Abstract
SUMMARY
Neisseria meningitidis is a Gram-negative microorganism that exists exclusively in humans and can cause devastating invasive disease. Although capsular polysaccharide-based vaccines against serogroups A, C, Y, and W135 are widely available, the pathway to a broadly protective vaccine against serogroup B has been more complex. The last 11 years has seen the discovery and development of the N. meningitidis serogroup B (MnB) outer membrane protein factor H binding protein (fHBP) as a vaccine component. Since the initial discovery of fHBP, a tremendous amount of work has accumulated on the diversity, structure, and regulation of this important protein. fHBP has proved to be a virulence factor for N. meningitidis and a target for functional bactericidal antibodies. fHBP is critical for survival of meningococci in the human host, as it is responsible for the primary interaction with human factor H (fH). Binding of hfH by the meningococcus serves to downregulate the host alternative complement pathway and helps the organism evade host innate immunity. Preclinical studies have shown that an fHBP-based vaccine can elicit serum bactericidal antibodies capable of killing MnB, and the vaccine has shown very encouraging results in human clinical trials. This report reviews our current knowledge of fHBP. In particular, we discuss the recent advances in our understanding of fHBP, its importance to N. meningitidis, and its potential role as a vaccine for preventing MnB disease.
INTRODUCTION
Neisseria meningitidis is a Gram-negative microorganism and an exclusive human pathogen. It usually exists in an asymptomatic nasopharyngeal carriage state. However, N. meningitidis can cause devastating invasive disease, such as septicemia or meningitis, following penetration of the mucosal tissue, invasion of the bloodstream, and colonization of the meninges. Person-to-person transmission occurs through aerosol droplets from close contact or human crowding in preschools, in university or military dormitories, or during international pilgrimages, e.g., the Hajj. Disease often progresses very rapidly and is therefore difficult to diagnose and treat. Disease rates vary from 1 to 1,000 per 100,000 (1, 2). The rates of disease are highest among infants, followed by a second peak during adolescence and early adulthood. The associated mortality is about 10%, and permanent sequelae such as hearing impairment, mental retardation, or limb loss are common in survivors. Goldschneider and coworkers demonstrated an inverse relationship between the incidence of meningococcal disease and the age-specific prevalence of serum bactericidal antibody activity (3). Exposure to the pathogen is initiated by carriage. Rates of carriage range from 10% to 35% in young adults, with most individuals being colonized at some time in their life (4, 5). In most people, carriage is an immunizing event resulting in stimulation of an adaptive immune response to the carriage strain (6).
Encapsulation is one of the hallmarks of meningococci that cause invasive disease, and the capsule is a known virulence factor. There are 12 known serogroups of N. meningitidis, based on different capsular polysaccharide structures, of which 5 (A, B, C, W135, and Y) are most commonly associated with significant clinical disease. Prevention of disease caused by serogroups A, C, W135, and Y is now possible due to the development of capsular polysaccharide-conjugated vaccines against these serogroups (7–9). Unfortunately, the capsular polysaccharide of serogroup B is poorly immunogenic, presumably due to its structural homology to polysialic acid on human neural cells (10–12). Efforts to develop vaccines based on the N. meningitidis serogroup B (MnB) capsular polysaccharide have failed (13). The lack of an effective MnB vaccine that provides broad coverage is reflected in the current meningococcal disease incidence. The majority of meningococcal disease in Europe is now caused by MnB, which also accounts for approximately one-third of cases in the United States. Therefore, there is a clear unmet medical need for a broadly protective vaccine to prevent disease caused by this pathogen. Outer membrane vesicle (OMV) vaccines have been employed in some countries in response to outbreaks caused by specific epidemic MnB strains (11). The major drawback with the OMV vaccines, however, is that the principal target of the serum bactericidal response in these vaccines is porin A (PorA), whose primary epitopes are highly variable among meningococci. Thus, OMV vaccines offer protection primarily by generating serum bactericidal antibodies against homologous PorA serosubtype-expressing strains (as was observed in the clinical trials in New Zealand) and are not broadly protective (11, 14). Multivalent PorA-based OMV vaccines have been used experimentally in several different age groups (15), but even a hexavalent PorA-based vaccine would be expected to have limited coverage in countries where disease is caused by strains expressing many different PorA serosubtypes. Other protein antigens have therefore been sought, with the goal of providing broader protection against all MnB strains.
During the last 11 years, the discovery and importance of the meningococcal factor H binding protein (fHBP) has led to the development of two recombinant MnB vaccines which either are directed to fHBP as the single target of the vaccine or contain fHBP as one of the components. Binding of human factor H (hfH) by meningococci downregulates the host alternative complement pathway and helps the organism to evade host innate immunity (16, 17). fHBP was identified as a vaccine candidate independently by two groups using different approaches, hence the different designations lipoprotein 2086 (LP2086) (18, 19) and genome-derived neisserial antigen 1870 (GNA1870) (20). One vaccine is composed of two lipidated variants of fHBP (the native form of the protein found on the bacterial surface and originally named LP2086) (21). The other vaccine is composed of a single nonlipidated fHBP variant (20), which is genetically fused to another inactive protein and used as part of a multiantigen formulation that also contains OMVs to enhance the immunogenicity of the vaccine (22). Both vaccines had advanced to clinical testing prior to knowledge of the role of fHBP as a human factor H binding protein. Preclinical studies showed that fHBP-based vaccines could elicit serum bactericidal antibodies capable of killing MnB strains in serum bactericidal assays (SBA) (20, 21, 23). These findings led to the advancement of fHBP-containing vaccines into clinical trials. The recent advances in our understanding of fHBP, its importance to N. meningitidis, and its role as a vaccine candidate are the subjects of this review.
CHARACTERIZATION OF fHBP
fHBP Discovery: Traditional and In Silico Approaches
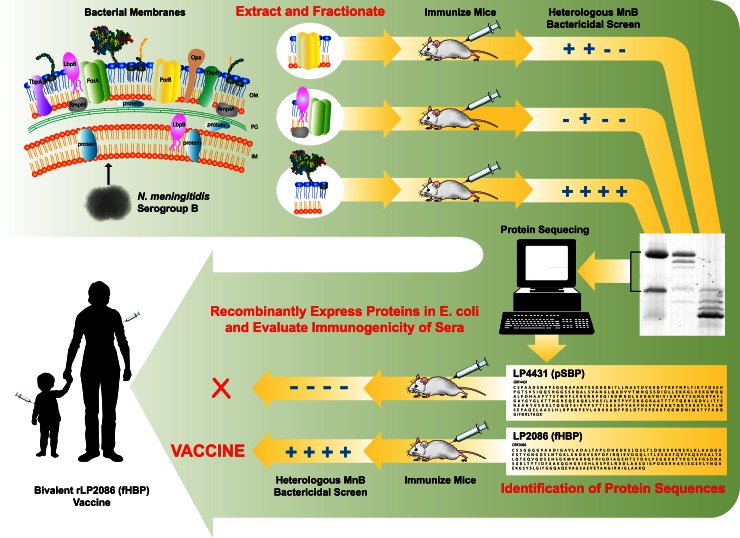
The search for a broadly protective vaccine that can prevent MnB disease has long been the goal of many scientists, clinicians, and public health representatives. Because serum bactericidal assays using human complement (hSBA) have been shown to correlate with protection in humans (24), the primary focus of vaccinologists in the field has been to identify potential vaccine components that will elicit antibody-dependent serum bactericidal activity. Different groups used different methods to discover and validate their targets. The vaccine potential of fHBP was first presented at the International Pathogenic Neisseria Conference in Oslo, Norway, in 2002 (18, 19) and later published (21) by a group of researchers from Wyeth (now Pfizer). They described a novel lipoprotein (LP2086, later identified as fHBP) identified by a process of differential detergent extraction of outer membrane proteins, separation by isoelectric focusing and ion-exchange chromatography, and sequential immunization of mice with different fractions to determine which proteins were capable of generating serum bactericidal antibodies that could kill genetically and phenotypically diverse meningococcal strains (Fig. 1) (18, 21). When more than one protein was obtained by the initial chromatographic separations, the individual proteins were recombinantly expressed, purified, and tested for their ability to elicit the bactericidal response. The vaccine candidate identified by this process that elicited the broadest bactericidal response was a meningococcal lipoprotein, fHBP. fHBP was cloned, recombinantly expressed in Escherichia coli, and purified for use in human clinical trials as a bacterial lipoprotein with the N-terminal tri-Pam-Cys-type modification common to bacterial native lipoproteins. The vaccine potential of fHBP was also identified by research groups at Chiron (now Novartis) and the Children's Hospital Oakland Research Institute (CHORI) using reverse vaccinology (20). The reverse vaccinology approach used computer algorithms to identify potential membrane protein open reading frames (ORFs) from bacterial genomes. These ORFs were then cloned, expressed in E. coli, and used to generate antibodies in mice. Antigens were selected that generated specific antibodies that bound to the cell surface of the bacteria and killed the bacteria in an SBA. Using this approach, approximately 20 other proteins that could elicit bactericidal antibodies in mice were identified (25). Genes of proteins that could elicit bactericidal antibodies were sequenced from diverse strains, and proteins with sequences more highly conserved than PorA were preferentially selected for further study. Although the last criterion was not met for fHBP, as sequence homology was in the range of 62 to 74% compared to porins which are >90% identical, the collaboration with the research group at CHORI resulted in the first publication identifying this family of proteins, initially called GNA1870, as a possible vaccine component (20).
Fig 1.
Biochemical approach resulting in the discovery of fHBP. The traditional biochemical approach is a proven method for the identification of vaccine candidates that provide broad protection against heterologous strains. The initial approach starts with the isolation of cell membranes containing surface antigens (top left). The proteins are extracted biochemically into separate fractions, with each fraction containing a different composition of membrane proteins. Each fraction is used to immunize mice, and the sera are tested for PorA-independent bactericidal antibody activity (SBA) against heterologous MnB strains (top right). Fractions that contain broadly reactive SBA activity are further fractionated and retested. The final fraction contains very few proteins but is highly enriched for SBA activity. These proteins are size separated and their amino acid sequences determined (bottom right). Using available genomic sequences, the corresponding gene is identified, cloned, and expressed in E. coli and the recombinant protein evaluated for SBA activity. The final proteins that elicit broad protection in preclinical studies are then candidates for clinical studies (bottom left).
fHBP Protein Description
The fHBP gene expresses a protein precursor which contains a lipoprotein signal motif, LXXC. The signal sequence is cleaved such that the cysteine (C) becomes the N terminus of the mature fHBP and is cotranslationally modified to a tri-Pam-Cys residue which serves to anchor the protein to the neisserial outer membrane (21, 26). Mature fHBP is 253 to 266 amino acids in length; most of the variation in size is a result of the variable length of a flexible segment or spacer, composed of 2 to 15 glycine and serine residues immediately following the N-terminal cysteine.
fHBP Nomenclature
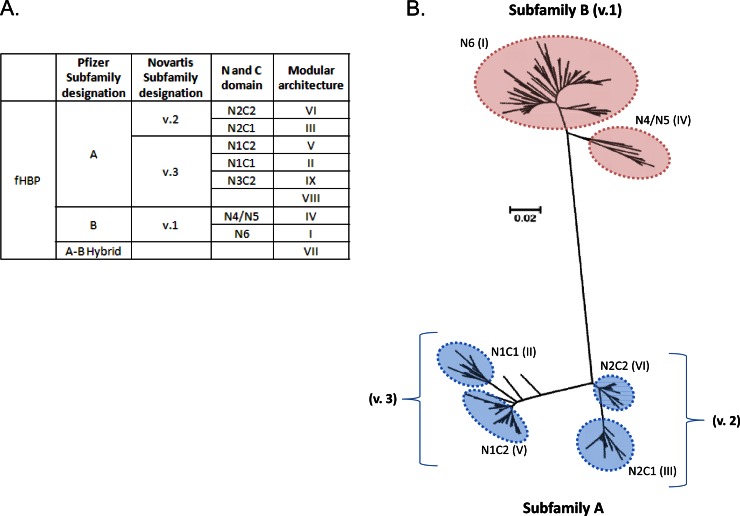
The fHBP nomenclature is somewhat confusing due to the independent discovery of fHBP by several groups and the resulting different approaches of sequence classification. Therefore, there is no accepted universal classification system for fHBP. In this section, we will attempt to clarify the relevance of the different naming systems and how they relate to each other. Schematics and a table that seek to clarify the naming systems are provided in Fig. 2. First, prior to the discovery that the natural ligand of the protein was human factor H, when it was therefore given the name factor H binding protein (fHBP), the gene was named according to the gene annotations from the strains in which it was discovered: ORF741 (27), LP2086 (21), and GNA1870 (20). Groups sequenced the gene from non-prevalence-based MnB collections and concluded from the sequences that the protein could be divided into groups: either 2 subfamilies (A and B) (21) or three major variants (1, 2, and 3) (20). Variant 1 corresponded to subfamily B, and variants 2 and 3 corresponded to subfamily A. LP2086 sequences were initially aligned and given the letter A or B according to the subfamily, and each protein sequence was given a unique number. Likewise, for the GNA1870 classification system, sequences were named according to the specific three major variants to which they belonged, followed by a period and a unique identifying number. For example, the subfamily A variant, A05, in the Pfizer classification system is called v3.45 in the Novartis classification system. These conflicting naming systems highlight the inherent difficulties when exploring genomic relationships within large data sets.
Fig 2.
Phylogenetic analysis of fHBP protein sequences. (A) Nomenclature used to describe fHBP, including Pfizer and Novartis subfamily designations, N- and C-terminal domains, and Pajon modular architecture assignations (20, 21, 29, 31). (B) Neighbor-joining tree of 569 fHBP variants from http://pubmlst.org/neisseria/fHbp/. The tree was generated in ClustalW (145) with 500 bootstraps and drawn using MEGA 5.05 (146). Subfamily designations are defined by Murphy et al. and Masignani et al. (20, 29). Subfamily A (variants 2 and 3) can be further subdivided into 4 major groupings based on the N- and C-terminal domains of fHBP (N1C1, N2C2, N1C2, and N2C1), and subfamily B can be divided into 3 groupings (N4, N5, and N6). Pajon et al. have made similar groupings based on five variable segments, dividing all fHBP variants into 9 modular groups (31). The six major modular groups are indicated by the roman numerals (the minor groups VIII and IX, each represented by a single variant, are not labeled on the tree). Not shown are several hybrid sequences that are composed of both subfamily A and subfamily B regions. N2C1 and N2C2 together are equivalent to variant 2 (v.2), and N1C2 and N1C1 are equivalent to variant 3 (v.3). The bar indicates genetic distance.
