Abstract
BACKGROUND
The goal of selecting a healthy blood donor is to safeguard donors and reduce the risks of infections and immunologic complications for recipients.
STUDY DESIGN AND METHODS
To evaluate the blood donor selection process, a survey was conducted in 28 blood transfusion centers located in 15 francophone African countries. Data collected included availability of blood products, risk factors for infection identified among blood donor candidates, the processing of the information collected before blood collection, the review process for the medical history of blood donor candidates, and deferral criteria for donor candidates.
RESULTS
During the year 2009, participating transfusion centers identified 366,924 blood donor candidates. A mean of 13% (range, 0%–36%) of the donor candidates were excluded based solely on their medical status. The main risk factors for blood-borne infections were having multiple sex partners, sexual intercourse with occasional partners, and religious scarification. Most transfusion centers collected this information verbally instead of having a written questionnaire. The topics least addressed were the possible complications relating to the donation, religious scarifications, and history of sickle cell anemia and hemorrhage. Only three centers recorded the temperature of the blood donors. The deferral criteria least reported were sickle cell anemia, piercing, scarification, and tattoo.
CONCLUSIONS
The medical selection process was not performed systemically and thoroughly enough, given the regional epidemiologic risks. It is essential to identify the risk factors specific to francophone African countries and modify the current medical history questionnaires to develop a more effective and relevant selection process.
Transfusion safety is one of the main challenges faced by blood transfusion centers in Africa due to the high prevalence of transfusion-transmissible infections (TTIs) and the high residual risk of transfusion contamination by these agents.1 This issue has been raised by numerous local and multicenter studies conducted in francophone African countries.2–7 An estimated 25% of blood units collected in sub-Saharan francophone Africa are contaminated by viral agents (human immunodeficiency virus [HIV], hepatitis B virus [HBV], hepatitis C virus [HCV], parasites [Plasmodium spp., microfilaria], and bacteria). Despite safety strategies recommended by the World Health Organization (WHO)8 and the support of international organizations participating in the transfusion safety of sub-Saharan Africa, the risk of TTIs is still high. Recent multicenter studies have shown that the risk of HIV transmission by transfusion ranges between 1 in 25,600 and 1 in 90,200, which is much higher than in industrialized countries.9
To increase the safety of blood products transfused in Africa, three strategies need to be considered: more effective medical selection of blood donors, better detection of TTIs, and physical and chemical treatment of blood products. Detection of TTIs has greatly improved in past years in Africa. Many studies have revealed that quick screening tests, validated through quality assurance, have reduced transfusion risks as was achieved by fourth-generation serologic tests.10,11 Viral detection through nucleic acid testing would certainly be most beneficial but it is still not feasible in most African countries due to financial and technical reasons.12 In addition, the efficacy of this biologic screening is limited by many factors such as genetic variation (e.g., HIV),13 test conditions, absence of quality assurance in many centers, and the inability to detect recently infected subjects.14 Physical and/or chemical treatment of blood products is still only feasible for platelet concentrates and plasma.15 However, blood products as a whole and red blood cells are the main blood-derived products used by a vast majority of sub-Saharan African countries. Furthermore, these countries cannot currently afford the cost of this type of physical and chemical blood treatment. It appears that the most efficient way to improve transfusion safety in Africa at this time is to improve the medical selection process to exclude subjects at high risks of transmitting blood-borne infectious pathogens from blood donation.
The medical selection step comes first in the overall process of collecting safe blood products. The identification and exclusion of donors with high-risk behaviors leads to a significant yet poorly quantified reduction in the risk of infections for the blood recipients and ensures donor safety as well.16 With the high TTI risk being associated with having multiple sex partners, sexual relationships between men, tattoos, or injection drug use, complementary strategies need to be used to exclude high-risk subjects in the selection of blood donors. Such strategies are: 1) predonation counseling, where the goal is to inform donor candidates on behaviors that are considered at risk, on the actual blood donation procedure, and the possibility of self-exclusion; 2) reviewing the donor candidate’s medical history with a physician to ensure the absence of symptoms (past or current) that may carry a risk for the donor or recipient; 3) physical examination, as certain physical signs and symptoms may contraindicate blood donation; and 4) infectious disease (ID) marker testing to identify infections.17,18 Maintaining a high level of vigilance after a blood donation is essential as the donor’s initial interview is audited in the hours or days after the donation. In developing countries, where resources are limited, the medical selection process is all the more critical as other strategies of ensuring safety of the blood products are difficult to achieve or insufficient on their own. This method is easy to achieve and does not require any specific equipment. The authors investigated the variability and the conditions in which this medical selection process was completed in various francophone African countries. The results of this study identify hurdles that need to be overcome and establish a baseline for the transfusion centers in Africa that would like to improve their current strategies in preventing ID.
