Abstract
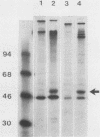
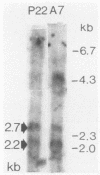
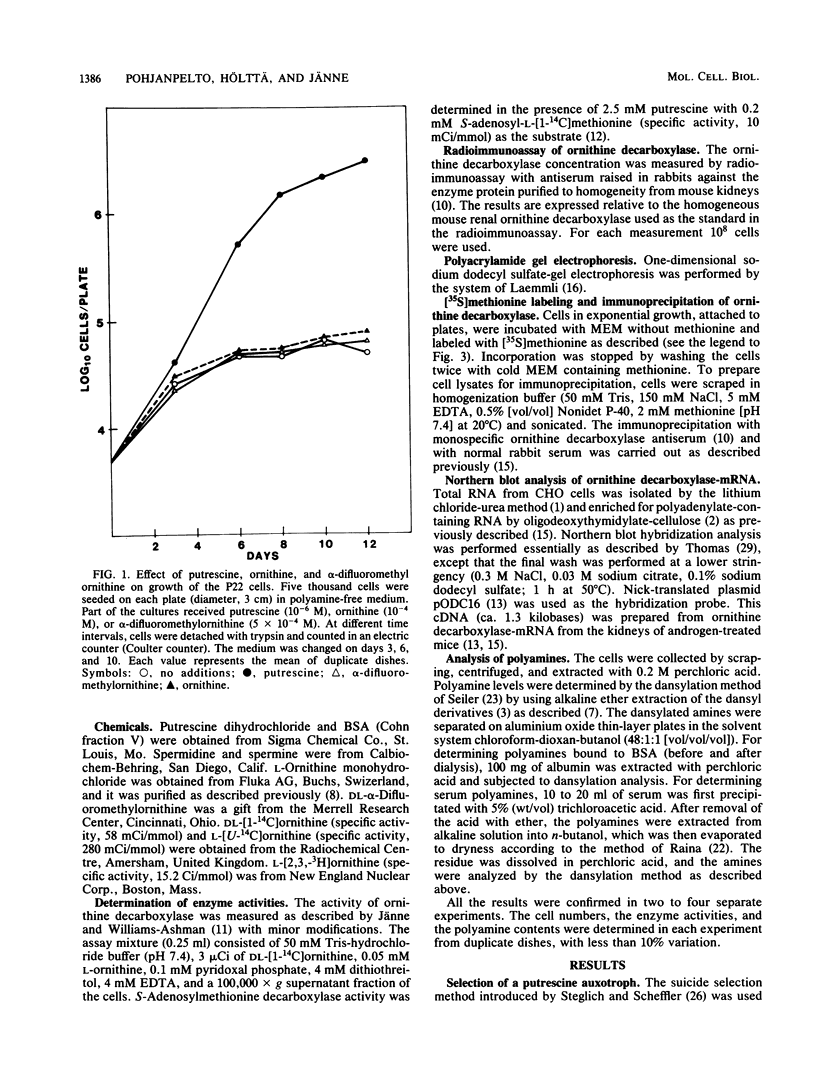
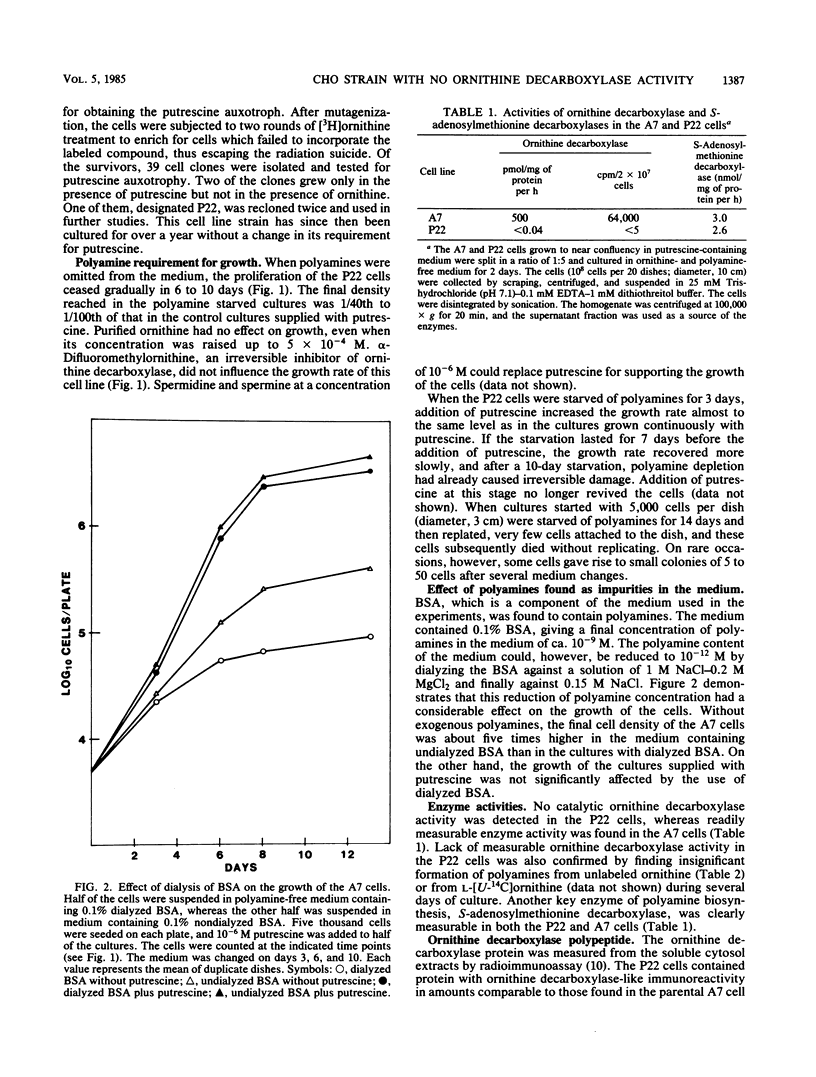
We previously described an arginase-deficient, polyamine-dependent Chinese hamster ovary cell line which grows in serum-free medium. From this strain we isolated a new mutant strain that has no detectable catalytic ornithine decarboxylase activity. The mutant cells contain, however, immunoreactive ornithine decarboxylase-like protein roughly in the same quantity as the parent strain. The mutant and the parent cell line strains also contain similar amounts of ornithine decarboxylase-mRNA hybridizable to a specific cDNA. If polyamines are omitted from the medium, proliferation of the mutant cells is considerably retarded and ceases in 6 to 10 days. Addition of ornithine or alpha-difluoromethylornithine, a specific inhibitor of ornithine decarboxylase, has no effect on these cells. Putrescine and spermidine decreased in the mutant cells to undetectable levels during polyamine starvation, whereas spermine was reduced to 1/5th of that found in the control cultures. Polyamines appear to be indispensable for the mutant strain, but this was obvious only after the amount of polyamines, found as impurities in bovine serum albumin used in the medium, was reduced by dialysis to 10(-12) M. Because sera contain polyamines, the ability of the mutant strain to grow in serum-free medium is a great advantage in elucidation of the mechanisms of polyamine function.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G., Dvir R., Harell A., Chayen R. Determination of polyamines in urine. Clin Chim Acta. 1973 Nov 23;49(1):65–72. doi: 10.1016/0009-8981(73)90344-6. [DOI] [PubMed] [Google Scholar]
- Hafner E. W., Tabor C. W., Tabor H. Mutants of Escherichia coli that do not contain 1,4-diaminobutane (putrescine) or spermidine. J Biol Chem. 1979 Dec 25;254(24):12419–12426. [PubMed] [Google Scholar]
- Harada J. J., Morris D. R. Cell cycle parameters of Chinese hamster ovary cells during exponential, polyamine-limited growth. Mol Cell Biol. 1981 Jul;1(7):594–599. doi: 10.1128/mcb.1.7.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heby O., Andersson G., Gray J. W. Interference with S and G2 phase progression by polyamine synthesis inhibitors. Exp Cell Res. 1978 Feb;111(2):461–464. doi: 10.1016/0014-4827(78)90192-1. [DOI] [PubMed] [Google Scholar]
