruun SV, Bech LM, Gjermansen C. 2002. The level of MXR1 gene expression in brewing yeast during beer fermentation is a major determinant for the concentration of dimethyl sulfide in beer. FEMS Yeast Res. 2:137–149 [DOI] [PubMed] [Google Scholar]
- 147. James M. 2012. Direct supplementation of yeast with lipids as a means to reduce sulfur dioxide formation. J. Am. Soc. Brew. Chem. 70:115–122 [Google Scholar]
- 148. Spiropoulos A, Bisson LF. 2000. MET17 and hydrogen sulfide formation in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 66:4421–4426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Duan WD, Roddick FA, Higgins VJ, Rogers PJ. 2004. Parallel analysis of H2S and SO2 formation by brewing yeast in response to sulfur-containing amino acids and ammonium ions. J. Am. Soc. Brew. Chem. 62:35–41 [Google Scholar]
- 150. Anness BJ, Bamforth CW. 1982. Dimethyl sulfide—a review. J. Inst. Brew. 88:244–252 [Google Scholar]
- 151. Bamforth CW. 1980. Dimethyl sulfoxide reductase of Saccharomyces spp. FEMS Microbiol. Lett. 7:55–59 [Google Scholar]
- 152. Saerens SMG, Duong CT, Nevoigt E. 2010. Genetic improvement of brewer's yeast: current state, perspectives and limits. Appl. Microbiol. Biotechnol. 86:1195–1212 [DOI] [PubMed] [Google Scholar]
- 153. Nevoigt E. 2008. Progress in metabolic engineering of Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 72:379–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Hammond JRM. 1995. Genetically-modified brewing yeasts for the 21st century. Progress to date. Yeast 11:1613–1627 [DOI] [PubMed] [Google Scholar]
- 155. Bamforth CW. 2003. Opportunities for newer technologies in the oldest biotechnology, brewing. Appl. Biotechnol. Food Sci. Policy 1:213–222 [Google Scholar]
- 156. Bokulich NA, Bamforth CW, Mills DA. 2012. A review of molecular methods for microbial community profiling of beer and wine. J. Am. Soc. Brew. Chem. 70:150–162 [Google Scholar]
- 157. Bokulich NA, Mills DA. 2012. Next-generation approaches to the microbial ecology of food fermentations. BMB Rep. 45:377–389 [DOI] [PubMed] [Google Scholar]
- 158. Noots I, Delcour JA, Michiels C. 1998. From field barley to malt: detection and specification of microbial activity for quality aspects. Crit. Rev. Microbiol. 25:121–153 [DOI] [PubMed] [Google Scholar]
- 159. Petters HI, Flannigan B, Austin B. 1988. Quantitative and qualitative studies of the microflora of barley malt production. J. Appl. Bacteriol. 65:279–297 [Google Scholar]
- 160. Wolf-Hall HM. 2007. Mold and mycotoxin problems encountered during malting and brewing. Int. J. Food Microbiol. 119:89–94 [DOI] [PubMed] [Google Scholar]
- 161. Scott PM. 1996. Mycotoxins transmitted into beer from contaminated grains during brewing. J. Assoc. Offic. Anal. Chem. Int. 79:875–882 [PubMed] [Google Scholar]
- 162. Kostelanska M, Hajslova J, Zachariasova M, Malachova A, Kalachova K, Poustka J, Fiala J, Scott PM, Berthiller F, Krska R. 2009. Occurrence of deoxynivalenol and its major conjugate, deoxynivalenol-3-glucoside, in beer and some brewing intermediates. J. Agric. Food Chem. 57:3187–3194 [DOI] [PubMed] [Google Scholar]
- 163. Schwartz PB, Casper HH, Barr JM. 1995. Survey of the natural occurrence of deoxynivalenol (vomitoxin) in barley grown in Minnesota, North Dakota and South Dakota during 1993. Tech. Q. Master Brew. Assoc. Am. 3:190–194 [Google Scholar]