This problem was revisited by Schwarz et al. by exploiting their novel sequence alignment method (28). In contrast to classical methods that derive an evolutionary network from a distance matrix, they embedded the sequence alignment data into high-dimensional vector space, resulting in the ability to identify sites of evolutionary divergence and also sites involved in recombination events. Both approaches suggested that fHBP can be divided into two major groups, the aforementioned subfamilies A and B. They also concluded that this divergence happened early in the evolution of this protein. Further divisions have evolved within these two major groups. Murphy et al. observed that recombination events have also occurred subsequently within the subfamilies and, though extremely rarely, also between the subfamilies (29). Variation in subfamily A proteins is based on the four possible combinations of interchangeable modules corresponding to the N and C domains of the proteins. Recombination between these modules or domains has led to four subgroups within subfamily A: N1C1, N2C1, N1C2, and N2C2. For subfamily B, there are three main subgroups associated with the N domain of the fHBP protein, i.e., N4, N5, and N6; however, no obvious modular structures or recombination regions are apparent for the C domain. N4 and N5 sequences are more closely related than N6 sequences. The rare variants that have arisen through recombination of the subfamilies are composed of the N6 module from subfamily B and the C2 module from subfamily A. The modular nature of fHBP sequences was further explored by Beernink et al., who identified five variable segments within the proteins (30). Phylogenetic analysis on 70 known fHBP sequence variants classified all variants into six different modular groups, designated I to VI (30). Pajon et al. (31) evaluated the 70 sequences studied by Beernink et al. plus 172 additional distinct sequences and identified three additional minor groups (VII, VIII, and IX), each represented by a single variant. Despite a greater number of consensus regions defined by Pajon et al. and Beernink al. than by Murphy et al., the same six major subgroups were identified (Fig. 2).
The complexity of the different nomenclatures was addressed by Brehony et al., who developed a publically available database (http://pubmlst.org/neisseria/fHbp/) where scientists can deposit their novel fHBP sequences and each can be given an independent number (32). This database provides an important and useful function by allowing investigators to identify sequences from either classification system and by tracking deposited sequences. Numbers are assigned sequentially and do not differentiate between subfamilies. This lack of differentiation has meant that the other two major naming systems (subfamily A and B and variants 1, 2, and 3) are still in use by different groups, as they are anchored to the investigational vaccines that they support. Importantly, subfamily A/variant 2 and 3 strains are immunologically related, as they elicit protective antibodies across the group (32), but do not cross-protect against subfamily B or variant 1 strains. These will be reviewed in “Preclinical Evaluation of the Potential Breadth of Coverage of fHBP-Based Vaccines” below.
Distribution and Diversity of fHBP
The epidemiology of fHBP from invasive and carriage isolates has been extensively studied in several geographic regions, providing a comprehensive view of the distribution and diversity of this protein. The largest of these studies included 1,263 systematically collected MnB disease-causing isolates from the years 2000 to 2006. The isolates were from the United States (ABC surveillance) and four European public health laboratories (those in France, Czech Republic, and Norway and the United Kingdom Health Protection Agency, which encompasses England, Wales, and Northern Ireland) (29, 33). Except for one strain in which the fHBP gene contained a premature stop codon at nucleotide 366, all the strains were found to encode a full-length fHBP protein; 197 unique protein sequences were identified in this collection. Phylogenetic analysis (Fig. 2) showed that the protein sequences fell into one of two groups, the aforementioned subfamilies A and B, with >83% sequence identity within each subfamily but only 60 to 75% identity between subfamilies (29). The subfamily distribution of the strains was approximately 30% subfamily A and 70% subfamily B (29). This overall distribution has remained constant, with regions such as Sweden (34) and Brazil (35) reporting similar values. South Africa, however, has a higher proportion of subfamily A variants reported (36). Interestingly, in a follow-up study by the Centers for Disease Control and Prevention in the United States, it was found that although there was a lower proportion of disease caused by fHBP subfamily A MnB isolates, infants and subjects ≥65 years of age were more likely to be infected with fHBP subfamily A strains, in contrast to adolescents and young adults, who acquire disease dominated by fHBP subfamily B strains (37). The same study also evaluated the distribution of fHBP proteins in other meningococcal serogroups (MnC, MnY, and MnW135) and showed that for these other serogroups the proportion of fHBP subfamily A variants was also increased. Relatively more subfamily A disease has also been identified for MnC isolates from South Africa and for W135 isolates from the sub-Saharan meningitis belt in Africa (36, 38, 39). Conversely, in South Africa, MnW135 is dominated by a higher proportion of fHBP subfamily B variants. MnA isolates obtained from sub-Saharan Africa were all fHBP subfamily B.
Meningococcal disease is preceded by pharyngeal carriage, and carriage rates are found to peak in adolescents and young adults (40). The fHBP distribution of carriage isolates from this age group has also been studied. These isolates have a higher proportion of fHBP subfamily A variants regardless of whether they possess a capsule locus (5) or not (41). These observations lead to an interesting proposition that isolates more associated with adolescent carriage or disease in the extreme age groups express fHBP subfamily A proteins, whereas isolates that cause disease in older children and adolescents are more associated with fHBP subfamily B. Though the fHBP gene has been detected in most isolates, the truncated fHBP gene identified by Murphy et al. (29) was also identified in MnC isolates (37, 42). However, in these MnC isolates, a subpopulation of cells with the mutation appeared to be expressing fHBP on the cell surface, which is suggestive of a compensatory expression mechanism (42). Four additional isolates carried the identical frameshift mutation but had acquired compensatory mutations restoring both the fHBP reading frame and fHBP surface expression (42), demonstrating a strong selection for retention or restoration of fHBP expression. fHBP confers a benefit for the bacteria in vivo, as binding human factor H protects the bacteria from complement attack (43). A small number of isolates that lack the fHBP gene have been identified (44). These isolates are estimated to represent 0.04% of all the isolates that caused disease in England, Wales, and Northern Ireland over a 35-year period. Similar strains have not been identified in other countries. Also, it is important to note that the immune status of the patients from whom these strains were isolated is unknown, and it cannot be ruled out that the patients were immunocompromised or had complement defects. Nevertheless, it remains to be determined whether NspA, another meningococcal surface protein that binds human fH, could substitute as a virulence factor and downregulate the alternative complement pathway in these fHBP gene-negative isolates or whether these isolates have other mechanisms to avoid the innate immune response (45).
Topology of fHBP on the N. meningitidis Cell Surface
fHBP is located on the surface of N. meningitidis, as depicted schematically in Fig. 3 (20, 21, 46). Its tri-Pam-Cys-modified N terminus serves to anchor the protein to the outer membrane (47). The subsequent Gly/Ser-rich segment forms a flexible and variable-length chain which tethers the main body of fHBP to the anchor, as seen in a recently solved three-dimensional (3D) structure (48, 49). fHBP presents a novel fold comprised chiefly of two domains, one dominated by a β-sheet and the other by a β-barrel. The N-terminal β-sheet is “taco shaped” and folded to itself with amino acid side chains from both edges coming into close contact. The C-terminal β-barrel is composed of consecutive antiparallel strands with a hydrogen-bonded backbone and is “cannoli like” in that the hydrophobic or fatty side chains fill the barrel. These features are shared by all fHBP structures solved so far. They include variants B01 (also known as variant 1.55) (48, 49) and B24 (variant 1.1) (50–52), as well as a B24-based artificial fHBP in which 22 amino acids were converted to A subfamily counterparts, mostly in the C-terminal domain (53). Protein variants of the two fHBP subfamilies share 60 to 75% sequence identity distributed throughout the protein, presumably allowing for conservation of structure and function, since variants of both subfamilies bind hfH.
Fig 3.
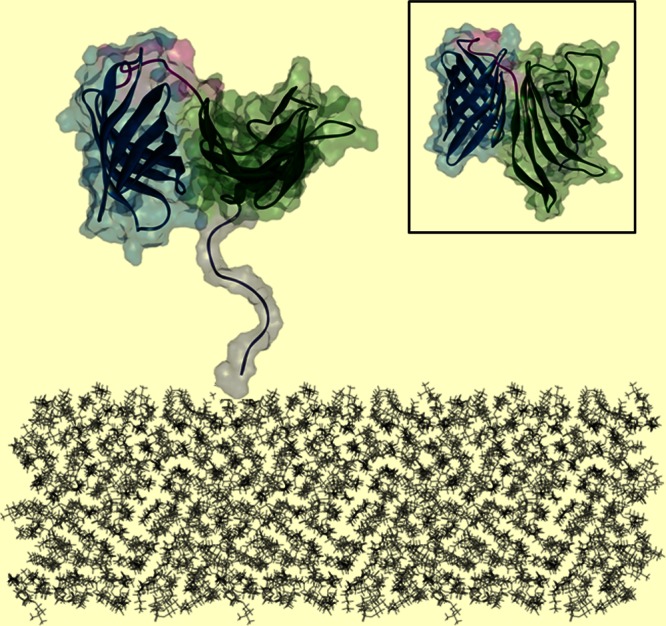
Schematic depiction of fHBP anchored on the surface of N. meningitidis. Shown is the B01 fHBP structure colored as follows: white, N-terminal flexible stem; green, N-terminal domain; blue, C-terminal domain; pink, linker between the β-structures of two domains. Inset, top view of fHBP demonstrating the surface proposed to face away from the bacterial surface.
The side-by-side spatial alignment of the two domains provides fHBP with a brick-shaped main body (Fig. 3, inset) which has a wider surface area that is accessible to antibodies (48). This upper surface is populated with residues that are either conserved across all fHBP variants or conserved within the A or B subfamily (Fig. 4) (29). Two nonbactericidal monoclonal antibodies that recognize free recombinant fHBP but do not bind to fHBP on intact meningococcal cells have been mapped to an area at the bottom surface of the N-terminal domain, illustrating that this area is less accessible to antibodies when the protein is expressed on the bacterial surface (48).
Fig 4.
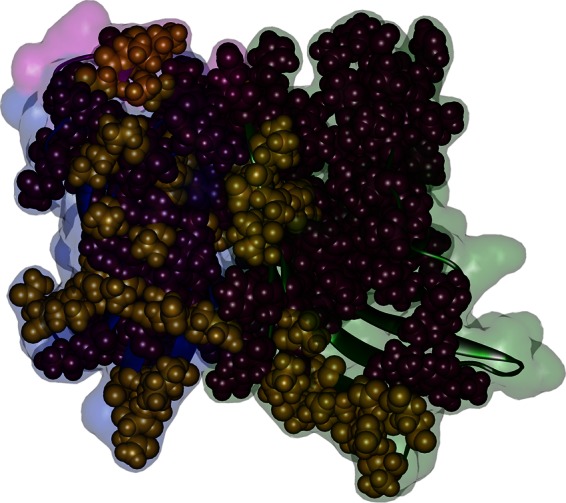
fHBP conservation within and across the A and B subfamilies. The residues defining the A and B subfamilies (gold) and the residues conserved in all A and B variants (purple) are shown as spheres on the B01 structure. Other residues are dimmed and colored according to structural regions: green, N-terminal domain; blue, C-terminal domain; pink, linker between the β-structures of two domains. The membrane-anchoring N-terminal flexible stem of fHBP is behind the structure.
Evidence that Human Factor H Is the Ligand for fHBP
The first indication that fH is the ligand for fHBP came from observations that strains in which fHBP had been deleted had reduced binding to fH and reduced survival in a human complement-mediated killing assay compared to the wild-type isogenic strains (16). The discovery that the natural ligand for fHBP was hfH revolutionized our understanding of the importance of this protein to N. meningitidis survival during infection.
Humans clear meningococci via both the classical and alternative complement pathways (24, 54–56), and it is widely accepted that host genetic factors play an important role in meningococcal susceptibility and disease outcome (56). Individuals with genetic defects in any of the complement activation pathways are at an increased risk of invasive meningococcal disease (IMD) (57). The alternative pathway involves deposition of C3b on the bacterial surface, leading to either direct lysis as a result of membrane attack complex formation or uptake by phagocytes. Cleavage of C3 to C3b by C3 convertase is controlled by host regulators such as fH. fH is the main inhibitor of the alternative complement pathway and a key discriminator between host and pathogen cells (58). Many pathogens, however, have evolved to evade this alternative complement pathway killing by binding fH on the bacterial surface (59). A well-characterized example is PorB.1A from Neisseria gonorrhoeae, which binds to fH short consensus repeat (SCR) domains 18 to 20 (58, 60–62). Although fH binding proteins have been identified in many pathogens, the sequence and structure of fHBP are unique to N. meningitidis and a few other neisserial species. Indeed, there was some early evidence of an immunoreactive fHBP-like protein in other neisserial strains, while the existence and expression of fHBP in these strains have not yet been demonstrated by isolating fHBP from these strains (20, 21).
Factor H is a 150-kDa glycoprotein typically present in human plasma at concentrations of 300 to 500 μg/ml (57, 63–65). It is composed of 20 repetitive domains, termed short consensus repeats (SCRs), each containing approximately 60 amino acids. The 3D structures of several SCRs have been determined, showing a globular structure with six-stranded antiparallel sheets connected with loops and turns. The protein is an extended molecule and appears to have a structure resembling beads on a string, where the SCRs exhibit different activities (66, 67). The binding of fH to specific host polyanions, such as heparin-like glycosaminoglycans (GAGs), can protect the host from complement attack (68), thus providing a mechanism for discrimination between invading microorganisms and the host (69).
Flow cytometric analysis has demonstrated that SCR domain 6 (SCR6) of fH is the main domain that interacts with fHBP on the surface of N. meningitidis (61). A cocrystal structure shows specific interactions involving both structural domains of fHBP (B24, variant 1.1) with the fH peptide that contains SCR domains 6 and 7 (fH67, one of the three SCR regions on fH involved in binding polyanions such as heparin), as shown in Fig. 5 (52). It is therefore no surprise that the interaction between fHBP and fH67 can be inhibited with a highly sulfated analogue of GAGs. This suggests that fHBPs may mimic GAGs and act similarly to host tissues by binding fH and preventing complement function on the bacterial surface. Therefore, recruitment of fH to the bacterial surface is one way in which N. meningitidis has evolved to counteract host innate immunity (17). A recent human genome-wide association study identified polymorphisms in the gene encoding fH that are associated with decreased host susceptibility to invasive meningococcal disease (70). This genetic variation in hfH may regulate its affinity to fHBP and therefore affect the ability of the bacteria to escape the host immune response.
Fig 5.
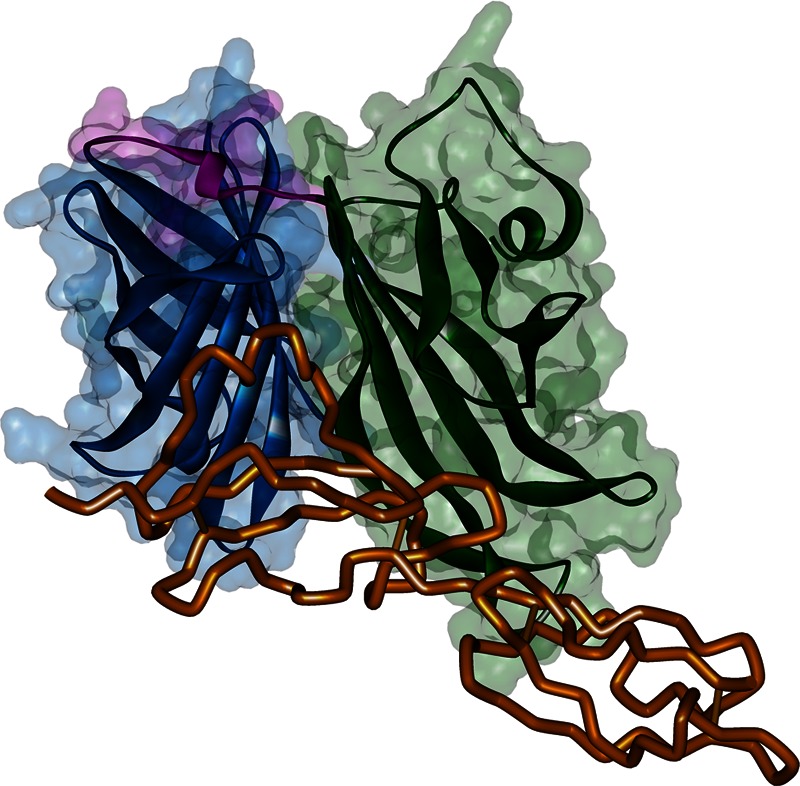
fH binding on fHBP. Shown is the cocrystal structure of human fH67 bound to B24 fHBP (52). fH67 is colored in orange, while fHBP is colored by domain: green, N-terminal domain; blue, C-terminal domain; pink, linker between the β-structures of two domains.
