Abstract
Cardiovascular calcification is a prominent feature of chronic inflammatory disorders — such as chronic kidney disease (CKD), type 2 diabetes (T2D), and atherosclerosis — that associate with significant morbidity and mortality. The concept that similar pathways control both bone remodeling and vascular calcification is widely accepted, but the precise mechanisms of calcification remain largely unknown. The central role of microRNAs (miRNA) as fine-tune regulators in the cardiovascular system and bone biology has gained acceptance and has raised the possibility for novel therapeutic targets. Additionally, circulating miRNAs have been proposed as biomarkers for a wide range of cardiovascular diseases, but knowledge of miRNA biology in cardiovascular calcification is very limited. This review focuses on the role of miRNA in cardiovascular disease, with emphasis on osteogenic processes. Herein, we discuss the current understanding of miRNAs in cardiovascular calcification. Furthermore, we identify a set of miRNAs common to diseases associated with cardiovascular calcification (CKD, T2D, and atherosclerosis), and we hypothesize that these miRNAs may provide a molecular signature for calcification. Finally, we discuss this novel hypothesis with emphasis on known biological and pathological osteogenic processes (e.g. osteogenic differentiation, release of calcifying matrix vesicles). The aim of this review is to provide an organized discussion of the known links between miRNA and calcification that provide emerging concepts for future studies on miRNA biology in cardiovascular calcification, which will be critical for developing new therapeutic strategies.
Keywords: miRNA, circulating miRNA, cardiovascular calcification, extracellular vesicles, cardiovascular disease, type 2 diabetes mellitus, chronic kidney disease, aortic stenosis, calcification, osteogenic, bone
Introduction
Micro-RNAs (miRNAs) are a large class of evolutionarily conserved, small, endogenous, non-coding RNAs serving as essential post-transcriptional modulators of gene expression that play a crucial role in normal physiology.1 miRNAs can be transcribed in parallel with host transcripts through two different transcription classes, exonic and intronic.2 Independent expression from intronic promoters could explain why host gene and miRNA expression do not always directly correlate.3 Another level of regulatory biological control of miRNAs are “sponge RNAs”, which act as a decoy for miRNAs.4 miRNAs regulate biological processes by binding to mRNA 3′-untranslated region (UTR) sequences to attenuate protein synthesis or mRNA stability.5 Acting as genetic switches or fine-tuners, miRNAs are key regulators of diverse biological and pathological processes, including development, organogenesis, apoptosis, and cell proliferation and differentiation. In addition to these known physiological roles, miRNA dysregulation often results in impaired cellular function and disease progression.5 The detailed regulation of miRNA biogenesis in cardiovascular disease is reviewed elsewhere.6 The central role of miRNAs as fine-tune regulators in the cardiovascular system is still under investigation; however, miRNAs have received little attention in cardiovascular calcification—one of the most severe pathophysiological outcomes associated with cardiovascular disease.
Cardiovascular calcification is a major characteristic of chronic inflammatory disorders — such as chronic kidney disease (CKD), type 2 diabetes (T2D), and atherosclerosis — that are associated with significant morbidity and mortality. The precise mechanisms of calcification within the vessel wall or heart valve leaflets remain largely unknown, but the concept that pathways controlling bone remodeling also occur in the cardiovascular system is well accepted. Indeed, vascular calcification associates with osteoporosis in humans and animal models7 — the so-called “calcification paradox”.8 The physiological balance between induction and inhibition of calcification becomes dysregulated in CKD, T2D, and atherosclerosis, leading to calcification at several sites in the cardiovascular system, including the intima and media of vessels and the cardiac valves.8 Atherosclerotic calcification occurs as a part of the atherogenic progress in the vessel intima with small hydroxyapatite mineral crystals (microcalcifications) that seem to associate with cholesterol crystals observed in early lesions.9 Medial calcification occurs primarily in association with CKD and T2D, independently of hypercholesterolemia. Calcification of the aortic valve is associated with many of the same risk factors as intimal and medial calcification and leads to impaired movement of aortic valve leaflets, resulting in valve dysfunction. All three of these processes share risk factors and etiological factors, including inflammation and oxidative stress that appear to lead to changes in phenotype of resident or circulating progenitor cells.
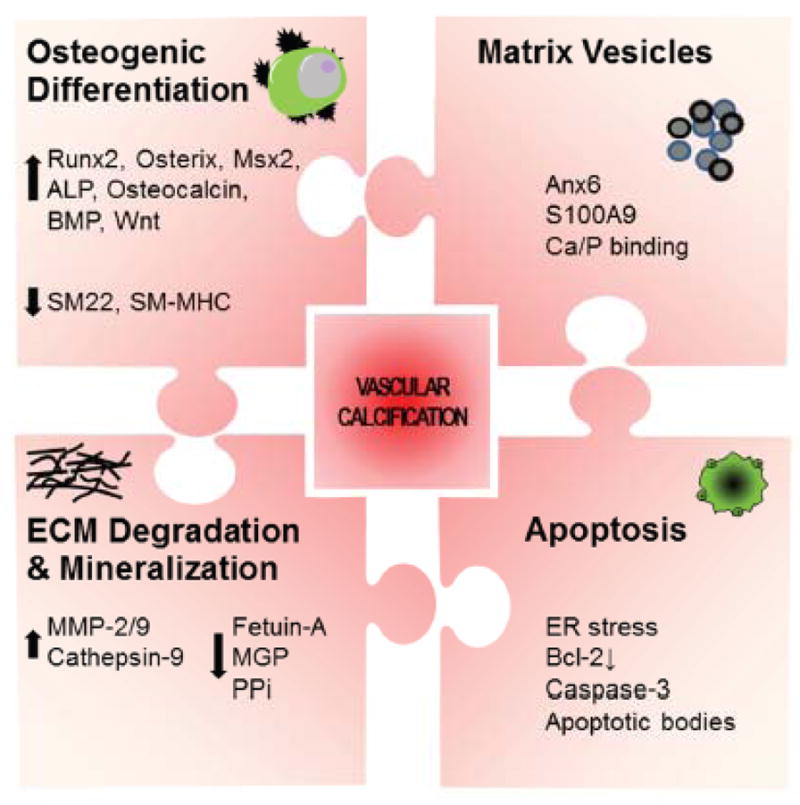
Vascular calcification is an active, cell-regulated process (Figure 1). Various studies provide evidence of phenotypic transition/dedifferentiation of mature smooth muscle cells (SMCs) into an osteogenic phenotype — a key feature in vascular calcification. Osteogenic transition of vascular SMCs or stem cells is induced by bone morphogenetic proteins, inflammation, oxidative stress, or high phosphate levels, and leads to a unique molecular pattern marked by osteogenic transcription factors.10 In medial calcification, SMCs undergo dedifferentiation from a contractile to a pro-atherogenic synthetic phenotype, lose the expression of their marker genes, acquire osteogenic markers, and deposit a mineralized bone-like matrix. The major lineage source of osteogenic cells within the calcified atherosclerotic intimal lesion are SMCs; however, circulating bone marrow-derived cells also contribute to early osteochondrogenic differentiation in atherosclerotic vessels.11 Recently, a novel concept emerged that circulating cells harboring osteogenic potential can home to atherosclerotic lesions and contribute to intimal calcification.12, 13 Independent of the source cell, it seems that master transcription factors, including Runx2/Cbfa1, Msx2, and osterix (SP7), designate cells for osteoblast lineages through the induction of downstream genes such as alkaline phosphatase (ALP), and osteocalcin. Runx2 acts as a critical regulator of osteogenic lineage and a modulator of bone-related genes14 and is essential and sufficient for driving SMC calcification.15,16, 17
Figure 1.
Major pathways/molecules involved in cardiovascular calcification.
Discovered in the bone biology field, a program of miRNAs controls Runx2 expression to prevent skeletal disorders.18 Three of these miRNAs (miR-133a, miR-135a, and miR-218) are altered in the circulation of patients with cardiovascular diseases.19, 20,21, 22 Additionally, circulating miRNAs have been proposed as biomarkers for a wide range of cardiovascular diseases, but knowledge of circulating miRNA in cardiovascular calcification is scant. No pattern of miRNA has been reported for vascular calcification; however, as new studies emerge, it may be helpful to understand the role of miRNAs in diseases and cellular signaling processes known to be associated with calcification. Therefore, in this review we begin by discussing the current, limited understanding of miRNA in cardiovascular calcification. We then document a set of circulating miRNAs, which is dysregulated in certain cardiovascular diseases (e.g., CKD, T2D, atherosclerosis), and we propose a potential circulating miRNA signature for vascular calcification. We discuss this novel hypothesis with emphasis on known biological and pathological osteogenic processes (e.g. osteogenic differentiation, release of calcifying matrix vesicles). Furthermore, we summarize and discuss miRNAs that control bone osteogenesis and link them to potential regulation of cardiovascular calcification.
miRNA in Cardiovascular Calcification and Osteogenesis
Evidence for the role of miRNA in cardiovascular calcification is very limited (Table 1). However, we recently provided the first miRNA-dependent mechanism in the progression of vascular calcification by demonstrating that miR-125b dysregulation leads to the transition of human coronary arterial SMCs into osteoblast-like cells partially by targeting the transcription factor osterix.23 Inhibition of miR-125b promoted ALP activity and matrix mineralization in vitro. Correspondingly, in vivo observations indicate that miR-125b decreased in calcified aortas of apolipoprotein-deficient (Apoe) mice fed a high fat diet for 26 weeks compared to those sacrificed after 10 weeks.23 Additionally, miRNA-processing enzymes DROSHA and DICER — essential for SMC function24 — were reduced in calcified SMCs.23
Table 1.