- 164. Clear RM, Patrick SK, Platford RG, Desjardins M. 1996. Occurrence and distribution of Fusarium species in barley and oat seed from Manitoba in 1993 and 1994. Can. J. Plant Pathol. 18:409–414 [Google Scholar]
- 165. Pestka JJ. 2010. Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 84:663–679 [DOI] [PubMed] [Google Scholar]
- 166. Boeira L, Bryce J, Stewart GG, Flannigan B. 1999. Inhibitory effect of Fusarium mycotoxins on growth of brewing yeasts. 2. Deoxynivalenol and nivalenol. J. Inst. Brew. 105:376–381 [Google Scholar]
- 167. Boeira L, Bryce J, Stewart GG, Flannigan B. 1999. Inhibitory effect of Fusarium mycotoxins on growth of brewing yeasts. 1. Zearalenone and fumonisin B1. J. Inst. Brew. 105:366–374 [DOI] [PubMed] [Google Scholar]
- 168. Deckers SM, Lorgouilloux Y, Gebruers K, Baggerman G, Verachtert H, Neven H, Michiels C, Derdelinckx G, Delcour JA, Martens J. 2011. Dynamic light scattering (DLS) as a tool to detect CO2-hydrophobin structures and study the primary gushing potential of beer. J. Am. Soc. Brew. Chem. 69:144–149 [Google Scholar]
- 169. Wosten HAB, van Wetter MA, Lugones LG, van der Mei HC, Busscher HJ, Wessels JGH. 1999. How a fungus escapes the water to grow into the air. Curr. Biol. 9:85–88 [DOI] [PubMed] [Google Scholar]
- 170. Reininkainen P, Peltola P, Lampinen R, Haikara A, Olkku J. 1999. Improving the quality of malting barley by employing microbial starter cultures in the field, p 551–558 In Proceedings of the 27th congress of the European Brewery Convention European Brewery Convention, Brussels, Belgium [Google Scholar]
- 171. Hippeli S, Elstner EF. 2002. Are hydrophobins and/or non-specific lipid transfer proteins responsible for gushing in beer? New hypotheses on the chemical nature of gushing inducing factors. Z. Naturforsch. 57:1–8 [DOI] [PubMed] [Google Scholar]
- 172. Gorjanović S. 2010. A review: the role of barley seed pathogenesis-related proteins (PRs) in beer production. J. Inst. Brew. 116:111–124 [Google Scholar]
- 173. Jegou S, Douliez Molle J-PD, Boivin P, Marion D. 2000. Purification and structural characterization of LTP1 polypeptides from beer. J. Agric. Food Chem. 48:5023–5029 [DOI] [PubMed] [Google Scholar]
- 174. Muthukrishnan S, Liang HG, Trick NH, Gill SB. 2001. Pathogenesis-related proteins and their genes in cereals. Plant Cell Tissue Organ Cult. 64:93–114 [Google Scholar]
- 175. Hecht D, Hippeli S. 2007. Role of ns-LTP1 in the development of primary gushing. Monatsschr. Brauwiss. 60:1–9 [Google Scholar]
- 176. Cvetkovic A, Blagojevic S, Hranisavijevic J. 1997. Effects of pathogen-related proteins from barley grain on brewers yeast. J. Inst. Brew. 103:183–186 [Google Scholar]
- 177. Gorjanović R, Beljanski M, Gavrović-Jankulović M, Gojgić-Cvijović G, Bejosano F. 2007. Antimicrobial activity of malting barley grain thaumatin-like protein isoforms, S and R. J. Inst. Brew. 113:206–212 [Google Scholar]
- 178. Gorjanović S, Sužnjević D, Beljanski M, Gorjanović SOR, Vrvić M. 2004. Effects of lipid transfer protein from malting barley on brewer's yeast fermentation. J. Inst. Brew. 110:297–302 [Google Scholar]