- Hölttä E., Pohjanpelto P. Polyamine dependence of Chinese hamster ovary cells in serum-free culture is due to deficient arginase activity. Biochim Biophys Acta. 1982 Dec 30;721(4):321–327. doi: 10.1016/0167-4889(82)90085-4. [DOI] [PubMed] [Google Scholar]
- Hölttä E., Pohjanpelto P. Polyamine starvation causes accumulation of cadaverine and its derivatives in a polyamine-dependent strain of Chinese-hamster ovary cells. Biochem J. 1983 Mar 15;210(3):945–948. doi: 10.1042/bj2100945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomaa V. V., Pajunen A. E., Bardin C. W., Jänne O. A. Ornithine decarboxylase in mouse kidney. Purification, characterization, and radioimmunological determination of the enzyme protein. J Biol Chem. 1983 Jun 10;258(11):6735–6740. [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. Dissociation of putrescine-activated decarboxylation of S-adenosyl-L-methionine from the enzymic synthesis of spermidine and spermine by purified prostatic enzyme preparations. Biochem Biophys Res Commun. 1971 Jan 22;42(2):222–229. [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. On the purification of L-ornithine decarboxylase from rat prostate and effects of thiol compounds on the enzyme. J Biol Chem. 1971 Mar 25;246(6):1725–1732. [PubMed] [Google Scholar]
- Jänne O. A., Kontula K. K., Isomaa V. V., Bardin C. W. Ornithine decarboxylase mRNA in mouse kidney: a low abundancy gene product regulated by androgens with rapid kinetics. Ann N Y Acad Sci. 1984;438:72–84. doi: 10.1111/j.1749-6632.1984.tb38277.x. [DOI] [PubMed] [Google Scholar]
- Knuutila S., Pohjanpelto P. Polyamine starvation causes parallel increase in nuclear and chromosomal aberrations in a polyamine-dependent strain of CHO. Exp Cell Res. 1983 Apr 15;145(1):222–226. doi: 10.1016/s0014-4827(83)80024-x. [DOI] [PubMed] [Google Scholar]
- Kontula K. K., Torkkeli T. K., Bardin C. W., Jänne O. A. Androgen induction of ornithine decarboxylase mRNA in mouse kidney as studied by complementary DNA. Proc Natl Acad Sci U S A. 1984 Feb;81(3):731–735. doi: 10.1073/pnas.81.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Pohjanpelto P., Knuutila S. Induction of major chromosome aberrations in Chinese hamster ovary cells by alpha-difluoromethylornithine. Cancer Res. 1984 Oct;44(10):4535–4539. [PubMed] [Google Scholar]