MATERIALS AND METHODS
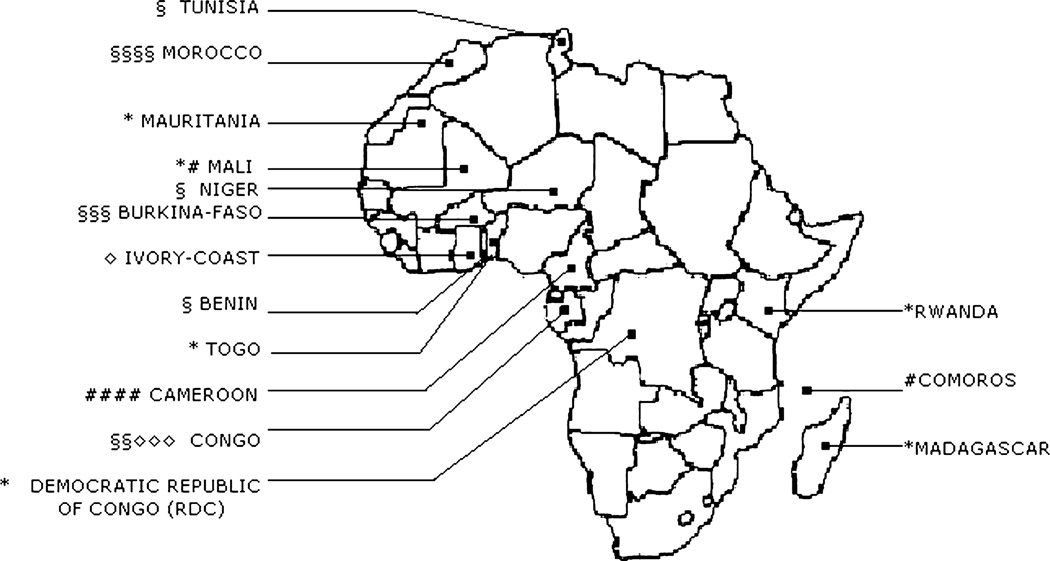
This descriptive multicenter study was completed in 28 transfusion centers located in 15 francophone countries in Western, Eastern, Central, and Northern Africa: one center in Benin (Service Départemental de Transfusion Sanguine de l’Atlantique et du Littoral); three centers in Burkina Faso (Centre Régional de Transfusion Sanguine de Bobo-Dioulasso, Centre Régional de Transfusion Sanguine de Fada N’Gourma, and Centre National de Transfusion Sanguine de Ouagadougou); four centers in Cameroon (Banque de Sang du Centre Hospitalier et Universitaire de Yaoundé, Banque de Sang de l’HôpitalCentral deYaoundé, Banque de Sang de l’Hôpital Général de Yaoundé, and Banque de Sang de l’Hôpital Laquintinie de Douala); one center in the Comoros (Centre Hospitalier d’El Maarouf); one in the Democratic Republic of Congo (Centre National de Transfusion Sanguine de Kinshasa); five centers in the Republic of Congo (Centre Régional Interdépartemental de Transfusion Sanguine de Brazzaville, Poste de Prélèvement du Réseau Transfusionnel Zone Sud Niari Lekoumou, Poste de Prélèvement du Réseau Transfusionnel Zone Nord, Poste de Prélèvement du Réseau Transfusionnel Zone Sud Bouenza, and Centre Régional Interdépartemental de Transfusion Sanguine de Pointe Noire); one center in Ivory Coast (Poste de Prélèvement de l’Hôpital Général de Yopougon Attie); one center in Madagascar (Centre National de Transfusion Sanguine d’Antananarivo); two centers in Mali (Antenne Transfusionnelle du Centre de Santé de Référence de Kidal and Centre National de Transfusion Sanguine de Bamako); four centers in Morocco (Centre Régional de Transfusion Sanguine de Casablanca, Centre Régional de Transfusion Sanguine de Marrakech, Centre Régional de Transfusion de Oujda, and Centre Régional de Transfusion Sanguine de Rabat); one center in Mauritania (Centre National de Transfusion Sanguine de Nouakchott); one center in Niger (Centre Régional de Transfusion Sanguine de Niamey); one center in Rwanda (Centre National de Transfusion Sanguine de Kigali); one in Togo (Centre National de Transfusion Sanguine de Lomé); and one in Tunisia (Centre Régional de Transfusion Sanguine de Sfax; Fig. 1).
