Abstract
SUMMARY
In both humans and animals, Clostridium perfringens is an important cause of histotoxic infections and diseases originating in the intestines, such as enteritis and enterotoxemia. The virulence of this Gram-positive, anaerobic bacterium is heavily dependent upon its prolific toxin-producing ability. Many of the ∼16 toxins produced by C. perfringens are encoded by large plasmids that range in size from ∼45 kb to ∼140 kb. These plasmid-encoded toxins are often closely associated with mobile elements. A C. perfringens strain can carry up to three different toxin plasmids, with a single plasmid carrying up to three distinct toxin genes. Molecular Koch's postulate analyses have established the importance of several plasmid-encoded toxins when C. perfringens disease strains cause enteritis or enterotoxemias. Many toxin plasmids are closely related, suggesting a common evolutionary origin. In particular, most toxin plasmids and some antibiotic resistance plasmids of C. perfringens share an ∼35-kb region containing a Tn916-related conjugation locus named tcp (transfer of clostridial plasmids). This tcp locus can mediate highly efficient conjugative transfer of these toxin or resistance plasmids. For example, conjugative transfer of a toxin plasmid from an infecting strain to C. perfringens normal intestinal flora strains may help to amplify and prolong an infection. Therefore, the presence of toxin genes on conjugative plasmids, particularly in association with insertion sequences that may mobilize these toxin genes, likely provides C. perfringens with considerable virulence plasticity and adaptability when it causes diseases originating in the gastrointestinal tract.
INTRODUCTION
The Gram-positive, anaerobic, spore-forming bacterium Clostridium perfringens is distributed ubiquitously throughout the environment, with a presence in soils, foods, sewage, feces, and the intestines of many healthy humans and animals (1–3). This bacterium also ranks among the most common and important pathogens of humans and livestock (1, 3, 4). C. perfringens causes histotoxic infections, including gas gangrene (clostridial myonecrosis), anaerobic cellulitis, and simple wound infections (3–5). It is also responsible for several human and animal diseases originating in the intestines; these illnesses typically manifest as enteritis or enterotoxemia, a condition where toxins produced in the intestines are absorbed into the circulation and then damage other internal organs such as the brain, lungs, or kidneys (3, 6).
The virulence of C. perfringens is attributable largely to its ability to produce at least 16 different toxins and extracellular enzymes (3, 7–11). However, no single strain produces this entire toxin panoply. A commonly used toxin typing classification system (1, 8, 9) assigns C. perfringens isolates to types A to E based upon their ability to produce four typing toxins, as indicated in Table 1. Besides expressing one or more of the typing toxins, some C. perfringens strains produce additional toxins, such as C. perfringens enterotoxin (CPE) or necrotic enteritis B-like toxin (NetB), which are also very important during certain diseases, as described below (1, 11, 12). Since the type A to E toxin typing scheme was developed before cpe or netB was identified, it does not address carriage of these (and other) toxin genes, indicating a need to update this historical classification system.
TABLE 1.
C. perfringens toxinotypes
| Type | Toxin produced |
|||
|---|---|---|---|---|
| Alpha | Beta | Epsilon | Iota | |
| A | + | − | − | − |
| B | + | + | + | − |
| C | + | + | − | − |
| D | + | − | + | − |
| E | + | − | − | + |
It has now become clear that many important C. perfringens toxins are encoded by large plasmids (13–24). Other recent studies, described later in this review, have provided important insights into the diversity of C. perfringens toxin plasmids, the critical importance of these plasmids for pathogenesis, and the ability of toxin plasmids to transfer among C. perfringens strains. Given this progress, it is timely to summarize and interpret this information. In response, this review will first introduce the C. perfringens toxins, with an emphasis on those toxins that can be plasmid encoded, and then briefly discuss the contributions of the key plasmid-encoded toxins to C. perfringens diseases. Our focus will then shift to discussing the current understanding of C. perfringens toxin plasmid biology, addressing such issues as toxin plasmid diversity, replication, conjugative transfer, plasmid compatibility, and evolution.
CLOSTRIDIUM PERFRINGENS TOXINS
Properties of the key C. perfringens toxins are highlighted in Table 2, and these toxins will now be briefly described.
TABLE 2.
Properties of the key C. perfringens toxinsc
| Toxin | Locationa | Molecular mass (kDa) | LD50b (mice) | Biological activity(ies) | Reaction to trypsin | Action(s) |
|---|---|---|---|---|---|---|
| CPA | C | 43 | 3 μg | Necrotizing, hemolytic, contraction of smooth muscle | Susceptible | Phospholipase C; activates host cell signaling |
| CPB | P | 35 | <400 ng | Dermonecrosis, edema, enterotoxic | Susceptible | Pore former |
| ETX | P | 34 | 100 ng | Dermonecrosis, edema, contraction of smooth muscle | Activation required | Pore former |
| ITX | P | Ia, 48; Ib, 72 | 40 μg | Necrotizing | Activation required | ADP-ribosylating action |
| PFO | C | 54 | 15 μg | Necrotizing | Susceptible | Pore former |
| CPE | C/P | 35 | 81 μg | Erythema, enterotoxic | Activation but not required | Pore former |
| CPB2 | P | 28 | 160 μg | Dermonecrosis, edema, enterotoxic | Susceptible | ? |
| TpeL | P | 191 | 600 μg | ? | ? | Glycosylates Ras |
| NetB | P | 33 | ? | Hemolytic | ? | Pore former |
Chromosomally Encoded Toxins
Alpha-toxin (CPA or PLC).
C. perfringens strains of all types can produce CPA, which is a zinc metallophospholipase C that has both phospholipase C (PLC) and sphingomyelinase activity (30, 31). Alpha-toxin cleaves charged phosphorylcholine head groups from the outer surface of host cell phospholipid bilayers, thereby disrupting the function of host cell membranes, leading to cell lysis and tissue necrosis.
Analysis of the CPA structure reveals that it has two biologically active domains (32): an N-terminal α-helical domain that includes the single active site of the enzyme and a C-terminal β-sandwich domain that is essential for both cytolytic and toxic activity. Both domains are immunogenic, but only the C-terminal domain stimulates a protective immune response (33, 34). The C-terminal domain of CPA has structural similarity to C2 lipid-binding domains of eukaryotic proteins such as synaptotagmin and pancreatic lipase (30), suggesting an explanation as to why this membrane binding domain of CPA is required for its toxicity and is immunoprotective.
The lipid-soluble products of these reactions, diacylglycerol and ceramide, are important in host cell signaling pathways (31, 35). Therefore, direct disruption of the host cell membrane is not the only mechanism by which CPA causes cell lysis. It has also been shown that CPA activates the MEK/extracellular signal-regulated kinase (ERK) pathway and thereby induces oxidative stress in affected cells (36, 37) and interleukin-8 (IL-8) production by stimulating both the ERK1/2 and p38 mitogen-activated protein kinase (MAPK) pathways (38). Recent studies suggested that CPA may induce signal transduction changes after binding to a ganglioside GM1 receptor (38).
Perfringolysin O.
Perfringolysin O (PFO) can be produced by all C. perfringens types; however, the pfoA gene is absent from many, if not all, type A food poisoning strains carrying a chromosomal enterotoxin gene (25, 39) and from Darmbrand-associated type C strains (40). PFO is a member of the cholesterol-dependent cytolysin (CDC) family of pore-forming toxins, which also includes listeriolysin O and streptolysin O (41–43). These CDCs are produced as soluble monomers, which oligomerize at the target cell surface to form a pore complex that then undergoes a conformational change and inserts into the membrane to form a large pore.
The mechanism by which PFO inserts into the host cell membrane is intriguing. The crystal structure of PFO reveals an elongated monomer that has three primarily β-sheet domains (D1, D2, and D4) and a domain (D3) with a core of four antiparallel β-sheets and four α-helices (44). Contact between D4 and the cell membrane leads to conformational changes in D3. The α-helices are converted into β-sheets that, together with the core D3 β-sheets, form two extended amphipathic transmembrane β-hairpins that, upon oligomerization, are capable of penetrating the cell membrane and forming a large pore that may be comprised of up to 50 monomeric subunits. In this process, the structure of each monomer is compressed by some 40 Å (45).
The formation of the PFO pore results in disruption of the cell's protective barrier, leading to an osmotic imbalance and ultimately to cell lysis. However, cell lysis may not be the major biological effect of PFO in an infected tissue. It is well established that both CPA and PFO are responsible for the characteristic lack of a leukocyte influx at the focus of a C. perfringens-mediated myonecrotic infection (46–48), and, like other CDCs, PFO is a Toll-like receptor 4 (TLR4) agonist that induces tumor necrosis factor alpha (TNF-α) and IL-6 expression and apoptosis in cultured macrophages by activating the p38 MAPK pathway (49).
Toxins That Can Be Either Chromosomally or Plasmid Encoded
C. perfringens enterotoxin.
C. perfringens enterotoxin (CPE) is produced by some type A, C, D, and E strains but not by any known type B isolates (14–16, 23, 50–52). The CPE primary amino acid sequence is (i) highly conserved, except for some type E strains that produce a slightly variant CPE (23), and (ii) unique, apart from some limited similarity (still of unknown significance) with the nonneurotoxic HA3 protein made by Clostridium botulinum (1, 53).
The CPE structure was recently solved by X-ray crystallography, which assigned this toxin to the aerolysin family of small pore-forming toxins (54, 55). Furthermore, those structural analyses, coupled with mutagenesis studies (56–61), indicated that CPE contains a C-terminal domain that binds to claudin receptors on host cells and an N-terminal domain, consisting of two halves, that is critical for pore formation by mediating oligomerization and membrane insertion.
CPE action begins by binding of the toxin to its receptors, which include certain members of the claudin tight junction protein family (62–68). Claudins are ∼20- to 25-kDa proteins that consist of four transmembrane domains and two extracellular loops (ECLs) (69, 70). CPE binds, via a pocket on its C-terminal domain, to the second ECL of claudin receptors (71). Particularly important for this receptor binding interaction are (i) an Asn residue located near the middle of ECL2 on receptor claudins and (ii) Tyr residues present at amino acids 306, 310, and 312 in the CPE C terminus (1, 65, 67, 72).
After binding, CPE first localizes in a small, ∼90-kDa complex (73). At 37°C, CPE in a small complex rapidly oligomerizes on the membrane surface to form a large (∼450-kDa) prepore complex named CPE hexamer 1 (CH-1) (59, 66, 74). In addition to six copies of the toxin, CH-1 contains both receptor and nonreceptor claudins (the presence of nonreceptor claudins in CH-1 likely reflects a propensity for claudin-claudin interactions) (66). The CH-1 prepore complex, which forms in both cultured Caco-2 cells and the small intestine, then inserts into membranes by using a β-hairpin formed by CPE amino acids 81 to 106 (59, 61). This process results in formation of a cation-selective CPE pore that is initially permeable to molecules of <200 Da (1, 75–77).
CPE pore formation elevates cytoplasmic Ca2+ levels, thereby triggering calmodulin- and calpain-dependent host cell death via either caspase 3-mediated apoptosis (low CPE doses) or oncosis (high CPE doses) (78, 79). Ca2+ entry also induces morphological damage that exposes the basolateral cell surface, allowing CPE to interact with claudins and another tight junction protein named occludin (74, 80). This process leads to formation of a second large (∼550-kDa) CPE complex, named CH-2, which contains six copies of CPE as well as occludin and both receptor and nonreceptor claudins (66). Whether CH-2 forms in the intestine is still unclear.
CPE induces necrosis, epithelial desquamation, and villus blunting in all sections of the small intestines, but it is particularly active in the ileum (8, 81). CPE-induced histological damage apparently causes intestinal fluid and electrolyte transport changes since (i) the onset of histological damage precedes the development of transport changes in CPE-treated rabbit small intestinal loops (82) and (ii) only those CPE doses causing histological damage are capable of producing fluid transport changes in rabbit small intestinal loops (81, 83). CPE effects on the colon appear to be more modest (84, 85), although this subject requires further study.
Plasmid-Encoded Toxins
Beta-toxin.
Beta-toxin (CPB) has 20 to 28% amino acid sequence similarity with several pore-forming toxins of Staphylococcus aureus (86). This toxin is exceptionally sensitive to trypsin (87, 88). While the CPB structure-versus-function relationship has not yet been well studied, an older site-directed mutagenesis study suggested that CPB receptor binding activity may be localized in the C-terminal region of the toxin (89).
CPB forms ∼12-Å channels that are selective for monovalent cations (90). The toxin shows specificity for only a few cultured cell lines, possibly due to the limited distribution of a still unidentified receptor. Evidence for CPB oligomer formation has been reported for beta-toxin-sensitive HL-60 cells (91).
In vivo, CPB causes necrotic enteritis, probably by targeting both enterocytes and endothelial cells (92). In addition, once produced in the intestines, CPB is absorbed (by unknown mechanisms) into the circulation to cause lethal enterotoxemia (3). The internal organs targeted by CPB during enterotoxemia are unknown.
Beta2-toxin.
Despite its name, beta2-toxin (CPB2) has <15% sequence identity with CPB (28). Two major variants (with many subvariants) have been identified for this toxin (20, 93), which can be produced by all C. perfringens types. Interestingly, some cpb2-positive strains have a premature stop codon in their cpb2 gene; however, in vitro aminoglycoside treatment induces ribosomal frameshifting to restore CPB2 expression by these strains (94). This observation may suggest that aminoglycoside treatment can sometimes stimulate CPB2 production in vivo, although there is still no conclusive evidence that CPB2 contributes to disease.
The cellular action and pathophysiological activity of CPB2 remain incompletely characterized. However, CPB2 is reportedly cytotoxic for CHO cells (28) although only at relatively high levels (20 μg/ml). This low potency of CPB2 may reflect its instability, perhaps due to protease susceptibility. This toxin can reportedly induce hemorrhagic necrosis in guinea pig intestine (28).
Epsilon-toxin.
Epsilon-toxin (ETX) ranks as the most potent clostridial toxin after botulinum and tetanus toxins (95, 96). The toxin is secreted as a 296-amino-acid prototoxin, which is then proteolytically activated by digestive proteases such as chymotrypsin and trypsin or in vitro by C. perfringens lambda-toxin (97, 98). Recently, an unusual C. perfringens strain that can use a cytoplasmic protease to partially activate ETX was identified (99). Optimal activation of prototoxin is achieved with a combination of trypsin and chymotrypsin, which removes 13 amino acids from the N terminus and 29 amino acids from the C terminus (97, 98). Removal of the C-terminal amino acids is critical for producing active ETX, probably because those residues block toxin oligomerization (97, 100).
Like CPE, ETX belongs to the aerolysin family of pore-forming toxins (101). The mature ETX protein is comprised of three structural domains (101). These domains include (i) the N-terminal domain, which is thought to be important for receptor binding; (ii) the middle domain, containing a β-hairpin loop that likely mediates toxin insertion during pore formation; and (iii) the C-terminal domain, proposed to function during toxin oligomerization.
Relatively few mammalian cell lines are sensitive to this toxin (102), suggesting that the as-yet-unidentified ETX receptor is not widely distributed among host cells. ETX was recently shown to bind in vitro to hepatitis A virus cellular receptor 1 (HAVCR-1) (103, 104), which is produced in the kidneys, testis, and, to a lesser extent, colon (105). This observation is interesting since ETX binds strongly to the kidneys (106) and HAVCR-1 is expressed by an ETX-sensitive kidney cell line but not by several ETX-insensitive human cell lines (103). However, whether HAVCR-1 functions as an ETX receptor during disease is not known.
Once bound, ETX uses lipid rafts to oligomerize into heptamers (107). Recent findings suggest that the ETX oligomeric complex is ∼700 kDa and contains, in addition to seven ∼30-kDa ETX monomers, mammalian proteins such as caveolin-1 and -2 (108, 109). ETX oligomerization initially occurs on the membrane surface (100); the ETX prepore then rapidly inserts into the membrane to form an active pore with a diameter of 0.4 to 1 nm and a slight selectivity for anions (100, 110, 111). Pore formation in ETX-treated host cells results in rapid loss of intracellular K+ and increased cytoplasmic levels of Cl− and Na+ (112). Unlike CPE, ETX causes only a slow increase in cytoplasmic Ca2+ levels in sensitive host cells (112). Instead, ETX-induced cytoplasmic K+ loss triggers rapid cell death due to a necrosis process involving ATP depletion. It was recently suggested that at low doses, ETX can be internalized into host cells (113), but the pathophysiological importance of this observation is unclear.
Through an undefined mechanism, ETX increases intestinal permeability (114), which allows entry of the toxin into the circulation. The absorbed toxin then affects various organs such as brain, kidneys, and lungs (3, 106). Effects observed in naturally or experimentally intoxicated animals include edema in multiple organs, which probably reflects the effects of ETX on endothelial cells (3). Intriguingly, most endothelial cell lines are not sensitive to ETX, perhaps because they have lost receptor expression during culture (95).
Iota-toxin.
Iota-toxin (ITX) is a member of the clostridial binary toxin family and consists of separate IA and IB proteins that are produced as proproteins and then proteolytically activated when their N-terminal sequences are removed by host proteases (e.g., chymotrypsin) or C. perfringens lambda-toxin (115–118). Mature IA consists of an N-terminal domain that interacts with IB and a C-terminal domain with ADP-ribosyltransferase activity. Mature IB exhibits some similarity with Bacillus anthracis protective antigen (PA) but not in the receptor binding domain, which is consistent with IB and PA recognizing different receptors (115–118). IB has four domains, which mediate (i) IA interactions, (ii) internalization into host cells, (iii) oligomerization, and (iv) binding to host cell receptors (115–118).
ITX action begins with IB binding to its receptor(s). The lipolysis-stimulated lipoprotein receptor (LSR) has been identified as an ITX receptor (119) as well as a receptor for some other clostridial binary toxins, including Clostridium difficile transferase and Clostridium spiroforme toxin but not C. botulinum C2 toxin (119, 120). However, recent studies suggested that the multifunctional mammalian surface protein CD44 may also function as an ITX receptor or coreceptor (121).
In lipid rafts, bound IB toxin oligomerizes as a heptamer, which then binds IA (122, 123). Once formed, the holotoxin is endocytosed, and IA translocates into the cytoplasm from early endosomes (124, 125). Inside the cytoplasm, IA exerts its enzymatic activity, which involves ADP-ribosylating actin at Arg-177 to disassemble the host cell cytoskeleton (126). ITX can persist for at least 24 h inside host cells, which results in a delayed apoptosis (127).
NetB.
The most recently identified toxin in the C. perfringens armory is NetB (11, 128), which is produced by many avian isolates of C. perfringens type A (129–132). Only one nonavian strain of C. perfringens has been shown to produce NetB, which is consistent with its key role in the pathogenesis of necrotic enteritis in chickens (11), as discussed below.
NetB is a 33-kDa secreted β-pore-forming toxin that is most closely related to CPB from C. perfringens, alpha-hemolysin from Staphylococcus aureus, and CytK from Bacillus cereus (11). Like most of these toxins, it is produced as a monomer and presumably oligomerizes on the host cell surface prior to membrane insertion, forming 1.6- to 1.8-nm pores in susceptible chicken leghorn male hepatoma (LMH) cells (11). The structures of both the soluble monomeric form of NetB (133) and a heptameric pore form of NetB (134) have recently been solved, and its structural similarity to S. aureus alpha-hemolysin was confirmed. Although the precise NetB receptor has not been identified, there is evidence for cell specificity, since not all chicken cell lines are susceptible to NetB (11). Recent studies have shown that NetB interacts with cholesterol to enhance pore formation (134) and that it formed pores with much higher single-channel conductance than alpha-hemolysin and varied in its ion selectivity, preferring cations over anions (133).
TpeL.
The gene (tpeL) encoding TpeL is carried by some type A, B, and C strains (10, 16, 17) and reportedly can be expressed during sporulation under the control of Spo0A and the sporulation-specific sigma factor, SigE (135). TpeL (toxin C. perfringens large cytotoxin) is the largest known C. perfringens toxin, although some strains produce a truncated (∼15-kDa-smaller), less active TpeL variant (10, 136). TpeL belongs to the clostridial glycosylating toxin (CGT) family, which includes toxins A and B of C. difficile as well as the lethal and hemorrhagic toxin of Clostridium sordellii and Clostridium novyi alpha-toxin. Like other CGTs, TpeL has an N-terminal domain mediating glycosyltransferase activity, a domain with autocatalytic activity, and a putative transmembrane domain that is thought to deliver the enzymatic domain into the cytoplasm (136). However, TpeL is distinguishable from other CGTs by its severely truncated C-terminal domain, which is notable since this region has been postulated to mediate CGT binding to cell surface receptors (10, 136, 137).