Specific miRNAs, their targets and effects in cardiovascular calcification
| miRNA/Regulation | Target | Cell source | Observation by using inhibitor/mimic | Reference number |
|---|---|---|---|---|
| miR-125b ↓ |
SP7 | Human CASMC | Inhibit ALP activity and matrix mineralization | 23 |
| miR-141 ↓ |
- | Porcine VIC | Blocks TGF-β-mediated ALP activity | 44 |
| miR-223 ↑ |
Mef2c RhoB SMα actin |
Human SMC | Enhance VSMC migration | 32 |
| miR-143 miR-145 ↓ |
KLF4 | Human SMC | - | 32 |
| miR-30d/e ↓ |
Runx2 | Human SMC | Inhibit ALP activity and matrix mineralization | 42 |
| miR-204 ↓ |
Runx2 | Mouse SMC | Inhibit ALP activity and osteocalcin secretion | 28 |
| miR-29a/b ↓ |
ADAMTS-7 | Rat SMC | Inhibit matrix mineralization | 52 |
| miR-135a*, miR-762, miR-714, or miR-712* ↑ |
NCX PMCA NCKX4 |
Mouse SMC | Promote matrix mineralization | 25 |
Bold; direct binding to 3′UTR not shown in this study, Arrow indicate regulation of miRNA
ALP, alkaline phosphatase; CASMC, coronary artery SMC
Using the Exiqon mercury Locked Nucleic Acids (LNA) microRNA array, Gui et al. found 20 altered miRNAs in the aortic media of klotho mutant (kl/kl) mice.25 Kl/kl mice display vascular calcification due to hyperphosphatemia and through a Runx2-dependent mechanism, presenting the symptoms of CKD-associated bone and mineral disorders.26 17 miRNAs were increased and 3 decreased (miR-1, miR-93, miR-302b) in kl/kl mice compared to wild type mice. An increased expression of miR-135a*, miR-762, miR-714, and miR-712* was detected in Ca/Pi-stimulated vascular SMC isolated from WT mice and within the aortic media of kl/kl mice and is correlated with decreased expression of their potential target genes NCX1, PMCA1, and NCKX4 – all Ca2+ efflux proteins. This disruption of Ca2+ transporters and the resultant increase in intracellular Ca2+ concentrations could be involved in medial SMC calcification.25 It is important, however, to mention that functional studies altering one single miRNA (miR-135a*, miR-762, miR-714, or miR-712*) by mimics or inhibitors failed to show an effect on SMC calcification; whereas inhibition of all 4 miRNAs together significantly reduce calcium content of SMC by 30%. miR-680 served as control, because it was the most highly up-regulated miRNA in the aortic media of kl/kl mice. The failure of miR-680 specific inhibitor to reduce SMC calcification resulted in the conclusion, that miR-135a*, miR-762, miR-714, and miR-712* are specifically involved in calcification. Indeed it has been shown that miRNAs often function in clusters; however, it is still unknown whether the cluster is limited to these 4 miRNAs. Additionally, the role of the other 15 highly regulated miRNAs in kl/kl mice needs to be determined.
miR-204, a known inhibitor of osteoblastogenesis,27 and one candidate of the Runx2-miRNA-cluster in osteoblasts18 was also recently found to contribute to SMC calcification in vitro and in vivo.28 In vitro, miR-204 was identified to be a negative regulator of SMC calcification through direct targeting the 3′UTR of the Runx2 gene in mouse SMCs cultured in calcifying media consisting of 10 mM b-glycerolphosphate.28 Similarly, in a mouse model of vitamin D3-induced vascular calcification, overexpression of miR-204 by agomiRs decreased medial calcification and Runx2 expression mostly to control level within 3 days of treatment.28
As mentioned above, osteogenic differentiation of SMCs is also characterized by a loss of traditional SMC markers. Changes in the expression levels of the miR-143/145 cluster promotes differentiation and represses proliferation of SMCs thereby maintaining the SMC phenotype; 29–31 and have also been linked to vascular calcification. Exposure of human primary SMC to pathophysiological levels of inorganic phosphate (Pi) decreased miR-143 and miR-145 expression. Similarly, these miRNAs are down-regulated in the aorta of 20 week old Apoe-deficient mice.32 Even though studies have yet to establish a direct functional role of miR-143 and miR-145 in vascular calcification, this hypothesis is supported by findings noted in literature of diseases associated with calcification. miR-145 promotes SMC differentiation by targeting Krüppel-like factor (KLF) 4, 30 and KLF4 mediates high phosphate-induced transition of SMCs into osteogenic cells.33 Inhibition of miR-143/145 promotes a phenotypic switch to the synthetic, pro-atherogenic SMC state,29 including the inhibition of SMC marker-like alpha-smooth muscle actin and smooth muscle myosin heavy chain34 — both diminished in osteogenic SMCs.35 Additionally, circulating miR-145 levels are reduced in patients with coronary artery disease (CAD).19 Moreover, miR-145 was identified as part of the specific miRNA profile of destabilized human plaques,36 a biomechanical failure of the plaque that may involve microcalcification.37, 38
In mediating these cellular changes, the paracrine osteogenic signals facilitated by morphogens of the bone morphogenetic protein (BMP) and wingless-type MMTV integration site family member (Wnt) superfamilies, are effective regulators of vascular and valvular calcification, but also necessary in controling skeletal osteogenesis.39 Two members of the BMP signaling pathway (reviewed in detail elsewhere),39 BMP-2 and BMP-4 are potent osteogenic differentiation factors detected in calcified areas of atherosclerotic lesions.40, 41 BMPs elicit their effects through activation of a receptor complex composed of type I and type II receptors and activate receptor-type–dependent and ligand-dependent Smad transcription factors, which modulate the expression of Runx2.39
A recent study indicates that BMP-2 promotes SMC calcification by decreasing the expression of miR-30b by 6.2-fold and miR-30e by 5.5-fold though an Smad-independent pathway that leads to a direct increase in Runx2.42 Using antagomirs to block these miRNAs it was found that downregulation of miR-30b and miR-30e in vitro is sufficient to increase Runx2 expression, even in the absence of BMP-2. Accordingly, calcified human coronary arteries demonstrate higher BMP-2 levels and lower levels of miR-30b than non-calcified coronary arteries. Similar miRNA modulations of BMP-2 signaling have been observed in studies of heart valve calcification. Nigam et al. recently identified a miRNA pattern specific to aortic stenosis (AS), which is typically caused by calcific aortic valve disease, using whole bicuspid valves.43 miR-30b was decreased in the aortic valves of patients requiring replacement due to AS, compared to those requiring replacement due to aortic insufficiency.43 Another group compared bicuspid and tricuspid aortic valve leaflets by miRNA microarray, and found a number of modulated miRNAs.44 Particularly, miR-141 had the most dramatic change, showing a 14.5-fold decrease in the bicuspid versus tricuspid valves; however the levels of calcification were comparable between the two groups. In vitro, miR-141 represses the valvular interstitial cell, the resident cell within cardiac valve leaflets, response to osteogenic stimuli, in part through blocking BMP2-dependent calcification.44 Likewise, Itoh et al. identified miR-141 as a pre-osteoblast differentiation-related miRNA, which modulated the BMP2-induced pre-osteoblast differentiation by direct translational repression of Dlx5, a transcription factor for osterix.45 miR-26a is another miRNA, which is repressed in aortic valve leaflets of patients with AS.43 miR-26a, was previously identified as a Smad-regulating miRNA related to osteoblastogenesis; it functionally represses osteoblast differentiation by targeting Smad1 and Smad5 expression.46 Human aortic valvular interstitial cells showed decreased mRNA levels of both BMP-2 and Smad1 when treated with a miR-26a mimic.
While BMP-2 is a known inducer of osteogenic differentiation, activation of Wnt signaling is crucial for controlling osteoblast function 47 and for the programming of vascular cells during cardiovascular calcification.39 Activation of the Wnt/β-catenin signaling pathway occurs in human calcified aortic valve stenosis,48 in low density lipoprotein-deficient mice, 49, 50 and in osteogenic SMCs in vitro.51 miR-29a potentiates osteoblastogenesis by modulating Wnt signaling. Interestingly, miR-29a/b was repressed in high-phosphate induced calcifying rat SMC, calcified abdominal aortas from rats with CKD induced by 5/6 nephrectomy and human radial arteries with chronic kidney failure.52 miR-29a/b directly targeted a disintegrin and metalloproteinase with thrombospondin motifs-7 (ADAMTS-7) as shown by luciferase assay. A miR-29a/b mimic inhibited, and a miR-29a/b inhibitor enhanced, high-phosphate-induced SMC calcification though alteration of ADAMTS-7, BMP-2, p-Smad 1/5/8 and Runx2 protein expression. Because miR-29 also associated with reduced extracellular matrix components, such as collagen and elastin,53 low cellular miR-29a/b levels may also cause vascular thickening, fibrosis and elastolysis, which accelerate arterial and aortic valve calcification.54
Likewise, the canonical Wnt signaling induces miR-29a expression, which negatively targets regulators of Wnt signaling, including Dickkopf (Dkk) 1, sFRP2, Kremen, and osteonectin.55, 56 Dkk1 is an extracellular antagonist of the canonical Wnt signaling that plays a crucial role in bone remodeling.57, 58 Dkk1 was also shown to prevent vascular calcification by preventing warfarin-induced activation of β-catenin, and osteogenic transdifferentiation of SMCs59 and tumor necrosis factor-α-induced induction of ALP activity.49 Dkk1 serum levels in CKD patients correlates negatively with arterial stiffness,60 and matrix metalloproteinase-2, another target of miR-29,61 promotes arterial calcification in CKD.62
Studies about miRNA expression in human calcified tissue are rare (Table 2). Li et al. analyzed the expression of miRNAs in patients with peripheral artery disease (arteriosclerosis obliterans), characterized by fibrosis of the tunica intima and calcification of the tunica media.63 miR-21, miR-130a, miR-27b, let-7f, and miR-210 were significantly increased, while miR-221 and miR-222 were decreased in the sclerotic intima samples, compared to normal vessels.63 Higher levels of miR-21 and miR-210 were confirmed in a study that compared atherosclerotic lesions with non-atherosclerotic left internal thoracic arteries.64 miR-210 is known to promote osteoblast differentiation though the inhibition of the BMP co-receptor activin type IB receptor (ALK4).65 In line with this evidence, activin — a ligand for ALK466 — inhibits SMC mineralization.67 Another study found a different miRNA pattern using atherosclerotic plaque material from the carotid artery, compared with a specimen from the arteria mammaria interna as control tissue.20 The healthy vessel expressed higher levels of miR-520b and miR-105, whereas miR-10b, miR-218, miR-30e, miR-26b, and miR-125a were predominantly expressed in atherosclerotic plaque.20 The investigators in these studies, however, did not examine calcification levels to determine a potential correlation with the observed changes in miRNA.