Fig. 1.
Number and type of participating blood centers for each country. The number of each type of symbol corresponds to the number of that type of blood center within the country participating in the study. *Number of national blood centers in the country. §Number of regional blood centers in the country. #Number of hospital-based blood banks in the country. ◇Number of collection units in the country.
To determine how the medical selection process was conducted, each participating center received a standardized questionnaire to complete and return with their medical selection questionnaire and the blood donor information sheet if available at their center. All the francophone blood centers for which we had an e-mail contact have been invited to participate in the study. No selection has been done among them. Regional and national blood centers of the survey represent the largest blood centers of their countries. The survey questions were aimed at identifying the following topics for the year 2009:
General information on how blood products were collected, including center address, type of organization (national center, regional center, hospital bank, collection unit); presence of a donor management center (blood donation promotion and organization); annual demand for blood products; number of donor candidates, actual donors, and donations collected during 2009; type of collection available at the center (mobile vs. fixed); proportion of mobile donations compared to total donations; strategies for education (posters, videos, movies), for increasing awareness and recruitment (phone, letter, newspaper, television, radio, posters, brochure, pamphlets), and motivation (gadgets, medals, diplomas, money, transportation costs, free care).
Personal characteristics of the blood donors: type of donation (voluntary, family or replacement, paid); donor (new, regular); age distribution; sex distribution; prevalence of TTIs (HIV, HBV, HCV, syphilis, malaria); the center’s impression of the most common high-risk behavior identified in donor candidates (homosexuality, drug use, sexual intercourse with partners having HIV, sexual intercourse with occasional partners, sexual intercourse with prostitutes, religious scarification, tattoos, piercing). The characteristics of the blood donor were obtained retrospectively from the centers’ 2009 databases.
Processing of donor information before the donation: staff involved (physician, laboratory technician, nurse) and information technology.
Donor candidates’ medical history interview.
Medical examination: measurements (blood pressure, temperature, weight, pulse, screening for anemia, height, physical examination), and values used to determine acceptable clinical or biologic variables (blood pressure, temperature, weight, hemoglobin [Hb] concentration, hematocrit [Hct]).
Reasons for deferral of donor candidates (behavior considered high risk for transmission of infectious agents by blood, history of blood transfusion, recent hospitalization or surgery, recent untreated symptoms, pregnancy, breastfeeding, recent fever, history of jaundice, cardiac, liver or kidney disease, diabetes, hypertension, hemorrhage, recent sexually transmissible infection, cutaneous eruptions, epilepsy, chronic cough, diarrhea, involuntary weight loss, recent vaccination, cancer, use of medication).
Use of ID marker tests before donation (viral serology, Hb concentration, or Hct).
Informed consent, verbal or written.
Use of biovigilance and an audit system after donation.
Cold storage of quarantined blood and duration of storage.
Responding centers had to choose among a list of potential risk factors, a list of frequently asked questions to blood donors, a list of clinical signs and variables, and a list of deferral reasons, which were observed and used in their blood center during medical selection of their blood donors.
We used standard summary statistics (proportion for categorical variables and mean or median with range for continuous variables) to describe both collection center and donor characteristics. Data summaries pertaining to donor characteristics were provided by each center using a questionnaire. Each center provided its own assessment of local blood requirements. Whether or not blood supplies were met in each center was quantified for each center by dividing the actual number of units collected in 2009 by the estimated need for blood units.