Many studies have provided a better understanding of the properties of fH binding to fHBP on the surface of bacteria, including fH affinity for fHBP and its role in mediating bacterial serum resistance. Human factor H was found to have different binding affinities for some fHBP-expressing MnB strains. However, it was not determined whether the differences in fH binding were sequence variant specific (71). A similar study looking at a different panel of variants confirmed that while fH bound to all strains tested, there were great differences in the fH dissociation kinetics among strains (72). This study also found no correlation between the level of fHBP expression and the level of fH binding. Strains which lack fHBP can be highly sensitive to killing by whole blood or human serum, as they are unable to bind fH and inhibit the alternative complement pathway (73). However, MnB strains that bind to fH, even with low affinity, are able to survive in human blood (71). Seib et al. confirmed this finding that serum resistance does not correlate with either fH-fHBP affinity or the level of fH binding (72). Although fH is required for bacterial survival in blood, the low affinity of fH for fHBP in some meningococcal strains is not a factor, likely because of the very high concentrations of fH in human blood, which leads to fH-fHBP binding saturation. The binding of fH to fHBP on the cell surface raises the concern that important vaccine epitopes may be shielded from bactericidal antibodies, both making a vaccine that targets fHBP less effective and possibly preventing the vaccine from generating an appropriate response. This is a hypothetical concern, as anti-fHBP antibodies are capable of both inhibiting binding of fH to fHBP and displacing fH from fHBP (74, 75). It has also been suggested that binding of fH to the fHBP in the vaccine might reduce the availability of epitopes during the immunization process. This was evaluated in a recent study by Beernink et al., who assessed the immunogenicity of fHBP-based vaccines in human fH transgenic mice that were immunized with fHBP or a mutant fHBP that eliminated fH binding (76). Though there was no statistically significant difference in SBA responses between the two vaccines, a logarithmic regression looked at the effect of the concentration of human factor H on immunogenicity and found that at high fH concentrations, the mutant vaccine generated slightly higher bactericidal responses. Nonetheless, humans vaccinated with wild-type fHBP generate substantial SBA titers (77–83), demonstrating that this is not a relevant concern for human vaccines. Recently, another meningococcal surface protein, NspA, was found to bind to human fH and contribute to bacterial complement resistance for some MnB strains (45). Capsule expression, however, interferes with the binding of fH to NspA, and thus the role of NspA in fH binding may be minimal.
Studies of binding of fHBP to its ligand fH have revealed how well adapted N. meningitidis is to its human host. fHBP binds preferentially to human fH, while chimpanzee, baboon, or rhesus macaque fHs show less binding or barely bind at all (84). The cocrystal structure of fHBP with human fH SCR6-7 peptide shows the host specificity between the divergent human and nonhuman primate fH SCR6 sequences that would interact with fHBP (61, 84). The specificity of fHBP for human fH also helps to explain the observation that bactericidal titers measured with rabbit complement are typically higher than those measured in the presence of human complement (85, 86). It is perhaps not surprising that N. meningitidis fHBP demonstrates a preference for human fH, as N. meningitidis is an obligate human pathogen that has coevolved with its human host.
Regulation of fHBP Expression
N. meningitidis surface antigens are known to undergo antigenic or phase-variable expression as a means to escape immune pressure and adapt to environmental conditions (87). Genomic analysis has revealed that for a number of genes, the genome contains short homologous DNA regions with the potential to be involved in antigenic variation. For other proteins, repetitive nucleotides result in phase-variable expression. Examples of surface proteins regulated by these mechanisms include neisserial pilin, PorA, and NadA (87, 88). The fHBP gene does not contain any of these intrinsic regulator sequences. Neisserial surface proteins can also be regulated by multicomponent regulatory systems or exogenous compounds. For example, FetA expression is regulated by iron (89), and an early report suggested that fHBP may also be regulated by iron due to the identification of a putative Fur box motif within its promoter region (20). More recently, it has been reported that fHBP gene transcription in vitro is regulated by iron availability, with the majority of strains upregulating fHBP gene transcription under iron-rich conditions. The biological relevance of this in vitro phenomenon is unknown, and clonal complexes that actually made less fHBP mRNA under conditions of increased iron in vitro were identified (90). It is also not known how transcription rates correspond to protein expression. Although free iron concentrations in the bloodstream are low, N. meningitidis has access to iron through iron binding proteins such as transferrin, lactoferrin, and hemoglobin. Oriente et al. demonstrated that fHBP expression is under the control of two independent promoters: a bicistronic transcript originating from an upstream gene and a monocistronic transcript from the promoter of fHBP, PfHBP (91). PfHBP contains a putative FNR box motif such that under oxygen limitation, transcription is upregulated from PfHBP in an FNR-dependent manner. This suggests that in microenvironments where oxygen is limiting, such as the bloodstream, N. meningitidis ensures the expression of fHBP, thereby enhancing resistance to complement.
Biological Properties of Monoclonal Antibodies Binding to fHBP on the Bacterial Surface
A number of studies have investigated the properties of monoclonal antibodies directed against fHBP (92–94). Diverse activities are described for these antibodies; some can inhibit fH binding, whereas others facilitate MnB killing either individually or in combination with other monoclonal antibodies (92, 95). Bactericidal killing can be observed with strains that express lower levels of fHBP using a combination of monoclonal antibodies that are synergistic (93). Recent data suggest that antibodies against two different meningococcal proteins, fHBP and neisserial heparin binding antigen (NHBA), can cooperate to induce a bactericidal response (96). A recent study demonstrated that some antibodies have the ability to block fH binding to fHBP and that these antibodies can enhance complement-mediated bactericidal activity when small amounts of fHBP are expressed on the bacterial surface (74). The effect of human IgG subclass on the functional activity of anti-fHBP antibodies was recently examined, and it was shown that IgG3 monoclonal antibodies had higher bactericidal activity than IgG1 and IgG2 antibodies against an MnB isolate, whereas IgG1 antibodies were similar or superior to IgG3 antibodies against a mutant with increased fHBP expression (97). The relevance of these findings for serum bactericidal activity in humans is unclear. However, since vaccination with fHBP will elicit polyclonal antibodies, it is reasonable to propose that multiple fHBP epitopes will be recognized and the complement cascade may be activated even when strains express lower levels of fHBP. In addition, polyclonal anti-fHBP antibodies can prevent the alternative complement pathway inhibitor, hfH, from binding to MnB strains, thus increasing their sensitivity to complement attack.
In addition to studies exploring the mode of bactericidal activity, several analytical approaches have been used to specifically map monoclonal antibody binding sites on fHBP. These include Western blotting, enzyme-linked immunosorbent assay (ELISA), flow cytometry, phage library screening, and nuclear magnetic resonance (NMR) and antibody structure modeling studies (16, 92, 93, 95, 98). Although these studies did not provide information as to whether the antibodies bind linear or conformational epitopes, they helped define the structure of fHBP on the bacterial surface. Figure 6A shows the locations of various monoclonal antibodies that have been mapped by these methods and in combination with structural studies indicating the positioning of fHBP above the bacterial outer membrane. The membrane-anchoring stem of fHBP would not be predicted to be accessible to the host humoral response due to hindrance of binding by the lipooligosaccharide (LOS) layer. fH and monoclonal antibodies do bind to the bacteria, demonstrating that fHBP is both surface exposed and accessible to antibodies (46, 99) (Fig. 6B). Importantly, bactericidal antibodies have been mapped to both the N and C termini of fHBP, highlighting the importance of both regions in eliciting bactericidal antibodies (98). Much of the protein, including the subfamily-defining surface, is oriented toward the extracellular space, where it can be subject to antibody recognition by broadly reactive but largely subfamily-specific antibodies (47, 48).
Fig 6.
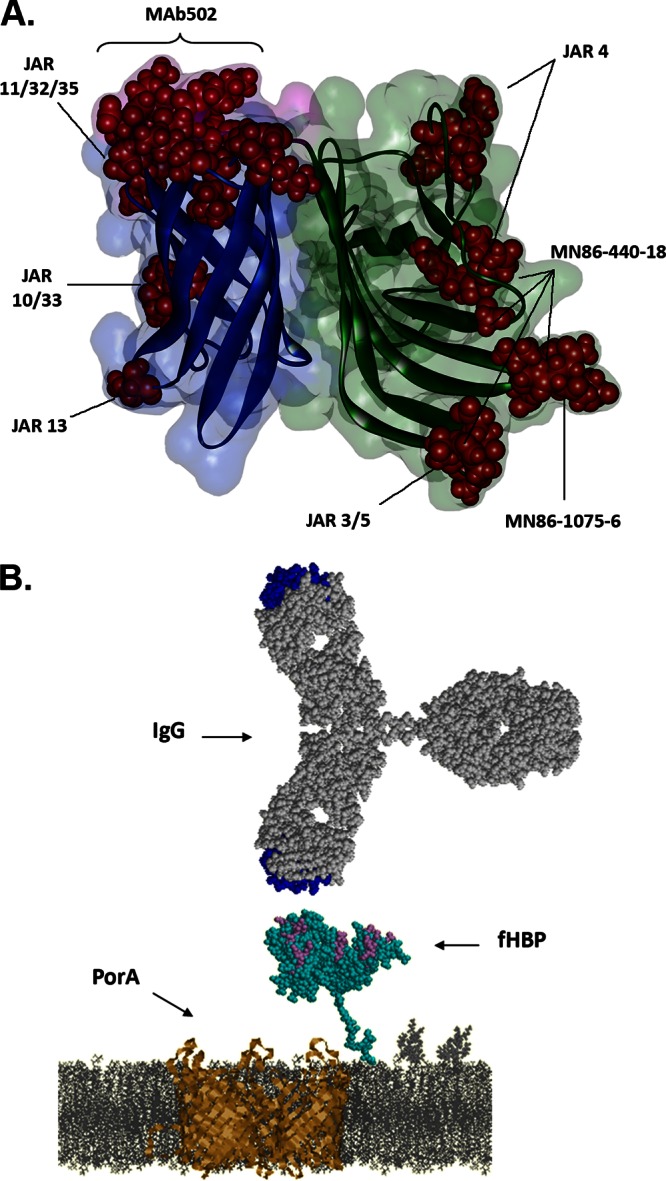
Antibody-fHBP interactions. (A) The binding residues on fHBP of bactericidal antibodies. Published bactericidal monoclonal antibody epitopes are highlighted as red spheres on the secondary structure diagram of B01 fHBP. fHBP domains are colored by domain (green, N-terminal domain; blue, C-terminal domain; pink, linker between the β-structures of two domains), with fH binding residues highlighted in purple. (B) Interaction between surface-exposed fHBP and IgG. Depicted schematically in the cell membrane are fHBP (cyan) and PorA (gold) expressed on the N. meningitidis surface; on the top, an IgG is drawn to scale. The fH binding residues on fHBP and the complementarity-determining regions on IgG are highlighted in purple and blue, respectively.
In Vitro fHBP Surface Expression
The importance of fHBP to N. meningitidis is reflected by the detection of the fHBP gene in essentially all MnB strains examined (29, 33, 37, 44). Of 1,263 U.S. and European MnB invasive strains from a prevalence-based study surveyed for surface expression of fHBP, fewer than 2% did not express fHBP levels above the limit of detection as measured using a universal monoclonal antibody, MN86-994-11, that binds to both subfamilies of fHBP (46). The apparent lack of binding was found to be dependent on the strain and not on the fHBP sequence. Recently, a similar cross-reactive antibody was identified by Vu et al., although only 21 different variants were tested (100). Isogenic fHBP mutants in which the fHBP gene was deleted are more sensitive to killing than the wild-type strains when grown in the presence of human sera (16, 93, 101). A biological explanation as to why fHBP is present in invasive MnB strains is that it prevents alternative pathway complement-dependent killing and/or killing by LL-37 (16, 101). LL-37 is a cathelicidin produced by various immune cells and epithelial cells in the nasopharynx, and it is postulated that fHBP prevents LL-37 from interacting with the bacterial cell surface by electrostatic interactions, as also observed with N. meningitidis capsule and LOS (101, 102).
In Vivo fHBP Surface Expression
As described above, surface expression of fHBP has been demonstrated by in vitro studies. However, there is less information on how the antigen is expressed in vivo. Indirect evidence can be drawn from small-animal infection studies where the host immune system interacts with surface-expressed fHBP in vivo. Studies in mice vaccinated with fHBP and then challenged with MnB showed reduced nasal colonization (103, 104). Passive protection studies in infant rats using antisera against fHBP demonstrated protection against homologous and heterologous strain challenge (105). Monoclonal antibodies to fHBP have also been shown to offer protection in infant rats, confirming that fHBP is expressed and accessible to antibody recognition in vivo (94).
Evidence of in vivo expression in humans comes from serological studies that assessed the presence of antibodies to fHBP in unvaccinated individuals. Jacobsson et al. demonstrated that titers to fHBP in children increase with age, with a brisk rise in titers in 10- to 19-year-olds and a peak in the 30- to 39-year-old age group (106). These observations correlate well with the age-related increases in nasopharyngeal acquisition and carriage of meningococci, with highest meningococcal acquisition being seen between 15 and 24 years of age (107). Titers to fHBP have also been detected in patients who have contracted invasive meningococcal disease. Litt et al. detected antibody reactivity to fHBP in children convalescing after meningococcal disease (27); however, levels of antibody to fHBP prior to infection were not assessed in the study. In contrast, Ala'aldeen et al. determined fHBP titers for subjects with invasive meningococcal disease (IMD) at the time of hospital admission and during early convalescence as well as for healthy subjects with and without meningococcal carriage (108). All patients with IMD had detectable levels of anti-fHBP IgG, with concentrations that increased during the convalescence period, providing strong evidence that fHBP is expressed in vivo during invasive disease. Furthermore, healthy carriers were also found to have anti-fHBP antibodies, at levels similar to those found in invasive disease patients and higher than those in noncarriers (108, 109). Finally, the existence of multiple variants and the antigenic diversity of fHBP, which may be a consequence of immune selection pressure in the human host, further imply that fHBP is expressed in vivo and recognized by the host immune system (32). Altogether, these studies provide evidence that fHBP is expressed in vivo in humans during bacterial colonization and invasive disease.
fHBPs AS ANTIGENS FOR BROADLY PROTECTIVE MnB PROPHYLACTIC VACCINES
Heterogeneity of fHBP Variants Expressed with Common N. meningitidis Epidemiological Markers
Multilocus sequence typing (MLST) of meningococci has been an important tool for the study of the population genetics and epidemiology of meningococcal carriage and disease isolates and in the understanding of the role of hypervirulent lineages (110, 111). However, MLST analysis of >1,000 MnB isolates revealed considerable heterogeneity of fHBP sequence variants within the common clonal complexes associated with invasive disease (29, 46). In addition, all major clonal complexes include strains expressing both fHBP subfamilies. Even the hyperinvasive ST32 complex, which is dominated by one subfamily B variant (B24, variant 1.1), contains at least 27 additional fHBP sequence variants (29). These results were also seen in other smaller, non-prevalence-based studies for MnB and in other N. meningitidis serogroups (32, 112, 113). Hao et al. recently conducted a comparative analysis of 38 Neisseria genomes and found that MLST could not predict virulence gene content or strain phenotype (114). Thus, while MLST remains a useful indicator for classification of strains and for tracking outbreaks, it is not a reliable predictor of the fHBP sequence type, nor is it predictive of whether an MnB strain can be killed by fHBP immune sera (23). As noted by Caugant and Maiden, the selection of vaccine targets requires a knowledge of the molecular epidemiology of the meningococcus, as this informs which targets may achieve broad strain coverage by serum bactericidal antibodies induced by the vaccine (4).