Table 2.
miRNAs expressed in human atherosclerotic/calcified tissue.
| miRNA | Disease | Tissue type | Finding | Control tissue | Reference number |
|---|---|---|---|---|---|
| miR-21, -34a, -146a, -146b-5p, -210 | CAD | Atherosclerotic arteries | Increased | Non-atherosclerotic left internal thoracic arteries | 64 |
| miR-105, -520b miR-10b, -26b, -30e, -125a, -218, |
CAD | Atherosclerotic carotid artery | Decreased Increased |
Arteriamamm ariainterna | 20 |
| miR-30b | CAD | Calcified carotid artery | Decreased | Non-calcified carotid artery from non CAD patients | 42 |
| miR-100, -127, -133a,b -145 | CAD | Destabilized plaque | Increased | Stabilized plaque | 21 |
|
miR-221, -222 miR-21, -27b, -210, -130a, let-7f |
AO | Sclerotic intima from lower extremities vessels | Decreased Increased |
Non-sclerotic intima from lower extremities vessels | 63 |
| miR-22, -27a, -141, -124, -125b, -185, -187, -194, -211, -330, -370, -449, -486, -551, -564, -575, -585, -622, -637, -648, -1202, -1282, -1469, -1908, -1972 miR-30e, -32, -145, -151, -152, -190, -373, -768 |
AS | Bicuspid aortic valve | Decreased Increased |
Tricuspid aortic valve | 44 |
| miR-26a, -30b, -195 | AS | Whole bicuspid valves | Decreased | Replacement due to aortic insufficiency | 43 |
Bold: Found in more than one study. Italic: Found also in circulation (Table 1).
CAD, coronary artery disease; AS, aortic stenosis; AO, arteriosclerosis obliterans.
In addition to the osteoblastogenesis of SMCs, the contribution of osteoclasts, bone resorbing cells that play an active role in normal bone physiology, to vascular calcification is controversial and poorly understood.12 The observation of osteoclast-like cells in calcified atherosclerotic lesions suggested this bone-related cell is active in the vessel wall. The evidence was strengthened recently by Sun et al., who demonstrated the functional role of SMC-derived Runx2 promoting infiltration of macrophages into the calcified lesion to form osteoclast-like cells —suggesting that the development of vascular calcification is coupled with the formation of osteoclast-like cells, paralleling the bone remodeling process.16 The receptor activator of the nuclear factor-kappa B (NF-kappa B) ligand (RANKL)/osteoprotegerin system controls proper osteoclastogenesis, and act as a biomarker for CAD.68, 69 Five miRNAs are linked to osteoclastogenesis as well as to cardiovascular disorders. (5) RANKL is a proposed target of miR-126, 70 which is decreased in the plasma of CAD 19 and T2D 71 patients. (2) miR-155, which is decreased in plasma of CKD72 and CAD19 patients, was shown to inhibit osteoclast function in Dicer-deficient osteoclasts.73 (3) miR-146a, highly expressed in atherosclerotic arteries,64 inhibits osteoclastogenesis by inhibition of the number of tartrate-resistant acid phosphatase-positive multinucleated cells.74 (4) miR-223, a key factor in osteoclastogenesis,75 was found to be increased by Pi treatment of human SMC and in the calcified aorta of Apoe-deficient mice.32 Overexpression of miR-223 enhanced SMC migration, decreased actin cytoskeleton and modulated target genes, like Mef-2 and RhoB. (5) Wang et al. demonstrated an association of miR-133a levels in circulating monocytes - osteoclast precursors - with postmenopausal osteoporosis.76 Women with low bone mineral density showed higher circulating miR-133a levels76, but the number of patients per group was small (n=10). Circulating miR-133a levels were also higher in patients with CAD.19 However, direct links between miR-133a and/or miR-223 and vascular calcification processes have not been reported.
More careful research needs to be conducted to establish direct links between miRNAs and known signaling pathways associated with osteogenic differentiation in the context of cardiovascular calcification. For example, despite the links from different studies noted above, the direct Pi – miR-143/145 – KLF4 axis in vascular calcification has not been directly examined. Additionally, future studies may also lead to a better understanding of the role of miRNA networks in controlling cellular differentiation and ultimate tissue level changes. For instance, though miR-204 is a known inhibitor of osteoblastogenesis, introduction by a mimic agomiR-204 does not alter bone formation in a mouse model of vitamin D3-induced vascular calcification.28 The observation that miRNAs function in clusters to control cellular and tissue homeostasis may help explain some of the current inconsistencies observed between normal bone formation and vascular calcification (e.g., the correlation between osteoporosis and calcification—“the calcification paradox”). Future miRNA studies may also better connect known signaling pathways associated with osteogenesis to the ultimate pathological outcomes, thereby leading to a deeper understanding of the cellular processes that lead to calcification.
Circulating miRNAs in Diseases Associated with Vascular Calcification – A Potential miRNA Signature
Our detailed investigation using currently published literature revealed common circulating miRNAs in diseases associated with vascular calcification. We compared miRNA signatures identified in 10 CAD, 4 T2D, and 1 CKD studies (Table 2). From this analysis, seven miRNAs (miR-21, miR-27, miR-34a, miR-126, miR-146a, miR-155, and miR210) were found to be useful biomarkers shared between at least two of the compared diseases, but only miR-21 was common among all three diseases.19,71,72 (Figure 2) miR-21 relates to key processes in the progression of atherosclerosis 77–79 and predicted targets of miR-21 include BMPR2, which has been linked to atherosclerotic calcification.80 Atherosclerotic arteries 64 and sclerotic intima from lower-extremity vessels 63 expressed higher miR-21 levels than did healthy vessels, whereas circulating levels of miR-21 in atherosclerosis (serum), T2D (plasma), and/or CKD (plasma) were reduced.19,71,72 The reason for this opposing observation is not fully understood, and requires further investigation. A recent report showed that miRNAs increased in atherosclerotic abdominal aortic aneurysm tissue, while reduced levels were found in the circulation.81 miRNAs packaged inside exsomes or apoptotic bodies may be specifically taken up by diseased tissue, which decreases circulating miRNAs.82, 83
Figure 2. Common circulating miRNA in diseases associated with vascular calcification.
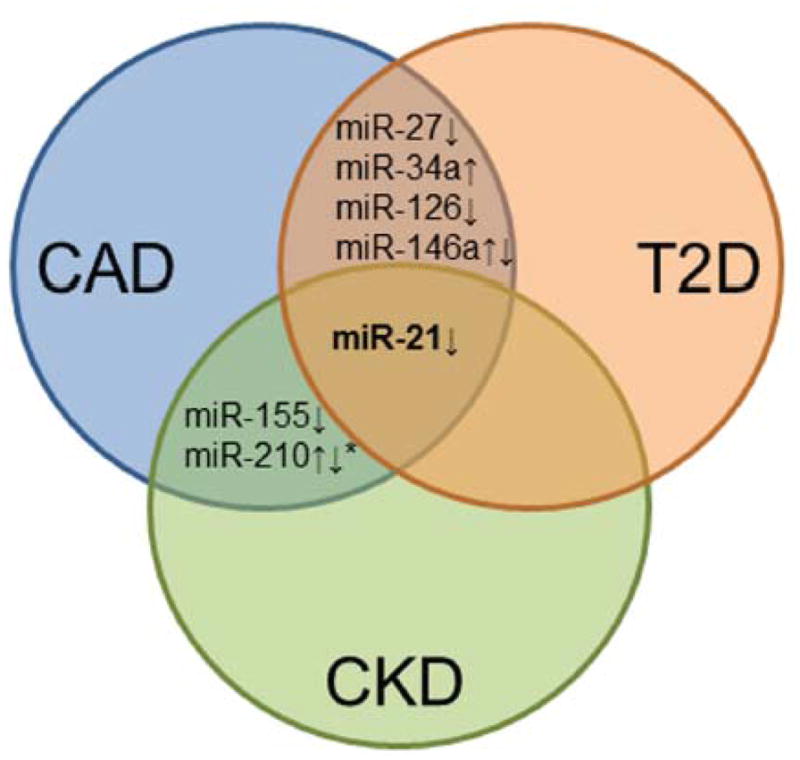
Venn diagram shows overlapping miRNAs identified in at least two of the three cardiovascular diseases (CRD, chronic renal disease; T2D, type 2 diabetes; CAD, coronary artery disease).*Also found in arteriosclerosis obliterans.