TpeL binds to unidentified receptors and is then endocytosed (136). After inositol hexakisphosphate-dependent cysteine protease cleavage and transport across the endocytic vesicle membrane, the enzymatic domain enters the cytoplasm from early endosomes. Due to its unique sugar binding motif, TpeL is the only CGT that can use both UDP-glucose and UDP-N-acetylglucosamine as donor substrates, although it prefers to utilize UDP-N-acetylglucosamine (136, 137). TpeL modifies the regulatory GTPase Ras at Thr35, which disrupts cell signaling, including Ras-Raf interactions and ERK activation (136). The role, if any, of TpeL in disease is still unclear, but it has been suggested that TpeL production might enhance virulence of avian necrotic enteritis strains (138).
Other toxins and secreted enzymes.
In addition to the toxins described above, C. perfringens produces a slew of other toxins and secreted enzymes. These include another plasmid-encoded toxin named delta-toxin and several chromosomally encoded toxins (e.g., kappa-toxin, a collagenase, and mu-toxin, a hyaluronidase) and enzymes (e.g., clostripain, a cysteine protease) (8, 139, 140). Lambda-toxin, a 36-kDa thermolysin-like protease, is plasmid encoded and (as mentioned above) can activate ETX and the IA or IB component of ITX in vitro (97), although the importance of lambda-toxin in disease is unclear. Finally, C. perfringens produces several chromosomally encoded sialidases that are not essential when C. perfringens type A strain 13 causes gas gangrene in a mouse myonecrosis model (141); however, the NanI sialidase may still contribute to the early stages of a gas gangrene infection. This enzyme may also be important for type B or D disease originating in the gastrointestinal (GI) tract, since it increases ETX binding and mediates the in vitro adherence of CN3718, a type D strain, to enterocyte-like Caco-2 cells (142).
REGULATION OF PLASMID-ENCODED TOXIN PRODUCTION
The VirS/VirR Regulatory System
The classical two-component global regulatory system VirS/VirR, consisting of the VirS membrane sensor histidine kinase and the VirR transcriptional regulator, was discovered nearly 20 years ago, when it was shown to regulate the production of PFO, CPA, and some extracellular enzymes by type A strain 13 (143, 144). Later studies demonstrated that VirS/VirR directly regulates PFO production when VirR binds to VirR boxes located upstream of the pfoA gene (145–147). In contrast, this two-component system was found to indirectly control CPA production via a regulatory RNA molecule named VR-RNA (148, 149).
Of relevance for this review, the chromosomal VirS/VirR system can also regulate the expression of several plasmid-carried toxin genes, as initially shown for cpb2 transcription in strain 13, where VirS/VirR works via VR-RNA (150). More recently, VirS/VirR was found to control NetB and CPB production by type A or C strains, respectively (151–153). Interestingly, close contact with enterocyte-like Caco-2 cells increases production of CPB by type C strain CN3685, and this effect requires VirS/VirR (153). Furthermore, this two-component system is required for type C strain CN3685 to produce CPB in vivo and cause either lethal enterotoxemia or necrotic enteritis in animal models (152). However, VirS/VirR is not necessary for production of all plasmid-carried toxin genes, since a VirS/VirR null mutant of type D strain CN3718 still produces wild-type levels of ETX (154).
The Agr-Like Regulatory System
C. perfringens carries a chromosomal operon with partial homology to the S. aureus operon encoding components of the Agr quorum-sensing (QS) system. This agr-like operon was shown to regulate CPA and PFO production by C. perfringens type A strain 13, presumably by encoding components of a similar QS system (155, 156). It also controls the production of several plasmid-encoded C. perfringens toxins, including CPB2 and CPE expression in type A strain F5603 (157), CPB production in type C strain CN3685 (158) and type B strains CN1793 and CN1795 (159), and ETX production in type D strain CN3718 (154). However, this Agr-like regulatory system is not required for wild-type levels of production of all C. perfringens toxins, since inactivating this system in type B strains CN1793 and CN1795 had no effect on their ETX or CPB2 production (159).
The Agr-like regulatory system plays a role in the virulence of some C. perfringens strains. Specifically, by using agrB null mutants and their complemented derivatives, it was demonstrated that the Agr-like regulatory system is essential for CN3685 to cause either lethal enterotoxemia or hemorrhagic necrotic enteritis in animal models (158). The dependency of CN3685 virulence on the Agr-like regulatory system was shown, at least in part, to involve this system regulating intestinal CPB production (158).
Since the highly conserved agr-like operon present among most or all C. perfringens strains apparently does not encode the AgrA/AgrC two-component system of the S. aureus Agr QS operon (155, 156), it was proposed that C. perfringens uses the VirS/VirR system for responding to Agr-like regulatory system signaling (155). While this putative relationship may yet explain the regulation of some toxins by some C. perfringens strains, recent results indicated that Agr-like regulatory system signaling in this bacterium does not always require the VirS/VirR system (154). Specifically, while type D strain CN3718 was shown to depend upon the Agr-like regulatory system to produce wild-type levels of ETX, inactivating VirS/VirR had no effect on ETX production levels (154). This finding suggests that CN3718 regulates ETX production by using another of the ∼20 C. perfringens two-component systems instead of, or in addition to, VirS/VirR.
C. PERFRINGENS DISEASES
The major diseases caused by C. perfringens are summarized in Table 3 and are briefly discussed below.
TABLE 3.
Main diseases associated with C. perfringens in human and animals
| Type | Major toxin(s) | Human disease(s) | Animal disease(s) |
|---|---|---|---|
| A | Alpha-toxin | Human myonecrosis (gas gangrene) | Gas gangrene in sheep, cattle, horses, and other spp.; yellow lamb disease in sheep |
| Alpha-toxin, CPE | Human food poisoning; non-food-borne GI diseases | Enteritis in dogs, pigs, horses, foals, and goats | |
| Alpha-toxin, NetB | Not reported | Necrotizing enteritis in chickens | |
| Alpha-toxin, CPB2 | Not reported | Possible enteritis in pigs; possible enterocolitis in horses | |
| B | Alpha-toxin, beta-toxin, epsilon-toxin | Not reported | Necrotizing enteritis and enterotoxemia in sheep, cattle, and horses |
| C | Alpha-toxin, beta-toxin | Human enteritis necroticans | Necrotizing enteritis and enterotoxemia in pigs, lambs, calves, foals, and other spp. (usually neonatal) |
| D | Alpha-toxin, epsilon-toxin | Not reported | Enterotoxemia in sheep, goats, and cattle |
| E | Alpha-toxin, iota-toxin | Not reported | Enteritis in rabbits, lambs, and cattle |
Diseases Involving Primarily Chromosomal Toxin Genes
Histotoxic infections of humans and animals.
C. perfringens type A causes gas gangrene (clostridial myonecrosis) in humans (160–162). The disease is instigated by the infection of a wound by C. perfringens spores from the soil or GI tract; it is a typical disease of war, with gunshot wounds being one of the major causes of the traumatic damage that leads to infection. Surgical wounds, particularly those that affect the bowel, are also major causes of gas gangrene infections. Irrespective of its cause, injury leads to disruption of blood flow to the tissues and localized tissue ischemia, creating the conditions required for the germination of C. perfringens spores and the subsequent growth of vegetative cells and extracellular toxin production (5, 163). The result is extensive tissue necrosis that is characterized by an absence of a leukocyte influx into the infection site (160, 164). Genetic studies, which involved the construction and subsequent analysis of isogenic plc and pfoA mutants of a gas gangrene strain of C. perfringens type A, showed that CPA (PLC) is essential for virulence in the mouse myonecrosis model and that PFO, although not essential for disease, acts synergistically with CPA (46, 165). Unless promptly treated by a combination of antibiotic therapy and surgical debridement, and potentially by amputation, the disease is almost invariably fatal.
Ruminants, horses, and swine are also highly susceptible to C. perfringens histotoxic infections, whereas carnivores are rarely affected (166). The main predisposing factors for gas gangrene in animals include castration, shearing, penetrating stake wounds, injury to the female reproductive tract during parturition, and injection sites (166–168). The typical gross appearance of these infections include severe edema, emphysema, discoloration of the overlying skin, coldness of the affected areas, and general signs of toxemia, while histologically, there is coagulation necrosis of tissues with marked leukostasis (166–168). Little information is available on the pathogenesis of naturally occurring gas gangrene in animals. However, CPA and PFO are presumably the main virulence factors, since gas gangrene in sheep, cattle, horses, and other animals presents with clinical, gross, and microscopic changes almost identical to those described for the mouse model of C. perfringens type A gas gangrene, where these two toxins are of paramount importance.
C. perfringens type A food poisoning.
C. perfringens type A food poisoning is a human syndrome that currently ranks as the second most common bacterial food-borne disease in the United States, where a million cases/year occur (1, 169). C. perfringens type A food poisoning usually develops when meat or poultry products become heavily contaminated with a CPE-positive type A strain. In ∼75 to 80% of characterized cases, the causative type A strain carries a chromosomal, rather than a plasmid-borne, cpe gene (1, 170). The specific association of type A chromosomal cpe isolates with food poisoning likely involves the exceptional resistance properties of their spores (171–175). One major contributor to this resistance phenotype is the ability of type A chromosomal cpe strains to produce a unique small acid-soluble protein 4 (SASP-4) variant that binds spore DNA more tightly than the SASP-4 made by most other C. perfringens strains, thus offering greater protection against heat and other food-associated stresses (176, 177). Other factors such as reduced spore core size, which is indicative of a more dehydrated (and thus more stress-resistant) core, further contribute to the extreme resistance phenotype of spores made by most type A chromosomal cpe strains (174, 175).
Upon ingestion of heavily contaminated food, vegetative cells of a chromosomal cpe strain survive passage into the intestines, where they initially multiply but then soon sporulate (1); Spo0A and alternate sigma factors control both in vivo sporulation and CPE production (178–181). The toxin accumulates in the mother cell until it is released at the completion of sporulation, when the mother cell lyses. The released toxin then acts, as described above, to damage the intestines and trigger diarrhea and abdominal cramping (1). C. perfringens type A food poisoning symptoms typically have a ∼12- to 16-h incubation period and then resolve within 24 h (1). However, fatalities can occur in the elderly or in patients with reduced intestinal activity from medication side effects (182, 183). It is thought that this lethality results when the medication reduces intestinal motility and interferes with CPE-induced diarrhea, thus prolonging contact between CPE and the intestinal mucosa. Based upon animal model studies (184), this longer presence of CPE in the intestines could facilitate absorption of the toxin into the circulation to cause a lethal enterotoxemia. The presence of CPE in the circulation leads to binding of the toxin to the kidneys and liver, causing a massive release of potassium, which can produce hyperkalemia-associated heart failure and death.
Diseases Involving Primarily Plasmid-Encoded Toxins
CPE-associated type A human non-food-borne gastrointestinal disease.
Type A strains carrying a CPE plasmid cause ∼5 to 10% of all cases of human non-food-borne GI diseases, including antibiotic-associated diarrhea or sporadic diarrhea (185). It was proposed that these cases involve true infections, but some could involve an overgrowth of normal C. perfringens flora, since type A strains harboring a cpe-carrying plasmid are present in the GI tract of some healthy people (186–188). These CPE-associated human non-food-borne GI diseases, which occur more frequently in the elderly, are typically more severe and longer-lasting than most cases of C. perfringens type A food poisoning (185). CPE is clearly important for the pathogenesis of these illnesses, as described below.
Type C enteritis necroticans of humans.
C. perfringens type C isolates cause food-borne enteritis necroticans, which currently occurs sporadically throughout much of Southeast Asia and less commonly elsewhere (92, 189, 190).
After World War II, type C strains caused enteritis necroticans outbreaks (termed Darmbrand) in malnourished people in Northern Germany (191). A recent study showed that these Darmbrand strains carry and express both plasmid-borne cpb and cpe genes (40), although multilocus sequence typing (MLST) analyses conducted during that work also indicated that Darmbrand strains are otherwise genetically related to type A food poisoning strains carrying a chromosomal cpe gene. Of particular note, Darmbrand strains produce the same variant small acid-soluble protein as type A chromosomal cpe food poisoning strains, which likely contributes to the ability of these type C strains to form exceptionally resistant spores and thus facilitates their survival in the food environment.
In the 1960s to 1970s, type C-induced enteritis necroticans (known locally as pigbel) was very common in Papua New Guinea (PNG), causing >50% of the deaths occurring in children between 5 and 10 years of age (189, 190). The disease is clinically characterized by abdominal pain that develops 1 to 5 days after eating a high-protein meal. Pathologically, pigbel involves severe mucosal necrosis of the jejunum or ileum. The pathogenesis of pigbel in PNG is associated with a low-protein diet, which leads to limited production of pancreatic proteases. In addition, the major dietary item in the PNG highlands is the sweet potato, which contains a trypsin inhibitor. Therefore, when a child eats a meal containing sweet potato and meat contaminated with C. perfringens type C, coupled with a dietary background of protein subnutrition, little trypsin activity is present in the gut to degrade CPB. In Pigbel, type C isolates are usually introduced into the gastrointestinal tract by consumption of a contaminated meat (typically pork).
Avian necrotic enteritis.
C. perfringens type A-mediated necrotic enteritis is of major importance to the poultry industry (192, 193). The onset of this disease usually requires predisposing conditions such as (i) switching the birds to a high-protein diet that favors the rapid growth of C. perfringens in the gastrointestinal tract or (ii) prior infection with Eimeria spp., which presumably facilitates access to the enterocytes of either C. perfringens cells or their toxins.
The mechanism of pathogenesis of avian necrotic enteritis has been the subject of some controversy. For many years, CPA was thought to be the major toxin required for virulence, but it has now been shown that a plc null mutant is virulent in a chicken necrotic enteritis model (194). Nonetheless, CPA may still play a role in the disease process since CPA has at least some immunoprotective properties (195, 196). The essential toxin in avian necrotic enteritis is now established as NetB based upon studies using netB null mutants (11) and recent vaccination studies that provide evidence that NetB is immunoprotective (197, 198).
C. perfringens enteritis/enterotoxemia of other (nonhuman) mammals.
(i) CPE-positive type A infections of animals.
Some case reports suggested that CPE also causes GI disease in domestic animals and possibly wild animals. For example, one study showed the presence of cpe-positive type A isolates and CPE in the small intestines of a goat kid suffering from necrotic enteritis (199). Additionally, fecal CPE and CPE-positive fecal isolates have been associated with canine diarrhea (200), and cpe-positive strains were suggested to cause recurrent diarrhea in dogs. In horses, fecal CPE was detected in ∼20% of adults with diarrhea and ∼30% of foals with diarrhea, while no fecal CPE was detected in healthy adult horses or foals (201).
(ii) CPE-negative C. perfringens type A.
Type A strains are rarely implicated in enteric disease of animals (22, 202), but they do cause yellow lamb disease (203), which is a rare form of acute enterotoxemia in lambs characterized by severe hemolysis, jaundice, and hemoglobinuric nephrosis. Most of the clinical signs and lesions of yellow lamb disease are attributed to the effects of CPA, although there is little evidence to support this claim. CPB2-producing C. perfringens type A has also been linked to disease in several animal species, including horses, sheep, and goats (94, 204–206); however, this association is circumstantial and based mainly upon isolation of CPB2-positive C. perfringens from sick animals. Similarly, some studies have reported more isolation of CPB2-positive type A strains from sick than from healthy pigs (204, 206).
(iii) C. perfringens type B.
Type B-mediated disease has been described in sheep, cattle, and horses; however, it is apparently restricted to parts of Europe, South Africa, and the Middle East (207). Disease by C. perfringens type B is characterized by sudden death or acute neurological signs with or without hemorrhagic diarrhea (3, 6, 208, 209).
Preliminary results suggest that both CPB and ETX are the most important toxins for the pathogenesis of type B infections in domestic animals (52). For example, without pretreatment with trypsin, CPB was found to be the main contributor to the lethal properties of type B supernatants using a mouse intravenous (i.v.) injection model, whereas seroneutralization studies with this model indicated that CPB and ETX are both important after trypsin pretreatment of type B supernatants (52). CPB is very sensitive to trypsin digestion, so animals with low levels of intestinal trypsin (such as neonates) are usually the most susceptible to infection by type B or C isolates (3, 6, 210). In contrast, ETX requires proteolytic activation via trypsin or other (intestinal or bacterial) proteases (97, 98). These opposing effects of trypsin on ETX and CPB activity suggest that when both toxins are present together in the intestine, such as during type B-associated infections, variations in intestinal conditions select for the predominant activity of ETX over CPB or vice versa. In animal model studies, at least some CPB produced by type B isolates remained active after trypsin treatment, but the overall lethality of most type B supernatants was lower after trypsin pretreatment (52).
(iv) C. perfringens type C.
Type C disease has been described for multiple animal species, including, but not limited to, sheep, cattle, horses, and pigs (3). Most type C infections occur in neonatal animals due, as mentioned above, to the lower trypsin levels in these animals, which favor CPB activity. Type C infection is characterized by sudden death or colic and diarrhea, with occasional neurological clinical signs observed. Histologically, the hallmark of type C infection is necrosis of the intestinal wall, which starts in the mucosa but usually progresses to affect all layers of the intestine. Fibrin thrombi occluding superficial arteries and veins of the lamina propria and submucosa are characteristic of this condition (207), and it was postulated (although not yet definitely proven) that vascular damage by CPB is an early event in type C infections (211, 212).
(v) C. perfringens type D.
Toxinotype D is by far the most common cause of clostridial enterotoxemia in sheep and goats and is occasionally the cause of clostridial enterotoxemia in other animal species (3). ETX is considered to mediate, in large part, the pathogenesis of C. perfringens type D disease; e.g., intravenous ETX injection in sheep and goats has been shown to reproduce most of the clinical signs and lesions of natural diseases in these species (213), and an intravenous ETX monoclonal antibody (MAb) was able to protect mice from intraduodenal challenge with type D strains (214). In enterotoxemia, ETX affects endothelial tight junctions in the brain (215), causing swelling and rupture of perivascular astrocyte processes (216). These effects are followed by increased capillary permeability (217), rapid extravasation of fluid (218), elevated intracerebral pressure, and parenchymal necrosis (215). In most animal species, type D disease is clinically characterized by neurological disease involving perivascular edema of the brain and, less frequently, by focal symmetrical encephalomalacia.
(vi) C. perfringens type E.
Toxinotype E has been linked to hemorrhagic enteritis and sudden death in beef calves and lambs (219). These strains may also cause enterotoxemia in rabbits, although suspected type E-induced disease in rabbits must be differentiated from that caused by C. spiroforme, which also produces a toxin similar to iota-toxin (220).
DEMONSTRATING THE PATHOGENIC ROLE OF PLASMID-BORNE TOXINS BY MOLECULAR KOCH'S POSTULATES
The association of each C. perfringens type with specific diseases strongly suggests that plasmid-borne toxins are important for pathogenesis, since most typing toxins are plasmid encoded. However, the application of molecular Koch's postulate analyses has now firmly demonstrated the involvement of several plasmid-encoded toxins in C. perfringens diseases, as described below. Although chromosomally encoded toxins are not the primary focus of this review, it should be noted that molecular Koch's postulates were first applied in C. perfringens research to demonstrate the pathogenic importance of (i) CPA and PFO for gas gangrene in mouse myonecrosis models (46, 165) and (ii) CPE when type A chromosomal cpe food poisoning strains cause gastrointestinal pathology in rabbit small intestinal loops (12).
CPE-Associated Type A Non-Food-Borne Human GI Disease
The application of molecular Koch's postulates definitively demonstrated that CPE is essential for the ability of the type A plasmid CPE sporadic diarrhea isolate F4969 to cause gastrointestinal pathology in animal models (12). Specifically, while sporulating culture lysates of wild-type F4969 caused fluid accumulation and histological damage in rabbit ileal loops, no intestinal pathology was observed by using similar sporulating culture lysates of an F4969 mutant in which the cpe gene had been inactivated by allelic exchange. The inability of the mutant lysates to cause intestinal pathology was attributable specifically to the loss of CPE expression, since pathogenicity could be restored by complementing the F4969 cpe mutant with a plasmid carrying the wild-type cpe gene.
Type A Avian Necrotic Enteritis
Analysis of a netB mutant derived by allelic replacement revealed that, unlike its isogenic parent strain, it was no longer able to cause disease in a chicken necrotic enteritis model. The ability to cause avian necrotic enteritis was restored when the mutation was complemented with the wild-type netB gene, providing clear evidence that NetB is a key toxin in the disease process (11).