- 179. Hegrova B, Farkova M, Macuchova S, Havel J, Preisler J. 2009. Investigation of relationships between barley stress peptides and beer gushing using SDS-PAGE and MS screening. J. Sep. Sci. 32:4247–4253 [DOI] [PubMed] [Google Scholar]
- 180. Stratford M, Carter AT. 1993. Yeast flocculation—lectin synthesis and activation. Yeast 9:371–378 [DOI] [PubMed] [Google Scholar]
- 181. Herrera VE, Axcell BC. 1991. Induction of premature yeast flocculation by a polysaccharide fraction isolated from malt husk. J. Inst. Brew. 97:359–366 [Google Scholar]
- 182. Panteloglou AG, Smart KA, Cook DJ. 2012. Malt-induced premature yeast flocculation: current perspectives. J. Ind. Microbiol. Biotechnol. 39:813–822 [DOI] [PubMed] [Google Scholar]
- 183. Herrera VE, Axcell B. 1989. The influence of barley lectins on yeast flocculation. J. Am. Soc. Brew. Chem. 47:29–34 [Google Scholar]
- 184. Koizumi H. 2008. Barley malt polysaccharides inducing premature yeast flocculation and their possible mechanisms. J. Am. Soc. Brew. Chem. 66:137–142 [Google Scholar]
- 185. Koizumi H. 2009. Structural features of barley malt polysaccharides inducing premature yeast flocculation. J. Am. Soc. Brew. Chem. 67:129–134 [Google Scholar]
- 186. van Nierop SNE, Cameron-Clarke A, Axcell BC. 2004. Enzymatic generation of factors from malt responsible for premature yeast flocculation. J. Am. Soc. Brew. Chem. 62:108–116 [Google Scholar]
- 187. O'Sullivan T, Walsh Y, O'Mahony A, Fitzgerald G, van Sinderen D. 1999. A comparative study of malthouse and brewhouse microflora. J. Inst. Brew. 105:55–61 [Google Scholar]
- 188. Briggs DE, McGuinness G. 1993. Microbes on barley grains. J. Inst. Brew. 99:249–255 [Google Scholar]
- 189. Doran P, Briggs DE. 1993. Microbes and grain germination. J. Inst. Brew. 99:165–170 [Google Scholar]
- 190. Kelly L, Briggs DE. 1992. The influence of the grain microflora on the germinative physiology of barley. J. Inst. Brew. 98:395–400 [Google Scholar]
- 191. Tuomi T, Laakso S, Rosenqvist H. 1995. Plant hormones in fungi and bacteria from malting barley. J. Inst. Brew. 101:351–357 [Google Scholar]
- 192. Laitila A, Kotaviita E, Peltola P, Home S, Wilhelmson A. 2007. Indigenous microbial community of barley greatly influences grain germination and malt quality. J. Inst. Brew. 113:9–20 [Google Scholar]
- 193. Boivin P, Malanda M. 1997. Improvement of malt quality and safety by adding starter culture during the malting process. Tech. Q. Master Brew. Assoc. Am. 34:96–101 [Google Scholar]
- 194. Laitila A, Alakomi HL, Raaska L, Mattila-Sandholm T, Haikara A. 2002. Antifungal activities of two Lactobacillus plantarum strains against Fusarium moulds in vitro and in malting of barley. J. Appl. Microbiol. 93:566–576 [DOI] [PubMed] [Google Scholar]
- 195. Mauch A, Jacob F, Coffey A, Arendt EK. 2011. Part I. The use of Lactobacillus plantarum starter cultures to inhibit rootlet growth during germination of barley, reducing malting loss, and its influence on malt quality. J. Am. Soc. Brew. Chem. 69:227–238 [Google Scholar]
- 196. Lowe DP, Ulmer HM, Barta RC, Goode DL, Arendt EK. 2005. Biological acidification of a mash containing 20% barley using Lactobacillus amylovorus FST 1.1: its effects on wort and beer quality. J. Am. Soc. Brew. Chem. 63:96–106 [Google Scholar]