Of note within the miRNAs shared between two of the diseases, miR-146a is an inflammation-related miRNA, implicated in atherosclerosis and osteoclastogenesis.74 While circulating miR-146a obtained from peripheral blood mononuclear cells (PBMC) is increased in CAD patients,84 T2D studies showed controversial results; miR-146a serum levels were increased,85 and levels in monocytes were decreased.86 In addition, miR-146a was more highly expressed in atherosclerotic arteries in an animal model64 and is associated with CKD in vivo.87 Additionally, miR-155, another inflammation-associated miRNA, was decreased in serum of CAD 19 and plasma of CKD patients.72 Deficiency of miR155 enhanced atherosclerotic plaque development and decreased plaque stability, 88 suggesting that it acts as an anti-inflammatory and atheroprotective miRNA. miR-155 is also highly expressed in endothelial cells (ECs) and SMCs, where it targets angiotensin-II receptor.89 The renin–angiotensin system participates in vascular calcification,90 and angiotensin-receptor blockade can inhibit arterial calcification by disrupting vascular osteogenesis in vivo.91 Furthermore, miR-155 repressed osteoblastogenesis by targeting Smad proteins.46 Thus, high expression of miR-155 may prevent vascular calcification by inhibiting the BMP signaling pathway or the renin–angiotensin system. Comparison of circulating miRNAs in published studies is challenging mainly because of the different sources of circulating miRNAs, which include serum, whole blood, PBMCs, endothelial progenitor cells, and platelets (Table 2) and employ different protocols. The miRNA profiles obtained from the different studies, therefore, are often not the same. Future studies need to take standardized approach to identify circulating miRNAs. In this context, a recent report suggested the necessity of careful selection for reference miRNAs by showing that hemolysis may significantly affect the levels of plasma miRNAs previously used as endogenous controls.92 Therefore, hemolysis should be determined, to avoid the phenomenon based on red blood cells.93 Alternatively, the determination of miRNAs known to be enriched in red blood cells, like miR-451 and miR-16 could be performed.92 A novel LNA™-based qPCR method can identify samples affected by sources of pre-analytical variation such as cellular contamination.93
Allowing for these limitations, a set of circulating miRNAs (consisting of miR-21, miR-27, miR-34a, miR-126, miR-146a, miR-155, and miR-210) is dysregulated in various pro-inflammatory diseases and may represent a miRNA signature for vascular calcification. Of note, systemic and local inflammation paradoxically affects cardiovascular calcification and bone loss, which supports the concept of inflammation-dependent cardiovascular calcification previously proposed by our group and others.9, 54, 94–96 Given the observation that miRNAs often function in clusters and networks,71 the subset of circulating miRNAs identified in our analysis may turn out to be a portion of a larger network that regulates cardiovascular calcification, wherein the up- or down- regulation of a single miRNA is less important than the function of the network as a whole. Using a network analysis, key miRNA clusters may then be identified as the core regulators of the calcification process. Future studies may be able to utilize this subset as a starting point when trying to compile the larger networks for calcification.
Extracellular Vesicles in miRNA Transport and Calcification
miRNAs are present in blood (plasma, platelets, erythrocytes, nucleated blood cells) with high stability that is conferred by encapsulation in extracellular vesicles,97, 98 association with a protein complex with the RNA-binding protein Argonaute (Ago) 2,124 or in lipoprotein complexes (HDL).99 These associations prevent the degradation of the miRNAs while in the circulation. However, the mechanism by which the majority of miRNAs are extracellular transported remains controversial. Exported miRNAs are found both within and outside of 16,500 and 120,000 × g centrifugation pellets, which contains most of the cell-derived vesicles.97 Other studies showed that the majority of miRNAs are independent of vesicles and co-purify with the Ago2 complex, which is known as a key intracellular effector protein of miRNA-mediated RNA silencing.100, 101 Arroy et al. quantified 88 plasma miRNAs (isolated by 120,000 × g centrifugation, 70 min) from healthy donors and found that 90% of them are present in a non-membrane-bound form.100 Another study compared microparticles isolated from a 16,000 × g (90 min) centrifugation of plasma from patients with stable CAD and patients with acute coronary syndrome as a control. In CAD patients, most plasma miRNAs associate with extracellular vesicles, and only a small amount are found in extracellular vesicle-free plasma.102 However, the number of cases is very small (n=5 per group) and a healthy control group is missing. To determine if the differing patterns of vesicle-associated miRNAs may be used to characterize different diseases, larger study collectives with standardized protocols are needed.
The miRNA pattern found within released vesicles is different from that associated with Ago2 complexes,100 implicating a cell-type-specific miRNA release utilizing different export systems. Depending on the size and type, extracellular vesicles are broadly classified as ectosomes (also called shedding microvesicles), exosomes, matrix vesicles, and apoptotic bodies. Ectosomes are large extracellular vesicles 50–100 nm in diameter. Exosomes are small membranous vesicles of endocytic origin with diameter of 40–100 nm. Matrix vesicles, which have been shown to calcify, are 30–300 nm in diameter and produced by blebbing of the plasma membrane. Apoptotic bodies, 500–1000 nm in diameter, are released from fragmented apoptotic cells. Cells may select miRNA and pre-miRNA for cellular release as cargo within these vesicles in a context dependent manner.103, 104 miRNA profiles of extracellular vesicles are different from their maternal cell profiles, indicating an active mechanism of selective miRNA packing from cells into vesicles,102 whereas Ago2–miRNA complexes may be passively produced by dead cells, released by live cells, or actively transported though cell-membrane–associated channels or receptors.105 Additionally, blockade of sphingomyelinase inhibits exosome generation, miRNA secretion, and subsequent intercellular miRNA transfer implicating a ceramide-dependent mechanism in miRNA packaging and release within extracellular vesicles.106, 107
Cells utilize extracellular vesicles to transport miRNA and mediate intercellular communication over long distances or on a local tissue level.98 Endothelial apoptotic bodies can convey miR-126 to atherosclerotic lesions, demonstrating unique paracrine-signaling function for miRNA during atherosclerosis.71, 83 A recent report provided evidence that miRNA-containing vesicles regulate intercellular communication between ECs and SMCs by selective packaging of the previously discussed regulators of SMC phenotype, miR-143/145, in EC-derived vesicles that are then transported to SMCs in the vessel wall.107
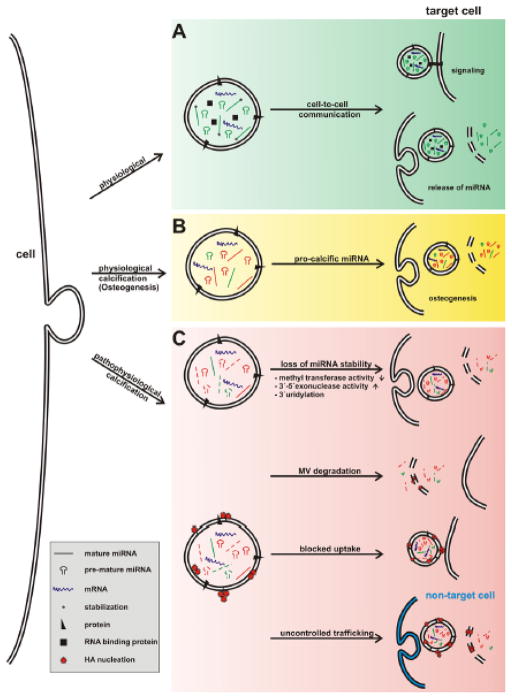
The mechanism by which miRNAs are received by target cells in a biologically active state is still unknown. In physiological conditions, extracellular vesicles may bind to the membrane proteins of the surface of target cells through receptor–ligand interaction, resulting in intracellular stimulation of genetic pathways. They can also fuse with cell–target membranes and release genetic content in a nonselective manner. Furthermore, vesicles can bind to surface receptors on target cells with endocytotic internalization by recipient cells, followed by fusion with the membranes, leading to a release of their content into the cytosol of target cells and allowing the vesicle contents to directly associate with intracellular components.108 (Figure 3A, 3B)
Figure 3. Alteration of matrix vesicle transport of circulating miRNAs in cardiovascular calcification.
(A)In physiological conditions or during (B) bone calcification (osteogenesis), extracellular vesicles bind to the membrane proteins of the surface of target cells through receptor–ligand interaction, causing signaling processes. They can also fuse with cell–target membrane or bind to surface receptors on target cells with endocytotic internalization by recipient cell, leading to a release of their content into the cytosol of target cells. (C) Mechanisms associated with extracellular vesicle-miRNA–mediated pathological calcification. Potential mechanisms associated with extracellular vesicle-miRNA–mediated pathological calcification include (1) a different miRNA packaging into vesicles due to the osteogenic environment; (2) increased degradation due to increased enzymatic activity within the vesicle; (3) blocked vesicle uptake into the target cell; and (4) uptake in non-target cells due to mineral nucleation on the outer membrane.
In addition to their known role as regulators of intercellular communication, extracellular vesicles have also been observed to participate in matrix mineralization. In non-pathological biomineralization in cartilage and bone, chrondrocytes and osteoblasts release extracellular vesicles, which serve as nucleation site for hydroxyapatite and initiate mineralization.109 These structures are also observed in ectopic calcification. The release of vesicles from SMC is an established key event in the initiation and promotion of SMC calcification.110 Treatment of SMCs with elevated calcium levels promotes the production of calcifying matrix vesicles, and the loss of fetuin-A, an inhibitor of mineral nucleation.111 These vesicles act as early nucleation sites for calcification. The phosphatidylserine-membrane complex from SMC-derived and macrophage-derived matrix vesicles redistributes and nucleates hydroxyapatite.112–114 In addition, hydroxyapatite nanocrystals shed from vesicles may further promote mineralization via direct effects on the SMC phenotype.115 Using a proteomic approach it was also shown that calcified extracellular vesicles-derived from SMC contain a specific protein profile compared with non-calcifying vesicles,110 which might be caused by a specific packaging mechanism.