Preclinical Evaluation of the Potential Breadth of Coverage of fHBP-Based Vaccines
fHBP has all of the attributes required for an effective MnB vaccine; it is cell surface exposed, present in >99% of all invasive MnB disease isolates, and expressed in vivo during invasive disease, and it generates protective bactericidal antibodies. Measurement of in vitro bactericidal activity by the SBA is the accepted correlate for protection and as such was used as a surrogate of efficacy for licensure of the MnC and MnACWY glycoconjugate vaccines (115–117). An effective fHBP MnB vaccine must protect not only against strains expressing the fHBP sequence present in the vaccine but also against strains expressing heterologous fHBP sequences. As outlined in “fHBP Nomenclature” above, fHBP can be divided and subdivided into different groups/subgroups on the basis of protein sequence. The relevance of the different subfamilies/variants to breadth of coverage has been assessed by several groups. To understand the breadth of protection, the sequence variation and surface expression of vaccine components expressed by the bacteria have to be understood, as well as the impact on the vaccine formulation on immunogenicity.
fHBP is naturally a lipoprotein, and early preclinical studies by Fletcher et al. clearly demonstrated that lipidated fHBP vaccines were more immunogenic than the same variants expressed without the lipidation tail (21). The breadth of coverage was also assessed in these studies; four fHBP variants from each subfamily were used to generate monovalent immune sera that were tested for serum bactericidal activity against nine isolates expressing heterologous fHBP sequences. It was observed that the subfamily A or B vaccine antigens elicited mainly subfamily-specific responses and that these were cross-protective against strains expressing variants from the same subfamily (21). This led to the hypothesis by the authors that two lipidated fHBPs (one from each subfamily) could be sufficient to develop a vaccine with the potential to provide broad coverage across MnB invasive strains. In the same time frame, Masignani et al. investigated the breadth of coverage of three nonlipidated fHBPs, two representing subfamily A (classified as variants 2 and 3) and one representing subfamily B (classified as variant 1) (Fig. 2A) (20). In these studies, only the vaccine homologous strains were used in the SBA and robust SBA titers were observed against the vaccine homologous strains. No SBA killing against the subfamily B isolate was observed using the subfamily A serum; however, some cross-protection was observed for the subfamily B vaccines and the subfamily A strains. Coverage studies for the nonlipidated variants have been further expanded and have demonstrated that as with lipidated proteins, SBA protection is subfamily specific; however, the breadth of coverage within the subfamily is not sufficiently covered when a single nonlipidated variant is used in the vaccine (20, 31, 113, 118).
The hypothesis that two lipidated proteins would be sufficient to provide broad coverage was further assessed by SBA using either rabbit or human immune serum generated using the lipidated variants B01 and A05 (23). One hundred MnB clinical isolates that represented the diversity of fHBP sequences were tested. The vast majority of the isolates were susceptible to both the rabbit and human antisera generated to the bivalent lipidated vaccine (23). These studies and the earlier epidemiological analyses (29) show that there is no link between the susceptibility of a strain to SBA and its particular fHBP protein or gene sequence provided that the antibodies were generated with a vaccine containing a lipidated fHBP variant from each subfamily. The factor that best predicted the susceptibility of MnB strains to killing in the SBA was in vitro fHBP surface expression. MnB strains show a wide range of fHBP surface expression, from below the limit of detection (mean fluorescence intensity [MFI] of <100) to very high levels (MFI of >30,000) (23, 46). In a panel of 100 diverse MnB strains, only 13 strains were not killed in the SBA using rabbit bivalent immune sera. All 13 strains expressed very low levels of fHBP in vitro. Forty-five diverse subfamily A and B strains were also tested in the SBA using adult human bivalent immune sera. Similar to the results with rabbit sera, the human sera could not kill strains that expressed very low levels of fHBP in vitro but could kill strains of many different fHBP sequence variants showing a wide range of fHBP surface expression (23). The bivalent fHBP vaccine induced immune sera in adults that were able to kill a broad range of variants, representing 84.5% of variants that cause IMD (23, 29) (Fig. 7). Another group also observed decreased killing against strains expressing low fHBP levels using mouse antisera (31). A further study from the same laboratory constructed isogenic mutants with different levels of fHBP expression and found that mouse fHBP antisera were not bactericidal against a mutant with low fHBP expression (39). Thus, the in vitro surface expression level of fHBP is a good predictor for whether a strain may be susceptible in the in vitro SBA.
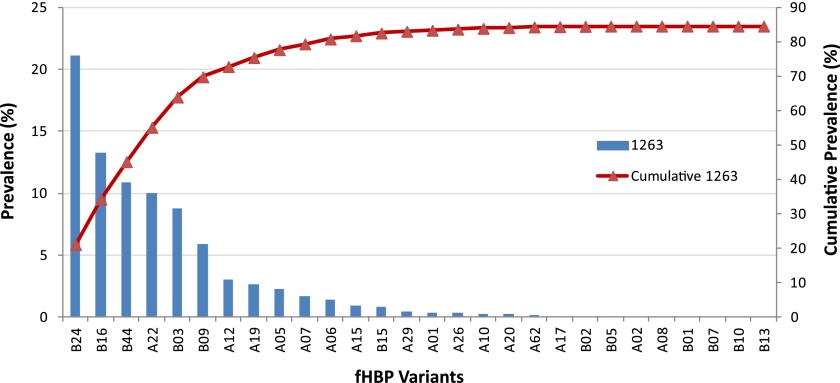
Fig 7.
Demonstration of the breadth of coverage afforded by the bivalent fHBP vaccine: cumulative coverage of fHBP variants. Variants shown to be killed by anti-fHBP bivalent immune sera from adults (23) are indicated on the x axis. The prevalence of these variants in a systematically collected set of 1,263 invasive MnB disease isolates from the United States and Europe (29) is shown on the left y axis, with the cumulative prevalence indicated by the red line (right y axis).
Several assays have been developed with the potential aim of estimating the breadth of coverage in conjunction with SBA. The meningococcal antigen surface expression (MEASURE) assay monitors fHBP surface expression and accessibility on intact bacteria by flow cytometry (119). This assay utilizes a broadly cross-reactive monoclonal antibody to a conserved epitope that binds to all fHBP variants regardless of subfamily. The MEASURE assay determines both fHBP surface expression and accessibility of fHBP to antibodies in the presence of other surface antigens, as it utilizes intact bacteria. The reactivity of the monoclonal antibody to the surface of meningococci correlated (correlation coefficient = 0.81) with the reactivity observed with polyclonal bivalent anti-fHBP sera and provided the ability to supply a more homogeneous reagent over time than with polyclonal sera (46). The recently developed meningococcal antigen typing system (MATS) assay evaluates total protein from a cell extract (120). The MATS assay is a sandwich ELISA that uses lysed bacterial extracts and rabbit polyclonal antibodies against fHBP, NHBA, or NadA. The MATS assay was found to correlate with susceptibility of MnB strains in the SBA. MnB strains that exceeded a threshold value for any of the three vaccine antigens had greater than an 80% chance of being killed in the SBA. However, both of these assays have limitations, as they measure in vitro expression levels of fHBP and do not provide an indication as to how different populations or individual subjects will respond to the vaccine. (This will be further reviewed in “Estimation of Vaccine Efficacy for fHBP-Containing Vaccines” below.) Therefore, the prediction of effectiveness needs to be done with caution and definitely has to be fully substantiated with large panels of epidemiologically relevant MnB strains in hSBA using individual subject sera, as only a positive response in hSBA correlated with protection (3). Ultimately, the data on how effective MnB vaccines will be in providing broad protection against meningococcal disease will be provided by surveillance studies monitoring the disease incidence over time, as with the implementation of the meningococcal conjugate vaccines (121, 122).
Estimation of Vaccine Efficacy for fHBP-Containing Vaccines
Seminal studies by Goldschneider et al. in the 1960s provided a link between serum bactericidal antibody titers and natural protection against meningococcal disease (3). As humans get older, meningococcal carriage rates increase, resulting in increased serum bactericidal antibody levels over time. Goldschneider et al. identified that humans with an hSBA titer of ≥4 were protected from meningococcal disease. Although meningococcal disease is devastating, the disease rates are generally too low to permit clinical efficacy testing with a disease endpoint. Meningococcal polysaccharide-based vaccines were therefore licensed based on serum bactericidal antibody levels as measured in SBA with either rabbit (rSBA) or human (hSBA) complement (123–125). True vaccine efficacy can then be monitored by postlicensure surveillance and is often comparable to the initial SBA rates determined during phase 3 clinical testing (126). Assessment of vaccine efficacy for surface-expressed proteins is much more complex than that for polysaccharide-based vaccines because of sequence heterogeneity, antigen accessibility, and antigen surface expression levels, which can vary widely among disease-causing MnB strains. The selection of clinical test strains used in hSBA to evaluate fHBP-containing vaccines must demonstrate that broad protection is achieved against epidemic and endemic invasive disease. The use of hSBA clinical test strains that carry only the homologous antigen sequence is not adequate, as they may not provide an indication as to whether the vaccine can be effective against more divergent sequences, as was observed with PorA-based vaccines (11, 14). A more representative, nonbiased approach that addresses several factors, including expression level and sequence variation, should be taken. It is also important to validate the approach by using both randomly selected and representative disease isolates for SBA. Thus, if strains expressing heterologous vaccine antigens at levels reflective of the majority of invasive MnB strains are used, then the efficacy of the MnB vaccines would likely represent the efficacy against the diversity of MnB invasive disease strains.
Development of fHBP Vaccines for Broad Protection against MnB Disease
Vaccine development for the prevention of MnB disease has proved to be a complex problem and has been under investigation for many decades. Promising candidates such as porin-based vaccines have fallen short due to the antigen sequence-specific protection that they afford. An MnB vaccine containing fHBP recently has been licensed, and there are two others, including one in late-stage clinical testing, that contain fHBP components that are intended to provide broad protection against invasive MnB disease. Two of the vaccines contain recombinant fHBP components produced in E. coli, while a third approach utilizes OMVs that have been engineered to express different fHBP sequences and different levels of fHBP expression. A challenge for all these programs will be the postlicensure demonstration of broad MnB coverage.
The 4CMenB vaccine (Novartis), which has been recently licensed in the European Union as Bexsero, is intended for individuals 2 months of age and older and is composed of 3 recombinant proteins expressing 5 MnB antigens (rMenB) together with an OMV component (MeNZB) which provides PorA coverage against the New Zealand outbreak strain NZ 98/254 (P1.7-2,4). Two of the recombinant proteins in the 4CMenB vaccine are fusion proteins containing sequence from four bacterial antigens (GNA2091 fused to fHBP and NHBA fused to GNA1030); the third recombinant protein is NadA (34). The fHBP included in this vaccine is a nonlipidated subfamily B (variant 1.1/B24) protein variant (34), a variant detected in about 17% of MnB strains collected in an MnB prevalence study of 1,263 isolates across Europe and the United States (29) and in 30% in a U.S.-only-based prevalence study (37). The 4CMenB vaccine contains 50 μg each of fHBP, NHBA, and NadA with 25 μg of the OMV.
The bivalent fHBP vaccine (rLP2086) (Pfizer) is designed around the premise that two fHBPs, one from each subfamily, A and B (B01 and A05), in their native lipidated form, are sufficient to provide broad coverage against MnB disease-causing strains (23).
Serum bactericidal data from clinical trials are available for both these vaccines. Both vaccine trials measure in vitro bactericidal activity with human complement (hSBA). A comparison of potential vaccine efficacy is difficult due to differences in vaccine composition and the fact that the numbers and types of MnB strains (and associated fHBP sequence variants) used to demonstrate the vaccine responses are very different. Table 1 provides details on the different MnB test strains used in the clinical trials for Novartis and Pfizer and the percentages of participants who responded with a positive hSBA titer against the test strain at 1 month after dose 2 and 1 month after dose 3. It is important to note that the immune responses obtained are specific for the age groups with which they are tested and cannot be compared.
TABLE 1.