- 197. Calderbank J, Hammond JRM. 1989. Influence of nitrate and bacterial-contamination on the formation of apparent total N-nitroso compounds (Atnc) during fermentation. J. Inst. Brew. 95:277–281 [Google Scholar]
- 198. Smith NA, Smith P. 1992. The role of Bacillus spp in N-nitrosamine formation during wort production. J. Inst. Brew. 98:409–414 [Google Scholar]
- 199. Hawthorne DB, Shaw RD, Davine DF, Kavanagh TE, Clarke BJ. 1993. Butyric acid off-flavors in beer: origins and control. J. Am. Soc. Brew. Chem. 49:4–8 [Google Scholar]
- 200. Haikara A, Home S. 1991. Mash filtration difficulties caused by split barley kernels: a microbiological problem, p 537–546 In Proceedings of the 23rd Congress of the European Brewery Convention, Lisbon European Brewery Convention, Brussels, Belgium [Google Scholar]
- 201. Evans D, Nischwitz R, Stewart D, Cole N, MacLeod L. 1999. The influence of malt foam-positive proteins and non-starch polysaccharides on beer foam quality. Monogr. Eur. Brew. Conv. 27:114–128 [Google Scholar]
- 202. Priest FG, Cowbourn MA, Hough JS. 1974. Wort enterobacteria. J. Inst. Brew. 80:342–356 [Google Scholar]
- 203. Van Vuuren HJJ, Cosser K, Prior BA. 1980. Influence of Enterobacter agglomerans on beer flavor. J. Inst. Brew. 86:31–33 [Google Scholar]
- 204. Jespersen L, Jakobsen M. 1996. Specific spoilage organisms in breweries and laboratory media for their detection. Int. J. Food Microbiol. 33:139–155 [DOI] [PubMed] [Google Scholar]
- 205. Martens H, Iserentant D, Verachtert H. 1997. Microbiological aspects of a mixed yeast-bacterial fermentation in the production of a special Belgian acidic ale. J. Inst. Brew. 103:85–91 [Google Scholar]
- 206. Dobson CM, Deneer H, Lee S, Hemmingsen S, Glaze S, Ziola B. 2002. Phylogenetic analysis of the genus Pediococcus, including Pediococcus claussenii sp. nov., a novel lactic acid bacterium isolated from beer. Int. J. Syst. Evol. Microbiol. 52:2003–2010 [DOI] [PubMed] [Google Scholar]
- 207. Priest FG, Campbell I. (ed). 2003. Brewing microbiology. Plenum Publishers, New York, NY [Google Scholar]
- 208. Ehrmann MA, Preissler P, Danne M, Vogel RF. 2010. Lactobacillus paucivorans sp. nov., isolated from a brewery environment. Int. J. Syst. Evol. Microbiol. 60:2353–2357 [DOI] [PubMed] [Google Scholar]
- 209. Suzuki K, Ozaki K, Yamashita H. 2004. Comparative analysis of conserved genetic markers and adjacent DNA regions identified in beer-spoilage lactic acid bacteria. Lett. Appl. Microbiol. 39:240–245 [DOI] [PubMed] [Google Scholar]
- 210. Bohak I, Back W, Richter L, Ehrmann M, Ludwig W, Schleifer KH. 1998. Lactobacillus amylolyticus sp. nov., isolated from beer malt and beer wort. Syst. Appl. Microbiol. 21:360–364 [DOI] [PubMed] [Google Scholar]
- 211. Back W, Bohak I, Ehrmann M, Ludwig W, Schleifer KH. 1996. Revival of the species Lactobacillus lindneri and the design of a species specific oligonucleotide probe. Syst. Appl. Microbiol. 19:322–325 [Google Scholar]
- 212. Curk MC, Hubert JC, Bringel F. 1996. Lactobacillus paraplantarum sp. nov., a new species related to Lactobacillus plantarum. Int. J. Syst. Bacteriol. 46:595–598 [DOI] [PubMed] [Google Scholar]
- 213. Back W. 1987. New description of a type of Lactobacillus harmful to beer—Lactobacillus brevisimilis spec nov. Monatsschr. Brauwiss. 40:484 [Google Scholar]