In a pro-osteogenic environment, the specific, physiologic vesicle-mediated transport of miRNA in the vasculature may be disturbed through differential miRNA packaging into the vesicles, decreased miRNA stability by increased enzymatic activity (methyl transferase, 3′-5′ exonuclease) within the vesicles, increased matrix vesicle degradation, or blocked or non-specific uptake of vesicle into the target cell due to mineral nucleation on the outer membrane (Figure 3C). In fact, SMC-derived matrix vesicles have been observed to calcify in a cell-independent manner when exposed to mineralizing conditions on a collagen I substrate.112 These results may explain the observations of an increased number of matrix vesicles 116 and numerous microcalcifications throughout the collagen I-rich fibrous caps of atherosclerotic plaques. 117 These microcalcifications have been hypothesized to contribute directly to plaque rupture that leads to thrombosis.118 Given these observations, miRNAs packed in extracellular vesicles may contribute to vascular calcification in two ways. First, vesicles packaged with miRNA for paracrine signaling within the plaque may become entrapped and form microcalcifications. Second, in turn, this may prevent the miRNAs within the vesicles from reaching the intended target cell, leading to phenotypic changes that promote further calcification. Therefore, insight into the underlying mechanism of selective packing of miRNAs into extracellular vesicles and selective uptake into the target cell will help increase understanding of the role of miRNA-containing vesicles in physiological intercellular communication as well as unintended disruption of this communication, which may prevent calcification in the vascular system.
Conclusion and emerging concepts
In this review, we have discussed an emerging role of miRNAs in cardiovascular calcification, and we have also analyzed the literature of diseases that are known to correlate with calcification in an attempt to establish a potential signature for cardiovascular calcification that should be investigated further. In vitro and in vivo studies have established miRNAs as biomarkers, thereby providing circulating miRNA signatures for different diseases. But these circulating miRNAs may not have biological functions while circulating. Instead, they may act as intercellular communicators, and this communication might be disturbed in calcification disorders, where calcifying matrix vesicles fail to properly delivery miRNAs to the target cell. Studies to fully exploit this potential novel mechanism of cardiovascular calcification are needed.
Moreover, miRNA biology is very complex. Multiple miRNAs can target the same gene (e.g., Runx2–miRNA cluster), and one miRNA might have several targets. Only a small amount of these fine-tuned targets may alter biological responses and phenotypes. Understanding the role of miRNA in vascular calcification may be helpful in considering the paradoxical clinical observations of the concurrence of cardiovascular calcification and osteoporosis. Despite its global clinical burden, no medical therapies are available to treat cardiovascular calcification. Targeting of miRNA represents a novel therapeutic opportunity for treating cardiovascular calcification. As cardiovascular calcification and bone remodeling share common mechanisms, we need an in-depth understanding of miRNA function and their association with the molecular pathogenesis of osteoporosis and vascular calcification. This knowledge will be critical for the developing of a more specific therapy for cardiovascular calcification that does not adversely affect physiological bone homoeostasis.
Table 3.
Circulating miRNA in diseases associated with vascular calcification.
| miRNA | Disease | Source | Finding | Reference number |
|---|---|---|---|---|
| miR-17, -21,-20a, -22a, -27a, -92a, -126, -145, -155,-221, -130a, -208b, let-7d miR-133a, -208a |
CAD | Serum | Decreased Increased |
19 |
| miR-146a/b | Increased | 84 | ||
| miR-34a | CAD | EPC | Increased | 119 |
| miR-221, -222 | CAD | EPC | Increased | 120 |
| miR-135a, -147 | CAD | PBMC | Decreased | 22 |
| miR-140, -182 | CAD | Whole blood | Decreased | 121 |
| miR-122, -370 | CAD | Plasma | Increased | 122 |
| miR-181a | CAD | Monocytes | Decreased | 123 |
| Let-7i | CAD | Monocytes | Decreased | 124 |
| miR-340, -624 | CAD | Platelets | Increased | 125 |
| miR-20b, -21, -24, -29b, -15a, -126, -150, -191, -197, -223, -320, -486 miR-28-3p |
T2D | Plasma | Decreased Increased |
71 |
| miR-146a | T2D | PBMC | Decreased | 86 |
| miR-21, -27a, b, -126, -130a | T2D | EPC | Decreased | 126 |
| miR-9, -29a, -30d, -34a, -124a, -146a, -375 | T2D | Serum | Increased | 85 |
| miR-16, -21, -155, -210, -638 | CKD | Plasma | Decreased | 72 |
| miR-188-5p, -135*, -323-3p, -509-3p, -520-3p, -572, -573, 629*, -632 miR-24, -106a, -191, -218, -222, -223, -342-3p, -412, let-7p |
HC | HDL | Decreased Increased |
99 |
| miR-21, -27b, -130a,-210 | AO | Serum | Increased | 63 |
Bold: Found in more than one study. Italic: Found also in tissue (Table 2).
CKD, chronic kidney disease; T2D, type 2 diabetes; CAD, coronary artery disease; AS, aortic stenosis; HC, familial hypercholesterolemia; AO, arteriosclerosis obliterans; PBMC, peripheral blood mononuclear cell; EPC, endothelial progenitor cell; HDL, high-density lipoprotein.
Acknowledgments
The authors thank Ms. Sara Karwacki for her editorial assistance.
Sources of Funding
This work was supported by an American Heart Association (AHA) Scientist Development Grant (0835460N) and by a grant from the National Institutes of Health (NIH) (R01HL114805-01), both to Dr. Aikawa.
Nonstandard Abbreviations
- Ago
Argonaute
- AS
aortic stenosis
- ALP
tissue non-specific alkaline phosphatase
- BMP
bone morphogenetic protein
- CAD
coronary artery disease
- CKD
chronic kidney disease
- Dkk-1
Dickkopf 1
- EC
endothelial cell
- KLF
krüppel-like factor
- miRNA
microRNA
- PBMC
peripheral blood mononuclear cells
- Pi
inorganic phosphate
- RANKL
receptor activator of the nuclear factor-kappa B (NF-kappa B) ligand
- SMC
smooth muscle cells
- T2D
type 2 diabetes
- UTR
untranslated region
- Wnt
wingless-type MMTV integration site family member
Footnotes
Disclosures
None.
References
- 1.Ambros V. The functions of animal micrornas. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microrna host genes and transcription units. Genome research. 2004;14:1902–1910. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang Y, Ridzon D, Wong L, Chen C. Characterization of microrna expression profiles in normal human tissues. BMC genomics. 2007;8:166. doi: 10.1186/1471-2164-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebert MS, Sharp PA. Emerging roles for natural microrna sponges. Current biology: CB. 2010;20:R858–861. doi: 10.1016/j.cub.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartel DP. Micrornas: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Small EM, Olson EN. Pervasive roles of micrornas in cardiovascular biology. Nature. 2011;469:336–342. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hjortnaes J, Bouten CV, Van Herwerden LA, Grundeman PF, Kluin J. Translating autologous heart valve tissue engineering from bench to bed. Tissue engineering Part B, Reviews. 2009;15:307–317. doi: 10.1089/ten.TEB.2008.0565. [DOI] [PubMed] [Google Scholar]
- 8.Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nature reviews Cardiology. 2010;7:528–536. doi: 10.1038/nrcardio.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 10.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: Pathobiological mechanisms and clinical implications. Circulation research. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 11.Naik V, Leaf EM, Hu JH, Yang HY, Nguyen NB, Giachelli CM, Speer MY. Sources of cells that contribute to atherosclerotic intimal calcification: An in vivo genetic fate mapping study. Cardiovascular research. 2012;94:545–554. doi: 10.1093/cvr/cvs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fadini GP, Rattazzi M, Matsumoto T, Asahara T, Khosla S. Emerging role of circulating calcifying cells in the bone-vascular axis. Circulation. 2012;125:2772–2781. doi: 10.1161/CIRCULATIONAHA.112.090860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doehring LC, Heeger C, Aherrahrou Z, Kaczmarek PM, Erdmann J, Schunkert H, Ehlers EM. Myeloid cd34+cd13+ precursor cells transdifferentiate into chondrocyte-like cells in atherosclerotic intimal calcification. The American journal of pathology. 2010;177:473–480. doi: 10.2353/ajpath.2010.090758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komori T. Regulation of bone development and extracellular matrix protein genes by runx2. Cell and tissue research. 2010;339:189–195. doi: 10.1007/s00441-009-0832-8. [DOI] [PubMed] [Google Scholar]
- 15.Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor runx2 by akt signaling. The Journal of biological chemistry. 2008;283:15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y, Byon C, Yuan K, Chen J, Mao X, Heath JM, Javed A, Zhang K, Anderson PG, Chen Y. Smooth muscle cell-specific runx2 deficiency inhibitsvascular calcification. Circulation research. 2012 doi: 10.1161/CIRCRESAHA.112.267237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Speer MY, Li X, Hiremath PG, Giachelli CM. Runx2/cbfa1, but not loss of myocardin, is required for smooth muscle cell lineage reprogramming toward osteochondrogenesis. Journal of cellular biochemistry. 2010;110:935–947. doi: 10.1002/jcb.22607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Xie RL, Croce CM, Stein JL, Lian JB, van Wijnen AJ, Stein GS. A program of micrornas controls osteogenic lineage progression by targeting transcription factor runx2. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9863–9868. doi: 10.1073/pnas.1018493108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Roxe T, Muller-Ardogan M, Bonauer A, Zeiher AM, Dimmeler S. Circulating micrornas in patients with coronary artery disease. Circulation research. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 20.Bidzhekov K, Gan L, Denecke B, Rostalsky A, Hristov M, Koeppel TA, Zernecke A, Weber C. Microrna expression signatures and parallels between monocyte subsets and atherosclerotic plaque in humans. Thrombosis and haemostasis. 2012;107:619–625. doi: 10.1160/TH11-09-0607. [DOI] [PubMed] [Google Scholar]