- Pohjanpelto P., Knuutila S. Polyamine deprivation causes major chromosome aberrations in a polyamine-dependent Chinese hamster ovary cell line. Exp Cell Res. 1982 Oct;141(2):333–339. doi: 10.1016/0014-4827(82)90221-x. [DOI] [PubMed] [Google Scholar]
- Pohjanpelto P. Putrescine shortens the S-period in human fibroblasts. Biomedicine. 1975 Nov 10;23(9):350–352. [PubMed] [Google Scholar]
- Pohjanpelto P., Virtanen I., Hölttä E. Polyamine starvation causes disappearance of actin filaments and microtubules in polyamine-auxotrophic CHO cells. Nature. 1981 Oct 8;293(5832):475–477. doi: 10.1038/293475a0. [DOI] [PubMed] [Google Scholar]
- RAINA A. STUDIES ON THE DETERMINATION OF SPERMIDINE AND SPERMINE AND THEIR METABOLISM IN THE DEVELOPING CHICK EMBRYO. Acta Physiol Scand Suppl. 1963:SUPPL 218–21981. [PubMed] [Google Scholar]
- Seiler N. Use of the dansyl reaction in biochemical analysis. Methods Biochem Anal. 1970;18:259–337. doi: 10.1002/9780470110362.ch5. [DOI] [PubMed] [Google Scholar]
- Seyfried C. E., Morris D. R. Relationship between inhibition of polyamine biosynthesis and DNA replication in activated lymphocytes. Cancer Res. 1979 Dec;39(12):4861–4867. [PubMed] [Google Scholar]
- Steglich C., Scheffler I. E. An ornithine decarboxylase-deficient mutant of Chinese hamster ovary cells. J Biol Chem. 1982 Apr 25;257(8):4603–4609. [PubMed] [Google Scholar]
- Sunkara P. S., Chang C. C., Prakash N. J. Role of polyamines during chromosome condensation of mammalian cells. Cell Biol Int Rep. 1983 Jun;7(6):455–465. doi: 10.1016/0309-1651(83)90135-2. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H., Tyagi A. K., Cohn M. S. The biochemistry, genetics, and regulation of polyamine biosynthesis in Saccharomyces cerevisiae. Fed Proc. 1982 Dec;41(14):3084–3088. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofilon P. J., Oredsson S. M., Deen D. F., Marton L. J. Polyamine depletion influences drug-induced chromosomal damage. Science. 1982 Sep 10;217(4564):1044–1046. doi: 10.1126/science.6810463. [DOI] [PubMed] [Google Scholar]
- Whitney P. A., Morris D. R. Polyamine auxotrophs of Saccharomyces cerevisiae. J Bacteriol. 1978 Apr;134(1):214–220. doi: 10.1128/jb.134.1.214-220.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]