MnB test strains used in clinical trialsa
| Company and target population | Trial details (age of participants, no. of participants, ClinicalTrials.gov identifier) | Strain name | Neisseria.org ID | Groupb | Sequence IDc | % Homology to fHBP vaccine antigend | % of responders after dosee: |
|
|---|---|---|---|---|---|---|---|---|
| 2 | 3 | |||||||
| Novartis | ||||||||
| Infants | 2 mo, 147, NCT00381615 | H44/76 | 1 | B, 1 | B24, 1.1 | 100 | 95 | 87f |
| M01240355 | 31 | A, 3 | A47, 3.31 | 65.4 | 0 | 0 | ||
| M01240101 | 15 | B, 1 | B44, 1.15 | 85.9 | 28 | 47 | ||
| 6–8 mo, 60, NCT00433914 | H44/76-SL | 1 | B, 1 | B24, 1.1 | 100 | 100 | 100g | |
| M01240101 | 15 | B, 1 | B44, 1.15 | 85.9 | 67 | 70 | ||
| M01240355 | 31 | A, 3 | A47, 3.31 | 65.4 | 7 | 17 | ||
| 2 mo, 1885, NCT00721396 | 44/76-SL | 1 | B, 1 | B24, 1.1 | 100 | NDh | 99i | |
| Adolescents | 11–17 yr, 1631, NCT00661713 | 44/76 | 1 | B, 1 | B24, 1.1 | 100 | 99 | 100j |
| 1000 | 25 | A, 2 | A15, 2.25 | 68.1 | 85 | 86k | ||
| 44/76 | 1 | B, 1 | B24, 1.1 | 100 | 100 | 100 | ||
| 95N477 | 22 | A, 2 | A10, 2.22 | 69.3 | 7 | 7 | ||
| Adults | 18–40 yr, 70 | M01240013 | 19 | A, 2 | A22, 2.19 | 72.4 | 81 | 79 |
| M1390 | 14 | B, 1 | B03, 1.14 | 91.8 | 96 | 96 | ||
| M3812 | 12 | B, 1 | B06, 1.12 | 93.7 | 82 | 79 | ||
| M4105 | 4 | B, 1 | B16, 1.4 | 96.1 | 100 | 100 | ||
| M4458 | 25 | A, 2 | A15, 2.25 | 68.1 | 79 | 70 | ||
| M6190 | 6 | B, 1 | B10, 1.6 | 94.5 | 39 | 43 | ||
| 18–50 yr, 54, NCT00560313 | 44/76-SL | 1 | B, 1 | B24, 1.1 | 100 | 100 | 97l | |
| Pfizer | ||||||||
| Toddlers | 18–36 mo, 99, NCT00387569 | PMB1745 | 45 | A, 3 | A05, 3.45 | 100 | 59 | 83m |
| PMB17 | 87 | B, 1 | B02, 1.87 | 92 | 38 | 89 | ||
| PMB663 | 19 | A | A22, 2.19 | 88.9 | 0 | 44 | ||
| PMB265 | 13 | B, 1 | B09, 1.13 | 88.1 | 0 | 11 | ||
| PMB3556 | 1 | B, 1 | B24, 1.1 | 86.2 | 7 | 20 | ||
| PMB1168 | 45 | A, 3 | A05, 3.45 | 100 | ND | 100 | ||
| PMB147 | 15 | B, 1 | B44, 1.15 | 91.6 | ND | 94 | ||
| PMB1256 | 14 | B, 1 | B03, 1.14 | 90.8 | ND | 82 | ||
| PMB1321 | 19 | A | A22, 2.19 | 88.9 | ND | 100 | ||
| Children and adolescents | 8–14 yr, 127, NCT00387725 | PMB1745 | 45 | A, 3 | A05, 3.45 | 100 | 86 | 91n |
| PMB663 | 19 | A | A22, 2.19 | 88.9 | 53 | 59 | ||
| PMB3556 | 1 | B, 1 | B24, 1.1 | 86.2 | 29 | 60 | ||
| PMB17 | 87 | B, 1 | B02, 1.87 | 92 | 69 | 98 | ||
| PMB265 | 13 | B, 1 | B09, 1.13 | 88.1 | 14 | 47 | ||
| Adolescents | 11–18 yr, 539, NCT00808028 | PMB3302 | 180 | A, 3 | A04, 3.180 | 96.6 | 97 | 98o |
| PMB1256 | 14 | B, 1 | B03, 1.14 | 90.8 | 30 | 68 | ||
| PMB2001 | 187 | A, 3 | A56, 3.187 | 98.1 | 97 | 96 | ||
| PMB2707 | 15 | B, 1 | B44, 1.15 | 91.6 | 70 | 87 | ||
| PMB1321 | 19 | A, 2 | A22, 2.19 | 88.9 | ND | 88 | ||
| PMB2948 | 1 | B, 1 | B24, 1.1 | 86.2 | ND | 84 | ||
| PMB1745 | 45 | A, 3 | A05, 3.45 | 100 | 95 | 97 | ||
| PMB17 | 87 | B, 1 | B02, 1.87 | 99 | 79 | 90 | ||
| Young adults | 18–25 yr, 103, NCT00297687 | PMB1745 | 45 | A, 3 | A05, 3.45 | 100 | 91 | 100p |
| PMB17 | 87 | B, 1 | B02, 1.87 | 99 | 82 | 88 | ||
| PMB267 | 49 | A, 2 | A17, 2.49 | 88.1 | 100 | 100 | ||
| PMB265 | 13 | B, 1 | B09, 1.13 | 88.1 | 97 | 96 | ||
| PMB663 | 19 | A | A22, 2.19 | 88.9 | 64 | 100 | ||
| PMB3556 | 45 | A, 3 | A05, 3.45 | 100 | 81 | 100 | ||
| Adults | 18–40 yr, 60, NCT00780806 | PMB1745 | 45 | A, 3 | A05, 3.45 | 100 | 75 | 94q |
| PMB17 | 87 | B, 1 | B02, 1.87 | 99 | 70 | 94 | ||
| PMB1321 | 19 | A, 2 | A22, 2.19 | 88.9 | 75 | 94 | ||
| PMB2707 | 15 | B, 1 | B44, 1.15 | 91.6 | 83 | 94 | ||
| PMB2948 | 1 | B, 1 | B24, 1.1 | 86.2 | 72 | 81 | ||
Strains that express the 4CMenB vaccine homologous PorA or NadA protein are not included in this table, which focuses on SBA responses to fHBP.
Pfizer subfamily, Novartis variant.
Pfizer variant number, Novartis subvariant number.
B24 (1.1) for Novartis, A05 (3.45) and B01 (1.55) for Pfizer.
Percentage of responders at 1 month after the indicated dose.
Percentage of participants with hSBA titers of ≥4 (77).
ND, not determined.
Percentage of participants with hSBA titers of ≥1:4 (83).
Percentage of participants with hSBA titers of ≥1:5 (78).
Percentage of participants with hSBA titers of ≥4 (82).
Percentage of participants with hSBA titers of ≥4 (127).
Percentage of participants with hSBA titers of ≥4 (79).
Percentage of participants with ≥4-fold rise in hSBA titer (128).
Percentage of participants with hSBA titers of ≥1:4 (129).
Percentage of participants with hSBA titers equal to or greater than the limit of quantitation (LOQ) (80).
Percentage of participants with hSBA titers of ≥1:4 (81).
Percentage of participants with hSBA titers of ≥1:4 (130).
The clinical studies that support the Novartis 4CMenB vaccine have been reviewed by Gorringe and Pajon (131). The 4CMenB vaccine has been studied in infants, adolescents, and adults. Novartis has licensed this vaccine by using four reference strains to determine bactericidal activity to each of the four vaccine antigens. For example, N. meningitidis strain 44/76 measures the individual contribution of the anti-fHBP response, NZ 98/254 the anti-PorA response, and 5/99 the anti-NadA response; therefore, each strain is specific for its respective antigens (127). In phase 1 (127) and 2 (79) clinical trials with healthy adults, the proportions of subjects with an hSBA titer of ≥1:4 at 1 month after dose 3 using a strain with the homologous fHBP variant were 96% and 100%, respectively. Moreover, a phase 2b/3 study in adolescents aged 11 to 17 years was able to demonstrate that similar high responses against the fHBP reference strain could be observed after two doses of the 4CMenB vaccine (82). hSBA data are available from some clinical trials and provide an indication of the breadth of coverage afforded by this vaccine by examining additional strains expressing heterologous fHBP variants. Toneatto et al. reported hSBA response rates of 7 to 100% for these fHBP heterologous strains using sera obtained from a phase 1 study in adults (n = 27) (127). Similar observations have been made for younger populations (infants and toddlers), with a trend for reduced immunogenicity in the very young. For example, 100% of 6- to 8-month-old infants vaccinated with 4CMenB had hSBA titers greater than or equal to 1:4 against the homologous fHBP strain, while a strain expressing the heterologous fHBP variant 1.15 (B44) had a responder rate of 70% (83). In a phase 2 clinical trial of healthy 2-month-old infants, 87% had an hSBA titer of ≥4 after the third dose against strains expressing the antigen homologous to the vaccine antigen, compared to 0 to 47% of subjects having SBA titers of ≥4 against strains with the heterologous fHBP variants 1.14 (B03), 1.15 (B44), and 1.4 (B16) (77). Gossger et al. demonstrated that response rates do not seem to be impacted by the concomitant administration of routine infant vaccines, with over 99% of vaccinated infants achieving an hSBA titer of 1:5 or greater against a strain expressing the fHBP variant homologous to the vaccine antigen (78).
The clinical development path for the Pfizer bivalent fHBP vaccine differs from that for the Novartis vaccine, as no immunogenicity data have been reported for infants. A small clinical study of the Pfizer vaccine in infants (132) yielded fever rates that, while not dissimilar to those observed with the Novartis vaccine (77, 78, 83, 132), were nonetheless higher than desired given that meningococcal serogroup B disease in young infants occurs predominantly in neonates. Accordingly, Pfizer has noted its intention to develop its vaccine for use in adolescents (133). Adolescents experience high rates of meningococcal B disease (134) and are important carriers of meningococci (40). In addition, immunization campaigns with meningococcal serogroup C vaccines in children and adolescents have resulted in herd immunity effects evident in infants and other unvaccinated populations (135, 136).
Published studies are available that describe the clinical trial results with both the initial (81, 128, 129) and final (80, 130, 137) formulations of Pfizer's bivalent rLP2086 vaccine. However, a comprehensive review of clinical findings for Pfizer's bivalent rLP2086 vaccine is not yet available. Therefore, a brief summary is provided here. Initial dose ranging studies with an initial formulation of the bivalent rLP2086 vaccine were conducted in adults (81), adolescents (129), and toddlers (128) using dose levels of 20 μg, 60 μg, and 200 μg of vaccine. An additional dose ranging phase 2 study with the final vaccine formulation (60 μg, 120 μg, and 200 μg) was conducted in adults (137) and adolescents (80). Based on the hSBA immunogenicity and tolerability profiles observed in the adolescent phase 2 study with the final vaccine formulation, the 120-μg dose level was selected for further clinical development (80). The Pfizer hSBA testing strategy was to select four primary strains (two from each subfamily) that are representative of fHBP diversity and prevalence and have low and medium fHBP surface expression. Additional exploratory strains were chosen for their divergence from the primary hSBA strains (strain information is listed in Table 1). For the early studies with the initial vaccine formulation in young adults at the 60-μg dose, the percentage of participants with hSBA titers of ≥1:4 was 60% after dose 2, rising to 98% after dose 3. For the highest dose level tested (200 μg) the response rates were 91% for the vaccine homologous strain after dose 2, rising to 100% after dose 3 (81). Five heterologous MnB strains were also tested in this study; the response rates after dose 3 ranged from 77 to 100% at the 60-μg dose and from 88 to 100% at the 200-μg dose. In another study in adults with the final bivalent rLP2086 vaccine at the 120-μg dose, the response rates after three doses were 81 to 94%, irrespective of whether the hSBA strain expressed homologous or heterologous fHBP (130). In adolescents and children over the age of 8 years, the response rate after dose 3 at the 200-μg dose was 91% for the vaccine homologous strain and ranged from 47 to 98% for vaccine heterologous strains (129). In another adolescent study using the final vaccine formulation and dose (120 μg), the response rate after two doses was 95% for the homologous strain and ranged from 30 to 97% for strains with heterologous variants (80). These response rates rose after 3 doses, to 68 to 98% for heterologous MnB strains and 97% for the homologous strain. These data highlight the importance of testing heterologous fHBP strains in the hSBA to understand the true MnB vaccine coverage. In addition to results for adults and adolescents, results from a clinical trial with the initial formulation of the bivalent rLP2086 vaccine conducted in healthy toddlers aged 18 to 36 months with evaluation of a 4-fold rise in hSBA titers have been published (128). The 200-μg response rate after dose 3 ranged from 44 to 100% for six heterologous MnB strains. Lower responses in the toddlers were observed against a B09 strain (variant 1.13) (11%) and a B24 strain (variant 1.1) (20%) (128). Furthermore, when different strains expressing the same variant were tested, different responses could be observed; for instance, an A22 (variant 2.19) strain had response rates of 44%, yet a second A22 (variant 2.19) strain had rates of 100%, emphasizing the complexity of monitoring the breadth of coverage.
Another approach to an MnB vaccine is to overexpress fHBP in OMVs. Phase 1 studies for an OMV-based vaccine that combines OMVs from three different strains that have been engineered to overexpress MnB antigens, including different fHBP variants, are in progress. Preclinical studies demonstrated that the vaccine generated serum bactericidal antibodies against homologous MnB strains (W. D. Zollinger, M. Donets, B. L. Brandt, B. Ionin, E. E. Moran, D. Schmiel, V. Pinto, M. Fisseha, J. Labrie, R. Marques, and P. Keiser, presented at the 16th International Pathogenic Neisseria Conference, Rotterdam, The Netherlands, 2008), and initial clinical findings indicate that the vaccine is safe and well tolerated (138). The same group has generated another genetically detoxified native OMV vaccine with overexpressed fHBP, stabilized OpcA, and expression of a second PorA. This vaccine has completed phase 1 clinical trials and was found to be safe and effective at generating cross-reactive bactericidal activity to different strains (139, 140). Recently, Koeberling et al. tested an OMV-based vaccine that expressed penta-acetylated LOS and overexpressed lipidated fHBP variants 1.1 (B24) and 2.77 (subfamily A) in infant rhesus macaque monkeys (141). The four vaccinated animals had serum bactericidal titers of ≥1:4 against nine diverse heterologous strains.
CONCLUDING REMARKS
The fHBP gene is found in all N. meningitidis genomes sequenced to date, in >99% of clinical MnB strains examined to date, and in MnB carriage isolates, suggesting that fHBP is a critical protein for this pathogen. In addition to MnB, other serogroups (A, C, W135, X, and Y) have been shown to contain fHBP (36, 38). The recent finding that fHBP gene transcription is expressed from two separate promoters, which are regulated by both oxygen and iron availability, further suggests the important role of fHBP in the virulence and pathogenesis of N. meningitidis (91). Although some sequence variation is exhibited by fHBP (32), the protein still maintains conserved regions for binding human fH and reactivity to protective immune sera. Taken together, all of the above information supports the classification of fHBP as a virulence factor involved in infection and possibly carriage.
fHBP is a vaccine candidate with great promise for the prevention of serogroup B meningococcal disease caused by diverse invasive MnB strains. This is exemplified by the following: (i) The fHBP gene is found in almost all MnB disease isolates, (ii) fHBP is expressed on the bacterial surface, (iii) protein sequences of fHBP can be divided into two distinct subfamilies containing a conserved subfamily-specific surface, (iv) fHBP is important for survival of N. meningitidis in ex vivo models, and (v) humans immunized with an fHBP-containing vaccine generate broadly protective bactericidal antibodies. In addition, fHBP-containing vaccines may have the opportunity to reduce MnB carriage and may elicit protection against meningococcal disease caused by other serogroups, since these strains also contain the fHBP gene.
UNCERTAINTIES
fHBP has great potential as a vaccine for the prevention of serogroup B meningococcal disease, yet several uncertainties remain.
Who Should Be Vaccinated?
While the disease burden in infants is high, it can be difficult to induce robust, broad, and sustained immune responses in the very young. This has now been observed for PorA, OMV, and the new generation of MnB vaccines (15, 77, 128, 142, 143). Though the incidence of serogroup B disease is high in infants, there are more cases in the adolescent and young adult group combined in the United States and most of Europe (134). In addition, adolescents clearly have a role in the carriage and transmission of meningococcal disease, and therefore vaccination of this age group may be important in both the control of outbreaks and overall disease prevention (40). The two advanced fHBP vaccines both induce robust responses against homologous strains in this age group, and if they are demonstrated to interrupt carriage, they may also have a profound effect on disease prevention in infants. However, whether the height of the immune response and the breadth of coverage will be sufficient to interrupt this transmission will be uncertain until postlicensure surveillance studies have been conducted. Another challenge for this population is the reduced rate of compliance to obtain a full multidose regimen (144).
Will These Vaccines Interrupt Carriage?