- 21.Cipollone F, Felicioni L, Sarzani R, Ucchino S, Spigonardo F, Mandolini C, Malatesta S, Bucci M, Mammarella C, Santovito D, de Lutiis F, Marchetti A, Mezzetti A, Buttitta F. A unique microrna signature associated with plaque instability in humans. Stroke; a journal of cerebral circulation. 2011;42:2556–2563. doi: 10.1161/STROKEAHA.110.597575. [DOI] [PubMed] [Google Scholar]
- 22.Hoekstra M, van der Lans CA, Halvorsen B, Gullestad L, Kuiper J, Aukrust P, van Berkel TJ, Biessen EA. The peripheral blood mononuclear cell microrna signature of coronary artery disease. Biochemical and biophysical research communications. 2010;394:792–797. doi: 10.1016/j.bbrc.2010.03.075. [DOI] [PubMed] [Google Scholar]
- 23.Goettsch C, Rauner M, Pacyna N, Hempel U, Bornstein SR, Hofbauer LC. Mir-125b regulates calcification of vascular smooth muscle cells. The American journal of pathology. 2011;179:1594–1600. doi: 10.1016/j.ajpath.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albinsson S, Suarez Y, Skoura A, Offermanns S, Miano JM, Sessa WC. Micrornas are necessary for vascular smooth muscle growth, differentiation, and function. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:1118–1126. doi: 10.1161/ATVBAHA.109.200873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gui T, Zhou G, Sun Y, Shimokado A, Itoh S, Oikawa K, Muragaki Y. Micrornas that target ca(2+) transporters are involved in vascular smooth muscle cell calcification. Laboratory investigation; a journal of technical methods and pathology. 2012 doi: 10.1038/labinvest.2012.85. [DOI] [PubMed] [Google Scholar]
- 26.Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, Hsiao LL. Vascular klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125:2243–2255. doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 27.Huang J, Zhao L, Xing L, Chen D. Microrna-204 regulates runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells. 2010;28:357–364. doi: 10.1002/stem.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui RR, Li SJ, Liu LJ, Yi L, Liang QH, Zhu X, Liu GY, Liu Y, Wu SS, Liao XB, Yuan LQ, Mao DA, Liao EY. Microrna-204 regulates vascular smooth muscle cell calcification in vitro and in vivo. Cardiovascular research. 2012 doi: 10.1093/cvr/cvs258. [DOI] [PubMed] [Google Scholar]
- 29.Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MV, Peterson KL, Indolfi C, Catalucci D, Chen J, Courtneidge SA, Condorelli G. The knockout of mir-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: Correlates with human disease. Cell death and differentiation. 2009;16:1590–1598. doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. Mir-145 and mir-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, Braun T. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the mir143/145 gene cluster. The Journal of clinical investigation. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rangrez AY, M’Baya-Moutoula E, Metzinger-Le Meuth V, Henaut L, Djelouat MS, Benchitrit J, Massy ZA, Metzinger L. Inorganic phosphate accelerates the migration of vascular smooth muscle cells: Evidence for the involvement of mir-223. PloS one. 2012;7:e47807. doi: 10.1371/journal.pone.0047807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida T, Yamashita M, Hayashi M. Kruppel-like factor 4 contributes to high phosphate-induced phenotypic switching of vascular smooth muscle cells into osteogenic cells. The Journal of biological chemistry. 2012 doi: 10.1074/jbc.M112.361360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, Deitch EA, Huo Y, Delphin ES, Zhang C. Microrna-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circulation research. 2009;105:158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM. Smooth muscle cell phenotypic transition associated with calcification: Upregulation of cbfa1 and downregulation of smooth muscle lineage markers. Circulation research. 2001;89:1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 36.Santovito D, Mezzetti A, Cipollone F. Micrornas and atherosclerosis: New actors for an old movie. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2012 doi: 10.1016/j.numecd.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Vengrenyuk Y, Carlier S, Xanthos S, Cardoso L, Ganatos P, Virmani R, Einav S, Gilchrist L, Weinbaum S. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14678–14683. doi: 10.1073/pnas.0606310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoshino T, Chow LA, Hsu JJ, Perlowski AA, Abedin M, Tobis J, Tintut Y, Mal AK, Klug WS, Demer LL. Mechanical stress analysis of a rigid inclusion in distensible material: A model of atherosclerotic calcification and plaque vulnerability. American journal of physiology. Heart and circulatory physiology. 2009;297:H802–810. doi: 10.1152/ajpheart.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bostrom KI, Rajamannan NM, Towler DA. The regulation of valvular and vascular sclerosis by osteogenic morphogens. Circulation research. 2011;109:564–577. doi: 10.1161/CIRCRESAHA.110.234278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. The Journal of clinical investigation. 1993;91:1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM, Vermeer C, Daemen MJ. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arteriosclerosis, thrombosis, and vascular biology. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 42.Balderman JAF, Lee HY, Mahoney CE, Handy DE, White K, Annis S, Lebeche D, Hajjar RJ, Loscalzo J, Leopold JA. Bone morphogenetic protein-2 decreases microrna -30b and microrna -30c to promote vascular smooth muscle cell calcification. Journal of the American Heart Association. 2012:1. doi: 10.1161/JAHA.112.003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nigam V, Sievers HH, Jensen BC, Sier HA, Simpson PC, Srivastava D, Mohamed SA. Altered micrornas in bicuspid aortic valve: A comparison between stenotic and insufficient valves. The Journal of heart valve disease. 2010;19:459–465. [PMC free article] [PubMed] [Google Scholar]
- 44.Yanagawa B, Lovren F, Pan Y, Garg V, Quan A, Tang G, Singh KK, Shukla PC, Kalra NP, Peterson MD, Verma S. Mirna-141 is a novel regulator of bmp-2-mediated calcification in aortic stenosis. The Journal of thoracic and cardiovascular surgery. 2012 doi: 10.1016/j.jtcvs.2011.10.097. [DOI] [PubMed] [Google Scholar]
- 45.Itoh T, Nozawa Y, Akao Y. Microrna-141 and -200a are involved in bone morphogenetic protein-2-induced mouse pre-osteoblast differentiation by targeting distal-less homeobox 5. The Journal of biological chemistry. 2009;284:19272–19279. doi: 10.1074/jbc.M109.014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taipaleenmaki H, Bjerre Hokland L, Chen L, Kauppinen S, Kassem M. Mechanisms in endocrinology: Micro-rnas: Targets for enhancing osteoblast differentiation and bone formation. European journal of endocrinology/European Federation of Endocrine Societies. 2012;166:359–371. doi: 10.1530/EJE-11-0646. [DOI] [PubMed] [Google Scholar]
- 47.Monroe DG, McGee-Lawrence ME, Oursler MJ, Westendorf JJ. Update on wnt signaling in bone cell biology and bone disease. Gene. 2012;492:1–18. doi: 10.1016/j.gene.2011.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. Journal of the American College of Cardiology. 2008;52:843–850. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J, Behrmann A, Cheng SL, Towler DA. Aortic msx2-wnt calcification cascade is regulated by tnf-alpha-dependent signals in diabetic ldlr−/− mice. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:2589–2596. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 50.Cheng SL, Shao JS, Halstead LR, Distelhorst K, Sierra O, Towler DA. Activation of vascular smooth muscle parathyroid hormone receptor inhibits wnt/beta-catenin signaling and aortic fibrosis in diabetic arteriosclerosis. Circulation research. 2010;107:271–282. doi: 10.1161/CIRCRESAHA.110.219899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faverman L, Mikhaylova L, Malmquist J, Nurminskaya M. Extracellular transglutaminase 2 activates beta-catenin signaling in calcifying vascular smooth muscle cells. FEBS letters. 2008;582:1552–1557. doi: 10.1016/j.febslet.2008.03.053. [DOI] [PubMed] [Google Scholar]
- 52.Du Y, Gao C, Liu Z, Wang L, Liu B, He F, Zhang T, Wang Y, Wang X, Xu M, Luo GZ, Zhu Y, Xu Q, Kong W. Upregulation of a disintegrin and metalloproteinase with thrombospondin motifs-7 by mir-29 repression mediates vascular smooth muscle calcification. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:2580–2588. doi: 10.1161/ATVBAHA.112.300206. [DOI] [PubMed] [Google Scholar]
- 53.Boon RA, Seeger T, Heydt S, Fischer A, Hergenreider E, Horrevoets AJ, Vinciguerra M, Rosenthal N, Sciacca S, Pilato M, van Heijningen P, Essers J, Brandes RP, Zeiher AM, Dimmeler S. Microrna-29 in aortic dilation: Implications for aneurysm formation. Circulation research. 2011;109:1115–1119. doi: 10.1161/CIRCRESAHA.111.255737. [DOI] [PubMed] [Google Scholar]