Type C Enteritis and Enterotoxemia
CPB is both sufficient and required for type C-induced enteric pathology, as shown recently by the use of purified CPB or isogenic toxin null mutants of type C isolate CN3685 (210, 221). Similar to natural type C infection, late-log-phase vegetative cultures of CN3685 cause necrotizing enteritis in rabbit small intestinal loops. When isogenic toxin null mutants were prepared by using TargeTron technology and then tested in the same model, a double cpa pfoA null mutant of CN3685 remained virulent. However, two independent cpb null mutants were completely attenuated for virulence in this animal model, and reversal of the cpb mutation restored CPB production and intestinal virulence. Additionally, preincubation of wild-type strain CN3685 with a CPB-neutralizing monoclonal antibody rendered the strain unable to cause intestinal pathology. Finally, highly purified CPB alone was able to reproduce the intestinal damage of wild-type CN3685, and this damage could be prevented by preincubating purified CPB with a CPB monoclonal antibody (210). Other studies using CN3685 and its isogenic derivatives later showed that CPB production is also very important for this type C strain to cause lethality in mouse and goat intraduodenal challenge models of type C enterotoxemia (222, 223).
TOXIN PLASMIDS OF C. PERFRINGENS
Plasmid Diversity
While early studies of C. perfringens plasmids focused primarily on antibiotic resistance and bacteriocin plasmids (224–232), the first linkage of C. perfringens toxin production with plasmids occurred over 30 years ago, when loss of CPB production was shown to correlate with the disappearance of a plasmid from a type C strain (233). Later studies then definitively localized several toxin genes to extrachromosomal DNA in a few C. perfringens strains (234). By using Southern blot analyses of pulsed-field gels, long-range and overlapping PCR techniques, and sequencing, it has now been firmly established (Fig. 1) that the genes encoding beta2-toxin (cpb2), epsilon-toxin (etx), iota-toxin (iap/ibp), beta-toxin (cpb), TpeL (tpeL), lambda-toxin (lam), NetB toxin (netB), and (sometimes) enterotoxin (cpe) are carried on large plasmids (13, 15–20, 40, 235).
Fig 1.
Size diversity of C. perfringens plasmids encoding key toxins. Shared colors (other than black) indicate a similar (if not identical) plasmid; e.g., the 65-kb etx- and cpb2-carrying plasmid of type B strains is also apparently present in some type D strains. “seq” denotes a sequenced plasmid.
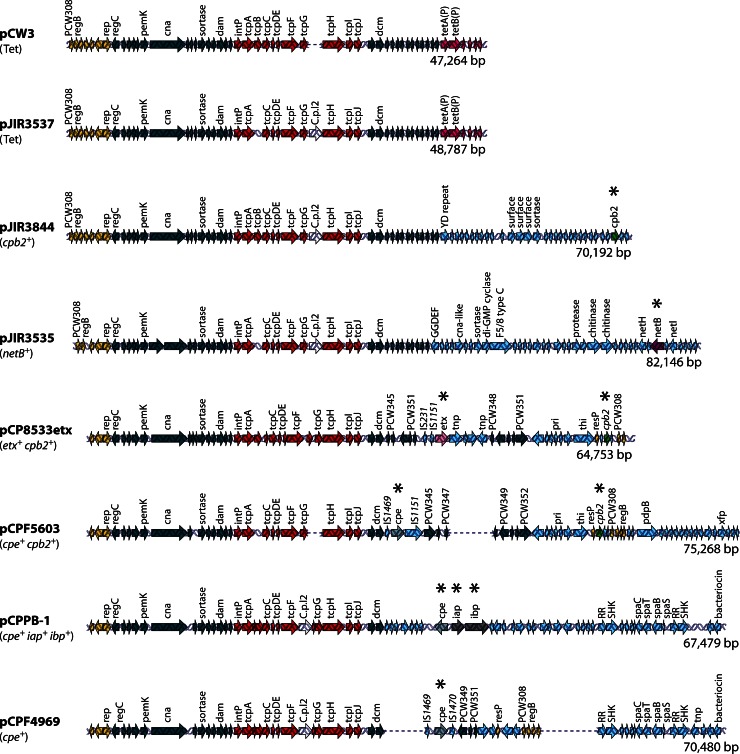
Complete sequencing of C. perfringens toxin plasmids remains challenging due to the presence of these plasmids at low copy numbers in C. perfringens cells and because these strains often contain several plasmids that are closely related. Therefore, to sequence a plasmid of interest, it is often necessary to first move that plasmid into a plasmid-free recipient strain. Nonetheless, when this review was being prepared, the complete sequences had been determined for three cpb2-carrying plasmids, a plasmid carrying both the etx and cpb2 genes, two cpe-carrying plasmids, two netB-carrying plasmids, and a plasmid carrying both the cpe and iab/ibp genes (Fig. 2) (13, 14, 19, 23, 235). The tetracycline resistance plasmid pCW3, which is often used as a paradigm plasmid for studying conjugative plasmid transfer in C. perfringens, has also been completely sequenced (Fig. 2) (236).
Fig 2.
Comparative alignment of sequenced C. perfringens plasmids. Shown are sequence alignments for pCW3 (236); pJIR3537 (tet+), pJIR3844 (cpb2+), and pJIR3535 (netB+) (19); pCP8533etx (etx+ cpb2+) (14); pCPF5603 (cpe+ cpb2+) (13); pCPPB-1 (cpe+ iota+) (23); and pCPF4969 (cpe+) (13). Each arrow represents an ORF; ORF arrows shown are as follows: red arrows, the conserved tcp locus (note the adjacent dcm ORF); dark blue arrows, other conserved ORFs shared by these plasmids; light purple arrows, tetracycline resistance gene; green arrows, the cpb2 toxin gene; purple arrows, the netB toxin gene; pink arrows, the etx gene; gray arrows, the cpe gene; dark gray arrows, the iota-toxin gene; yellow arrows, plasmid replication region; light blue arrows, regions unique to each plasmid. Asterisks denote a toxin gene. The GenBank accession numbers for the plasmid sequences are DQ366035 for pCW3, JN689220 for pJIR3537, JN689217 for pJIR3844, JN689219 for pJIR3536, AB444205 for pCP8533etx, AB236337 for pCPF5603, AB604032 for pCPPB-1, and AB236336 for pCPF4969. RR refers to response regulator, and SHK refers to sensor histidine kinase.
The cpe-carrying plasmids of type A strains.
The first sequenced C. perfringens plasmids (Fig. 2) carrying functional toxin genes were the CPE-encoding plasmids from two type A strains causing non-food-borne human gastrointestinal (GI) diseases (13). The 75.3-kb cpe-carrying plasmid (pCPF5603) of type A sporadic diarrhea isolate F5603 was shown to carry both cpe and cpb2 toxin genes, whereas the ∼70-kb plasmid pCPF4969 from type A sporadic diarrhea isolate F4969 lacks the cpb2 gene.
Overlapping PCR surveys and pulsed-field Southern blot analyses established that most type A CPE-associated non-food-borne human GI disease isolates carry either a pCPF5603-like or a pCPF4969-like cpe-carrying plasmid (13, 237). These two cpe-carrying plasmid families share a ∼35-kb conserved region encoding the tcp (transfer of clostridial plasmids) region, which can mediate C. perfringens toxin plasmid transfer, as discussed below. The pCPF4969 variable region contains genes encoding two putative bacteriocins and a two-component regulator similar to VirS/VirR, while the pCPF5603 variable region contains the functional cpb2 gene and several metabolic genes. Some isolates carrying a pCPF4969-like plasmid also possess a second plasmid encoding CPB2 (13, 20).
The netB- and cpb2-carrying plasmids of netB-positive avian type A strains.
A recent study (19) determined that NetB is encoded on a large conjugative plasmid in the type A avian necrotic enteritis strain EHE-NE18, which also carries two other large plasmids. High-throughput sequencing identified three closely related conjugative plasmids in this strain, including (i) the 82-kb plasmid pJIR3535, which encodes the netB gene and other potential virulence genes (Fig. 2); (ii) the 70-kb plasmid pJIR3844, which carries the cpb2 gene (Fig. 2); and (iii) a 49-kb tetracycline resistance plasmid, pJIR3537, that is very closely related to pCW3 (Fig. 2). Each of these three plasmids contains a highly conserved 40-kb region encoding plasmid replication and transfer functions, including a tcp conjugation locus similar to that found in pCW3 and pCPF5603-like and pCPF4969-like cpe-carrying plasmids. Other workers (226, 235) determined the sequences of two plasmids from a different necrotic enteritis-causing strain of C. perfringens, CP1. These plasmids, pNetB-NE10 and pCpb2-CP1, had the same genetic organization and 99.1% and 97.9% identity to pJIR3535 and pJIR3844, respectively. These data provide evidence that the netB- and cpb2-carrying plasmids present in necrotic enteritis strains of C. perfringens are highly conserved. This conservation extends to the pathogenicity locus NELoc1 (located on netB-carrying plasmids) and the locus NELoc3 (located on cpb2-carrying plasmids), which were previously shown to be associated with necrotic enteritis strains (24). Analysis of other necrotic enteritis strains (235) showed that NELoc1 was more highly conserved than NELoc3, which is consistent with the fact that it carries the netB gene. These data also confirmed that the chromosomal NELoc2 region is associated with necrotic enteritis-causing strains, as originally suggested (24).
The toxin plasmids of type B strains.
Type B strain CN8533 produces the two most lethal C. perfringens toxins, i.e., CPB and ETX. Sequencing (14) determined that this strain carries a ∼64.7-kb etx-carrying plasmid, named pCP8533etx, with the tcp conjugative transfer region and open reading frames (ORFs) encoding additional potential virulence factors such as CPB2 or collagen adhesion protein (Fig. 2). Notably, the cpb gene is not carried by this plasmid. Interestingly, nearly 80% of the pCP8533etx ORFs are also present on pCPF5603 (Fig. 2). Furthermore, Southern blot analyses and overlapping PCR results indicated that most, if not all, type B isolates carry an etx-carrying plasmid that is very similar, if not identical, to pCP8533etx (14, 16).
The cpb gene has been localized, by Southern blotting analyses of pulsed-field gels, to ∼90-kb plasmids in most type B isolates, although a few type B isolates carry a ∼65-kb cpb-carrying plasmid that is distinct from their etx-carrying plasmid (16). The cpb-carrying plasmids of type B strains were also shown to possess the tcp locus, suggesting that they are conjugative (16). Overlapping PCR analysis revealed that the tpeL toxin gene is located ∼3 kb downstream from the cpb gene in these plasmids (16). Finally, most type B isolates were shown to possess a third virulence plasmid carrying genes encoding urease and lambda-toxin (16).
The toxin plasmids of type C strains.
While type B strains carry either 65-kb or 90-kb cpb-carrying plasmids (16), the cpb-carrying plasmids of type C isolates exhibit greater size diversity, ranging from ∼65 kb to ∼110 kb (17). Note that almost all large toxin plasmids in type C isolates carry the tcp genes, suggesting that they are conjugative (17). Southern blot analyses of pulsed-field gels run with restriction enzyme-digested DNA showed that these ∼65-kb and ∼90-kb cpb-carrying plasmids of some type C isolates resemble the equivalent-sized cpb-carrying plasmids of type B isolates; e.g., these two cpb-carrying plasmids also carry a tpeL gene ∼3 kb upstream from their cpb gene (16, 17). However, in other tpeL-positive type C strains, the tpeL gene is located on a different plasmid from the cpb-carrying plasmid (17).
Some type C isolates possess ∼75- or ∼85-kb cpb-carrying plasmids that also carry the cpe gene (17). However, a few type C strains have their cpe gene on an ∼110-kb plasmid that is distinct from their cpb-carrying plasmid (17, 40). Interestingly, among surveyed type C strains, no cpe-positive isolates were found to carry the tpeL gene (17). While some type C strains possess cpb2 genes on plasmids ranging in size from ∼65 to ∼90 kb, those cpb2-carrying plasmids are distinct from the cpb-carrying plasmid present in these isolates (17).
Toxin plasmids of type D strains.
Unlike type B etx-carrying plasmids, the etx-carrying plasmids of type D strains exhibit considerable size diversity (15). For type D isolates lacking the cpe or cpb2 gene, the etx gene is generally present on an ∼48-kb plasmid, although a few type D strains carry larger (∼73- to 75-kb) etx-carrying plasmids (15). For type D isolates possessing the cpe and/or the cpb2 gene, the etx gene is located on large plasmids ranging in size from ∼75 to 110 kb (15). In these type D isolates, their cpb2 gene is present on ∼45- to 85-kb plasmids, most commonly 75-kb plasmids, while their cpe gene is carried on large plasmids ranging from 75 kb to, most commonly, ∼110 kb (15). A few type D strains apparently carry the same 65-kb etx- and cpb2-carrying plasmid found in type B strains (14). For most type D isolates, their toxin plasmids also have the tcp locus genes essential for conjugative transfer (15), and conjugative transfer has been demonstrated for two type D etx-carrying plasmids (21).
Toxin plasmids of type E strains.
Two major families of iota-toxin plasmids have been identified, the first of which includes large plasmids, varying in size from ∼97 kb to ∼135 kb, with a pCPF5603 backbone (18). These iota-toxin plasmids carry functional iap/ibp genes, but their adjacent cpe sequences are silent due to extensive mutations in the cpe gene (18, 22). This iap/iab-carrying plasmid family also encodes urease and lambda-toxin (18). The second iota-toxin plasmid family, which includes the recently sequenced plasmid pCPPB-1, carries expressed iap/ibp and cpe genes (23). This ∼65-kb plasmid has a pCPF4969 backbone but does not encode lambda-toxin or urease (23). In all examined type E isolates, the iap/ibp-carrying plasmid has a tcp locus, strongly suggesting that these plasmids are conjugative (18, 23).
Relationship between C. perfringens toxin plasmids.
Emerging evidence indicates that many, although not all, C. perfringens toxin plasmids are related to either pCPF5603 or pCPF4969 and carry the same tcp sequences also found in some conjugative antibiotic resistance plasmids, e.g., pCW3. For example, the etx-carrying plasmid present in most or all type B isolates, and a few type D isolates, resembles pCPF5603 (13, 14). Similarly, the netB-derived plasmids pJIR3536 and pNetB-NE10 share ∼35 kb of conserved backbone (Fig. 2) with pCPF5603 and pCW3 (13, 19, 235, 236). As mentioned above, some type E iota-toxin-encoding plasmids share substantial similarity with pCPF5603 (18), while others more closely resemble pCPF4969 (23).
The similarity of many C. perfringens toxin plasmids may impact plasmid carriage and, by extension, toxin production and virulence. For example, no C. perfringens isolate has been found to carry both iap/ibp genes and the cpb or etx gene, suggesting fundamental plasmid incompatibility issues. However, some toxin plasmid combinations can be stably maintained in a single C. perfringens cell; e.g., some chicken necrotic enteritis strains can carry three related plasmids, including two different toxin plasmids, while type B isolates carry their cpb and etx genes on separate plasmids (13, 16, 19). In this regard, it is notable that the cpb-carrying plasmids and etx-carrying plasmids in type B strains are much less diverse than the cpb-carrying plasmids in type C strains or the etx-carrying plasmids of type D strains (15–17), further suggesting that only certain plasmid combinations can be stably maintained in the same C. perfringens cell.
As mentioned above, most of the examined C. perfringens toxin plasmids carry the tcp locus, which mediates conjugative transfer of C. perfringens plasmids (see below). Therefore, when different C. perfringens strains make physical contact, conjugative exchange of their toxin plasmids may occur, which may sometimes be followed by the loss of one toxin plasmid in a recipient strain due to plasmid incompatibility. However, in certain situations (e.g., the type A cpe-positive strains that carry cpe and cpb2 on separate plasmids, type B strains, and type A avian necrotic enteritis strains), the two toxin plasmids can be stably maintained together, thus enhancing virulence diversity.
Association of C. perfringens Toxin Genes with Insertion Sequences
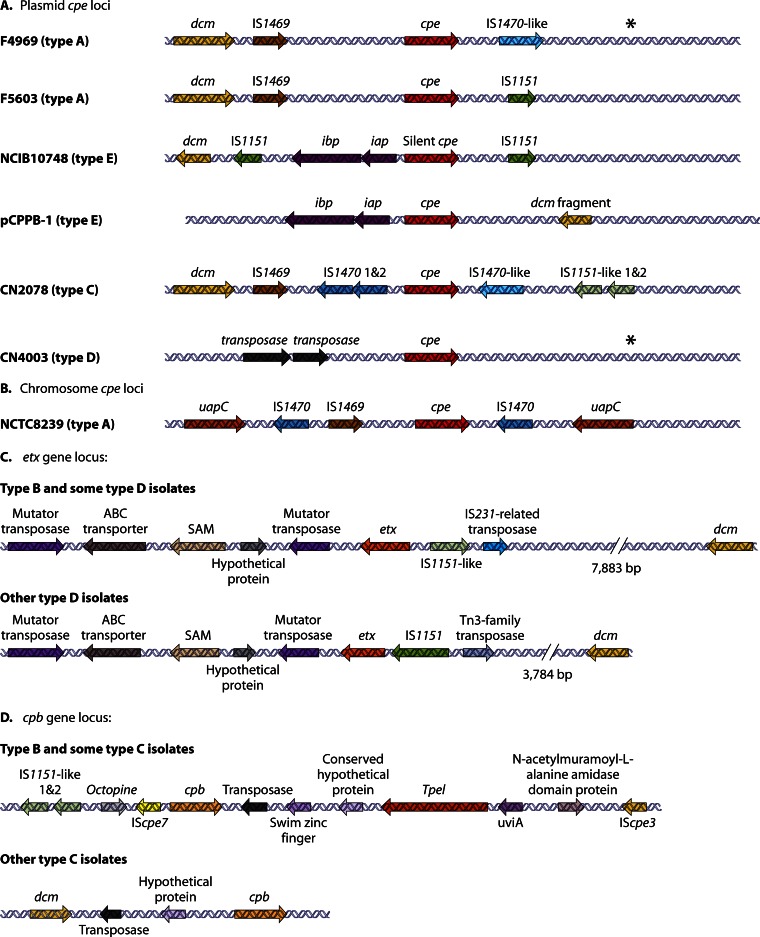
As mentioned above, in 75 to 80% of type A food poisoning isolates, the cpe gene is chromosomal (1, 170, 187, 237) and located near an upstream IS1469 sequence and flanking IS1470 sequences (Fig. 3). This structure resembles that of a compound transposon (238); however, IS1470-mediated transposition of the cpe gene has not yet been demonstrated. This genetic organization differs from that of the plasmid-determined cpe loci (Fig. 3); i.e., in pCPF5603-like plasmids, the cpe gene is flanked by an upstream IS1469 sequence and a downstream IS1151 sequence, while the cpe gene in the pCPF4969 plasmid family is flanked by an upstream IS1469 sequence and a downstream IS1470-like sequence (13).
Fig 3.
Organization of toxin (cpe, etx, and cpb) loci in type A, B, C, D, and E strains of C. perfringens. (A) Organization of plasmid-borne cpe loci in type A, E, C, and D strains. (B) Organization of the type A chromosome cep locus. (C) Organization of plasmid-borne etx loci in type B and D strains. (D) Organization of plasmid-borne cpb loci in type B and C isolates. Each arrow represents an ORF. Asterisks indicate a region with similarity to sequences present downstream of the cpe gene in F4969, except for the absence of an IS1470-like gene. (Panels A and B adapted from reference 26; panel C adapted from reference 14; panel D adapted from references 16 and 17.)
Approximately 15% and 25% of type C and D isolates, respectively, carry plasmid-borne cpe genes that are identical to the type A cpe gene (26). However, the genetic organization of the cpe locus varies between these type C and D strains and the plasmid cpe locus found in type A strains (Fig. 3). Most cpe-positive type C isolates possess a cpe locus similar to that found in the chromosomal cpe locus of type A isolates, except that (i) the IS1469 sequence is located upstream of an IS1470 sequence and (ii) there is an IS1151-like sequence located downstream of the cpe gene in these type C strains (26). One unusual type C cpe locus that is missing the two copies of IS1470 found in the cpe locus of most type C cpe-positive strains has been identified (26).
The type D cpe locus (Fig. 3) has a unique genetic organization (26). There are two copies of an ORF with 67% identity to a Tn1456-like transposase gene (COG4644) located upstream of the cpe gene. The region downstream of the cpe gene is organized similarly to the sequences downstream of the cpe gene in type A isolate F4969, except for the absence of an IS1470-like insertion sequence (IS) (26).
In all studied cpe-positive type E isolates, the iota-toxin genes are located in close proximity to the cpe promoter region, suggesting an insertional event (Fig. 3). In pCPF5603-like iota-toxin plasmids, this putative insertion appears to have silenced the cpe promoter, leading to a loss of cpe expression (18, 22). In these type E strains, the locus carrying iab/ibp genes and silent cpe sequences lies between two IS1151-like insertion sequences, but again, there is no direct experimental evidence that this putative compound transposon can transpose (18, 22). In contrast, for the pCPPB-1 family of iota-toxin plasmids, only one of three cpe promoters was apparently inactivated by insertion of the iap/ibp genes, so the cpe gene is still transcribed (23).