It is now well established that polysaccharide-based vaccines can interrupt carriage; however, the mechanism of action of anti-fHBP antibodies differs from that of both anticapsule and anti-PorA antibodies, which function strictly via the classical complement pathway. Antibodies to fHBP can block hfH binding and/or induce bactericidal activity, thereby utilizing a combination of the alternative and complement pathways (74).
Will the Vaccines Have Different Efficacy in the Field?
Preclinical data obtained for animals have indicated that lipoproteins induce more robust bactericidal responses, yet how these results will translate to human populations is uncertain (21) and cannot be assessed in the absence of head-to-head clinical studies. It is also unclear whether the MATS and MEASURE assays can predict individual or population coverage, as they both measure only fHBP expression levels, which can predict whether a strain is killed in hSBA but do not predict vaccine responses in individuals or a population, unlike the hSBA response, the true correlate of protection. Therefore, ongoing epidemiological surveillance will be required to confirm vaccine effectiveness and monitor sustained effectiveness within immunized populations.
ACKNOWLEDGMENTS
We thank Emilio Emini, Bruce Green, Joseph Eiden, Laura York, Robert Donald, Li Hao, John Perez, Judith Abselon, Tom Jones, and Kena Swanson for invaluable discussions and comments and Alexey Gribenko and Dawn DeThomas for help with the artwork (all are employees of Pfizer Inc.).
A.S.A., L.K.M., G.W.Z., and K.U.J. are employees of Pfizer Inc., and R.J.Z., S.L.L., E.M., and S.K.H. were previously employed by Pfizer Inc. at the time of manuscript development. As such, all authors acknowledge that they have received payments from Pfizer Inc. in the form of salaries and have either in the past owned or currently own Pfizer Inc. stock. Pfizer Inc. is currently developing a vaccine for the prevention of N. meningitidis serogroup B disease.
This paper was written by Pfizer research scientists and as such was financed by Pfizer.
REFERENCES
- 1. Stephens DS. 2007. Conquering the meningococcus. FEMS Microbiol. Rev. 31:3–14 [DOI] [PubMed] [Google Scholar]
- 2. Wilder-Smith A. 2009. Meningococcal vaccines: a neglected topic in travel medicine? Expert Rev. Vaccines 8:1343–1350 [DOI] [PubMed] [Google Scholar]
- 3. Goldschneider I, Gotschlich EC, Artenstein MS. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caugant DA, Maiden MCJ. 2009. Meningococcal carriage and disease—population biology and evolution. Vaccine 27:B64–B70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Claus H, Maiden MC, Wilson DJ, McCarthy ND, Jolley KA, Urwin R, Hessler F, Frosch M, Vogel U. 2005. Genetic analysis of meningococci carried by children and young adults. J. Infect. Dis. 191:1263–1271 [DOI] [PubMed] [Google Scholar]
- 6. Stephens DS. 1999. Uncloaking the meningococcus: dynamics of carriage and disease. Lancet 353:941–942 [DOI] [PubMed] [Google Scholar]
- 7. Healy CM, Baker CJ. 2005. The future of meningococcal vaccines. Pediatr. Infect. Dis. J. 24:175–176 [DOI] [PubMed] [Google Scholar]
- 8. Knuf M, Baine Y, Bianco V, Boutriau D, Miller JM. 2012. Antibody persistence and immune memory 15 months after priming with an investigational tetravalent meningococcal tetanus toxoid conjugate vaccine (MenACWY-TT) in toddlers and young children. Hum. Vaccin. Immunother. 8:866–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. 2001. Meningococcal disease. N. Engl. J. Med. 344:1378–1388 [DOI] [PubMed] [Google Scholar]
- 10. Finne J, Leinonen M, Makela PH. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet ii:355–357 [DOI] [PubMed] [Google Scholar]
- 11. Holst J, Martin D, Arnold R, Huergo CC, Oster P, O'Hallahan J, Rosenqvist E. 2009. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine 27:B3–B12 [DOI] [PubMed] [Google Scholar]
- 12. Wyle FA, Artenstein MS, Brandt BL, Tramont EC, Kasper DL, Altieri PL, Berman SL, Lowenthal JP. 1972. Immunologic response of man to group B meningococcal polysaccharide vaccines. J. Infect. Dis. 126:514–521 [DOI] [PubMed] [Google Scholar]
- 13. Bruge J, Bouveret-Le Cam N, Danve B, Rougon G, Schulz D. 2004. Clinical evaluation of a group B meningococcal N-propionylated polysaccharide conjugate vaccine in adult, male volunteers. Vaccine 22:1087–1096 [DOI] [PubMed] [Google Scholar]
- 14. Tappero JW, Lagos R, Maldonado Ballesteros A, Plikaytis B, Williams D, Dykes J, Gheesling LL, Carlone GM, Hoiby EA, Holst J, Nokleby H, Rosenqvist E, Sierra G, Campa C, Sotolongo F, Vega J, Garcia J, Herrera P, Poolman JT, Perkins BA. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 281:1520–1527 [DOI] [PubMed] [Google Scholar]
- 15. Cartwright K, Morris R, Rumke H, Fox A, Borrow R, Begg N, Richmond P, Poolman J. 1999. Immunogenicity and reactogenicity in UK infants of a novel meningococcal vesicle vaccine containing multiple class 1 (PorA) outer membrane proteins. Vaccine 17:2612–2619 [DOI] [PubMed] [Google Scholar]
- 16. Madico G, Welsch JA, Lewis LA, McNaughton A, Perlman DH, Costello CE, Ngampasutadol J, Vogel U, Granoff DM, Ram S. 2006. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J. Immunol. 177:501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schneider MC, Exley RM, Chan H, Feavers I, Kang Y-H, Sim RB, Tang CM. 2006. Functional significance of factor H binding to Neisseria meningitidis. J. Immunol. 176:7566–7575 [DOI] [PubMed] [Google Scholar]
- 18. Bernfield L, Fletcher LD, Howell A, Farley JE, Zagursky R, Knauf M, Zlotnick G. 2002. Identification of a novel vaccine candidate for group B Neisseria meningitidis, p 116 Proc. 13th Int. Pathog. Neisseria Conf., Oslo, Norway [Google Scholar]
- 19. Farley J, Fletcher L, Bernfield L, Howell A, Knauf M, Weise P, Xie X, Zhang Y. 2002. Characterization, cloning and expression of different subfamilies of the ORF 2086 gene from Neisseria meningitidis, p 124 Proc. 13th Int. Pathog. Neisseria Conf., Oslo, Norway [Google Scholar]
- 20. Masignani V, Comanducci M, Giuliani MM, Bambini S, Adu-Bobie J, Arico B, Brunelli B, Pieri A, Santini L, Savino S, Serruto D, Litt D, Kroll S, Welsch JA, Granoff DM, Rappuoli R, Pizza M. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J. Exp. Med. 197:789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fletcher LD, Bernfield L, Barniak V, Farley JE, Howell A, Knauf M, Ooi P, Smith RP, Weise P, Wetherell M, Xie X, Zagursky R, Zhang Y, Zlotnick GW. 2004. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect. Immun. 72:2088–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giuliani MM, Adu-Bobie J, Comanducci M, Arico B, Savino S, Santini L, Brunelli B, Bambini S, Biolchi A, Capecchi B, Cartocci E, Ciucchi L, Di Marcello F, Ferlicca F, Galli B, Luzzi E, Masignani V, Serruto D, Veggi D, Contorni M, Morandi M, Bartalesi A, Cinotti V, Mannucci D, Titta F, Ovidi E, Welsch JA, Granoff D, Rappuoli R, Pizza M. 2006. A universal vaccine for serogroup B meningococcus. Proc. Natl. Acad. Sci. U. S. A. 103:10834–10839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jiang HQ, Hoiseth SK, Harris SL, McNeil LK, Zhu D, Tan C, Scott AA, Alexander K, Mason K, Miller L, DaSilva I, Mack M, Zhao XJ, Pride MW, Andrew L, Murphy E, Hagen M, French R, Arora A, Jones TR, Jansen KU, Zlotnick GW, Anderson AS. 2010. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine 28:6086–6093 [DOI] [PubMed] [Google Scholar]
- 24. Frasch CE, Borrow R, Donnelly J. 2009. Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease. Vaccine 27:B112–B116 [DOI] [PubMed] [Google Scholar]
- 25. Pizza M, Scarlato V, Masignani V, Giuliani MM, Arico B, Comanducci M, Jennings GT, Baldi L, Bartolini E, Capecchi B, Galeotti CL, Luzzi E, Manetti R, Marchetti E, Mora M, Nuti S, Ratti G, Santini L, Savino S, Scarselli M, Storni E, Zuo P, Broeker M, Hundt E, Knapp B, Blair E, Mason T, Tettelin H, Hood DW, Jeffries AC, Saunders NJ, Granoff DM, Venter JC, Moxon ER, Grandi G, Rappuoli R. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287:1816–1820 [DOI] [PubMed] [Google Scholar]
- 26. Wu HC. 1996. Biosynthesis of lipoproteins, p 1005–1014 In Neidhardt FC, et al. (ed), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed, vol 1 ASM Press, Washington, DC [Google Scholar]
- 27. Litt D, Savino S, Beddek A, Comanducci M, Sandiford C, Stevens J, Levin M, Ison C, Pizza M, Rappuoli R, Kroll JS. 2004. Putative vaccine antigens from Neisseria meningitidis recognized by serum antibodies of young children convalescing after meningococcal disease. J. Infect. Dis. 190:1488–1497 [DOI] [PubMed] [Google Scholar]
- 28. Schwarz R, Seibel PN, Rahmann S, Schoen C, Huenerberg M, Muller-Reible C, Dandekar T, Karchin R, Schultz J, Muller T. 2009. Detecting species-site dependencies in large multiple sequence alignments. Nucleic Acids Res. 37:5959–5968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murphy E, Andrew L, Lee KL, Dilts DA, Nunez L, Fink PS, Ambrose K, Borrow R, Findlow J, Taha MK, Deghmane AE, Kriz P, Musilek M, Kalmusova J, Caugant DA, Alvestad T, Mayer LW, Sacchi CT, Wang X, Martin D, von Gottberg A, du Plessis M, Klugman KP, Anderson AS, Jansen KU, Zlotnick GW, Hoiseth SK. 2009. Sequence diversity of the factor H binding protein vaccine candidate in epidemiologically relevant strains of serogroup B Neisseria meningitidis. J. Infect. Dis. 200:379–389 [DOI] [PubMed] [Google Scholar]
- 30. Beernink PT, Granoff DM. 2009. The modular architecture of meningococcal factor H-binding protein. Microbiology 155:2873–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pajon R, Beernink PT, Harrison LH, Granoff DM. 2010. Frequency of factor H-binding protein modular groups and susceptibility to cross-reactive bactericidal activity in invasive meningococcal isolates. Vaccine 28:2122–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brehony C, Wilson DJ, Maiden MCJ. 2009. Variation of the factor H-binding protein of Neisseria meningitidis. Microbiology 155:4155–4169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anderson A, Hoiseth S, Murphy E, Andrew L, McNeil L, Abad R, Vazquez J, Vogel U, Frosch M, Jansen K. 2009. Epidemiology of the serogroup B N. meningitidis (MNB) factor H binding protein and implications for vaccine development, p P505 Abstr. 27th Annu. Meet. Eur. Soc. Paediatr. Infect. Dis., Brussels, Belgium [Google Scholar]
- 34. Jacobsson S, Hedberg ST, Molling P, Unemo M, Comanducci M, Rappuoli R, Olcen P. 2009. Prevalence and sequence variations of the genes encoding the five antigens included in the novel 5CVMB vaccine covering group B meningococcal disease. Vaccine 27:1579–1584 [DOI] [PubMed] [Google Scholar]
- 35. de Filippis I, de Lemos AP, Hostetler JB, Wollenberg K, Sacchi CT, Harrison LH, Bash MC, Prevots DR. 2012. Molecular epidemiology of Neisseria meningitidis serogroup B in Brazil. PLoS One 7:e33016 doi: 10.1371/journal.pone.0033016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mothibeli KM, du Plessis M, von Gottberg A, Murphy E, Hoiseth SK, Zlotnick G, Klugman KP. 2011. Distribution of factor H binding protein beyond serogroup B: variation among five serogroups of invasive Neisseria meningitidis in South Africa. Vaccine 29:2187–2192 [DOI] [PubMed] [Google Scholar]
- 37. Wang X, Cohn A, Comanducci M, Andrew L, Zhao X, MacNeil JR, Schmink S, Muzzi A, Bambini S, Rappuoli R, Pizza M, Murphy E, Hoiseth SK, Jansen KU, Anderson AS, Harrison LH, Clark TA, Messonnier NE, Mayer LW. 2011. Prevalence and genetic diversity of candidate vaccine antigens among invasive Neisseria meningitidis isolates in the United States. Vaccine 29:4739–4744 [DOI] [PubMed] [Google Scholar]
- 38. Beernink PT, Caugant DA, Welsch JA, Koeberling O, Granoff DM. 2009. Meningococcal factor H-binding protein variants expressed by epidemic capsular group A, W-135, and X strains from Africa. J. Infect. Dis. 199:1360–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pajon R, Fergus AM, Koeberling O, Caugant DA, Granoff DM. 2011. Meningococcal factor H binding proteins in epidemic strains from Africa: implications for vaccine development. PLoS Negl. Trop. Dis. 5:e1302 doi: 10.1371/journal.pntd.0001302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Christensen H, May M, Bowen L, Hickman M, Trotter CL. 2010. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect. Dis. 10:853–861 [DOI] [PubMed] [Google Scholar]
- 41. Marsh JW, Shutt KA, Pajon R, Tulenko MM, Liu S, Hollick RA, Kiehlbauch JA, Clark TA, Stephens DS, Arnold KE, Myers RA, Mayer LW, Harrison LH. 2011. Diversity of factor H-binding protein in Neisseria meningitidis carriage isolates. Vaccine 29:6049–6058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Harris SL, Zhu D, Murphy E, McNeil LK, Wang X, Mayer LW, Harrison LH, Jansen KU, Anderson AS. 2011. Preclinical evidence for the potential of a bivalent fHBP vaccine to prevent Neisseria meningitidis serogroup C. Dis. Hum. Vaccin. 7(Suppl.):68–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vu DM, Shaughnessy J, Lewis LA, Ram S, Rice PA, Granoff DM. 2011. Enhanced bacteremia in human factor H transgenic rats infected by Neisseria meningitidis. Infect. Immun. 80:643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lucidarme J, Tan L, Exley RM, Findlow J, Borrow R, Tang CM. 2011. Characterization of Neisseria meningitidis isolates that do not express the virulence factor and vaccine antigen factor H binding protein. Clin. Vaccine Immunol. 18:1002–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lewis LA, Ngampasutadol J, Wallace R, Reid JE, Vogel U, Ram S. 2010. The meningococcal vaccine candidate neisserial surface protein A (NspA) binds to factor H and enhances meningococcal resistance to complement. PLoS Pathog. 6:e1001027 doi: 10.1371/journal.ppat.1001027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McNeil LK, Murphy E, Zhao X-J, Guttmann S, Harris SL, Scott AA, Tan C, Mack M, DaSilva I, Alexander K, Mason K, Jiang H-Q, Zhu D, Mininni TL, Zlotnick GW, Hoiseth SK, Jones TR, Pride MW, Jansen KU, Anderson AS. 2009. Detection of LP2086 on the cell surface of Neisseria meningitidis and its accessibility in the presence of serogroup B capsular polysaccharide. Vaccine 27:3417–3421 [DOI] [PubMed] [Google Scholar]