- 214. Russell C, Walker TK. 1953. Lactobacillus malefermentans n.sp. isolated from beer. J. Gen. Microbiol. 8:160–162 [DOI] [PubMed] [Google Scholar]
- 215. Pittet V, Morrow K, Ziola B. 2011. Ethanol tolerance of lactic acid bacteria, including relevance of the exopolysaccharide gene gtf. J. Am. Soc. Brew. Chem. 69:57–61 [Google Scholar]
- 216. Van Oevelen D, Verachtert H. 1979. Slime production by brewery strains of Pediococcus cerevisiae. J. Am. Soc. Brew. Chem. 37:34–37 [Google Scholar]
- 217. Back W. 1981. Beer spoilage bacteria. Taxonomy of beer spoilage bacteria. Gram positive species. Monatsschr. Brauwiss. 34:267–276 [Google Scholar]
- 218. Haakensen M, Ziola B. 2008. Identification of novel horA-harbouring bacteria capable of spoiling beer. Can. J. Microbiol. 54:321–325 [DOI] [PubMed] [Google Scholar]
- 219. Behr J, Vogel RF. 2009. Mechanisms of hop inhibition: hop ionophores. J. Agric. Food Chem. 57:6074–6081 [DOI] [PubMed] [Google Scholar]
- 220. Sakamoto K, Konings WN. 2003. Beer spoilage bacteria and hop resistance. Int. J. Food Microbiol. 89:105–124 [DOI] [PubMed] [Google Scholar]
- 221. Simpson WJ. 1993. Studies on the sensitivity of lactic acid bacteria to hop bitter acids. J. Inst. Brew. 99:405–411 [Google Scholar]
- 222. Behr J, Vogel RF. 2010. Mechanisms of hop inhibition include the transmembrane redox reaction. Appl. Environ. Microbiol. 76:142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Sakamoto K, Margolles A, van Veen HW, Konings WN. 2001. Hop resistance in the beer spoilage bacterium Lactobacillus brevis is mediated by the ATP-binding cassette multidrug transporter HorA. J. Bacteriol. 183:5371–5375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Hayashi N, Ito M, Horiike S, Taguchi H. 2001. Molecular cloning of a putative divalent-cation transporter gene as a new genetic marker for the identification of Lactobacillus brevis strains capable of growing in beer. Appl. Microbiol. Biotechnol. 55:596–603 [DOI] [PubMed] [Google Scholar]
- 225. Behr J, Israel L, Ganzle MG, Vogel RF. 2007. Proteomic approach for characterization of hop-inducible proteins in Lactobacillus brevis. Appl. Environ. Microbiol. 73:3300–3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Shimwell JL. 1937. On the relation between the staining properties of bacteria and their reaction towards hop antiseptic. J. Inst. Brew. 43:191–195 [Google Scholar]
- 227. Hazelwood LA, Walsh MC, Pronk JT, Daran JM. 2010. Involvement of vacuolar sequestration and active transport in tolerance of Saccharomyces cerevisiae to hop iso-alpha-acids. Appl. Environ. Microbiol. 76:318–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Bokulich NA, Bamforth CW, Mills DA. 2012. Brewhouse-resident microbiota are responsible for multi-stage fermentation of American coolship ale. PLoS One 7:e35507 doi: 10.1371/journal.pone.0035507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Chowdhury I, Watier D, Leguerinel I, Hornez JP. 1997. Effect of Pectinatus cerevisiiphilus on Saccharomyces cerevisiae concerning its growth and alcohol production in wort medium. Food Microbiol. 14:265–272 [Google Scholar]