Two variations of the etx gene locus have been identified (Fig. 3). Most type B strains, and a few type D strains, have an etx locus similar to the pCPF5603 cpe locus, with IS1151-like and IS231-related transposase gene sequences located upstream of the etx gene. In contrast, the etx locus of most type D strains contains an IS1151 sequence and a Tn3-like transposase gene upstream of the etx gene. All etx loci have the same mutator transposase sequence located downstream of their etx gene (14).
Similarly, all type B strains and some type C isolates have a similar cpb locus (16, 17), with the cpb gene downstream of IScpe7 and IS1151 sequences but upstream of a Tn3-like transposase gene. The tpeL gene is also located downstream of this cpb gene (Fig. 3). In addition, another IScpe3 sequence gene is present upstream of the tpeL gene. Other type C strains have the same upstream IScpe7 and IS1151-like sequence but lack the downstream tpeL gene.
Evolution of Characterized C. perfringens Toxin Plasmids
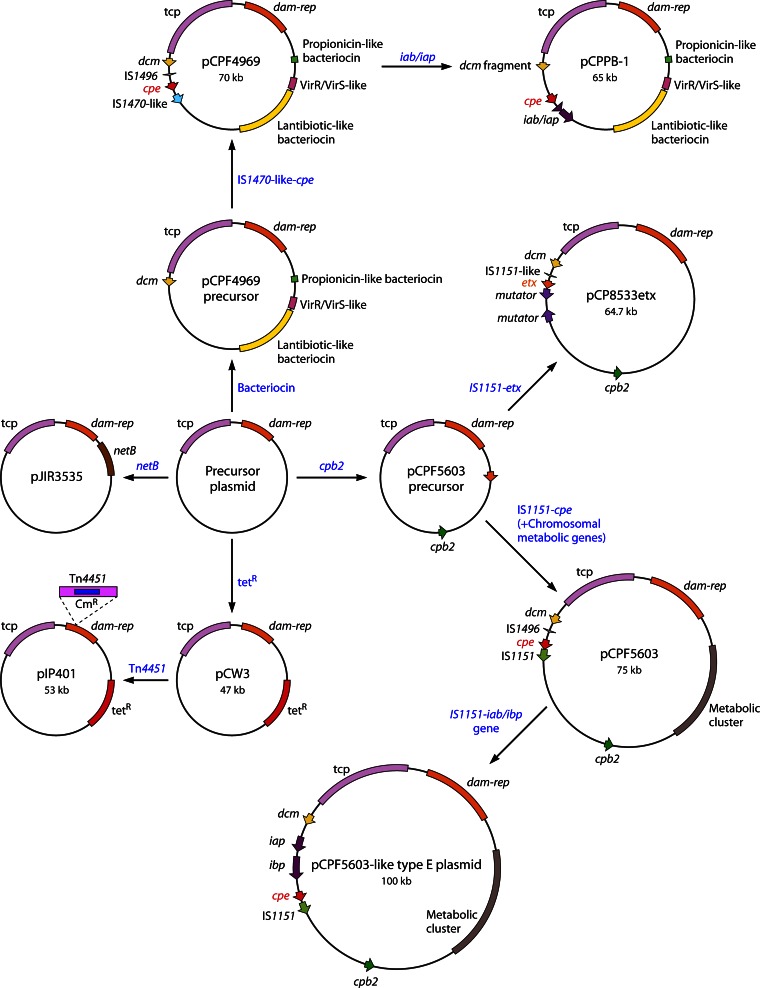
Many C. perfringens toxin gene loci are located near the dcm gene (Fig. 3), which may represent a hot spot region for the insertion of toxin gene-carrying mobile genetic elements (13–18, 26). Some indirect evidence supports this hypothesis. For example, although IS-mediated movement of plasmid-borne C. perfringens toxin genes from one location to another has not been directly demonstrated, toxin gene-carrying circular DNA molecules that potentially represent transposition intermediates have been detected (15, 16, 18, 26, 40, 238). Specifically, those circular intermediates can carry the cpe genes of type A, C, and D isolates, the iota-toxin genes of type E isolates, the cpb-tpeL genes of type B isolates, or the etx genes of type D isolates. We postulate that IS-mediated movement of toxin genes may help to explain why some C. perfringens toxin genes are found on different plasmid backbones.
While overlapping PCR analyses have strongly suggested that some C. perfringens toxin plasmids have a different (but as-yet-uncharacterized) backbone from the pCPF5603- or pCPF4969-like toxin plasmids (15, 17), all of the sequenced toxin plasmids share considerable homology with these two cpe-carrying plasmid families and pCW3, the paradigm conjugative plasmid from C. perfringens. This observation provides considerable insight into the possible origin and evolution of the C. perfringens toxin plasmids (Fig. 4). Both the pCPF5603- and pCPF4969-like toxin plasmids contain two regions (dam-rep) and tcp, which are also present on the pCW3 tetracycline resistance plasmid (13, 14, 18, 23, 227, 235, 236). Since the tcp region has homology with Tn916, which is a conjugative transposon, it is conceivable that a Tn916-like transposon may have integrated into a plasmid, creating a conjugation-capable precursor plasmid (13, 236).
Fig 4.
Model for evolution of characterized C. perfringens toxin plasmids. See the text for discussion of the possible evolution of pIP401 (230), pCW3 (236), pJIR3535 (netB+) (19), pCP8533etx (etx+ cpb2+) (14), pCPF5603 (cpe+ cpb2+) (13), pCPPB-1 (cpe+ iota+) (23), and pCPF4969 (cpe+) (13). Each box color depicts a different region of importance on the toxin plasmids, as indicated.
This putative conjugative precursor plasmid, which has not yet been identified, may then have acquired or lost genes by transposition or recombination events. In some cases, the acquired genes encoded antibiotic resistance. For example, the first C. perfringens plasmids shown to be capable of conjugative transfer, i.e., pCW3 (239) and pIP401 (226), both encode tetracycline resistance but lack toxin genes. Furthermore, pIP401 is a pCW3-like plasmid that acquired the chloramphenicol resistance transposon Tn4451 (240).
At other times, the putative conjugative precursor plasmid may have acquired mobile genetic elements carrying toxin genes. For example, if a mobile element carrying both IS1470-like sequences and the cpe gene integrated into the precursor plasmid, the result would have been a pCPF4969 toxin family plasmid. Alternatively, if this precursor plasmid acquired a mobile element carrying IS1151-cpe sequences or IS1151-etx sequences, it would have given rise to pCPF5603-like cpe-carrying plasmids or the pCP8533 etx-carrying plasmids, respectively. In one C. perfringens strain carrying pCPF5603, an IS1151–iota-toxin element apparently then inserted into the cpe promoter, silencing the cpe gene and creating the pCPF5603-like family of iota-toxin plasmids. In another C. perfringens strain carrying pCPF4969, we postulate that a similar mobile element carrying IS1151–iota-toxin genes inserted slightly upstream of the cpe gene, giving rise to the pCPBB-1 family of type E toxin plasmids carrying functional iota-toxin genes and cpe genes.
Conjugative Transfer of Toxin Plasmids
To date, five toxin plasmids have been shown experimentally to be conjugative, but virtually all of the large toxin plasmids of C. perfringens carry a tcp conjugation locus that is very closely related to the tcp conjugation region of pCW3 and therefore are highly likely to be conjugative. The first toxin plasmid shown to be conjugative was pMRS4969, a genetically marked derivative of the CPE plasmid pCPF4969 (241). Mixed-plate matings into suitable recipient strains of C. perfringens were used to demonstrate that pMRS4969 transferred by conjugation at a high frequency (2.0 × 10−2 to 4.6 × 10−4 transconjugants per donor cell). Cell-to-cell contact was required for transfer. The resultant transconjugants carried the same plasmid that was present in the donor strain and could also act as a conjugation donor, at a similarly high frequency, providing evidence that this plasmid carried a functional conjugation locus. Finally, Southern blots provided evidence that pMRS4969 carried regions that were also present on pCW3, which at the time had not been sequenced. It was postulated that these regions were involved in conjugative transfer, a postulate that was subsequently validated (236).
More recent studies (21) have demonstrated that the etx-carrying plasmids from two C. perfringens type D strains, CN1020 and CN3718, are also conjugative. Initial mating experiments using etx-carrying plasmid derivatives in which the etx gene was insertionally inactivated by the catP gene showed that both strains contained plasmids that also transferred at very high frequencies (2.9 ×10−1 to 3.8 × 10−2). Once more, the transconjugants could act as donors in subsequent matings. These transfer frequencies were so high that further matings conducted with one of the wild-type type D strains yielded detectable transfer frequencies in the absence of any antibiotic selection (21). These experiments have shown that a toxinotype A strain can be converted to a genotypic toxinotype D strain by conjugation, a process which we postulate is likely to occur in the gastrointestinal tract, with potential disease significance (see Concluding Remarks). These results also illustrate the genetic plasticity of toxin types, since most toxin type genes are probably located on conjugative elements.
Finally, as described above, a chicken necrotic enteritis strain, EHE-NE18, has been shown to carry three closely related plasmids, encoding NetB toxin production, CPB2 toxin production, and tetracycline resistance, respectively (19). It was a relatively straightforward process to show that the tetracycline resistance plasmid, which was almost identical to pCW3, was conjugative. In addition, by separately genetically marking the netB and cbp2 toxin genes, it was demonstrated that their host plasmids also were independently conjugative. Cotransfer experiments showed that when the transfer of the netB-carrying plasmid was selected, the rate of cotransfer of the tetracycline resistance plasmid was very high (90%), but when transfer of tetracycline resistance was selected, cotransfer of the netB-carrying plasmid was only 1% (19). Sequence analysis showed that all three plasmids carried a pCW3-like tcp conjugation locus. To our knowledge, this was the first time that a bacterial strain had been shown to carry three independently conjugative plasmids that all have virtually the same conjugation locus. A similar situation is also probably common among C. perfringens type B, C, and D strains, since they often carry two or more toxin plasmids with a tcp locus (15–17).
All conjugative C. perfringens plasmids identified to date have the tcp locus, which has been demonstrated to be essential for conjugative transfer of pCW3 (236, 242–245). Furthermore, either sequence analysis (13, 14, 23) or Southern hybridization analysis (14–18) indicated that many C. perfringens type B to E strains contain multiple large plasmids carrying toxin genes (cpb, etx, iapA/iapB, cpb2, and tpeL) and a tcp locus, which we assume is a reasonable predictor of their conjugative potential. Similarly, it has been shown that necrotic enteritis strains of C. perfringens type A also carry multiple plasmids that all have the tcp locus (19, 235).
Functional Analysis of the tcp Conjugation Locus
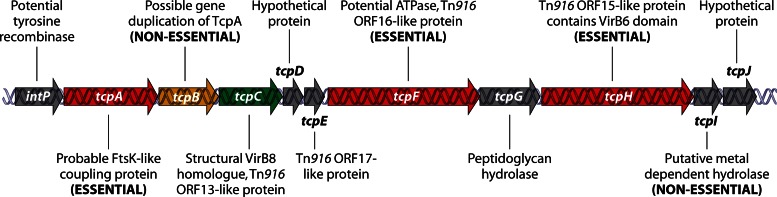
Analysis of the conjugation mechanism in C. perfringens has focused on the tetracycline resistance plasmid pCW3 (224, 227, 228), which is 47,263 bp and encodes 51 potential open reading frames (236). As mentioned above, a conjugation locus of 11 genes, intP to tcpJ, has been designated the transfer of clostridial plasmid (tcp) locus. This locus is present in all known conjugative C. perfringens plasmids and is related to the conjugation locus from the Tn916 conjugative transposon family. Detailed mutagenesis and complementation studies (244) have shown that many of these tcp locus genes are required for the optimal conjugative transfer of pCW3 (Fig. 5).
Fig 5.
The pCW3 tcp locus. The genetic organization and protein products of the tcp locus are shown. (Adapted from reference 244 with permission of the publisher [copyright 2012 Blackwell Publishing Ltd.].)
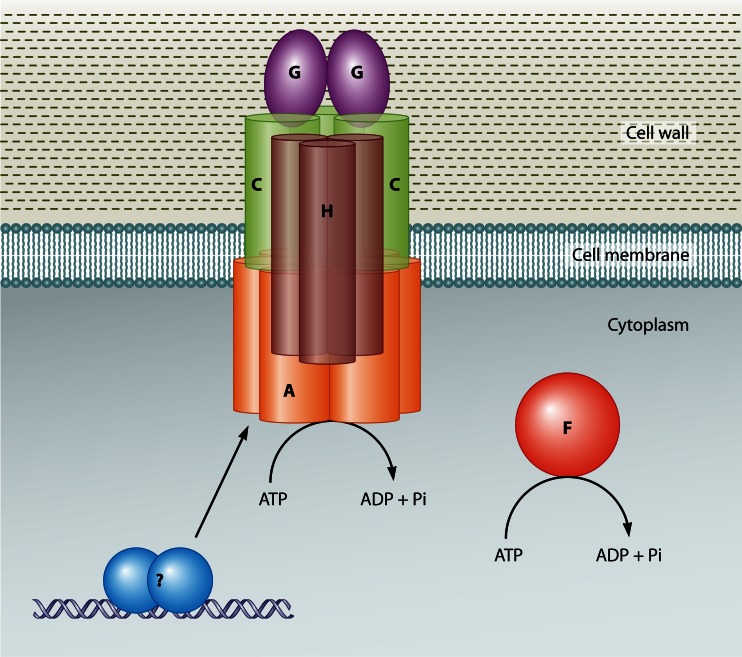
Plasmid conjugation systems generally consist of two components: (i) a relaxosome-DNA complex that includes a plasmid-encoded relaxase enzyme, which binds to the plasmid and nicks one strand at the origin of transfer (oriT) site, and (ii) a membrane-bound transfer apparatus through which a relaxase–single-stranded-DNA (ssDNA) complex passes from the donor strain into the recipient (246). There is no apparent relaxase gene carried on pCW3 (236), but the first gene in the putative tcp operon is intP, which likely encodes a tyrosine recombinase that may act as the functional equivalent to a relaxase in the pCW3 transfer process.
The tcpA gene product is essential for conjugation: tcpA mutants cannot transfer, and conjugation proficiency is restored by complementation with the wild-type tcpA gene (243). The next gene, tcpB, appears to be a truncated variant of tcpA that is probably derived from a gene duplication event. It is not present in many of the conjugative C. perfringens plasmids and is not required for conjugative transfer. TcpA apparently functions as the coupling protein that docks the putative relaxosome complex to the conjugation apparatus. It has two N-terminal transmembrane domains and a cytoplasmic domain that includes an FtsK-like ATPase domain found in proteins of the DNA translocase superfamily (247). These proteins include FtsK and SpoIIIE, which are involved in double-stranded-DNA (dsDNA) translocation, and coupling proteins from plasmid conjugation systems. Mutagenesis studies showed that both the ATPase motifs present in TcpA and an FtsK-like RAAG motif are essential for TcpA function (243).
Since TcpA was proposed to act as a coupling protein, it was anticipated that it should undergo protein-protein interactions with other components of the C. perfringens conjugation apparatus. TcpA forms homodimers and interacts with TcpC, TcpG, and TcpH, all of which are encoded within the tcp locus (248). Mutation of the ATPase domain of TcpA reduced TcpA homodimer formation, and deletion of the putative TcpA N-terminal transmembrane domains also affected plasmid transfer. Analysis of the latter derivative showed that it had reduced TcpA self-interaction as well as less interaction with TcpC and TcpH.
Mutagenesis of the tcpC gene revealed that it was required for optimal conjugative transfer; a tcpC mutant has a transfer frequency that is 5 orders of magnitude lower than that of the wild type. Again, complementation restored activity to wild-type levels (244). The TcpC protein has 24% amino acid sequence identity to ORF13 from Tn916 (236), but neither of these proteins has any domains suggesting protein function. However, TcpC does have an N-terminal transmembrane domain, and it was shown that this domain is required for TcpC function, for its independent localization to the C. perfringens cell envelope, and for its protein-protein interactions (244). The soluble C-terminal region of TcpC has been purified, and its crystal structure was determined to a resolution of 1.8 Å (244). TcpC crystallized as a trimer, with each monomer consisting of two domains: the central and C-terminal domains. These domains are structurally related to each other and to the structure of the periplasmic region of VirB8, which is an important component of the type IV T-DNA conjugation system from Agrobacterium tumefaciens. VirB8 is thought to function as a scaffolding protein that promotes transfer complex assembly and stabilization (249, 250). Note that although TcpC and VirB8 have structural similarity, they have no amino acid sequence identity. Protein-protein interaction studies showed that TcpC interacts with itself, TcpA, TcpG, and TcpH. Within the structure of the TcpC trimer, the central domains are located internally and form most of the contacts within the trimer, which is consistent with this domain being important for TcpC homooligomerization. In contrast, the C-terminal domain occupies most of the external surfaces, and protein-protein interaction analysis showed that this domain is most important for interactions with TcpA, TcpG, and TcpH (244). These results suggest that TcpC may play a role similar to that of VirB8 in the assembly and stability of the pCW3 conjugation apparatus.
TcpD and TcpE are small putative transmembrane proteins of 115 and 122 amino acids, respectively (236). TcpD has no sequence similarity to proteins in the database, but TcpE has 27% identity to the ORF17 conjugation protein from Tn916, the precise function of which is unknown. Similarly, the role of the tcpD and tcpE genes, as well as the tcpJ gene, which encodes a hypothetical protein, during the conjugative transfer of pCW3 is unclear.
The TcpF protein contains a conserved ATPase domain, is related to ORF16 from Tn916, and is likely to provide at least some energy required to drive plasmid DNA transfer. It is essential for conjugation, since mutagenesis of the tcpF gene eliminates pCW3 transfer, which subsequently can be restored by complementation (236). Immunofluorescence studies have shown that TcpF and TcpH are localized to the poles of donor cells, suggesting that pCW3 is transferred through a conjugation apparatus located at the cell poles (242).
TcpH is a large 832-amino-acid protein that has similarity to ORF15 from Tn916 and has been shown to be essential for pCW3 transfer (236). It has an N-terminal region with eight putative transmembrane domains, including a VirB6-like region, and a putative cytoplasmic C-terminal domain. TcpH is located in the cell envelope of C. perfringens (242). Mutagenesis studies have shown that the N-terminal domain (amino acids 1 to 581), a conserved 242VQQPW246 motif, and transmembrane domains 5 to 8 (from amino acids 311 to 450), which include the VirB6-like domain, are essential for TcpH function (242). A combination of bacterial two-hybrid experiments and protein-protein interaction studies showed that TcpH interacts with itself, TcpA, and TcpC (242, 248) and that the N-terminal domain, but not the VQQPW motif, is required for these interactions (242). It is proposed that TcpH is the major structural protein of the pCW3 conjugation apparatus and that it forms the transmembrane channel through which the plasmid DNA complex passes from the donor to the recipient cell.
Peptidoglycan hydrolases are commonly associated with bacterial conjugation systems, presumably facilitating the formation of the conjugation apparatus in the cell wall. Unusually, the tcp locus appears to encode two functionally distinct peptidoglycan hydrolases, TcpG and TcpI. Mutagenesis studies have shown that TcpG, but not TcpI, is required for optimal transfer of pCW3 (245). TcpG has peptidoglycan hydrolase-like activity. It has two putative catalytic domains, an N-terminal muramidase-like FlgJ glucosaminidase domain and a C-terminal NlpC/P60 endopeptidase domain, both of which have been shown to be functional (245). TcpG interacts with TcpA (248) and TcpC (244) but not with TcpH (242). Based on these data, a model for the pCW3 conjugation apparatus has been proposed (244, 248), as shown in Fig. 6. In this model, the VirB6-like TcpC protein acts as a scaffolding protein that helps form a complex at the cell envelope. This complex includes TcpC, the coupling protein TcpA, the peptidoglycan hydrolase TcpG, and TcpH, which is proposed to form the cell wall pore through which the plasmid DNA is transported into the recipient cell. Further genetic and structural studies are required to determine the roles of the putative IntP, TcpD, and TcpE proteins and to determine how these proteins, and TcpF, interact with the conjugation apparatus.
Fig 6.
Putative model of the pCW3 conjugation apparatus. The relative locations and known protein-protein interactions of the TcpA (A) (orange), TcpH (H) (brown), TcpC (C) (green), TcpG (purple), and TcpF (F) (red) proteins are based on data from previous studies (236, 242, 244, 245, 248). (Based on a model prepared by Jessica Wisniewski, Monash University.)
Replication of Toxin Plasmids
An important factor in the ability of plasmids to replicate autonomously is the self-encoded plasmid replication initiator protein, often referred to as the Rep protein. This protein recognizes plasmid-specific DNA sequences and determines the point from which replication starts. Rep proteins generally share signature domains that enable them to be assigned to one of several replication initiation families (251).