- 54.Aikawa E, Aikawa M, Libby P, Figueiredo JL, Rusanescu G, Iwamoto Y, Fukuda D, Kohler RH, Shi GP, Jaffer FA, Weissleder R. Arterial and aortic valve calcification abolished by elastolytic cathepsin s deficiency in chronic renal disease. Circulation. 2009;119:1785–1794. doi: 10.1161/CIRCULATIONAHA.108.827972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kapinas K, Kessler C, Ricks T, Gronowicz G, Delany AM. Mir-29 modulates wnt signaling in human osteoblasts through a positive feedback loop. The Journal of biological chemistry. 2010;285:25221–25231. doi: 10.1074/jbc.M110.116137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kapinas K, Kessler CB, Delany AM. Mir-29 suppression of osteonectin in osteoblasts: Regulation during differentiation and by canonical wnt signaling. Journal of cellular biochemistry. 2009;108:216–224. doi: 10.1002/jcb.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 58.Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, Niehrs C, Izpisua Belmonte JC, Westphal H. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Developmental cell. 2001;1:423–434. doi: 10.1016/s1534-5807(01)00041-7. [DOI] [PubMed] [Google Scholar]
- 59.Beazley KE, Deasey S, Lima F, Nurminskaya MV. Transglutaminase 2-mediated activation of beta-catenin signaling has a critical role in warfarin-induced vascular calcification. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:123–130. doi: 10.1161/ATVBAHA.111.237834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thambiah S, Roplekar R, Manghat P, Fogelman I, Fraser WD, Goldsmith D, Hampson G. Circulating sclerostin and dickkopf-1 (dkk1) in predialysis chronic kidney disease (ckd): Relationship with bone density and arterial stiffness. Calcified tissue international. 2012;90:473–480. doi: 10.1007/s00223-012-9595-4. [DOI] [PubMed] [Google Scholar]
- 61.Jones JA, Stroud RE, O’Quinn EC, Black LE, Barth JL, Elefteriades JA, Bavaria JE, Gorman JH, 3rd, Gorman RC, Spinale FG, Ikonomidis JS. Selective microrna suppression in human thoracic aneurysms: Relationship of mir-29a to aortic size and proteolytic induction. Circulation. Cardiovascular genetics. 2011;4:605–613. doi: 10.1161/CIRCGENETICS.111.960419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen NX, O’Neill KD, Chen X, Kiattisunthorn K, Gattone VH, Moe SM. Activation of arterial matrix metalloproteinases leads to vascular calcification in chronic kidney disease. American journal of nephrology. 2011;34:211–219. doi: 10.1159/000330175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li T, Cao H, Zhuang J, Wan J, Guan M, Yu B, Li X, Zhang W. Identification of mir-130a, mir-27b and mir-210 as serum biomarkers for atherosclerosis obliterans. Clinica chimica acta; international journal of clinical chemistry. 2011;412:66–70. doi: 10.1016/j.cca.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 64.Raitoharju E, Lyytikainen LP, Levula M, Oksala N, Mennander A, Tarkka M, Klopp N, Illig T, Kahonen M, Karhunen PJ, Laaksonen R, Lehtimaki T. Mir-21, mir-210, mir-34a, and mir-146a/b are up-regulated in human atherosclerotic plaques in the tampere vascular study. Atherosclerosis. 2011;219:211–217. doi: 10.1016/j.atherosclerosis.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 65.Shin V, Zebboudj AF, Bostrom K. Endothelial cells modulate osteogenesis in calcifying vascular cells. Journal of vascular research. 2004;41:193–201. doi: 10.1159/000077394. [DOI] [PubMed] [Google Scholar]
- 66.Itoh S, Itoh F, Goumans MJ, Ten Dijke P. Signaling of transforming growth factor-beta family members through smad proteins. European journal of biochemistry/FEBS. 2000;267:6954–6967. doi: 10.1046/j.1432-1327.2000.01828.x. [DOI] [PubMed] [Google Scholar]
- 67.Eijken M, Swagemakers S, Koedam M, Steenbergen C, Derkx P, Uitterlinden AG, van der Spek PJ, Visser JA, de Jong FH, Pols HA, van Leeuwen JP. The activin a-follistatin system: Potent regulator of human extracellular matrix mineralization. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2007;21:2949–2960. doi: 10.1096/fj.07-8080com. [DOI] [PubMed] [Google Scholar]
- 68.Mohammadpour AH, Shamsara J, Nazemi S, Ghadirzadeh S, Shahsavand S, Ramezani M. Evaluation of rankl/opg serum concentration ratio as a new biomarker for coronary artery calcification: A pilot study. Thrombosis. 2012;2012:306263. doi: 10.1155/2012/306263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kiechl S, Schett G, Schwaiger J, Seppi K, Eder P, Egger G, Santer P, Mayr A, Xu Q, Willeit J. Soluble receptor activator of nuclear factor-kappa b ligand and risk for cardiovascular disease. Circulation. 2007;116:385–391. doi: 10.1161/CIRCULATIONAHA.106.686774. [DOI] [PubMed] [Google Scholar]
- 70.Dombkowski AA, Sultana Z, Craig DB, Jamil H. In silico analysis of combinatorial microrna activity reveals target genes and pathways associated with breast cancer metastasis. Cancer informatics. 2011;10:13–29. doi: 10.4137/CIN.S6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A, Willeit J, Mayr M. Plasma microrna profiling reveals loss of endothelial mir-126 and other micrornas in type 2 diabetes. Circulation research. 2010;107:810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 72.Neal CS, Michael MZ, Pimlott LK, Yong TY, Li JY, Gleadle JM. Circulating microrna expression is reduced in chronic kidney disease. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2011;26:3794–3802. doi: 10.1093/ndt/gfr485. [DOI] [PubMed] [Google Scholar]
- 73.Mizoguchi F, Izu Y, Hayata T, Hemmi H, Nakashima K, Nakamura T, Kato S, Miyasaka N, Ezura Y, Noda M. Osteoclast-specific dicer gene deficiency suppresses osteoclastic bone resorption. Journal of cellular biochemistry. 2010;109:866–875. doi: 10.1002/jcb.22228. [DOI] [PubMed] [Google Scholar]
- 74.Nakasa T, Shibuya H, Nagata Y, Niimoto T, Ochi M. The inhibitory effect of microrna-146a expression on bone destruction in collagen-induced arthritis. Arthritis and rheumatism. 2011;63:1582–1590. doi: 10.1002/art.30321. [DOI] [PubMed] [Google Scholar]
- 75.Sugatani T, Hruska KA. Microrna-223 is a key factor in osteoclast differentiation. Journal of cellular biochemistry. 2007;101:996–999. doi: 10.1002/jcb.21335. [DOI] [PubMed] [Google Scholar]
- 76.Wang Y, Li L, Moore BT, Peng XH, Fang X, Lappe JM, Recker RR, Xiao P. Mir-133a in human circulating monocytes: A potential biomarker associated with postmenopausal osteoporosis. PloS one. 2012;7:e34641. doi: 10.1371/journal.pone.0034641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weber M, Baker MB, Moore JP, Searles CD. Mir-21 is induced in endothelial cells by shear stress and modulates apoptosis and enos activity. Biochemical and biophysical research communications. 2010;393:643–648. doi: 10.1016/j.bbrc.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou J, Wang KC, Wu W, Subramaniam S, Shyy JY, Chiu JJ, Li JY, Chien S. Microrna-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10355–10360. doi: 10.1073/pnas.1107052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fleissner F, Jazbutyte V, Fiedler J, Gupta SK, Yin X, Xu Q, Galuppo P, Kneitz S, Mayr M, Ertl G, Bauersachs J, Thum T. Short communication: Asymmetric dimethylarginine impairs angiogenic progenitor cell function in patients with coronary artery disease through a microrna-21-dependent mechanism. Circulation research. 2010;107:138–143. doi: 10.1161/CIRCRESAHA.110.216770. [DOI] [PubMed] [Google Scholar]
- 80.Li X, Yang HY, Giachelli CM. Bmp-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis. 2008;199:271–277. doi: 10.1016/j.atherosclerosis.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kin K, Miyagawa S, Fukushima S, Shirakawa Y, Torikai K, Shimamura K, Daimon T, Kawahara Y, Kuratani T, Sawa Y. Tissue- and plasma-specific microrna signatures for atherosclerotic abdominal aortic aneurysm. Journal of the American Heart Association. 2012:1. doi: 10.1161/JAHA.112.000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ohyashiki K, Umezu T, Yoshizawa S, Ito Y, Ohyashiki M, Kawashima H, Tanaka M, Kuroda M, Ohyashiki JH. Clinical impact of down-regulated plasma mir-92a levels in non-hodgkin’s lymphoma. PloS one. 2011;6:e16408. doi: 10.1371/journal.pone.0016408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, Hristov M, Koppel T, Jahantigh MN, Lutgens E, Wang S, Olson EN, Schober A, Weber C. Delivery of microrna-126 by apoptotic bodies induces cxcl12-dependent vascular protection. Science signaling. 2009;2:ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 84.Takahashi Y, Satoh M, Minami Y, Tabuchi T, Itoh T, Nakamura M. Expression of mir-146a/b is associated with the toll-like receptor 4 signal in coronary artery disease: Effect of renin-angiotensin system blockade and statins on mirna-146a/b and toll-like receptor 4 levels. Clin Sci (Lond) 2010;119:395–405. doi: 10.1042/CS20100003. [DOI] [PubMed] [Google Scholar]
- 85.Kong L, Zhu J, Han W, Jiang X, Xu M, Zhao Y, Dong Q, Pang Z, Guan Q, Gao L, Zhao J, Zhao L. Significance of serum micrornas in pre-diabetes and newly diagnosed type 2 diabetes: A clinical study. Acta diabetologica. 2011;48:61–69. doi: 10.1007/s00592-010-0226-0. [DOI] [PubMed] [Google Scholar]
- 86.Balasubramanyam M, Aravind S, Gokulakrishnan K, Prabu P, Sathishkumar C, Ranjani H, Mohan V. Impaired mir-146a expression links subclinical inflammation and insulin resistance in type 2 diabetes. Molecular and cellular biochemistry. 2011;351:197–205. doi: 10.1007/s11010-011-0727-3. [DOI] [PubMed] [Google Scholar]
- 87.Ichii O, Otsuka S, Sasaki N, Namiki Y, Hashimoto Y, Kon Y. Altered expression of microrna mir-146a correlates with the development of chronic renal inflammation. Kidney international. 2012;81:280–292. doi: 10.1038/ki.2011.345. [DOI] [PubMed] [Google Scholar]