- 47. Mascioni A, Jacob J, Moy F, Dilts D, Fink P, Malakian K, Sigethy S, Wen Y, Novikova E, Zlotnick GW, Tsao DH. 2009. Backbone and side-chain assignment of the lipidated and non-lipidated forms of the meningococcal outer membrane protein LP2086. Biomol. NMR Assign. 3:111–113 [DOI] [PubMed] [Google Scholar]
- 48. Mascioni A, Bentley BE, Camarda R, Dilts DA, Fink P, Gusarova V, Hoiseth SK, Jacob J, Lin SL, Malakian K, McNeil LK, Mininni T, Moy F, Murphy E, Novikova E, Sigethy S, Wen Y, Zlotnick GW, Tsao DHH. 2009. Structural basis for the immunogenic properties of the meningococcal vaccine candidate LP2086. J. Biol. Chem. 284:8738–8746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mascioni A, Novikova E, Bentley BE, McNeil LK, Murphy E, Camarda R, Lin S, Ddilts Fink P, Mininni TL, Hoiseth S, Tsao D, Zlotnick GW. 2008. Determination of the domain and solution structure of rLP2086, a meningococcal vaccine candidate and human factor H binding protein, p O61 Proc. 16th Int. Pathog. Neisseria Conf., Rotterdam, The Netherlands [Google Scholar]
- 50. Cantini F, Veggi D, Dragonetti S, Savino S, Scarselli M, Romagnoli G, Pizza M, Banci L, Rappuoli R. 2009. Solution structure of the factor H-binding protein, a survival factor and protective antigen of Neisseria meningitidis. J. Biol. Chem. 284:9022–9026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cendron L, Veggi D, Girardi E, Zanotti G. 2011. Structure of the uncomplexed Neisseria meningitidis factor H-binding protein fHbp (rLP2086). Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 67:531–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schneider MC, Prosser BE, Caesar JJE, Kugelberg E, Li S, Zhang Q, Quoraishi S, Lovett JE, Deane JE, Sim RB, Roversi P, Johnson S, Tang CM, Lea SM. 2009. Neisseria meningitidis recruits factor H using protein mimicry of host carbohydrates. Nature 458:890–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Scarselli M, Aricò B, Brunelli B, Savino S, Di Marcello F, Palumbo E, Veggi D, Ciucchi L, Cartocci E, Bottomley MJ, Malito E, Lo Surdo P, Comanducci M, Giuliani MM, Cantini F, Dragonetti S, Colaprico A, Doro F, Giannetti P, Pallaoro M, Brogioni B, Tontini M, Hilleringmann M, Nardi-Dei V, Banci L, Pizza M, Rappuoli R. 2011. Rational design of a meningococcal antigen inducing broad protective immunity. Sci. Transl. Med. 3:91ra62 doi: 10.1126/scitranslmed.3002234 [DOI] [PubMed] [Google Scholar]
- 54. Emonts M, Hazelzet JA, de Groot R, Hermans PWM. 2003. Host genetic determinants of Neisseria meningitidis infections. Lancet Infect. Dis. 3:565–577 [DOI] [PubMed] [Google Scholar]
- 55. Lo H, Tang CM, Exley RM. 2009. Mechanisms of avoidance of host immunity by Neisseria meningitidis and its effect on vaccine development. Lancet Infect. Dis. 9:418–427 [DOI] [PubMed] [Google Scholar]
- 56. Wright V, Hibberd M, Levin M. 2009. Genetic polymorphisms in host response to meningococcal infection: the role of susceptibility and severity genes. Vaccine 27:B90–B102 [DOI] [PubMed] [Google Scholar]
- 57. Haralambous E, Dolly SO, Hibberd ML, Litt DJ, Udalova IA, O'Dwyer C, Langford PR, Simon Kroll J, Levin M. 2006. Factor H, a regulator of complement activity, is a major determinant of meningococcal disease susceptibility in UK Caucasian patients. Scand. J. Infect. Dis. 38:764–771 [DOI] [PubMed] [Google Scholar]
- 58. Pangburn MK. 2000. Host recognition and target differentiation by factor H, a regulator of the alternative pathway of complement. Immunopharmacol. 49:149–157 [DOI] [PubMed] [Google Scholar]
- 59. Lambris JD, Ricklin D, Geisbrecht BV. 2008. Complement evasion by human pathogens. Nat. Rev. Microbiol. 6:132–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ram S, McQuillen DP, Gulati S, Elkins C, Pangburn MK, Rice PA. 1998. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J. Exp. Med. 188:671–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shaughnessy J, Lewis LA, Jarva H, Ram S. 2009. Functional comparison of the binding of factor H short consensus repeat 6 (SCR 6) to factor H binding protein from Neisseria meningitidis and the binding of factor H SCR 18 to 20 to Neisseria gonorrhoeae porin. Infect. Immun. 77:2094–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Welsch JA, Ram S. 2008. Factor H and neisserial pathogenesis. Vaccine 26(Suppl. 8):I40–I45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kraiczy P, Wurzner R. 2006. Complement escape of human pathogenic bacteria by acquisition of complement regulators. Mol. Immunol. 43:31–44 [DOI] [PubMed] [Google Scholar]
- 64. Pio R, Elsassr TH, Martinez A, Cuttitta F. 2002. Identification, characterization, and physiological actions of factor H as an adrenomedullin binding protein present in human plasma. Microsc. Res. Tech. 57:23–27 [DOI] [PubMed] [Google Scholar]
- 65. Zipfel PF. 2001. Complement factor H: physiology and pathophysiology. Semin. Thromb. Hemost. 27:191–199 [DOI] [PubMed] [Google Scholar]
- 66. DiScipio RG. 1992. Ultrastructures and interactions of complement factors H and I. J. Immunol. 149:2592–2599 [PubMed] [Google Scholar]
- 67. Rodríguez de Córdoba S, Esparza-Gordillo J, Goicoechea de Jorge E, Lopez-Trascasa M, Sánchez-Corral P. 2004. The human complement factor H: functional roles, genetic variations and disease associations. Mol. Immunol. 41:355–367 [DOI] [PubMed] [Google Scholar]
- 68. Pangburn MK, Atkinson MA, Meri S. 1991. Localization of the heparin-binding site on complement factor H. J. Biol. Chem. 266:16847–16853 [PubMed] [Google Scholar]
- 69. Kazatchkine MD, Fearon DT, Austen KF. 1979. Human alternative complement pathway: membrane-associated sialic acid regulates the competition between B and beta1H for cell-bound C3b. J. Immunol. 122:75–81 [PubMed] [Google Scholar]
- 70. Davila S, Wright VJ, Khor CC, Sim KS, Binder A, Breunis WB, Inwald D, Nadel S, Betts H, Carrol ED, de Groot R, Hermans PW, Hazelzet J, Emonts M, Lim CC, Kuijpers TW, Martinon-Torres F, Salas A, Zenz W, Levin M, Hibberd ML. 2010. Genome-wide association study identifies variants in the CFH region associated with host susceptibility to meningococcal disease. Nat. Genet. 42:772–776 [DOI] [PubMed] [Google Scholar]
- 71. Dunphy KY, Beernink PT, Brogioni B, Granoff DM. 2011. Effect of factor H-binding protein sequence variation on factor H binding and survival of Neisseria meningitidis in human blood. Infect. Immun. 79:353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Seib KL, Brunelli B, Brogioni B, Palumbo E, Bambini S, Muzzi A, DiMarcello F, Marchi S, van der Ende A, Arico B, Savino S, Scarselli M, Comanducci M, Rappuoli R, Giuliani MM, Pizza M. 2011. Characterization of diverse subvariants of the meningococcal factor H (fH) binding protein for their ability to bind fH, to mediate serum resistance, and to induce bactericidal antibodies. Infect. Immun. 79:970–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Seib KL, Oriente F, Adu-Bobie J, Montanari P, Ferlicca F, Giuliani MM, Rappuoli R, Pizza M, Delany I. 2010. Influence of serogroup B meningococcal vaccine antigens on growth and survival of the meningococcus in vitro and in ex vivo and in vivo models of infection. Vaccine 28:2416–2427 [DOI] [PubMed] [Google Scholar]
- 74. Giuntini S, Reason DC, Granoff DM. 2011. Complement-mediated bactericidal activity of anti-factor H binding protein monoclonal antibodies against the meningococcus relies upon blocking factor H binding. Infect. Immun. 79:3751–3759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. McNeil LK, Zlotnick GW, Zhao X, Anderson AS, Jansen KU. 2010. Anti-fHBP antibodies elicited after immunization with a recombinant fHBP vaccine candidate (rLP2086) can displace human factor H from the surface of serogroup B meningococci, p 94 Proc. 18th Int. Pathog. Neisseria Conf., Banff, Canada [Google Scholar]
- 76. Beernink PT, Shaughnessy J, Braga EM, Liu Q, Rice PA, Ram S, Granoff DM. 2011. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J. Immunol. 186:3606–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Findlow J, Borrow R, Snape MD, Dawson T, Holland A, John TM, Evans A, Telford KL, Ypma E, Toneatto D, Oster P, Miller E, Pollard AJ. 2010. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin. Infect. Dis. 51:1127–1137 [DOI] [PubMed] [Google Scholar]
- 78. Gossger N, Snape MD, Yu LM, Finn A, Bona G, Esposito S, Principi N, Diez-Domingo J, Sokal E, Becker B, Kieninger D, Prymula R, Dull P, Ypma E, Toneatto D, Kimura A, Pollard AJ. 2012. Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: a randomized controlled trial. JAMA 307:573–582 [DOI] [PubMed] [Google Scholar]
- 79. Kimura A, Toneatto D, Kleinschmidt A, Wang H, Dull P. 2011. Immunogenicity and safety of a multicomponent meningococcal serogroup B vaccine and a quadrivalent meningococcal CRM197 conjugate vaccine against serogroups A, C, W-135, and Y in adults who are at increased risk for occupational exposure to meningococcal isolates. Clin. Vaccine Immunol. 18:483–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Richmond PC, Marshall HS, Nissen MD, Jiang Q, Jansen KU, Garces-Sanchez M, Martinon-Torres F, Beeslaar J, Szenborn L, Wysocki J, Eiden J, Harris SL, Jones TR, Perez JL. 2012. Safety, immunogenicity, and tolerability of meningococcal serogroup B bivalent recombinant lipoprotein 2086 vaccine in healthy adolescents: a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 12:597–607 [DOI] [PubMed] [Google Scholar]
- 81. Richmond PC, Nissen MD, Marshall HS, Lambert SB, Roberton D, Gruber WC, Jones TR, Arora A. 2012. A bivalent Neisseria meningitidis recombinant lipidated factor H binding protein vaccine in young adults: Results of a randomised, controlled, dose-escalation phase 1 trial. Vaccine 30:6163–6174 [DOI] [PubMed] [Google Scholar]
- 82. Santolaya ME, O'Ryan ML, Valenzuela MT, Prado V, Vergara R, Munoz A, Toneatto D, Grana G, Wang H, Clemens R, Dull PM. 2012. Imunogenicity and tolerability of a mulicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile: a phase 2b/3 randomised, observer-blind, placebo-controlled study. Lancet 379:617–624 [DOI] [PubMed] [Google Scholar]
- 83. Snape MD, Dawson T, Oster P, Evans A, John TM, Ohene-Kena B, Findlow J, Yu LM, Borrow R, Ypma E, Toneatto D, Pollard AJ. 2010. Immunogenicity of two investigational serogroup B meningococcal vaccines in the first year of life: a randomized comparative trial. Pediatr. Infect. Dis. J. 29:e71–79 [DOI] [PubMed] [Google Scholar]
- 84. Granoff DM, Welsch JA, Ram S. 2009. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect. Immun. 77:764–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Santos GF, Deck RR, Donnelly J, Blackwelder W, Granoff DM. 2001. Importance of complement source in measuring meningococcal bactericidal titers. Clin. Diagn. Lab. Immunol. 8:616–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zollinger WD, Mandrell RE. 1983. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect. Immun. 40:257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Snyder LAS, Butcher SA, Saunders NJ. 2001. Comparative whole-genome analyses reveal over 100 putative phase-variable genes in the pathogenic Neisseria spp. Microbiology 147:2321–2332 [DOI] [PubMed] [Google Scholar]
- 88. Comanducci M, Bambini S, Brunelli B, Adu-Bobie J, Arico B, Capecchi B, Giuliani MM, Masignani V, Santini L, Savino S, Granoff DM, Caugant DA, Pizza M, Rappuoli R, Mora M. 2002. NadA, a novel vaccine candidate of Neisseria meningitidis. J. Exp. Med. 195:1445–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bennett JS, Thompson EA, Kriz P, Jolley KA, Maiden MC, Bennett JS, Thompson EAL, Kriz P, Jolley KA, Maiden MCJ. 2009. A common gene pool for the Neisseria FetA antigen. Int. J. Med. Microbiol. 299:133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sanders H, Brehony C, Maiden MC, Vipond C, Feavers IM. 2012. The effect of iron availability on transcription of the Neisseria meningitidis fHbp gene varies among clonal complexes. Microbiology 158:869–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Oriente F, Scarlato V, Delany I. 2010. Expression of factor H binding protein of meningococcus responds to oxygen-limitation through a dedicated FNR-regulated promoter. J. Bacteriol. 192:691–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Beernink PT, Welsch JA, Bar-Lev M, Koeberling O, Comanducci M, Granoff DM. 2008. Fine antigenic specificity and cooperative bactericidal activity of monoclonal antibodies directed at the meningococcal vaccine candidate factor H-binding protein. Infect. Immun. 76:4232–4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Welsch JA, Ram S, Koeberling O, Granoff DM. 2008. Complement-dependent synergistic bactericidal activity of antibodies against factor H-binding protein, a sparsely distributed meningococcal vaccine antigen. J. Infect. Dis. 197:1053–1061 [DOI] [PubMed] [Google Scholar]
- 94. Welsch JA, Rossi R, Comanducci M, Granoff DM. 2004. Protective activity of monoclonal antibodies to genome-derived neisserial antigen 1870, a Neisseria meningitidis candidate vaccine J. Immunol. 172:5606–5615 [DOI] [PubMed] [Google Scholar]
- 95. Scarselli M, Cantini F, Santini L, Veggi D, Dragonetti S, Donati C, Savino S, Giuliani MM, Comanducci M, Marcello FD, Romagnoli G, Pizza M, Banci L, Rappuoli R. 2009. Epitope mapping of a bactericidal monoclonal antibody against the factor H binding protein of Neisseria meningitidis. J. Mol. Biol. 386:97–108 [DOI] [PubMed] [Google Scholar]