Unexpectedly, analysis of the nucleotide sequences of the large conjugative clostridial plasmids, including pCW3, failed to identify a potential replication protein based on amino acid sequence identity or domain searches. Subcloning of pCW3 and analysis of the replication ability of the resultant derivatives identified a 3,918-bp fragment that encoded the ability to replicate independently in C. perfringens (236). Subsequent transposon mutagenesis studies of a recombinant shuttle plasmid containing this region then led to the identification of the rep gene carried by pCW3. Transposon insertions that mapped to the rep gene resulted in an inability of the shuttle plasmid to replicate in C. perfringens. The region upstream of the rep gene contained four 17-bp direct repeats that were postulated to act as the iteron-like sequences that presumably would be required for the Rep-mediated initiation of plasmid replication (236). The putative Rep protein had no similarity to proteins or motifs of known function in the databases but had a pI of 10, in keeping with its proposed function as a DNA binding protein. An almost identical Rep protein, with 95 to 100% amino acid sequence identity, is encoded by all of the sequenced conjugative toxin and resistance plasmids described in this review, providing evidence that all of these plasmids replicate by the same mechanism. The fact that these Rep proteins are unique to C. perfringens may explain why this family of plasmids has not been detected in any other species (236). The tetracycline and chloramphenicol resistance plasmid pIP401, which is closely related to pCW3, can transfer by conjugation from C. perfringens to C. difficile, but the resultant transconjugants appear to be unstable (252), presumably because the Rep protein is not functional in C. difficile. Nonetheless, such conjugation events may explain why very closely related mobilizable chloramphenicol resistance transposons, Tn4451 and Tn4453, are found in C. perfringens and C. difficile, respectively (253), and how the pCW3-encoded Tet(P) tetracycline resistance determinant has moved to Clostridium septicum and Clostridium sordellii (254). Similar events could explain the presence of related toxin genes in several different clostridial species (see Concluding Remarks).
Finally, it is very common for C. perfringens strains to carry several very closely related, but independently conjugative, plasmids that carry different toxin or resistance genes (15–19). There is no real precedent for this observation in other bacterial species, which raises the question as to what is the basis for the compatibility of these plasmids. It was suggested (19) that differences in a parRMC locus located upstream of the common rep gene may be responsible. In agreement with this hypothesis, other workers (235) analyzed the known parRMC loci of all of the sequenced plasmids that carry the tcp locus and divided them into four distinct groups. These groups are consistent with the known compatibility of the conjugative plasmids, but it is essential that this hypothesis be verified experimentally.
CONCLUDING REMARKS
It has now been established that C. perfringens maintains a large pool of closely related plasmids that are potentially moving from one cell to another via conjugation. These plasmids also have regions that seem to act as hotspots for the integration of mobile elements that are associated with plasmid-carried toxin genes. The net consequence is that some C. perfringens cells now carry multiple (at least up to three) different toxin plasmids. However, plasmid incompatibility issues apparently place some limitations on the total repertoire of toxin plasmids that can be maintained by a single C. perfringens bacterium. Perhaps the best evidence for toxin plasmid incompatibility issues is the absence of certain toxin plasmid combinations; e.g., C. perfringens strains carrying both iota-toxin- and cpb-harboring plasmids are never identified.
It is also now clear that a single toxin gene can reside on many different plasmids among the C. perfringens population. Since these plasmids often share large (∼35 kb) regions of identical sequences, there must be strong selective pressure to maintain this large pool of different toxin plasmids, or, considering their extensive shared regions of sequence identity, homologous recombination would otherwise rapidly lead to the evolution of plasmids that carry the conjugation locus and numerous toxin genes. However, there does appear to be some evolutionary movement toward that eventual outcome, as individual C. perfringens plasmids that can carry up to three different toxin genes have been identified.
C. perfringens likely maintains a large number of toxin genes on different conjugative plasmids because this strategy offers enormous virulence plasticity and adaptability. One example illustrating this principle would be the presence of cpb and etx genes on two different plasmids. Type C strains carrying only a CPB plasmid cause disease in hosts with lower intestinal trypsin levels due to age, diet, or disease, which allows CPB to persist and act for a longer duration in the intestines. In contrast, type D strains carrying an ETX plasmid cause illness in animals with normal protease levels, which proteolytically activates ETX. Type B strains, which have acquired both the CPB and ETX plasmids, have the versatility to cause disease at either low or normal intestinal protease levels.
C. perfringens is not the only pathogenic clostridial species that utilizes toxin plasmids for virulence. The neurotoxins of Clostridium botulinum and Clostridium tetani can also be plasmid encoded, and some botulinum toxin-encoding plasmids were recently shown to be conjugative, possibly involving a truncated tcp-like locus (255, 256). However, C. perfringens is remarkable for carrying so many different plasmid-encoded toxins. Why?
Studies are now revealing that the ability to produce plasmid-encoded toxins extends the disease spectrum of C. perfringens; i.e., these toxins are often important when this bacterium causes enteritis or enterotoxemia. Simple type A isolates (i.e., those strains producing chromosomally encoded PFO and CPA but no plasmid-encoded toxins) are virulent, since they cause histotoxic infections (4, 257). However, these simple type A strains rarely cause enteritis or enterotoxemia. The limited intestinal pathogenicity of type A strains producing only PFO and CPA is consistent with the common presence of these strains as innocuous normal intestinal flora and studies showing that inactivation of pfoA or plc genes in type C strains (210), or plc genes in NetB-producing type A strains (194), has little effect on the ability of those toxin null mutants to cause infections originating in the intestines.
Instead, when causing enteritis or enterotoxemia, C. perfringens usually relies upon plasmid-encoded toxins; the chromosomal cpe type A strains causing most cases of C. perfringens type A food poisoning represent the exception to this generalization, but even these strains use a toxin gene that is apparently associated with a mobile element (238) that may have mobilized from a plasmid (40). This strong association between C. perfringens plasmid-encoded toxins and enteritis or enterotoxemia likely involves, at least in part, the conjugative nature of the many toxin plasmids carried by this bacterium; that is, when a C. perfringens cell carrying a conjugative toxin plasmid is introduced into the intestines, it may then transfer its toxin plasmid to the normal resident C. perfringens strains. This in vivo plasmid transfer would likely impact the virulence properties of the recipient strain, as we have demonstrated in vitro for transconjugants receiving an etx-carrying plasmid (21), where the type A recipient strains were converted to ETX-producing type D strains. It is important that conjugative transfer of toxin plasmids between invading and resident C. perfringens strains be demonstrated experimentally in vivo, but this is not a simple task.
Since C. perfringens strains in normal flora are presumably under selective pressure for colonization and persistence in the intestines, this putative conjugative transfer of toxin plasmids to colonization-proficient C. perfringens strains in normal flora should help to establish and amplify infections originating in the intestines. For example, this effect could explain why the symptoms of CPE-associated non-food-borne GI diseases, which are caused by type A plasmid cpe strains, are more severe and of longer duration than the symptoms of C. perfringens type A food poisoning, which typically involves type A strains carrying a chromosomal cpe gene (241).
The putative in vivo augmentation of pathogenicity virulence by conjugative toxin plasmid transfer is likely to be important for establishing C. perfringens diseases originating in the intestines but should typically represent only one early step in pathogenesis. Factors altering the intestinal host defenses, the normal flora microbiome, or the intestinal environment also contribute to most cases of C. perfringens enteritis or enterotoxemia. For example, age, diet, or disease can reduce trypsin activity in the intestines, prolonging the presence of active CPB in the intestines (3). Alternatively, changing the normal intestinal flora by diet, antibiotic use, or coinfections with other pathogens is often necessary for a toxin plasmid-carrying C. perfringens strain to multiply sufficiently to reach pathogenic levels in the intestines or to gain access to the intestinal mucosa (3).
The presence of many C. perfringens toxin genes on conjugative plasmids may also have far-ranging virulence consequences. Some plasmid-borne toxin genes may have conjugatively transferred to other clostridial spp. Perhaps, in combination with the mobilization of these toxin genes by associated insertion sequences, this interspecies plasmid transfer may have enhanced the pathogenicity of the recipients. For example, this process could explain the presence of ITX-like binary toxins in several other pathogenic clostridial spp., e.g., C. difficile and Clostridium spiroforme. Similarly, the presence of tpeL genes on conjugative plasmids in C. perfringens is notable given the widespread distribution among pathogenic clostridial spp. of genes encoding large glycosylating toxins. Conceivably, future toxin plasmid transfers could create additional strains with unique or enhanced virulence attributes.
Finally, while virulence plasmids clearly play a major role in many C. perfringens infections, it is becoming apparent that strain clonality is also an important contributing factor for pathogenicity. The concept of distinct C. perfringens lineages was first reported after MLST of type A chromosomal cpe strains versus other C. perfringens strains (39), and this conclusion has been supported by additional MLST studies (258) and DNA microarray analyses (259). Later, Darmbrand strains were shown to share a close genetic relationship with type A chromosomal cpe strains, even though these type C strains carry plasmid-borne cpe and cpb genes (40). The shared genomic background between the chromosomal cpe type A and type C Darmbrand strains allows production of exceptionally resistant spores, which in turn likely increases the transmissibility of these strains during food-borne illness. Similarly, it is now being established that type A avian necrotic enteritis strains are another distinct C. perfringens lineage. Besides carrying several virulence-associated plasmids, including the netB-carrying plasmid, type A necrotic enteritis strains also typically possess the unique NeLoc2 chromosomal pathogenicity locus (235, 260). It is not yet clear whether NeLoc2 directly enhances the virulence fitness of these strains or instead helps to retain these virulence plasmids.
While much has been learned recently regarding the biology and virulence contributions of C. perfringens toxin plasmids, many important questions remain unanswered regarding these plasmids and their toxins. For example, the receptors for most of the plasmid-encoded toxins have not yet been identified. In addition, the structure-versus-function relationship for many of the plasmid-encoded toxins is incompletely understood. With respect to the toxin plasmids themselves, further insights will be gained by sequencing and studying those toxin plasmids that are not closely related to pCPF5603 or pCPF4969. Similarly, the issue of toxin plasmid incompatibility remains to be elucidated experimentally, and the possible interspecies transfer of conjugative C. perfringens toxin plasmids should be investigated. The contribution of C. perfringens clonality to toxin-mediated diseases is an emerging topic that requires mechanistic study. Finally, further studies are needed to evaluate whether the many non-toxin-encoding genes carried on toxin plasmids also contribute to virulence and whether plasmids encode other, still unrecognized, toxins. These and many other intriguing issues are the subject of planned future studies in our laboratories.
ACKNOWLEDGMENTS
Preparation of this review was generously supported, in part, by U.S. Public Health Service grants R37AI19844-30 (B.A.M.) and R01AI056177-09 (B.A.M., J.I.R., and F.A.U.) and by a project grant awarded to B.A.M. from the Middle Atlantic Regional Centers of Excellence-2 (MARCE-2; funded by U.S. Public Health Service grant 2U54AI57168-09 [M. Levine, overall principal investigator]). The Monash group (J.I.R.) also acknowledges the support of the Australian Research Council (ARC) through funding of the ARC Centre of Excellence in Structural and Functional Microbial Genomics.
Biographies

Jihong Li, Ph.D., is a Research Instructor at the University of Pittsburgh School of Medicine. Her research interests involve the pathogenesis of Clostridium perfringens diseases, including the mechanism of action of C. perfringens toxins, virulence plasmid genetics, and toxin gene regulation. Her research has been published in many peer-reviewed journals (PLoS Pathogens, PLoS One, Infection and Immunity, and Journal of Bacteriology, etc.) and presented at numerous national and international conferences. Her work has also produced several book chapters and reviews about C. perfringens. She is a member of the American Society for Microbiology.

Vicki Adams conducted her Ph.D. studies in the Microbiology Department of Monash University, Melbourne, Australia. She has worked for many years on mobile genetic elements from the clostridia, including several transposons and transposon-like elements. Her studies have included the biochemistry of large serine site-specific recombinases and most recently have included the study of the conjugative toxin plasmids of Clostridium perfringens.

Trudi L. Bannam, Ph.D., is a Research Scientist with Monash University, Australia. Her research interests include molecular microbiology, plasmid biology, and microbial pathogenesis. Dr. Bannam has published on diverse aspects of Clostridium perfringens biology, including conjugative plasmid biology, molecular tool building, antibiotic resistance, mobile genetic elements, as well as structure-function analysis of toxins involved in pathogenesis.

Kazuaki Miyamoto, M.D., Ph.D., is a Research and Clinical Microbiologist in the Department of Microbiology, Faculty of Pharmaceutical Sciences, Tokushima Bunri University and Mahara Hospital. His research interests include basic microbiology and medical microbiology. Dr. Miyamoto has published extensively on toxin plasmid genetics of type A to E Clostridium perfringens. His research has also addressed the identification and detection of toxins using molecular assays. Dr. Miyamoto's other research interests include nosocomial outbreaks and chemotherapy for rarely identified spotted fever group rickettsiosis in Japan. Dr. Miyamoto is a member of the American Society for Microbiology and the Japanese Society for Bacteriology.

Jorge P. García, D.V.M., is a Research Associated Specialist with the California Animal Health and Food Safety Laboratory, UC Davis. His research interests include animal models for human clostridial diseases and the mechanism of action of Clostridium perfringens toxins. Dr. García has published on clostridial and other diseases of animals, with special emphasis on food animals and animal models for the study of these diseases.

Francisco A. Uzal, D.V.M., M.Sc., Ph.D., Dipl. A.C.V.P., is a Professor of Diagnostic Pathology with the California Animal Health and Food Safety, UC Davis. His research focuses on pathogenesis and diagnostics of clostridial diseases, including animal models for human clostridial diseases. Dr. Uzal has published extensively on clostridial and other diseases of animals, with special emphasis on food animals. He is a member of the American College of Veterinary Pathology and of the American Association of Veterinary Laboratory Diagnosticians. Dr. Uzal is an editor of the Journal of Veterinary Diagnostic Investigation and serves as an ad hoc reviewer for numerous per-reviewed journals, including Infection and Immunity, Microbes and Infection, Veterinary Pathology, Anaerobe, and others. He is a member of the Organizing Committee of Clostpath, the International Conference on the Molecular Genetics and Pathogenesis of the Clostridia.

Julian I. Rood, Ph.D., is a Professor and Chief Investigator of the Australian Research Council Centre of Excellence in Structural and Functional Microbial Genomics and Deputy Head of the Department of Microbiology at Monash University in Melbourne, Australia. His research group has worked for many years on the genetics and pathogenesis of anaerobic bacteria, particularly the pathogenic clostridia and Dichelobacter nodosus, the causative agent of ovine footrot. His research has focused on the role of toxins in clostridial diseases, the regulation of virulence gene expression, and the role of conjugative plasmids and transposons in the spread of toxin genes and antibiotic resistance determinants in the pathogenic clostridia. He is a Past President and Fellow of the Australian Society for Microbiology, a Fellow of the American Academy of Microbiology, and the Ambassador to Australia of the American Society for Microbiology. He is Editor-in-Chief of the journal Plasmid.

Bruce A. McClane, Ph.D., is a Professor in the Department of Microbiology and Molecular Genetics at the University of Pittsburgh School of Medicine. His research interests include bacterial pathogenesis and bacterial toxins. Dr. McClane has published extensively on Clostridium perfringens and its toxins. His research has provided important insights into the genetics, structure-function relationships, action, and role in pathogenesis of C. perfringens toxins involved in enteric disease. Dr. McClane's research has also focused on understanding nontoxin aspects of C. perfringens pathogenicity, including spore resistance properties and adherence. He is a member of the American Society for Microbiology and a Fellow of the American Academy of Microbiology. Dr. McClane has received a Merit Award from the National Institutes of Health. He has served on the Editorial Board of Infection and Immunity since 1992 and has reviewed grant applications for the NIH, U.S. Department of Agriculture, and many other granting agencies.