- 230. Juvonen R, Suihko ML. 2006. Megasphaera paucivorans sp. nov., Megasphaera sueciensis sp. nov. and Pectinatus haikarae sp. nov., isolated from brewery samples, and emended description of the genus Pectinatus. Int. J. Syst. Evol. Microbiol. 56:695–702 [DOI] [PubMed] [Google Scholar]
- 231. Richards M, Corbey DA. 1974. Isolation of Zymomonas mobilis from primed beer. J. Inst. Brew. 80:241–244 [Google Scholar]
- 232. van der Aa Kuhle A, Jespersen L. 1998. Detection and identification of wild yeasts in lager breweries. Int. J. Food Microbiol. 43:205–213 [DOI] [PubMed] [Google Scholar]
- 233. Meaden PG, Taylor NR. 1991. Cloning of a yeast gene which causes phenolic off-flavours in beer. J. Inst. Brew. 97:353–357 [Google Scholar]
- 234. Aguilar-Uscanga MG, Delia ML, Strehaiano P. 2003. Brettanomyces bruxellensis: effect of oxygen on growth and acetic acid production. Appl. Microbiol. Biotechnol. 61:157–162 [DOI] [PubMed] [Google Scholar]
- 235. Daenen L, Sterckx F, Delvaux FR, Verachtert H, Derdelinckx G. 2008. Evaluation of the glycoside hydrolase activity of a Brettanomyces strain on glycosides from sour cherry (Prunus cerasus L.) used in the production of special fruit beers. FEMS Yeast Res. 8:1103–1114 [DOI] [PubMed] [Google Scholar]
- 236. Andrews J, Gilliland R. 1952. Super-attenuation of beer: a study of three organisms capable of causing abnormal attenuations. J. Inst. Brew. 58:189–196 [Google Scholar]
- 237. Timke M, Wang-Lieu NQ, Altendorf K, Lipski A. 2008. Identity, beer spoiling and biofilm forming potential of yeasts from beer bottling plant associated biofilms. Antonie Van Leeuwenhoek 93:151–161 [DOI] [PubMed] [Google Scholar]
- 238. Loret S, Deloyer P, Dandrifosse G. 2005. Levels of biogenic amines as a measure of the quality of the beer fermentation process: data from Belgian samples. Food Chem. 89:519–525 [Google Scholar]
- 239. Romero R, Bagur MG, Sanchez-Vinas M, Gazquez D. 2003. The influence of the brewing process on the formation of biogenic amines in beers. Anal. Bioanal. Chem. 376:162–167 [DOI] [PubMed] [Google Scholar]
- 240. Slomkowska A, Ambroziak W. 2002. Biogenic amine profile of the most popular Polish beers. Eur. Food Res. Technol. 215:380–383 [Google Scholar]
- 241. Izquierdo-Pulido M, Marine-Font A, Vidal-Carou MC. 1994. Biogenic amines formation during malting and brewing. J. Food Sci. 59:1104–1107 [Google Scholar]
- 242. Izquierdo-Pulido M, Marine-Font A, Vidal-Carou MC. 2000. Effect of tyrosine on tyramine formation during beer fermentation. Food Chem. 2000:329–332 [DOI] [PubMed] [Google Scholar]
- 243. Kalac P, Savel J, Krizek M, Pelikanova T, Prokopova M. 2002. Biogenic amine formation in bottled beer. Food Chem. 79:431–434 [Google Scholar]
- 244. Kalac P, Krizek M. 2003. A review of biogenic amines and polyamines in beer. J. Inst. Brew. 109:123–128 [Google Scholar]
- 245. Shalaby AR. 1996. Significance of biogenic amines to food safety and human health. Food Res. Int. 29:675–690 [Google Scholar]
- 246. Kalac P, Krausova P. 2005. A review of dietary polyamines: formation, implications for growth and health and occurrence in foods. Food Chem. 90:219–230 [Google Scholar]
- 247. Vally H, Thompson PJ. 2003. Allergic and asthmatic reactions to alcoholic drinks. Addict. Biol. 8:3–11 [DOI] [PubMed] [Google Scholar]
- 248. Peatfield RC. 1995. Relationships between food, wine, and beer-precipitated migrainous headaches. Headache 35:355–357 [DOI] [PubMed] [Google Scholar]