- 96. Vu DM, Wong TT, Granoff DM. 2011. Cooperative serum bactericidal activity between human antibodies to meningococcal factor H binding protein and neisserial heparin binding antigen. Vaccine 29:1968–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Giuntini S, Reason DC, Granoff DM. 2012. Combined roles of human IgG subclass, alternative complement pathway activation, and epitope density in the bactericidal activity of antibodies to meningococcal factor H binding protein. Infect. Immun. 80:187–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Beernink PT, LoPasso C, Angiolillo A, Felici F, Granoff D. 2009. A region of the N-terminal domain of meningococcal factor H-binding protein that elicits bactericidal antibody across antigenic variant groups. Mol. Immunol. 46:1647–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Madico G, Ngampasutadol J, Gulati S, Vogel U, Rice PA, Ram S. 2007. Factor H binding and function in sialylated pathogenic neisseriae is influenced by gonococcal, but not meningococcal, porin. J. Immunol. 178:4489–4497 [DOI] [PubMed] [Google Scholar]
- 100. Vu DM, Pajon R, Reason DC, Granoff DM. 2012. A broadly cross-reactive monoclonal antibody against an epitope on the N-terminus of meningococcal fHbp. Sci. Rep. 2:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Seib KL, Serruto D, Oriente F, Delany I, Adu-Bobie J, Veggi D, Arico B, Rappuoli R, Pizza M. 2009. Factor H-binding protein is important for meningococcal survival in human whole blood and serum and in the presence of the antimicrobial peptide LL-37. Infect. Immun. 77:292–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Jones A, Georg M, Maudsdotter L, Jonsson A-B. 2009. Endotoxin, capsule, and bacterial attachment contribute to Neisseria meningitidis resistance to the human antimicrobial peptide LL-37. J. Bacteriol. 191:3861–3868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhu D, Barniak V, Zhang Y, Green B, Zlotnick G. 2006. Intranasal immunization of mice with recombinant lipidated P2086 protein reduces nasal colonization of group B Neisseria meningitidis. Vaccine 24:5420–5425 [DOI] [PubMed] [Google Scholar]
- 104. Zhu D, Zhang Y, Barniak V, Bernfield L, Howell A, Zlotnick G. 2005. Evaluation of recombinant lipidated P2086 protein as a vaccine candidate for group B Neisseria meningitidis in a murine nasal challenge model. Infect. Immun. 73:6838–6845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Pillai S, Howell A, Alexander K, Bentley BE, Jiang H-Q, Ambrose K, Zhu D, Zlotnick G. 2005. Outer membrane protein (OMP) based vaccine for Neisseria meningitidis serogroup B. Vaccine 23:2206–2209 [DOI] [PubMed] [Google Scholar]
- 106. Jacobsson S, Mölling P, Olcén P. 2009. Seroprevalence of antibodies against fHbp and NadA, two potential vaccine antigens for Neisseria meningitidis. Vaccine 27:5755–5759 [DOI] [PubMed] [Google Scholar]
- 107. Yazdankhah SP, Caugant DA. 2004. Neisseria meningitidis: an overview of the carriage state. J. Med. Microbiol. 53:821–832 [DOI] [PubMed] [Google Scholar]
- 108. Ala'aldeen DA, Flint M, Oldfield NJ, Omer SA, McNeil LK, Jiang Q, Murphy E, Giardina PC, Novikova EG, Dodge-Scully IL, Bayliss CD, Turner DP, Neal KR, Hoiseth SK, Jansen KU, Anderson AS. 2010. Human antibody responses to the meningococcal factor H binding protein (LP2086) during invasive disease, colonization and carriage. Vaccine 28:7667–7675 [DOI] [PubMed] [Google Scholar]
- 109. Anderson AS. 2009. Development of a factor H binding protein vaccine for broad protection against invasive Neisseria meningitidis serogroup B (MNB) disease, p O026 Abstr. 10th Eur. Meningococci Dis. Soc. Meet., Manchester, United Kingdom [Google Scholar]
- 110. Caugant DA. 2008. Genetics and evolution of Neisseria meningitidis: importance for the epidemiology of meningococcal disease. Infect. Genet. Evol. 8:558–565 [DOI] [PubMed] [Google Scholar]
- 111. Maiden MCJ, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, Zhang Q, Zhou J, Zurth K, Caugant DA, Feavers IM, Achtman M, Spratt BG. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic-microorganisms. Proc. Natl. Acad. Sci. U. S. A. 95:3140–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bambini S, Muzzi A, Olcen P, Rappuoli R, Pizza M, Comanducci M. 2009. Distribution and genetic variability of three vaccine components in a panel of strains representative of the diversity of serogroup B meningococcus. Vaccine 27:2794–2803 [DOI] [PubMed] [Google Scholar]
- 113. Beernink PT, Welsch JA, Harrison LH, Leipus A, Kaplan SL, Granoff DM. 2007. Prevalence of factor H-binding protein variants and NadA among meningococcal group B isolates from the United States: implications for the development of a multicomponent group B vaccine. J. Infect. Dis. 195:1472–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hao W, Ma JH, Warren K, Tsang RS, Low DE, Jamieson FB, Alexander DC. 2011. Extensive genomic variation within clonal complexes of Neisseria meningitidis. Genome Biol. Evol. 3:1406–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. English M, MacLennan JM, Bowen-Morris JM, Deeks J, Boardman M, Brown K, Smith S, Buttery J, Clarke J, Quataert S, Lockhart S, Moxon ER. 2000. A randomised, double-blind, controlled trial of the immunogenicity and tolerability of a meningococcal group C conjugate vaccine in young British infants. Vaccine 19:1232–1238 [DOI] [PubMed] [Google Scholar]
- 116. MacLennan JM, Shackley F, Heath PT, Deeks JJ, Flamank C, Herbert M, Griffiths H, Hatzmann E, Goilav C, Moxon ER. 2000. Safety, immunogenicity, and induction of immunologic memory by a serogroup C meningococcal conjugate vaccine in infants: a randomized controlled trial. JAMA 283:2795–2801 [DOI] [PubMed] [Google Scholar]
- 117. Pichichero M, Casey J, Blatter M, Rothstein E, Ryall R, Bybel M, Gilmet G, Papa T. 2005. Comparative trial of the safety and immunogenicity of quadrivalent (A, C, Y, W-135) meningococcal polysaccharide-diphtheria conjugate vaccine versus quadrivalent polysaccharide vaccine in two- to ten-year-old children. Pediatr. Infect. Dis. J. 24:57–62 [DOI] [PubMed] [Google Scholar]
- 118. Koeberling O, Giuntini S, Seubert A, Granoff DM. 2009. Meningococcal outer membrane vesicle vaccines derived from mutant strains engineered to express factor H binding proteins from antigenic variant groups 1 and 2. Clin. Vaccine Immunol. 16:156–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. McNeil LK, Zlotnick G, Camposano E, Logan SM, Novikova EG, Zhao XJ, Anderson AS, Pride MW, Jansen KU. 2011. Development of a meningococcal antigen surface expression (Measure) assay for the phenotypic characterization of fHBP expression by Neisseria meningitidis, p 19–20 Abstr. 11th EMGM Meeting, Ljubljana, Slovenia [Google Scholar]
- 120. Donnelly J, Medini D, Boccadifuoco G, Biolchi A, Ward J, Frasch C, Moxon ER, Stella M, Comanducci M, Bambini S, Muzzi A, Andrews W, Chen J, Santos G, Santini L, Boucher P, Serruto D, Pizza M, Rappuoli R, Giuliani MM. 2010. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc. Natl. Acad. Sci. U. S. A. 107:19490–19495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Campbell H, Andrews N, Borrow R, Trotter C, Miller E. 2010. Updated postlicensure surveillance of the meningococcal C conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modeling predictions of the duration of herd immunity. Clin. Vaccine Immunol. 17:840–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Cohn AC, MacNeil JR, Harrison LH, Hatcher C, Theodore J, Schmidt M, Pondo T, Arnold KE, Baumbach J, Bennett N, Craig AS, Farley M, Gershman K, Petit S, Lynfield R, Reingold A, Schaffner W, Shutt KA, Zell ER, Mayer LW, Clark T, Stephens D, Messonnier NE. 2010. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998-2007: implications for prevention of meningococcal disease. Clin. Infect. Dis. 50:184–191 [DOI] [PubMed] [Google Scholar]
- 123. Artenstein MS. 1975. Control of meningococcal meningitis with meningococcal vaccines. Yale J. Biol. Med. 48:197–200 [PMC free article] [PubMed] [Google Scholar]
- 124. Artenstein MS, Gold R, Zimmerly JG, Wyle FA, Schneider H, Harkins C. 1970. Prevention of meningococcal disease by group C polysaccharide vaccine. N. Engl. J. Med. 282:417–420 [DOI] [PubMed] [Google Scholar]
- 125. Gold R, Artenstein MS. 1971. Meningococcal infections. 2. Field trial of group C meningococcal polysaccharide vaccine in 1969-70. Bull. World Health Organ. 45:279–282 [PMC free article] [PubMed] [Google Scholar]
- 126. Macneil JR, Cohn AC, Zell ER, Schmink S, Miller E, Clark T, Messonnier NE. 2011. Early estimate of the effectiveness of quadrivalent meningococcal conjugate vaccine. Pediatr. Infect. Dis. J. 30:451–455 [DOI] [PubMed] [Google Scholar]
- 127. Toneatto D, Ismaili S, Ypma E, Vienken K, Oster P, Dull P. 2011. The first use of an investigational multicomponent meningococcal serogroup B vaccine (4CMenB) in humans. Hum. Vaccin. 7:646–653 [DOI] [PubMed] [Google Scholar]
- 128. Marshall HS, Richmond PC, Nissen MD, Jiang Q, Anderson AS, Jansen KU, Reynolds G, Ziegler JB, Harris SL, Jones TR, Perez JL. 2012. Safety and immunogenicity of a meningococcal B bivalent rLP2086 vaccine in healthy toddlers aged 18–36 months: a phase 1 randomized-controlled clinical trial. Pediatr. Infect. Dis. J. 31:1061–1068 [DOI] [PubMed] [Google Scholar]
- 129. Nissen MD, Marshall HS, Richmond PC, Jiang Q, Harris SL, Jones TR, Jansen KU, Perez JL. 30 October 2012. A randomized, controlled, phase 1/2 trial of a Neisseria meningitidis serogroup B bivalent rLP2086 vaccine in healthy children and adolescents. Pediatr. Infect. Dis. J. [Epub ahead of print.] doi: 10.1097/INF.0b013e31827b0d24 [DOI] [PubMed] [Google Scholar]
- 130. Marshall HS, Richmond PC, Nissen MD, Wouters A, Baber J, Jiang Q, Anderson AS, Jones TR, Harris SL, Jansen KU, Perez JL. 24 January 2013. A phase 2 open-label safety and immunogenicity study of a meningococcal B bivalent rLP2086 vaccine in healthy adults. Vaccine [Epub ahead of print.] doi: 10.1016/j.vaccine.2013.01.021 [DOI] [PubMed] [Google Scholar]
- 131. Gorringe AR, Pajon R. 2012. Bexsero: a multicomponent vaccine for prevention of meningococcal disease. Hum. Vaccin. Immunother. 8:174–183 [DOI] [PubMed] [Google Scholar]
- 132. Pfizer 2011, posting date Study evaluating safety, tolerability, and immunogenicity of meningococcal B vaccine in healthy infants. http://clinicaltrials.gov/ct2/show/NCT00798304?term=NCT00798304&rank=1
- 133. Jansen KU. 2011, posting date Vaccines and Related Biological Products Advisory Committee meeting presentation: VRBPAC meeting to discuss approaches to licensure of meningococcal serogroup B vaccines. http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/ucm251454.htm
- 134. Anderson AS, Hao L, Jiang Q, Harris SL, Jones TR, Perez JL, York L, Eiden J, Jansen KU. 2013. Potential impact of the bivalent rLP2806 vaccine on Neisseria meningitidis carriage and invasive serogroup B disease. Hum. Vaccin. Immunother. 9:471–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Trotter CL, Maiden MC. 2009. Meningococcal vaccines and herd immunity: lessons learned from serogroup C conjugate vaccination programs. Expert Rev. Vaccines 8:851–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. van der Avoort HGAM, Berbers GAM, van Binnendijk RS, Boot HJ, Feltkamp MCW, Friesema IHM, de Greeff SC, Hahné SJM, Kemmeren JM, van der Klis FRM, Kramer MA, van Lier EA, van der Maas NAT, de Melker HE, Mooi FR, Reubsaet F, Schouls LM, De Voer RR. 2010. The National Immunisation Programme in the Netherlands. Developments in 2009. RIVM, National Institute for Public Health and the Environment; http://www.rivm.nl/bibliotheek/rapporten/210021012.pdf [Google Scholar]
- 137. Sheldon EA, Schwartz H, Jiang Q, Giardina PC, Perez JL. 2012. A phase 1, randomized, open-label, active-controlled trial to assess the safety of a meningococcal serogroup B bivalent rLP2086 vaccine in healthy adults. Hum. Vaccin Immunother. 8:888–895 [DOI] [PubMed] [Google Scholar]
- 138. Keiser PB, Miller LB, Biggs-Cicatelli S, Zollinger WD. 2009. Plasma fibrinogen levels after vaccination with a native outer membrane vesicle vaccine for Neisseria meningitidis. Vaccine 27:6809–6813 [DOI] [PubMed] [Google Scholar]
- 139. Keiser PB, Biggs-Cicatelli S, Moran EE, Schmiel DH, Pinto VB, Burden RE, Miller LB, Moon JE, Bowden RA, Cummings JF, Zollinger WD. 2011. A phase 1 study of a meningococcal native outer membrane vesicle vaccine made from a group B strain with deleted lpxL1 and synX, over-expressed factor H binding protein, two PorAs and stabilized OpcA expression. Vaccine 29:1413–1420 [DOI] [PubMed] [Google Scholar]
- 140. Keiser PB, Gibbs BT, Coster TS, Moran EE, Stoddard MB, Labrie JE, 3rd, Schmiel DH, Pinto V, Chen P, Zollinger WD. 2010. A phase 1 study of a group B meningococcal native outer membrane vesicle vaccine made from a strain with deleted lpxL2 and synX and stable expression of opcA. Vaccine 28:6970–6976 [DOI] [PubMed] [Google Scholar]
- 141. Koeberling O, Seubert A, Santos G, Colaprico A, Ugozzoli M, Donnelly J, Granoff DM. 2011. Immunogenicity of a meningococcal native outer membrane vesicle vaccine with attenuated endotoxin and over-expressed factor H binding protein in infant rhesus monkeys. Vaccine 29:4728–4734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Brunelli B, Del Tordello E, Palumbo E, Biolchi A, Bambini S, Comanducci M, Muzzi A, Pizza M, Rappuoli R, Donnelly JJ, Giuliani MM, Serruto D. 2011. Influence of sequence variability on bactericidal activity sera induced by factor H binding protein variant 1.1. Vaccine 29:1072–1081 [DOI] [PubMed] [Google Scholar]
- 143. Ruijne N, Lea RA, O'Hallahan J, Oster P, Martin D. 2006. Understanding the immune responses to the meningococcal strain-specific vaccine MeNZB measured in studies of infants. Clin. Vaccine Immunol. 13:797–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Stokley S, Cohn A, Dorell C, Hariri S, Yankey D, Messonnier N, Wortley PM. 2011. Adolescent vaccination-coverage levels in the United States: 2006-2009. Pediatrics 128:1078–1086 [DOI] [PubMed] [Google Scholar]
- 145. Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. 2003. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 31:3497–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]