REFERENCES
- 1. McClane BA, Robertson SL, Li J. 2013. Clostridium perfringens, p 465–489 In Doyle MP, Buchanan RL. (ed), Food microbiology: fundamentals and frontiers, 4th ed ASM Press, Washington, DC [Google Scholar]
- 2. Labbe RG. 1989. Clostridium perfringens, p 192–234 In Doyle MP. (ed), Foodborne bacterial pathogens. Marcel Decker, New York, NY [Google Scholar]
- 3. McClane BA, Uzal FA, Miyakawa MF, Lyerly D, Wilkins TD. 2006. The enterotoxic clostridia, p 688–752 In Dworkin M, Falkow S, Rosenburg E, Schleifer H, Stackebrandt E. (ed), The prokaryotes: a handbook on the biology of bacteria, 3rd ed Springer, New York, NY [Google Scholar]
- 4. Rood JI. 2006. Clostridium perfringens and histotoxic disease, p 753–770 In Dworkin M, Falkow S, Rosenburg E, Schleifer H, Stackebrandt E. (ed), The prokaryotes: a handbook on the biology of bacteria, 3rd ed Springer, New York, NY [Google Scholar]
- 5. Titball RW, Rood JI. 2002. Clostridium perfringens: wound infections, p 1875–1903 In Sussman M. (ed), Molecular medical microbiology. Academic Press, London, England [Google Scholar]
- 6. Songer JG. 1996. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9:216–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hatheway C. 1990. Toxigenic clostridia. Clin. Microbiol. Rev. 3:66–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McDonel JL. 1986. Toxins of Clostridium perfringens types A, B, C, D, and E, p 477–517 In Dorner F, Drews H. (ed), Pharmacology of bacterial toxins. Pergamon Press, Oxford, England [Google Scholar]
- 9. Petit L, Gilbert M, Popoff M. 1999. Clostridium perfringens: toxinotype and genotype. Trends Microbiol. 7:104–110 [DOI] [PubMed] [Google Scholar]
- 10. Animoto K, Noro T, Oishi E, Shimizu M. 2007. A novel toxin homologous to large clostridial cytotoxins found in culture supernatant of Clostridium perfringens type C. Microbiology 153:1198–1206 [DOI] [PubMed] [Google Scholar]
- 11. Keyburn AL, Boyce JD, Vaz P, Bannam TL, Ford ME, Parker D, Di Rubbo A, Rood JI, Moore RJ. 2008. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 4:e26 doi: 10.1371/journal.ppat.0040026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sarker MR, Carman RJ, McClane BA. 1999. Inactivation of the gene (cpe) encoding Clostridium perfringens enterotoxin eliminates the ability of two cpe-positive C. perfringens type A human gastrointestinal disease isolates to affect rabbit ileal loops. Mol. Microbiol. 33:946–958 [DOI] [PubMed] [Google Scholar]
- 13. Miyamoto K, Fisher DJ, Li J, Sayeed S, Akimoto S, McClane BA. 2006. Complete sequencing and diversity analysis of the enterotoxin-encoding plasmids in Clostridium perfringens type A non-food-borne human gastrointestinal disease isolates. J. Bacteriol. 188:1585–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miyamoto K, Li J, Sayeed S, Akimoto S, McClane BA. 2008. Sequencing and diversity analyses reveal extensive similarities between some epsilon-toxin-encoding plasmids and the pCPF5603 Clostridium perfringens enterotoxin plasmid. J. Bacteriol. 190:7178–7188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sayeed S, Li J, McClane BA. 2007. Virulence plasmid diversity in Clostridium perfringens type D isolates. Infect. Immun. 75:2391–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sayeed S, Li J, McClane BA. 2010. Characterization of virulence plasmid diversity among Clostridium perfringens type B isolates. Infect. Immun. 78:495–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gurjar A, Li J, McClane BA. 2010. Characterization of toxin plasmids in Clostridium perfringens type C isolates. Infect. Immun. 78:4860–4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li J, Miyamoto K, McClane BA. 2007. Comparison of virulence plasmids among Clostridium perfringens type E isolates. Infect. Immun. 75:1811–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bannam T, Yan X, Harrison P, Seemann T, Keyburn A, Stubenrauch C, Weeramantri L, Chueng J, McClane B, Boyce J, Moore R, Rood J. 2011. Necrotic enteritis-derived Clostridium perfringens strain with three closely related independently conjugative toxin and antibiotic plasmids. mBio 2(5):e00190–11 doi: 10.1128/mBio.00190-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fisher DJ, Miyamoto K, Harrison B, Akimoto S, Sarker MR, McClane BA. 2005. Association of beta2 toxin production with Clostridium perfringens type A human gastrointestinal disease isolates carrying a plasmid enterotoxin gene. Mol. Microbiol. 56:747–762 [DOI] [PubMed] [Google Scholar]
- 21. Hughes ML, Poon R, Adams V, Sayeed S, Saputo J, Uzal FA, McClane BA, Rood JI. 2007. Epsilon-toxin plasmids of Clostridium perfringens type D are conjugative. J. Bacteriol. 189:7531–7538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Billington SJ, Wieckowski EU, Sarker MR, Bueschel D, Songer JG, McClane BA. 1998. Clostridium perfringens type E animal enteritis isolates with highly conserved, silent enterotoxin sequences. Infect. Immun. 66:4531–4536 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23. Miyamoto K, Yumine N, Mimura K, Nagahama M, Li J, McClane BA, Akimoto S. 2011. Identification of novel Clostridium perfringens type E strains that carry an iota toxin plasmid with a functional enterotoxin gene. PLoS One 6:e20376 doi: 10.1371/journal.pone.0020376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lepp D, Roxas B, Parreira V, Marri P, Rosey E, Gong J, Songer J, Vedantam G, Prescott J. 2010. Identification of novel pathogenicity loci in Clostridium perfringens strains that cause avian necrotic enteritis. PLoS One 5:e10795 doi: 10.1371/journal.pone.0010795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Myers GS, Rasko DA, Cheung JK, Ravel J, Seshadri R, DeBoy RT, Ren Q, Varga J, Awad MM, Brinkac LM, Daugherty SC, Haft DH, Dodson RJ, Madupu R, Nelson WC, Rosovitz MJ, Sullivan SA, Khouri H, Dimitrov GI, Watkins KL, Mulligan S, Benton J, Radune D, Fisher DJ, Atkins HS, Hiscox T, Jost BH, Billington SJ, Songer JG, McClane BA, Titball RW, Rood JI, Melville SB, Paulsen IT. 2006. Skewed genomic variability in strains of the toxigenic bacterial pathogen, Clostridium perfringens. Genome Res. 16:1031–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li J, Miyamoto K, Sayeed S, McClane BA. 2010. Organization of the cpe locus in CPE-positive Clostridium perfringens type C and D isolates. PLoS One 5:e10932 doi: 10.1371/journal.pone.0010932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shimizu T, Ohtani K, Hirakawa H, Ohshima K, Yamashita A, Shiba T, Ogasawara N, Hattori M, Kuhara S, Hayashi H. 2002. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc. Natl. Acad. Sci. U. S. A. 99:996–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gibert M, Jolivet-Reynaud C, Popoff MR. 1997. Beta2 toxin, a novel toxin produced by Clostridium perfringens. Gene 203:65–73 [DOI] [PubMed] [Google Scholar]
- 29. Gill DM. 1982. Bacterial toxins: a table of lethal amounts. Microbiol. Rev. 46:86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Titball RW, Naylor CE, Basak AK. 1999. The Clostridium perfringens alpha-toxin. Anaerobe 5:51–64 [DOI] [PubMed] [Google Scholar]
- 31. Sakurai J, Nagahama M, Oda M. 2004. Clostridium perfringens alpha-toxin: characterization and mode of action. J. Biochem. (Tokyo) 136:569–574 [DOI] [PubMed] [Google Scholar]
- 32. Naylor CE, Eaton JT, Howells A, Justin N, Moss DS, Titball RW, Basak AK. 1998. Structure of the key toxin in gas gangrene. Nat. Struct. Biol. 5:738–746 [DOI] [PubMed] [Google Scholar]
- 33. Williamson ED, Titball RW. 1993. A genetically engineered vaccine against the alpha-toxin of Clostridium perfringens protects mice against experimental gas gangrene. Vaccine 11:1253–1258 [DOI] [PubMed] [Google Scholar]
- 34. Neeson BN, Clark GC, Atkins HS, Lingard B, Titball RW. 2007. Analysis of protection afforded by a Clostridium perfringens alpha-toxoid against heterologous clostridial phospholipases C. Microb. Pathog. 43:161–165 [DOI] [PubMed] [Google Scholar]
- 35. Goni FM, Alonso A. 2002. Sphingomyelinases: enzymology and membrane activity. FEBS Lett. 531:38–46 [DOI] [PubMed] [Google Scholar]
- 36. Monturiol-Gross L, Flores-Diaz M, Araya-Castillo C, Pineda-Padilla MJ, Clark GC, Titball RW, Alape-Giron A. 2012. Reactive oxygen species and the MEK/ERK pathway are involved in the toxicity of Clostridium perfringens alpha-toxin, a prototype bacterial phospholipase C. J. Infect. Dis. 206:1218–1226 [DOI] [PubMed] [Google Scholar]
- 37. Oda M, Ikari S, Matsuno T, Morimune Y, Nagahama M, Sakurai J. 2006. Signal transduction mechanism involved in Clostridium perfringens alpha-toxin-induced superoxide anion generation in rabbit neutrophils. Infect. Immun. 74:2876–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oda M, Kabura M, Takagishi T, Suzue A, Tominaga K, Urano S, Nagahama M, Kobayashi K, Furukawa K, Sakurai J. 2012. Clostridium perfringens alpha-toxin recognizes the GM1a-TrkA complex. J. Biol. Chem. 287:33070–33079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Deguchi A, Miyamoto K, Kuwahara T, Kaneko I, Li J, McClane BA, Akimoto S. 2009. Genetic characterization of type A entertoxigenic Clostridium perfringens strains. PLoS One 4:e5598 doi: 10.1371/journal.pone.0005598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ma M, Li J, McClane B. 2012. Genotypic and phenotypic characterization of Clostridium perfringens isolates from Darmbrand cases in post-World War II Germany. Infect. Immun. 80:4354–4363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tweten RK. 2005. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect. Immun. 73:6199–6209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gilbert RJ. 2010. Cholesterol-dependent cytolysins. Adv. Exp. Med. Biol. 677:56–66 [DOI] [PubMed] [Google Scholar]
- 43. Dunstone MA, Tweten RK. 2012. Packing a punch: the mechanism of pore formation by cholesterol dependent cytolysins and membrane attack complex/perforin-like proteins. Curr. Opin. Struct. Biol. 22:342–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rossjohn J, Feil SC, McKinstry WJ, Tweten RK, Parker MW. 1997. Structure of a cholesterol-binding, thiol-activated cytolysin and a model of its membrane form. Cell 89:685–692 [DOI] [PubMed] [Google Scholar]
- 45. Shatursky O, Heuck AP, Shepard LA, Rossjohn J, Parker MW, Johnson AE, Tweten RK. 1999. The mechanism of membrane insertion for a cholesterol-dependent cytolysin: a novel paradigm for pore-forming toxins. Cell 99:293–299 [DOI] [PubMed] [Google Scholar]
- 46. Awad MM, Bryant AE, Stevens DL, Rood JI. 1995. Virulence studies on chromosomal alpha-toxin and theta-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of alpha-toxin in Clostridium perfringens-mediated gas gangrene. Mol. Microbiol. 15:191–202 [DOI] [PubMed] [Google Scholar]
- 47. Bryant AE, Chen RY, Nagata Y, Wang Y, Lee CH, Finegold S, Guth PH, Stevens DL. 2000. Clostridial gas gangrene. II. Phospholipase C-induced activation of platelet gpIIbIIIa mediates vascular occlusion and myonecrosis in Clostridium perfringens gas gangrene. J. Infect. Dis. 182:808–815 [DOI] [PubMed] [Google Scholar]
- 48. Bryant AE, Chen RY, Nagata Y, Wang Y, Lee CH, Finegold S, Guth PH, Stevens DL. 2000. Clostridial gas gangrene. I. Cellular and molecular mechanisms of microvascular dysfunction induced by exotoxins of Clostridium perfringens. J. Infect. Dis. 182:799–807 [DOI] [PubMed] [Google Scholar]
- 49. Park JM, Ng VH, Maeda S, Rest RF, Karin M. 2004. Anthrolysin O and other gram-positive cytolysins are Toll-like receptor 4 agonists. J. Exp. Med. 200:1647–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fisher DJ, Fernandez-Miyakawa ME, Sayeed S, Poon R, Adams V, Rood JI, Uzal FA, McClane BA. 2006. Dissecting the contributions of Clostridium perfringens type C toxins to lethality in the mouse intravenous injection model. Infect. Immun. 74:5200–5210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Collie RE, McClane BA. 1998. Evidence that the enterotoxin gene can be episomal in Clostridium perfringens isolates associated with nonfoodborne human gastrointestinal diseases. J. Clin. Microbiol. 36:30–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fernandez-Miyakawa ME, Fisher DJ, Poon R, Sayeed S, Adams V, Rood JI, McClane BA, Uzal FA. 2007. Both epsilon-toxin and beta-toxin are important for the lethal properties of Clostridium perfringens type B isolates in the mouse intravenous injection model. Infect. Immun. 75:1443–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Czeczulin JR, Hanna PC, McClane BA. 1993. Cloning, nucleotide sequencing, and expression of the Clostridium perfringens enterotoxin gene in Escherichia coli. Infect. Immun. 61:3429–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Briggs DC, Naylor CE, Smedley JG, III, Lukoyanova N, Robertson S, Moss DS, McClane BA, Basak AK. 2011. Structure of the food-poisoning Clostridium perfringens enterotoxin reveals similarity to the aerolysin-like pore-forming toxins. J. Mol. Biol. 413:138–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kitadokoro K, Nishimura K, Kamitani S, Fukui-Miyazaki A, Toshima H, Abe H, Kamata Y, Sugita-Konishi Y, Yamamoto S, Karatani H, Horiguchi Y. 2011. Crystal structure of Clostridium perfringens enterotoxin displays features of beta-pore-forming toxins. J. Biol. Chem. 286:19549–19555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hanna PC, Wnek AP, McClane BA. 1989. Molecular cloning of the 3′ half of the Clostridium perfringens enterotoxin gene and demonstration that this region encodes receptor-binding activity. J. Bacteriol. 171:6815–6820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hanna PC, Mietzner TA, Schoolnik GK, McClane BA. 1991. Localization of the receptor-binding region of Clostridium perfringens enterotoxin utilizing cloned toxin fragments and synthetic peptides. The 30 C-terminal amino acids define a functional binding region. J. Biol. Chem. 266:11037–11043 [PubMed] [Google Scholar]
- 58. Smedley JG, III, McClane BA. 2004. Fine-mapping of the N-terminal cytotoxicity region of Clostridium perfringens enterotoxin by site-directed mutagenesis. Infect. Immun. 72:6914–6923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Smedley JG, III, Uzal FA, McClane BA. 2007. Identification of a prepore large-complex stage in the mechanism of action of Clostridium perfringens enterotoxin. Infect. Immun. 75:2381–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kokai-Kun JF, Benton K, Wieckowski EU, McClane BA. 1999. Identification of a Clostridium perfringens enterotoxin region required for large complex formation and cytotoxicity by random mutagenesis. Infect. Immun. 67:5634–5641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen J, Theoret JR, Shrestha A, Smedley JG, III, McClane BA. 2012. Cysteine-scanning mutagenesis supports the importance of Clostridium perfringens enterotoxin amino acids 80 to 106 for membrane insertion and pore formation. Infect. Immun. 80:4078–4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Katahira J, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. 1997. Molecular cloning and functional characterization of the receptor for Clostridium perfringens enterotoxin. J. Cell Biol. 136:1239–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Katahira J, Sugiyama H, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. 1997. Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J. Biol. Chem. 272:26652–26658 [DOI] [PubMed] [Google Scholar]
- 64. Fujita K, Katahira J, Horiguchi Y, Sonoda N, Furuse M, Tskuita S. 2000. Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction membrane protein. FEBS Lett. 476:258–261 [DOI] [PubMed] [Google Scholar]
- 65. Robertson S, Smedley JG, III, McClane BA. 2010. Identification of a claudin-4 residue important for mediating the host cell binding and action of Clostridium perfringens enterotoxin. Infect. Immun. 78:505–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Robertson SL, Smedley JG, III, Singh U, Chakrabarti G, Van Itallie CM, Anderson JM, McClane BA. 2007. Compositional and stoichiometric analysis of Clostridium perfringens enterotoxin complexes in Caco-2 cells and claudin 4 fibroblast transfectants. Cell. Microbiol. 9:2734–2755 [DOI] [PubMed] [Google Scholar]
- 67. Veshnyakova A, Piontek J, Protze J, Waziri N, Heise I, Krause G. 2012. Mechanism of Clostridium perfringens enterotoxin interaction with claudin-3/-4 protein suggests structural modifications of the toxin to target specific claudins. J. Biol. Chem. 287:1698–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shrestha A, McClane BA. 2013. Human claudin-8 and -14 are receptors capable of conveying the cytotoxic effects of Clostridium perfringens enterotoxin. mBio 4(1):e000594–12 doi: 10.1128/mBio.00594-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Anderson JM, Van Itallie CM. 2009. Physiology and function of the tight junction. Cold Spring Harb. Perspect. Biol. 1:a002584 doi: 10.1101/cshperspect.a002584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Van Itallie CM, Anderson JM. 2006. Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 68:403–429 [DOI] [PubMed] [Google Scholar]
- 71. Van Itallie CM, Betts L, Smedley JG, III, McClane BA, Anderson JM. 2008. Structure of the claudin-binding domain of Clostridium perfringens enterotoxin. J. Biol. Chem. 283:268–274 [DOI] [PubMed] [Google Scholar]
- 72. Takahashi A, Komiya E, Kakutani H, Yoshida T, Fujii M, Horiguchi Y, Mizuguchi H, Tsutsumi Y, Tsunoda S, Koizumi N, Isoda K, Yagi K, Watanabe Y, Kondoh M. 2008. Domain mapping of a claudin-4 modulator, the C-terminal region of C-terminal fragment of Clostridium perfringens enterotoxin, by site-directed mutagenesis. Biochem. Pharmacol. 75:1639–1648 [DOI] [PubMed] [Google Scholar]
- 73. Wieckowski EU, Wnek AP, McClane BA. 1994. Evidence that an ∼50kDa mammalian plasma membrane protein with receptor-like properties mediates the amphiphilicity of specifically-bound Clostridium perfringens enterotoxin. J. Biol. Chem. 269:10838–10848 [PubMed] [Google Scholar]
- 74. Singh U, Van Itallie CM, Mitic LL, Anderson JM, McClane BA. 2000. Caco-2 cells treated with Clostridium perfringens enterotoxin form multiple large complex species, one of which contains the tight junction protein occludin. J. Biol. Chem. 275:18407–18417 [DOI] [PubMed] [Google Scholar]
- 75. McClane BA, Rood JI. 2001. Clostridial toxins involved in human enteric and histotoxic infections, p 169–209 In Bahl H, Duerre P. (ed), Clostridia: biotechnology and medical applications. Wiley-VCH, Weinheim, Germany [Google Scholar]
- 76. Hardy SP, Denmead M, Parekh N, Granum PE. 1999. Cationic currents induced by Clostridium perfringens type A enterotoxin in human intestinal Caco-2 cells. J. Med. Microbiol. 48:235–243 [DOI] [PubMed] [Google Scholar]
- 77. Hardy SP, Ritchie C, Allen MC, Ashley RH, Granum PE. 2001. Clostridium perfringens type A enterotoxin forms mepacrine-sensitive pores in pure phospholipid bilayers in the absence of putative receptor proteins. Biochim. Biophys. Acta 1515:38–43 [DOI] [PubMed] [Google Scholar]
- 78. Chakrabarti G, McClane BA. 2005. The importance of calcium influx, calpain, and calmodulin for the activation of Caco-2 cell death pathways by Clostridium perfringens enterotoxin. Cell. Microbiol. 7:129–146 [DOI] [PubMed] [Google Scholar]
- 79. Chakrabarti G, Zhou X, McClane BA. 2003. Death pathways activated in Caco-2 cells by Clostridium perfringens enterotoxin. Infect. Immun. 71:4260–4270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Singh U, Mitic LL, Wieckowski E, Anderson JM, McClane BA. 2001. Comparative biochemical and immunochemical studies reveal differences in the effects of Clostridium perfringens enterotoxin on polarized Caco-2 cells versus Vero cells. J. Biol. Chem. 276:33402–33412 [DOI] [PubMed] [Google Scholar]
- 81. McDonel JL. 1980. Binding of Clostridium perfringens 125I-enterotoxin to rabbit intestinal cells. Biochemistry 21:4801–4807 [DOI] [PubMed] [Google Scholar]
- 82. Sherman S, Klein E, McClane BA. 1994. Clostridium perfringens type A enterotoxin induces concurrent development of tissue damage and fluid accumulation in the rabbit ileum. J. Diarrhoeal Dis. Res. 12:200–207 [PubMed] [Google Scholar]
- 83. Smedley JG, III, Saputo J, Parker JC, Fernandez-Miyakawa ME, Robertson SL, McClane BA, Uzal FA. 2008. Noncytotoxic Clostridium perfringens enterotoxin (CPE) variants localize CPE intestinal binding and demonstrate a relationship between CPE-induced cytotoxicity and enterotoxicity. Infect. Immun. 76:3793–3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. McDonel JL, Demers GW. 1982. In vivo effects of enterotoxin from Clostridium perfringens type A in rabbit colon: binding vs biologic activity. J. Infect. Dis. 145:490–494 [DOI] [PubMed] [Google Scholar]
- 85. Fernandez Miyakawa ME, Pistone Creydt V, Uzal FA, McClane BA, Ibarra C. 2005. Clostridium perfringens enterotoxin damages the human intestine in vitro. Infect. Immun. 73:8407–8410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hunter SEC, Brown JE, Oynston PCF, Sakurai J, Titball RW. 1993. Molecular genetic analysis of beta-toxin of Clostridium perfringens reveals sequence homology with alpha-toxin, gamma-toxin, and leukocidin of Staphylococcus aureus. Infect. Immun. 61:3958–3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Macias Rioseco M, Beingesser J, Uzal FA. 2012. Freezing or adding trypsin inhibitor to equine intestinal contents extends the lifespan of Clostridium perfringens beta toxin for diagnostic purposes. Anaerobe 18:357–360 [DOI] [PubMed] [Google Scholar]