- 88.Donners MM, Wolfs IM, Stoger LJ, van der Vorst EP, Pottgens CC, Heymans S, Schroen B, Gijbels MJ, de Winther MP. Hematopoietic mir155 deficiency enhances atherosclerosis and decreases plaque stability in hyperlipidemic mice. PloS one. 2012;7:e35877. doi: 10.1371/journal.pone.0035877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu N, Zhang D, Chen S, Liu X, Lin L, Huang X, Guo Z, Liu J, Wang Y, Yuan W, Qin Y. Endothelial enriched micrornas regulate angiotensin ii-induced endothelial inflammation and migration. Atherosclerosis. 2011;215:286–293. doi: 10.1016/j.atherosclerosis.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 90.Jia G, Stormont RM, Gangahar DM, Agrawal DK. Role of matrix gla protein in angiotensin ii-induced exacerbation of vascular stiffness. American journal of physiology. Heart and circulatory physiology. 2012 doi: 10.1152/ajpheart.00826.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Armstrong ZB, Boughner DR, Drangova M, Rogers KA. Angiotensin ii type 1 receptor blocker inhibits arterial calcification in a pre-clinical model. Cardiovascular research. 2011;90:165–170. doi: 10.1093/cvr/cvq391. [DOI] [PubMed] [Google Scholar]
- 92.Kirschner MB, Kao SC, Edelman JJ, Armstrong NJ, Vallely MP, van Zandwijk N, Reid G. Haemolysis during sample preparation alters microrna content of plasma. PloS one. 2011;6:e24145. doi: 10.1371/journal.pone.0024145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blondal T, Jensby Nielsen S, Baker A, Andreasen D, Mouritzen P, Wrang Teilum M, Dahlsveen IK. Assessing sample and mirna profile quality in serum and plasma or other biofluids. Methods. 2012 doi: 10.1016/j.ymeth.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 94.New SE, Aikawa E. Molecular imaging insights into early inflammatory stages of arterial and aortic valve calcification. Circulation research. 2011;108:1381–1391. doi: 10.1161/CIRCRESAHA.110.234146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hjortnaes J, Butcher J, Figueiredo JL, Riccio M, Kohler RH, Kozloff KM, Weissleder R, Aikawa E. Arterial and aortic valve calcification inversely correlates with osteoporotic bone remodelling: A role for inflammation. European heart journal. 2010;31:1975–1984. doi: 10.1093/eurheartj/ehq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Geng Y, Hsu JJ, Lu J, Ting TC, Miyazaki M, Demer LL, Tintut Y. Role of cellular cholesterol metabolism in vascular cell calcification. The Journal of biological chemistry. 2011;286:33701–33706. doi: 10.1074/jbc.M111.269639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of micrornas and microrna-protective protein by mammalian cells. Nucleic acids research. 2010;38:7248–7259. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mrnas and micrornas is a novel mechanism of genetic exchange between cells. Nature cell biology. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 99.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. Micrornas are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nature cell biology. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating micrornas independent of vesicles in human plasma. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microrna. Nucleic acids research. 2011;39:7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Diehl P, Fricke A, Sander L, Stamm J, Bassler N, Htun N, Ziemann M, Helbing T, El-Osta A, Jowett JB, Peter K. Microparticles: Major transport vehicles for distinct micrornas in circulation. Cardiovascular research. 2012;93:633–644. doi: 10.1093/cvr/cvs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pigati L, Yaddanapudi SC, Iyengar R, Kim DJ, Hearn SA, Danforth D, Hastings ML, Duelli DM. Selective release of microrna species from normal and malignant mammary epithelial cells. PloS one. 2010;5:e13515. doi: 10.1371/journal.pone.0013515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-micrornas. Nucleic acids research. 2010;38:215–224. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Creemers EE, Tijsen AJ, Pinto YM. Circulating micrornas: Novel biomarkers and extracellular communicators in cardiovascular disease? Circulation research. 2012;110:483–495. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 106.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of micrornas in living cells. The Journal of biological chemistry. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S. Atheroprotective communication between endothelial cells and smooth muscle cells through mirnas. Nature cell biology. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 108.Meckes DG, Jr, Raab-Traub N. Microvesicles and viral infection. Journal of virology. 2011;85:12844–12854. doi: 10.1128/JVI.05853-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Anderson HC. Molecular biology of matrix vesicles. Clinical orthopaedics and related research. 1995:266–280. [PubMed] [Google Scholar]
- 110.Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circulation research. 2011;109:697–711. doi: 10.1161/CIRCRESAHA.110.234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: A potential mechanism for accelerated vascular calcification in esrd. Journal of the American Society of Nephrology: JASN. 2004;15:2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 112.Chen NX, O’Neill KD, Chen X, Moe SM. Annexin-mediated matrix vesicle calcification in vascular smooth muscle cells. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2008;23:1798–1805. doi: 10.1359/JBMR.080604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kapustin AN, Davies JD, Reynolds JL, McNair R, Jones GT, Sidibe A, Schurgers LJ, Skepper JN, Proudfoot D, Mayr M, Shanahan CM. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circulation research. 2011;109:e1–12. doi: 10.1161/CIRCRESAHA.110.238808. [DOI] [PubMed] [Google Scholar]
- 114.New SE, Marchini JF, Aikawa M, Shanahan CM, Croce K, Aikawa E. Novel role of macrophage-derived matrix vesicles in arterial microcalcification. Circulation. 2011;124:A10866. doi: 10.1161/CIRCRESAHA.113.301036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sage AP, Lu J, Tintut Y, Demer LL. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney international. 2011;79:414–422. doi: 10.1038/ki.2010.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bobryshev YV, Killingsworth MC, Lord RS, Grabs AJ. Matrix vesicles in the fibrous cap of atherosclerotic plaque: Possible contribution to plaque rupture. Journal of cellular and molecular medicine. 2008;12:2073–2082. doi: 10.1111/j.1582-4934.2008.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maldonado N, Kelly-Arnold A, Vengrenyuk Y, Laudier D, Fallon JT, Virmani R, Cardoso L, Weinbaum S. A mechanistic analysis of the role of microcalcifications in atherosclerotic plaque stability: Potential implications for plaque rupture. American journal of physiology. Heart and circulatory physiology. 2012;303:H619–628. doi: 10.1152/ajpheart.00036.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maldonado N, Kelly-Arnold A, Cardoso L, Weinbaum S. The explosive growth of small voids in vulnerable cap rupture; cavitation and interfacial debonding. Journal of biomechanics. 2012 doi: 10.1016/j.jbiomech.2012.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tabuchi T, Satoh M, Itoh T, Nakamura M. Microrna-34a regulates the longevity-associated protein sirt1 in coronary artery disease: Effect of statins on sirt1 and microrna-34a expression. Clin Sci (Lond) 2012;123:161–171. doi: 10.1042/CS20110563. [DOI] [PubMed] [Google Scholar]
- 120.Minami Y, Satoh M, Maesawa C, Takahashi Y, Tabuchi T, Itoh T, Nakamura M. Effect of atorvastatin on microrna 221/222 expression in endothelial progenitor cells obtained from patients with coronary artery disease. European journal of clinical investigation. 2009;39:359–367. doi: 10.1111/j.1365-2362.2009.02110.x. [DOI] [PubMed] [Google Scholar]
- 121.Taurino C, Miller WH, McBride MW, McClure JD, Khanin R, Moreno MU, Dymott JA, Delles C, Dominiczak AF. Gene expression profiling in whole blood of patients with coronary artery disease. Clin Sci (Lond) 2010;119:335–343. doi: 10.1042/CS20100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gao W, He HW, Wang ZM, Zhao H, Lian XQ, Wang YS, Zhu J, Yan JJ, Zhang DG, Yang ZJ, Wang LS. Plasma levels of lipometabolism-related mir-122 and mir-370 are increased in patients with hyperlipidemia and associated with coronary artery disease. Lipids in health and disease. 2012;11:55. doi: 10.1186/1476-511X-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hulsmans M, Sinnaeve P, Van der Schueren B, Mathieu C, Janssens S, Holvoet P. Decreased mir-181a expression in monocytes of obese patients is associated with the occurrence of metabolic syndrome and coronary artery disease. The Journal of clinical endocrinology and metabolism. 2012;97:E1213–1218. doi: 10.1210/jc.2012-1008. [DOI] [PubMed] [Google Scholar]
- 124.Satoh M, Tabuchi T, Minami Y, Takahashi Y, Itoh T, Nakamura M. Expression of let-7i is associated with toll-like receptor 4 signal in coronary artery disease: Effect of statins on let-7i and toll-like receptor 4 signal. Immunobiology. 2012;217:533–539. doi: 10.1016/j.imbio.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 125.Sondermeijer BM, Bakker A, Halliani A, de Ronde MW, Marquart AA, Tijsen AJ, Mulders TA, Kok MG, Battjes S, Maiwald S, Sivapalaratnam S, Trip MD, Moerland PD, Meijers JC, Creemers EE, Pinto-Sietsma SJ. Platelets in patients with premature coronary artery disease exhibit upregulation of mirna340* and mirna624*. PloS one. 2011;6:e25946. doi: 10.1371/journal.pone.0025946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Meng S, Cao JT, Zhang B, Zhou Q, Shen CX, Wang CQ. Downregulation of microrna-126 in endothelial progenitor cells from diabetes patients, impairs their functional properties, via target gene spred-1. Journal of molecular and cellular cardiology. 2012;53:64–72. doi: 10.1016/j.yjmcc.2012.04.003. [DOI] [PubMed] [Google Scholar]