- 249. Shulman KI, Tailor SAN, Walker SE, Gardner DM. 1997. Tap (draft) beer and monoamine oxidase inhibitor dietary restrictions. Can. J. Psychiatry 42:310–312 [DOI] [PubMed] [Google Scholar]
- 250. Tailor SAN, Shulman KI, Walker SE, Moss J, Gardner D. 1994. Hypertensive episode associated with phenelzine and tap beer—a reanalysis of the role of pressor amines in beer. J. Clin. Psychopharmacol. 14:5–14 [PubMed] [Google Scholar]
- 251. Gasarasi G, Kelgtermans M, Verstrepen KJ, Van Roy J, Delvaux F, Derdelinckx G. 2003. Occurrence of biogenic amines in beer: causes and proposals of remedies. Monatsschr. Brauwiss. 56:58–63 [Google Scholar]
- 252. Halasz A, Barath A, Holzapfel WH. 1999. The biogenic amine content of beer; the effect of barley, malting and brewing on amine concentration. Z. Lebensm. Unters. Forsch. A 208:418–423 [Google Scholar]
- 253. Storgards E, Tapani K, Hartwall P, Saleva R, Suihko ML. 2006. Microbial attachment and biofilm formation in brewery bottling plants. J. Am. Soc. Brew. Chem. 64:8–15 [Google Scholar]
- 254. Martens H, Dawoud E, Verachtert H. 1991. Wort enterobacteria and other microbial populations involved during the first month of lambic fermentation. J. Inst. Brew. 97:435–439 [Google Scholar]
- 255. Martens H, Dawoud E, Verachtert H. 1992. Synthesis of aroma compounds by wort enterobacteria during the first stage of lambic fermentation. J. Inst. Brew. 98:421–425 [Google Scholar]
- 256. Spaepen M, Vanoevelen D, Verachtert H. 1978. Fatty acids and esters produced during spontaneous fermentation of lambic and gueuze. J. Inst. Brew. 84:278–282 [Google Scholar]
- 257. Van Oevelen D, Delescaille F, Verachtert H. 1976. Synthesis of aroma compounds during spontaneous fermentation of lambic and gueuze. J. Inst. Brew. 82:322–326 [Google Scholar]
- 258. Verachtert H, Shanta Kumara HMC, Dawoud E. 1990. Yeasts in mixed cultures, p 429–478 In Verachtert H, De Mot R. (ed), Yeast biotechnology and biocatalysis. Marcel Dekker, New York, NY [Google Scholar]
- 259. Lyumugabe F, Kamaliza G, Bajyana E, Thonart PH. 2010. Microbiological and physico-chemical characteristic of Rwandese traditional beer “Ikigage.” Afr. J. Biotechnol. 9:4241–4246 [Google Scholar]
- 260. Maoura N, Mbaiguinam M, Nguyen HV, Gaillardin C, Pourquie J. 2005. Identification and typing of the yeast strains isolated from bili bili, a traditional sorghum beer of Chad. Afr. J. Biotechnol. 4:646–656 [Google Scholar]
- 261. Kayode APP, Vieira-Dalode G, Linneman AR, Kotchoni SO, Hounhouigan AJD, van Boekel MAJS, Nout MJ. 2011. Diversity of yeasts involved in the fermentation of tchoukoutou, an opaque sorghum beer from Benin. Afr. J. Microbiol. Res. 5:2737–2742 [Google Scholar]
- 262. N′Guessan KF, Brou K, Jacques N, Casaregola S, Dje KM. 2011. Identification of yeasts during alcoholic fermentation of tchapalo, a traditional sorghum beer from Cote d'Ivoire. Antonie Van Leeuwenhoek 99:855–864 [DOI] [PubMed] [Google Scholar]
- 263. Sawadogo-Lingani H, Lei V, Diawara B, Nielsen DS, Møller PL, Traoré AS, Jakobsen M. 2007. The biodiversity of predominant lactic acid bacteria in dolo and pito wort for the production of sorghum beer. J. Appl. Microbiol. 103:765–777 [DOI] [PubMed] [Google Scholar]