- 88. Sakurai J, Duncan CL. 1978. Some properties of the beta-toxin produced by Clostridium perfringens type C. Infect. Immun. 21:678–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Steinthorsdottir V, Fridriksdottir V, Gunnarsson E, Andresson OS. 1998. Site-directed mutagenesis of Clostridium perfringens beta-toxin: expression of wild-type and mutant toxins in Bacillus subtillis. FEMS Microbiol. Lett. 158:17–23 [DOI] [PubMed] [Google Scholar]
- 90. Shatursky O, Bayles R, Rogers M, Jost BH, Songer JG, Tweten RK. 2000. Clostridium perfringens beta-toxin forms potential-dependent, cation-selective channels in lipid bilayers. Infect. Immun. 68:5546–5551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nagahama M, Hayashi S, Morimitsu S, Sakurai J. 2003. Biological activities and pore formation of Clostridium perfringens beta toxin in HL 60 cells. J. Biol. Chem. 278:36934–36941 [DOI] [PubMed] [Google Scholar]
- 92. Uzal FA, McClane BA. 2011. Recent progress in understanding the pathogenesis of Clostridium perfringens type C infections. Vet. Microbiol. 153:37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jost BH, Billington SJ, Trinh HT, Bueschel DM, Songer JG. 2005. Atypical cpb2 genes, encoding beta2-toxin in Clostridium perfringens isolates of nonporcine origin. Infect. Immun. 73:652–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Vilei EM, Schlatter Y, Perreten V, Straub R, Popoff MR, Gibert M, Grone A, Frey J. 2005. Antibiotic-induced expression of a cryptic cpb2 gene in equine beta2-toxigenic Clostridium perfringens. Mol. Microbiol. 57:1570–1581 [DOI] [PubMed] [Google Scholar]
- 95. Popoff MR. 2011. Epsilon toxin: a fascinating pore-forming toxin. FEBS J. 278:4602–4615 [DOI] [PubMed] [Google Scholar]
- 96. Bokori-Brown M, Savva CG, Fernandes da Costa SP, Naylor CE, Basak AK, Titball RW. 2011. Molecular basis of toxicity of Clostridium perfringens epsilon toxin. FEBS J. 278:4589–4601 [DOI] [PubMed] [Google Scholar]
- 97. Minami J, Katayama S, Matsushita O, Matsushita C, Okabe A. 1997. Lambda-toxin of Clostridium perfringens activates the precursor of epsilon-toxin by releasing its N- and C-terminal peptides. Microbiol. Immunol. 41:527–535 [DOI] [PubMed] [Google Scholar]
- 98. Miyata S, Matsushita O, Minami J, Katayama S, Shimamoto S, Okabe A. 2001. Cleavage of a C-terminal peptide is essential for heptamerization of Clostridium perfringens epsilon-toxin in the synaptosomal membrane. J. Biol. Chem. 276:13778–13783 [DOI] [PubMed] [Google Scholar]
- 99. Harkness JM, Li J, McClane BA. 2012. Identification of a lambda toxin-negative Clostridium perfringens strain that processes and activates epsilon prototoxin intracellularly. Anaerobe 18:546–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Robertson SL, Li J, Uzal FA, McClane BA. 2011. Evidence for a prepore stage in the action of Clostridium perfringens epsilon toxin. PLoS One 6:e22053 doi: 10.1371/journal.pone.0022053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Cole AR, Gibert M, Popoff M, Moss DS, Titball RW, Basak AK. 2004. Clostridium perfringens epsilon-toxin shows structural similarity to the pore-forming toxin aerolysin. Nat. Struct. Mol. Biol. 11:797–798 [DOI] [PubMed] [Google Scholar]
- 102. Shortt SJ, Titball RW, Lindsay CD. 2000. An assessment of the in vitro toxicology of Clostridium perfringens type D epsilon-toxin in human and animal cells. Hum. Exp. Toxicol. 19:108–116 [DOI] [PubMed] [Google Scholar]
- 103. Ivie SE, Fennessey CM, Sheng J, Rubin DH, McClain MS. 2011. Gene-trap mutagenesis identifies mammalian genes contributing to intoxication by Clostridium perfringens epsilon-toxin. PLoS One 6:e17787 doi: 10.1371/journal.pone.0017787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ivie SE, McClain MS. 2012. Identification of amino acids important for binding of Clostridium perfringens epsilon toxin to host cells and to HAVCR1. Biochemistry 51:7588–7595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Feigelstock D, Thompson P, Mattoo P, Zhang Y, Kaplan GG. 1998. The human homolog of HAVCR-1 codes for a hepatitis A virus cellular receptor. J. Virol. 72:6621–6628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tamai E, Ishida T, Miyata S, Matsushita O, Suda H, Kobayashi S, Sonobe H, Okabe A. 2003. Accumulation of Clostridium perfringens epsilon-toxin in the mouse kidney and its possible biological significance. Infect. Immun. 71:5371–5375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Miyata S, Minami J, Tamia E, Matsushita P, Shimamoto S, Okabe A. 2002. Clostridium perfringens epsilon-toxin forms a hepatmeric pore within the detergent-insoluble microdomains of Madin-Darby canine kidney cells and rat synaptosomes. J. Biol. Chem. 277:39463–39468 [DOI] [PubMed] [Google Scholar]
- 108. Fennessey CM, Sheng J, Rubin DH, McClain MS. 2012. Oligomerization of Clostridium perfringens epsilon toxin is dependent upon caveolins 1 and 2. PLoS One 7:e46866 doi: 10.1371/journal.pone.0046866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Shimada H, Kitada S. 2011. Mega assemblages of oligomeric aerolysin-like toxins stabilized by toxin-associating membrane proteins. J. Biochem. 149:103–115 [DOI] [PubMed] [Google Scholar]
- 110. Knapp O, Maier E, Benz R, Geny B, Popoff MR. 2009. Identification of the channel-forming domain of Clostridium perfringens epsilon-toxin (ETX). Biochim. Biophys. Acta 1788:2584–2593 [DOI] [PubMed] [Google Scholar]
- 111. Nestorovich EM, Karginov VA, Bezrukov SM. 2010. Polymer partitioning and ion selectivity suggest asymmetrical shape for the membrane pore formed by epsilon toxin. Biophys. J. 99:782–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Petit L, Maier E, Gibert M, Popoff MR, Benz R. 2001. Clostridium perfringens epsilon toxin induces a rapid change of cell membrane permeability to ions and forms channels in artificial lipid bilayers. J. Biol. Chem. 276:15736–15740 [DOI] [PubMed] [Google Scholar]
- 113. Nagahama M, Itohayashi Y, Hara H, Higashihara M, Fukatani Y, Takagishi T, Oda M, Kobayashi K, Nakagawa I, Sakurai J. 2011. Cellular vacuolation induced by Clostridium perfringens epsilon-toxin. FEBS J. 278:3395–3407 [DOI] [PubMed] [Google Scholar]
- 114. Goldstein J, Morris WE, Loidl CF, Tironi-Farinatti C, McClane BA, Uzal FA, Fernandez Miyakawa ME. 2009. Clostridium perfringens epsilon toxin increases the small intestinal permeability in mice and rats. PLoS One 4:e7065 doi: 10.1371/journal.pone.0007065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sakurai J, Nagahama M, Oda M, Tsuge H, Kobayashi K. 2009. Clostridium perfringens iota-toxin: structure and function. Toxins 1:208–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Stiles BG, Wigelsworth DJ, Popoff MR, Barth H. 2011. Clostridial binary toxins: iota and C2 family portraits. Front. Cell. Infect. Microbiol. 1:11 doi: 10.3389/fcimb.2011.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Barth H, Stiles BG. 2008. Binary actin-ADP-ribosylating toxins and their use as molecular Trojan horses for drug delivery into eukaryotic cells. Curr. Med. Chem. 15:459–469 [DOI] [PubMed] [Google Scholar]
- 118. Aktories K, Schwan C, Papatheodorou P, Lang AE. 2012. Bidirectional attack on the actin cytoskeleton. Bacterial protein toxins causing polymerization or depolymerization of actin. Toxicon 60:572–581 [DOI] [PubMed] [Google Scholar]
- 119. Papatheodorou P, Carette JE, Bell GW, Schwan C, Guttenberg G, Brummelkamp TR, Aktories K. 2011. Lipolysis-stimulated lipoprotein receptor (LSR) is the host receptor for the binary toxin Clostridium difficile transferase (CDT). Proc. Natl. Acad. Sci. U. S. A. 108:16422–16427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Papatheodorou P, Wilczek C, Nolke T, Guttenberg G, Hornuss D, Schwan C, Aktories K. 2012. Identification of the cellular receptor of Clostridium spiroforme toxin. Infect. Immun. 80:1418–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wigelsworth DJ, Ruthel G, Schnell L, Herrlich P, Blonder J, Veenstra TD, Carman RJ, Wilkins TD, Van Nhieu GT, Pauillac S, Gibert M, Sauvonnet N, Stiles BG, Popoff MR, Barth H. 2012. CD44 promotes intoxication by the clostridial iota-family toxins. PLoS One 7:e51356 doi: 10.1371/journal.pone.0051356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hale ML, Marvaud JC, Popoff MR, Stiles BG. 2004. Detergent-resistant membrane microdomains facilitate Ib oligomer formation and biological activity of Clostridium perfringens iota-toxin. Infect. Immun. 72:2186–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Nagahama M, Yamaguchi A, Hagiyama T, Ohkubo N, Kobayashi K, Sakurai J. 2004. Binding and internalization of Clostridium perfringens iota-toxin in lipid rafts. Infect. Immun. 72:3267–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Marvaud JC, Stiles BG, Chenal A, Gillet D, Gibert M, Smith LA, Popoff MR. 2002. Clostridium perfringens iota toxin. Mapping of the Ia domain involved in docking with Ib and cellular internalization. J. Biol. Chem. 277:43659–43666 [DOI] [PubMed] [Google Scholar]
- 125. Gibert M, Monier MN, Ruez R, Hale ML, Stiles BG, Benmerah A, Johannes L, Lamaze C, Popoff MR. 2011. Endocytosis and toxicity of clostridial binary toxins depend on a clathrin-independent pathway regulated by Rho-GDI. Cell. Microbiol. 13:154–170 [DOI] [PubMed] [Google Scholar]
- 126. Schering B, Barmann M, Chhatwal GS, Geipel U, Aktories K. 1988. ADP-ribosylation of skeletal muscle and non-muscle actin by Clostridium perfringens iota toxin. Eur. J. Biochem. 171:225–229 [DOI] [PubMed] [Google Scholar]
- 127. Hilger H, Pust S, von Figura G, Kaiser E, Stiles BG, Popoff MR, Barth H. 2009. The long-lived nature of Clostridium perfringens iota toxin in mammalian cells induces delayed apoptosis. Infect. Immun. 77:5593–5601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Keyburn AL, Bannam TL, Moore RJ, Rood JI. 2010. NetB, a pore-forming toxin from necrotic enteritis strains of Clostridium perfringens. Toxins 2:1913–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Keyburn AL, Yan XX, Bannam TL, Van Immerseel F, Rood JI, Moore RJ. 2010. Association between avian necrotic enteritis and Clostridium perfringens strains expressing NetB toxin. Vet. Res. 41:21 doi: 10.1051/vetres/2009069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Martin TG, Smyth JA. 2009. Prevalence of netB among some clinical isolates of Clostridium perfringens from animals in the United States. Vet. Microbiol. 136:202–205 [DOI] [PubMed] [Google Scholar]
- 131. Abildgaard L, Sondergaard TE, Engberg RM, Schramm A, Hojberg O. 2010. In vitro production of necrotic enteritis toxin B, NetB, by netB-positive and netB-negative Clostridium perfringens originating from healthy and diseased broiler chickens. Vet. Microbiol. 144:231–235 [DOI] [PubMed] [Google Scholar]
- 132. Engstrom BE, Johansson A, Aspan A, Kaldhusdal M. 2012. Genetic relatedness and netB prevalence among environmental Clostridium perfringens strains associated with a broiler flock affected by mild necrotic enteritis. Vet. Microbiol. 159:260–264 [DOI] [PubMed] [Google Scholar]
- 133. Yan X, Porter CJ, Hardy SP, Steer D, Smith AI, Quinsey NS, Hughes V, Cheung JK, Keyburn AL, Kaldhusdal M, Moore RJ, Bannam TL, Whisstock JC, Rood JI. 2013. Structural and functional analysis of the pore-forming toxin NetB from Clostridium perfringens. mBio 4(1):e00019–13 doi: 10.1128/mBio.00019-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Savva CG, Fernandes da Costa SP, Bokori-Brown M, Naylor CE, Cole AR, Moss DS, Titball RW, Basak AK. 2013. Molecular architecture and functional analysis of NetB, a pore-forming toxin from Clostridium perfringens. J. Biol. Chem. 288:3512–3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Paredes-Sabja D, Sarker N, Sarker MR. 2011. Clostridium perfringens tpeL is expressed during sporulation. Microb. Pathog. 51:384–388 [DOI] [PubMed] [Google Scholar]
- 136. Guttenberg G, Hornei S, Jank T, Schwan C, Lu W, Einsle O, Papatheodorou P, Aktories K. 2012. Molecular characteristics of Clostridium perfringens TpeL toxin and consequences of mono-O-GlcNAcylation of Ras in living cells. J. Biol. Chem. 287:24929–24940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Nagahama M, Ohkubo A, Oda M, Kobayashi K, Amimoto K, Miyamoto K, Sakurai J. 2011. Clostridium perfringens TpeL glycosylates the Rac and Ras subfamily proteins. Infect. Immun. 79:905–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Coursodon CF, Glock RD, Moore KL, Cooper KK, Songer JG. 2012. TpeL-producing strains of Clostridium perfringens type A are highly virulent for broiler chicks. Anaerobe 18:117–121 [DOI] [PubMed] [Google Scholar]
- 139. Manich M, Knapp O, Gibert M, Maier E, Jolivet-Reynaud C, Geny B, Benz R, Popoff MR. 2008. Clostridium perfringens delta toxin is sequence related to beta toxin, NetB, and Staphylococcus pore-forming toxins, but shows functional differences. PLoS One 3:e3764 doi: 10.1371/journal.pone.0003764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Adams JJ, Gregg K, Bayer EA, Boraston AB, Smith SP. 2008. Structural basis of Clostridium perfringens toxin complex formation. Proc. Natl. Acad. Sci. U. S. A. 105:12194–12199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Chiarezza M, Lyras D, Pidot SJ, Flore-Diaz M, Awad MM, Kennedy CL, Cordner LM, Phumoonna T, Poon R, Hughes ML, Emmins JJ, Alape-Giron A, Rood JI. 2009. The NanI and NanJ sialidases of Clostridium perfringens are not essential for virulence. Infect. Immun. 77:4421–4428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Li J, Sayeed S, Robertson S, Chen J, McClane BA. 2011. Sialidases affect the host cell adherence and epsilon toxin-induced cytotoxicity of Clostridium perfringens type D strain CN3718. PLoS Pathog. 7:e1002429 doi: 10.1371/journal.ppat.1002429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Shimizu T, Ba-Thein W, Tamaki M, Hayashi H. 1994. The virR gene, a member of a class of two-component response regulators, regulates the production of perfringolysin O, collagenase, and hemagglutinin in Clostridium perfringens. J. Bacteriol. 176:1616–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Lyristis M, Bryant AE, Sloan J, Awad MM, Nisbet IT, Stevens DL, Rood JI. 1994. Identification and molecular analysis of a locus that regulates extracellular toxin production in Clostridium perfringens. Mol. Microbiol. 12:761–777 [DOI] [PubMed] [Google Scholar]
- 145. Cheung JK, Awad MM, McGowan S, Rood JI. 2009. Functional analysis of the VirSR phosphorelay from Clostridium perfringens. PLoS One 4:e5849 doi: 10.1371/journal.pone.0005849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Cheung JK, Dupuy B, Deveson DS, Rood JI. 2004. The spatial organization of the VirR boxes is critical for VirR-mediated expression of the perfringolysin O gene, pfoA, from Clostridium perfringens. J. Bacteriol. 186:3321–3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. McGowan S, Lucet IS, Cheung JK, Awad MM, Whisstock JC, Rood JI. 2002. The FxRxHrS motif: a conserved region essential for DNA binding of the VirR response regulator from Clostridium perfringens. J. Mol. Biol. 322:997–1011 [DOI] [PubMed] [Google Scholar]
- 148. Shimizu T, Yaguchi H, Ohtani K, Banu S, Hayashi H. 2002. Clostridial VirR/VirS regulon involves a regulatory RNA molecule for expression of toxins. Mol. Microbiol. 43:257–265 [DOI] [PubMed] [Google Scholar]
- 149. Shimizu T, Shima K, Yoshino K, Yonezawa K, Hayashi H. 2002. Proteome and transcriptome analysis of the virulence genes regulated by the VirR/VirS system in Clostridium perfringens. J. Bacteriol. 184:2587–2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ohtani K, Kawsar HI, Okumura K, Hayashi H, Shimizu T. 2003. The VirR/VirS regulatory cascade affects transcription of plasmid-encoded putative virulence genes in Clostridium perfringens. FEMS Microbiol. Lett. 222:137–141 [DOI] [PubMed] [Google Scholar]
- 151. Cheung JK, Keyburn AL, Carter G, Lanckriet A, Van Immerseel F, Moore R, Rood JI. 2010. The VirSR two-component signal transduction system regulates NetB toxin production in Clostridium perfringens. Infect. Immun. 78:3064–3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Ma M, Vidal J, Saputo J, McClane BA, Uzal F. 2011. The VirS/VirR two-component system regulates the anaerobic cytotoxicity, intestinal pathogenicity, and enterotoxemic lethality of type C isolate CN3685. mBio 2(1):e00338–10 doi: 10.1128/mBio.00338-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Vidal JE, Ohtani K, Shimizu T, McClane BA. 2009. Contact with enterocyte-like Caco-2 cells induces rapid upregulation of toxin production by Clostridium perfringens type C isolates. Cell. Microbiol. 11:1306–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Chen J, Rood JI, McClane BA. 2011. Epsilon-toxin production by Clostridium perfringens type D strain CN3718 is dependent upon the agr operon but not the VirS/VirR two-component regulatory system. mBio 2(6):e00275–11 doi: 10.1128/mBio.00275-11 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 155. Ohtani K, Yuan Y, Hassan S, Wang R, Wang Y, Shimizu M. 2009. Virulence gene regulation by the agr system in Clostridium perfringens. J. Bacteriol. 191:3919–3927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Vidal JE, Chen J, Li J, McClane BA. 2009. Use of an EZ-Tn5-based random mutagenesis system to identify a novel toxin regulatory locus in Clostridium perfringens strain 13. PLoS One 4:e6232 doi: 10.1371/journal.pone.0006232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Li J, Chen J, Vidal JE, McClane BA. 2011. The Agr-like quorum-sensing system regulates sporulation and production of enterotoxin and beta2 toxin by Clostridium perfringens type A non-food-borne human gastrointestinal disease strain F5603. Infect. Immun. 79:2451–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Vidal JE, Ma M, Saputo J, Garcia J, Uzal FA, McClane BA. 2011. Evidence that the Agr-like quorum sensing system regulates the toxin production, cytotoxicity and pathogenicity of Clostridium perfringens type C isolate CN3685. Mol. Microbiol. 83:179–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Chen J, McClane BA. 2012. Role of the agr-like quorum-sensing system in regulating toxin production by Clostridium perfringens type B strains CN1793 and CN1795. Infect. Immun. 80:3008–3017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. McNee JW, Dunn JS. 1917. The method of spread of gas gangrene into living muscle. Br. Med. J. i:726–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Maclennan JD. 1962. The histotoxic clostridial infections of man. Bacteriol. Rev. 26:177–276 [PMC free article] [PubMed] [Google Scholar]
- 162. Stevens DL, Rood JI. 2006. Histotoxic clostridia, p 715–725 In Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Rood JI. (ed), Gram-positive pathogens, 2nd ed ASM Press, Washington, DC [Google Scholar]
- 163. Rood JI. 2007. Clostridium perfringens and histotoxic disease, p 753–770 In Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E. (ed), The prokaryotes: a handbook on the biology of bacteria, 3rd ed Springer, New York, NY [Google Scholar]
- 164. Stevens DL, Tweten RK, Awad MM, Rood JI, Bryant AE. 1997. Clostridial gas gangrene: evidence that alpha and theta toxins differentially modulate the immune response and induce acute tissue necrosis. J. Infect. Dis. 176:189–195 [DOI] [PubMed] [Google Scholar]
- 165. Awad MM, Ellemor DM, Boyd RL, Emmins JJ, Rood JI. 2001. Synergistic effects of alpha-toxin and perfringolysin O in Clostridium perfringens-mediated gas gangrene. Infect. Immun. 69:7904–7910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Parish S, Valberg S. 2009. Inflammatory myopathies. Clostridial myonecrosis, p 1400–1492 In Smith BP. (ed), Large animal internal medicine. Mosby-Elsevier, St Louis, MO [Google Scholar]
- 167. Harwood DG. 1984. Apparent iatrogenic clostridial myositis in cattle. Vet. Rec. 115:412. [DOI] [PubMed] [Google Scholar]
- 168. Odani JS, Blanchard PC, Adaska JM, Moeller RB, Uzal FA. 2009. Malignant edema in postpartum dairy cattle. J. Vet. Diagn. Invest. 21:920–924 [DOI] [PubMed] [Google Scholar]
- 169. Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M, Roy S, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Grant K, Kenyon S, Nwafor I, Plowman J, Ohai C, Halford-Maw R, Peck M, McLauchlin J. 2008. The identification and characterization of Clostridium perfringens by real-time PCR, location of enterotoxin gene, and heat resistance. Foodborne Pathog. Dis. 5:629–639 [DOI] [PubMed] [Google Scholar]
- 171. Sarker MR, Shivers RP, Sparks SG, Juneja VK, McClane BA. 2000. Comparative experiments to examine the effects of heating on vegetative cells and spores of Clostridium perfringens isolates carrying plasmid versus chromosomal enterotoxin genes. Appl. Environ. Microbiol. 66:3234–3240 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 172. Li J, McClane BA. 2006. Further comparison of temperature effects on growth and survival of Clostridium perfringens type A isolates carrying a chromosomal or plasmid-borne enterotoxin gene. Appl. Environ. Microbiol. 72:4561–4568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Li J, McClane BA. 2006. Comparative effects of osmotic, sodium nitrite-induced, and pH-induced stress on growth and survival of Clostridium perfringens type A isolates carrying chromosomal or plasmid-borne enterotoxin genes. Appl. Environ. Microbiol. 72:7620–7625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Novak JS, Juneja VK, McClane BA. 2003. An ultrastructural comparison of spores from various strains of Clostridium perfringens and correlations with heat resistance parameters. Int. J. Food Microbiol. 86:239–247 [DOI] [PubMed] [Google Scholar]
- 175. Orsburn B, Melville SB, Popham D. 2008. Factors contributing to heat resistance of Clostridium perfringens endospores. Appl. Environ. Microbiol. 74:3328–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Li J, McClane BA. 2008. A novel small acid soluble protein variant is important for spore resistance of most Clostridium perfringens food poisoning isolates. PLoS Pathog. 4:e1000056 doi: 10.1371/journal.ppat.1000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Li J, Paredes-Sabja D, Sarker MR, McClane BA. 2009. Further charactrization of Clostridium perfringens small acid soluble protein-4 (Ssp4) properties and expression. PLoS One 4:e6249 doi: 10.1371/journal.pone.0006249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Li J, McClane BA. 2010. Evaluating the involvement of alternative sigma factors SigF and SigG in Clostridium perfringens sporulation and enterotoxin synthesis. Infect. Immun. 78:4286–4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Harry KH, Zhou R, Kroos L, Melville SB. 2009. Sporulation and enterotoxin (CPE) synthesis are controlled by the sporulation-specific factors SigE and SigK in Clostridium perfringens. J. Bacteriol. 191:2728–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Zhao Y, Melville SB. 1998. Identification and characterization of sporulation-dependent promoters upstream of the enterotoxin gene (cpe) of Clostridium perfringens. J. Bacteriol. 180:136–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Huang IH, Waters M, Grau RR, Sarker MR. 2004. Disruption of the gene (spo0A) encoding sporulation transcription factor blocks endospore formation and enterotoxin production in enterotoxigenic Clostridium perfringens type A. FEMS Microbiol. Lett. 233:233–240 [DOI] [PubMed] [Google Scholar]
- 182. Bos J, Smithee L, McClane BA, Distefano RF, Uzal F, Songer JG, Mallonee S, Crutcher JM. 15 April 2005. Fatal necrotizing enteritis following a foodborne outbreak of enterotoxigenic Clostridium perfringens type A infection. Clin. Infect. Dis. 15:e78–e83 [DOI] [PubMed] [Google Scholar]
- 183. CDC 2012. Fatal foodborne Clostridium perfringens illness at a state psychiatric hospital—Louisiana, 2010. MMWR Morb. Mortal. Wkly. Rep. 61:605–608 [PubMed] [Google Scholar]
- 184. Caserta JA, Robertson SL, Saputo J, Shrestha A, McClane BA, Uzal FA. 2011. Development and application of a mouse interstinal loop model to study the in vivo action of Clostridium perfringens enterotoxin. Infect. Immun. 79:3020–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Carman RJ. 1997. Clostridium perfringens in spontaneous and antibiotic-associated diarrhoea of man and other animals. Rev. Med. Microbiol. 8(Suppl 1):S43–S45 [Google Scholar]
- 186. Carman RJ, Sayeed S, Li J, Genheimer CW, Hiltonsmith MF, Wilkins TD, McClane BA. 2008. Clostridium perfringens toxin genotypes in the feces of healthy North Americans. Anaerobe 14:102–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Heikinheimo A, Lindstrom M, Granum PE, Korkeala H. 2006. Humans as reservoir for enterotoxin gene-carrying Clostridium perfringens type A. Emerg. Infect. Dis. 12:1724–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Borriello SP, Barclay FE, Welch AR, Stringer MF, Watson GN, Williams RKT, Seal DV, Sullens K. 1985. Epidemiology of diarrhea caused by enterotoxigenic Clostridium perfringens. J. Med. Microbiol. 20:363–372 [DOI] [PubMed] [Google Scholar]
- 189. Johnson S, Gerding DN. 1997. Enterotoxemic infections, p 117–140 In Rood JI, McClane BA, Songer JG, Titball RW. (ed), The clostridia: molecular biology and pathogenesis. Academic Press, London, England [Google Scholar]
- 190. Lawrence GW. 1997. The pathogenesis of enteritis necroticans, p 197–207 In Rood JI, McClane BA, Songer JG, Titball RW. (ed), The clostridia: molecular biology and pathogenesis. Academic Press, London, England [Google Scholar]
- 191. Zeissler J, Rassfeld-Sternberg L. 1949. Enteritis necroticans due to Clostridium welchii type F. Br. Med. J. i:267–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Van Immerseel F, Rood JI, Moore RJ, Titball RW. 2009. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 17:32–36 [DOI] [PubMed] [Google Scholar]
- 193. Cooper KK, Songer JG. 2009. Necrotic enteritis in chickens: a paradigm of enteric infection by Clostridium perfringens type A. Anaerobe 15:55–60 [DOI] [PubMed] [Google Scholar]
- 194. Keyburn AL, Sheedy SA, Ford ME, Williamson MM, Awad MM, Rood JI, Moore RJ. 2006. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect. Immun. 74:6496–6500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Kulkarni RR, Parreira VR, Sharif S, Prescott JF. 2007. Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis. Clin. Vaccine Immunol. 14:1070–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Zekarias B, Mo H, Curtiss R., III 2008. Recombinant attenuated Salmonella enterica serovar Typhimurium expressing the carboxy-terminal domain of alpha toxin from Clostridium perfringens induces protective responses against necrotic enteritis in chickens. Clin. Vaccine Immunol. 15:805–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Lee KW, Lillehoj HS, Park MS, Jang SI, Ritter GD, Hong YH, Jeong W, Jeoung HY, An DJ, Lillehoj EP. 2012. Clostridium perfringens alpha-toxin and NetB toxin antibodies and their possible role in protection against necrotic enteritis and gangrenous dermatitis in broiler chickens. Avian Dis. 56:230–233 [DOI] [PubMed] [Google Scholar]
- 198. Jang SI, Lillehoj HS, Lee SH, Lee KW, Lillehoj EP, Hong YH, An DJ, Jeong W, Chun JE, Bertrand F, Dupuis L, Deville S, Arous JB. 2012. Vaccination with Clostridium perfringens recombinant proteins in combination with Montanide ISA 71 VG adjuvant increases protection against experimental necrotic enteritis in commercial broiler chickens. Vaccine 30:5401–5406 [DOI] [PubMed] [Google Scholar]
- 199. Miyakawa ME, Saputo J, Leger JS, Puschner B, Fisher DJ, McClane BA, Uzal FA. 2007. Necrotizing enterocolitis and death in a goat kid associated with enterotoxin (CPE)-producing Clostridium perfringens type A. Can. Vet. J. 48:1266–1269 [PMC free article] [PubMed] [Google Scholar]
- 200. Marks S, Kather E, Kass P, Melli A. 2002. Genotypic and phenotypic characterization of Clostridium perfringens and Clostridium difficile in diarrheic and healthy dogs. J. Vet. Intern. Med. 16:533–540 [DOI] [PubMed] [Google Scholar]
- 201. Weese JS, Staempfli HR, Prescott JF. 2001. A prospective study of the roles of Clostridium difficile and enterotoxigenic Clostridium perfringens in equine diarrhoea. Equine Vet. J. 33:403–409 [DOI] [PubMed] [Google Scholar]
- 202. Guss SB. 1977. Management and diseases of dairy goats, p 106–109 Dairy Goat Journal Publishing Corp., Scottsdale, AZ [Google Scholar]
- 203. McGowan B, Moulton JE, Rood SE. 1958. Lamb losses associated with Clostridium perfringens type A. J. Am. Vet. Med. Assoc. 133:219–221 [PubMed] [Google Scholar]
- 204. Bueschel DM, Jost BH, Billington SJ, Trinh HT, Songer JG. 2003. Prevalence of cpb2, encoding beta2 toxin, in Clostridium perfringens field isolates: correlation of genotype with phenotype. Vet. Microbiol. 94:121–129 [DOI] [PubMed] [Google Scholar]
- 205. Dray T. 2004. Clostridium perfringens type A and beta2 toxin associated with enterotoxemia in a 5-week-old goat. Can. Vet. J. 45:251–253 [PMC free article] [PubMed] [Google Scholar]
- 206. Waters M, Savoie A, Garmory HS, Bueschel D, Popoff MR, Songer JG, Titball RW, McClane BA, Sarker MR. 2003. Genotyping and phenotyping of beta2-toxigenic Clostridium perfringens fecal isolates associated with gastrointestinal diseases in piglets. J. Clin. Microbiol. 41:3584–3591 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 207. Songer JG, Uzal FA. 2005. Clostridial enteric infections in pigs. J. Vet. Diagn. Invest. 17:528–536 [DOI] [PubMed] [Google Scholar]
- 208. Songer JG. 1998. Clostridial diseases of small ruminants. Vet. Res. 29:219–232 [PubMed] [Google Scholar]
- 209. Lewis CJ. 2000. Clostridial diseases, p 131–142 In Martin WB, Aitken ID. (ed), Diseases of sheep. Blackwell Science, Oxford, England [Google Scholar]
- 210. Sayeed S, Uzal FA, Fisher DJ, Saputo J, Vidal JE, Chen Y, Gupta P, Rood JI, McClane BA. 2008. Beta toxin is essential for the intestinal virulence of Clostridium perfringens type C disease isolate CN3685 in a rabbit ileal loop model. Mol. Microbiol. 67:15–30 [DOI] [PubMed] [Google Scholar]
- 211. Gurtner C, Popescu F, Wyder M, Sutter E, Zeeh F, Frey J, von Schubert C, Posthaus H. 2010. Rapid cytopathic effects of Clostridium perfringens beta-toxin on porcine endothelial cells. Infect. Immun. 78:2966–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Schumacher VL, Martel A, Pasmans F, Van Immerseel F, Posthaus H. Endothelial binding of beta toxin to small intestinal mucosal endothelial cells in early stages of experimentally induced Clostridium perfringens type C enteritis in pigs. Vet. Pathol., in press [DOI] [PubMed] [Google Scholar]
- 213. Uzal FA, Kelly WR. 1997. Effects of the intravenous administration of Clostridium perfringens type D epsilon toxin on young goats and lambs. J. Comp. Pathol. 116:63–71 [DOI] [PubMed] [Google Scholar]
- 214. Sayeed S, Fernandez-Miyakawa ME, Fisher DJ, Adams V, Poon R, Rood JI, Uzal FA, McClane BA. 2005. Epsilon-toxin is required for most Clostridium perfringens type D vegetative culture supernatants to cause lethality in the mouse intravenous injection model. Infect. Immun. 73:7413–7421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Buxton D, Linklater KA, Dyson DA. 1978. Pulpy kidney disease and its diagnosis by histological examination. Vet. Rec. 102:241. [DOI] [PubMed] [Google Scholar]
- 216. Finnie JW. 1984. Histopathological changes in the brain of mice given Clostridium perfringens type D epsilon toxin. J. Comp. Pathol. 94:363–370 [DOI] [PubMed] [Google Scholar]
- 217. Finnie JW. 1984. Ultrastructural changes in the brain of mice given Clostridium perfringens type D epsilon toxin. J. Comp. Pathol. 94:445–452 [DOI] [PubMed] [Google Scholar]
- 218. Finnie JW, Hajduk P. 1992. An immunohistochemical study of plasma albumin extravasation in the brain of mice after the administration of Clostridium perfringens type D epsilon toxin. Aust. Vet. J. 69:261–262 [DOI] [PubMed] [Google Scholar]
- 219. Uzal FA, Songer G. 2008. Diagnosis of Clostridium perfringens intestinal infections in sheep and goat. J. Vet. Diagn. Invest. 20:253–265 [DOI] [PubMed] [Google Scholar]
- 220. Songer JG, Miskimmins DW. 2004. Clostridium perfringens type E enteritis in calves: two cases and a brief review of the literature. Anaerobe 10:239–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Vidal JE, McClane BA, Saputo J, Parker J, Uzal FA. 2008. Effects of Clostridium perfringens beta-toxin on the rabbit small intestine and colon. Infect. Immun. 76:4396–4404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Garcia JP, Beingesser J, Fisher DJ, Sayeed S, McClane BA, Posthaus H, Uzal FA. 2012. The effect of Clostridium perfringens type C strain CN3685 and its isogenic beta toxin null mutant in goats. Vet. Microbiol. 157:412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Uzal FA, Saputo J, Sayeed S, Vidal JE, Fisher DJ, Poon R, Adams V, Fernandez-Miyakawa ME, Rood JI, McClane BA. 2009. Development and application of new mouse models to study the pathogenesis of Clostridium perfringens type C enterotoxemias. Infect. Immun. 77:5291–5299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Abraham LJ, Wales AJ, Rood JI. 1985. Worldwide distribution of the conjugative Clostridium perfringens tetracycline resistance plasmid, pCW3. Plasmid 14:37–46 [DOI] [PubMed] [Google Scholar]
- 225. Rokos EA, Rood JI, Duncan CL. 1978. Multiple plasmids in different toxigenic types of Clostridium perfringens. FEMS Microbiol. Lett. 4:323–326 [Google Scholar]
- 226. Brefort G, Magot M, Ionesco H, Sebald M. 1977. Characterization and transferability of Clostridium perfringens plasmids. Plasmid 1:52–66 [DOI] [PubMed] [Google Scholar]
- 227. Rood JI, Maher EA, Somers EB, Campos E, Duncan CL. 1978. Isolation and characterization of multiply antibiotic-resistant Clostridium perfringens strains from porcine feces. Antimicrob. Agents Chemother. 13:871–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Abraham LJ, Rood JI. 1985. Cloning and analysis of the Clostridium perfringens tetracycline resistance plasmid, pCW3. Plasmid 13:155–162 [DOI] [PubMed] [Google Scholar]
- 229. Garnier T, Saurin W, Cole ST. 1987. Molecular characterization of the resolvase gene, res, carried by a multicopy plasmid from Clostridium perfringens: common evolutionary origin for prokaryotic site-specific recombinases. Mol. Microbiol. 1:371–376 [DOI] [PubMed] [Google Scholar]
- 230. Garnier T, Cole ST. 1988. Complete nucleotide sequence and genetic organization of the bacteriocinogenic plasmid, pIP404, from Clostridium perfringens. Plasmid 19:134–150 [DOI] [PubMed] [Google Scholar]
- 231. Garnier T, Cole ST. 1988. Identification and molecular genetic analysis of replication functions of the bacteriocinogenic plasmid pIP404 from Clostridium perfringens. Plasmid 19:151–160 [DOI] [PubMed] [Google Scholar]
- 232. Garnier T, Cole ST. 1986. Characterization of a bacteriocinogenic plasmid from Clostridium perfringens and molecular genetic analysis of the bacteriocin-encoding gene. J. Bacteriol. 168:1189–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Duncan CL, Rokos EA, Christenson CM, Rood JI. 1978. Multiple plasmids in different toxigenic types of Clostridium perfringens: possible control of beta toxin production, p 246–248 In Schlessinger D. (ed), Microbiology—1978. American Society for Microbiology, Washington, DC [Google Scholar]
- 234. Katayama SI, Dupuy B, Daube G, China B, Cole ST. 1996. Genome mapping of Clostridium perfringens strains with I-Ceu I shows many virulence genes to be plasmid-borne. Mol. Gen. Genet. 251:720–726 [DOI] [PubMed] [Google Scholar]
- 235. Parreira VR, Costa M, Eikmeyer F, Blom J, Prescott JF. 2012. Sequence of two plasmids from Clostridium perfringens chicken necrotic enteritis isolates and comparison with C. perfringens conjugative plasmids. PLoS One 7:e49753 doi: 10.1371/journal.pone.0049753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Bannam TL, Teng WL, Bulach D, Lyras D, Rood JI. 2006. Functional identification of conjugation and replication regions of the tetracycline resistance plasmid pCW3 from Clostridium perfringens. J. Bacteriol. 188:4942–4951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Miyamoto K, Wen Q, McClane BA. 2004. Multiplex PCR genotyping assay that distinguishes between isolates of Clostridium perfringens type A carrying a chromosomal enterotoxin gene (cpe) locus, a plasmid cpe locus with an IS1470-like sequence, or a plasmid cpe locus with an IS1151 sequence. J. Clin. Microbiol. 41:1552–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Brynestad S, Granum PE. 1999. Evidence that Tn5565, which includes the enterotoxin gene in Clostridium perfringens, can have a circular form which may be a transposition intermediate. FEMS Microbiol. Lett. 170:281–286 [DOI] [PubMed] [Google Scholar]
- 239. Rood JI, Scott VN, Duncan CL. 1978. Identification of a transferable tetracycline resistance plasmid (pCW3) from Clostridium perfringens. Plasmid 1:563–570 [DOI] [PubMed] [Google Scholar]
- 240. Abraham LJ, Rood JI. 1987. Identification of Tn4451 and Tn4452, chloramphenicol resistance transposons from Clostridium perfringens. J. Bacteriol. 169:1579–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Brynestad S, Sarker MR, McClane BA, Granum PE, Rood JI. 2001. The enterotoxin (CPE) plasmid from Clostridium perfringens is conjugative. Infect. Immun. 69:3483–3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Teng WL, Bannam TL, Parsons JA, Rood JI. 2008. Functional characterization and localization of the TcpH conjugation protein from Clostridium perfringens. J. Bacteriol. 190:5075–5086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Parsons JA, Bannam TL, Devenish RJ, Rood JI. 2007. TcpA, an FtsK/SpoIIIE homolog, is essential for transfer of the conjugative plasmid pCW3 in Clostridium perfringens. J. Bacteriol. 189:7782–7790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Porter CJ, Bantwal R, Bannam TL, Rosado CJ, Pearce MC, Adams V, Lyras D, Whisstock JC, Rood JI. 2012. The conjugation protein TcpC from Clostridium perfringens is structurally related to the type IV secretion system protein VirB8 from Gram-negative bacteria. Mol. Microbiol. 83:275–288 [DOI] [PubMed] [Google Scholar]
- 245. Bantwal R, Bannam TL, Porter CJ, Quinsey NS, Lyras D, Adams V, Rood JI. 2012. The peptidoglycan hydrolase TcpG is required for efficient conjugative transfer of pCW3 in Clostridium perfringens. Plasmid 67:139–147 [DOI] [PubMed] [Google Scholar]
- 246. Llosa M, Gomis-Ruth FX, Coll M, de la Cruz F. 2002. Bacterial conjugation: a two-step mechanism for DNA transport. Mol. Microbiol. 45:1–8 [DOI] [PubMed] [Google Scholar]
- 247. Iyer LM, Makarova KS, Koonin EV, Aravind L. 2004. Comparative genomics of the FtsK-HerA superfamily of pumping ATPases: implications for the origins of chromosome segregation, cell division and viral capsid packaging. Nucleic Acids Res. 32:5260–5279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Steen JA, Bannam TL, Teng WL, Devenish RJ, Rood JI. 2009. The putative coupling protein TcpA interacts with other pCW3-encoded proteins to form an essential part of the conjugation complex. J. Bacteriol. 191:2926–2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Kumar RB, Xie YH, Das A. 2000. Subcellular localization of the Agrobacterium tumefaciens T-DNA transport pore proteins: VirB8 is essential for the assembly of the transport pore. Mol. Microbiol. 36:608–617 [DOI] [PubMed] [Google Scholar]
- 250. Sivanesan D, Hancock MA, Villamil Giraldo AM, Baron C. 2010. Quantitative analysis of VirB8-VirB9-VirB10 interactions provides a dynamic model of type IV secretion system core complex assembly. Biochemistry 49:4483–4493 [DOI] [PubMed] [Google Scholar]
- 251. del Solar G, Giraldo R, Ruiz-Echevarria MJ, Espinosa M, Diaz-Orejas R. 1998. Replication and control of circular bacterial plasmids. Microbiol. Mol. Biol. Rev. 62:434–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Ionesco H. 1980. Transferable tetracycline resistance in “Clostridium difficile”. Ann. Microbiol. (Paris) 131A:171–179 (In French.) [PubMed] [Google Scholar]
- 253. Lyras D, Storie C, Huggins AS, Crellin PK, Bannam TL, Rood JI. 1998. Chloramphenicol resistance in Clostridium difficile is encoded on Tn4453 transposons that are closely related to Tn4451 from Clostridium perfringens. Antimicrob. Agents Chemother. 42:1563–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Sasaki Y, Yamamoto K, Tamura Y, Takahashi T. 2001. Tetracycline-resistance genes of Clostridium perfringens, Clostridium septicum and Clostridium sordellii isolated from cattle affected with malignant edema. Vet. Microbiol. 83:61–69 [DOI] [PubMed] [Google Scholar]
- 255. Raffestin S, Marvaud JC, Cerrato R, Dupuy B, Popoff MR. 2004. Organization and regulation of the neurotoxin genes in Clostridium botulinum and Clostridium tetani. Anaerobe 10:93–100 [DOI] [PubMed] [Google Scholar]
- 256. Marshall KM, Bradshaw M, Johnson EA. 2010. Conjugative botulinum neurotoxin-encoding plasmids in Clostridium botulinum. PLoS One 5:e11087 doi: 10.1371/journal.pone.0011087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. McClane BA, Lyerly DM, Wilkins TD. 2006. Enterotoxic clostridia: Clostridium perfringens type A and Clostridium difficile, p 703–714 In Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Rood J. (ed), Gram-positive pathogens, 3rd ed ASM Press, Washington, DC [Google Scholar]
- 258. Xiao Y, Wagendorp A, Moezelaar R, Abee T, Wells-Bennik MH. 2012. A wide variety of Clostridium perfringens type A food-borne isolates that carry a chromosomal cpe gene belong to one multilocus sequence typing cluster. Appl. Environ. Microbiol. 78:7060–7068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Lahti P, Lindstrom M, Somervuo P, Heikinheimo A, Korkeala H. 2012. Comparative genomic hybridization analysis shows different epidemiology of chromosomal and plasmid-borne cpe-carrying Clostridium perfringens type A. PLoS One 7:e46162 doi: 10.1371/journal.pone.0046162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Lepp D, Gong J, Songer JG, Boerlin P, Parreira VR, Prescott JF. 2013. Identification of accessory genome regions in poultry Clostridium perfringens isolates carrying the NetB plasmid. J. Bacteriol. 195:1152–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]