RESULTS
The collection unit is the simplest type of center in a remote area in charge of collecting blood from donors and sending it to a bigger blood center (regional or national). They are not supposed to screen for TTI or to prepare blood products. Among the participating centers in this study, 12 were regional, six were national, six were hospital blood banks, and four were blood collection units only (Table 1). Among 14 centers that provided data on blood unit deficit, the majority (64%) reported a shortage of 1000 to 9999 units. Of 28 centers, 23 (82%) had a recruitment department to promote blood donation and organize blood collection. Among 24 centers who responded to the question regarding number of donor candidates for year 2009, 59% reported an estimated 10,000 or more donors in 2009, and 33% centers reported fewer than 5000 donors. Of 21 centers, 19 (90%) had excluded fewer than 5000 donors per year. Twenty centers provided the demand of blood products, which added up to 370,373 units in 2009, for a supply of 302,856 donations (representing only 82% [range, 27%–100%] fulfillment of the demand). The total numbers of units collected by each type of blood center were 98,445, 171,055, 20,622, and 12,734, respectively, for the national blood center, the regional blood center, the hospital-based blood center, and the collection unit. The median numbers (range) of units collected were, respectively, 21,630 [7104–29,045], 15,651 [7956–44,135], 2537 [161–12,500], and 2201 [1701–6632]. A median of 23% (range, 0%–96%) of blood products were obtained through mobile blood collection.
TABLE 1.
Collection characteristics among 28 participating blood donation centers in francophone Africa in 2009
| Characteristics | Number (%) of centers |
|---|---|
| Type of center | |
| Regional | 12 (43) |
| National | 6 (21) |
| Hospital based | 6 (21) |
| Collection unit | 4 (14) |
| Total number of units collected | |
| <1,000 | 1 (4) |
| 1,000–4,999 | 6 (26) |
| 5,000–9,999 | 3 (13) |
| 10,000–19,999 | 7 (31) |
| 20,000 or more | 6 (26) |
| Proportion (%) of units collected on units required | |
| <25 | 0 (0) |
| 25–49.9 | 2 (10) |
| 50–74.9 | 8 (38) |
| ≥75 | 11 (52) |
| Blood unit shortage (number per year) | |
| <1,000 | 1 (7) |
| 1,000–4,999 | 6 (43) |
| 5,000–9,999 | 3 (21) |
| 10,000–19,999 | 4 (29) |
| Blood unit surplus (number per year) | |
| <1,000 | 2 (29) |
| 1,000–4,999 | 3 (42) |
| 5,000–9,999 | 2 (29) |
| Centers with a donor management unit | 23 (82) |
| Donor candidates per year | |
| <5,000 | 8 (33) |
| 5,000–9,999 | 2 (8) |
| 10,000–19,999 | 9 (38) |
| 20,000–39,000 | 3 (13) |
| 40,000 or more | 2 (8) |
| Donors excluded by medical interview per year | |
| <5,000 | 19 (90) |
| 5,000–9,999 | 1 (5) |
| 10,000–19,999 | 1 (5) |
| Proportion (%) of donors excluded by medical interview per year | |
| <10 | 10 (48) |
| 10–19.9 | 4 (19) |
| 20–29.9 | 6 (28) |
| 30 or more | 1 (5) |
The total number of donor candidates among all centers from January 1, 2009, to December 31, 2009, totaled 366,924 (Table 2). Among the candidates, a median of 12% (range, 0%–36%) were deferred based solely on the medical selection process. Voluntary non-remunerated blood donors constituted 61% of all blood donors. No center mentioned having paid blood donors. Only nine blood centers of 22 using the questionnaire asked the question on type of donation during interview. All of them (22) excluded paid donors (Tables 4 and 5). The others six centers did not mention paid donation in the interview nor during donor deferral. Repeat donors constituted 24% of all donors. The median age of donors (provided by 15 centers) was 35 years old (range, 25–42 years). All of the blood centers performed the test for HIV, HBV, HCV, and syphilis infections. The proportions of donors across centers having TTIs were 1.8, 6.8, 2.5, and 1.7% for HIV, HBV, HCV, and syphilis infections, respectively. Blood centers excluding more than 20% of their blood donor candidates did not have a lower prevalence of ID markers.
TABLE 2.
Blood donor characteristics among 366,924 donor candidates in 28 participating blood donation centers in francophone Africa in 2009
| Characteristics | Number of donors | Percentage (range) per blood center |
|---|---|---|
| Type of donor | ||
| Voluntary | 154,291 | 61 (1.2–100) |
| Familial | 98,934 | 39 (0–98.8) |
| Total | 253,225 | |
| Blood donation frequency | ||
| First-time donors | 173,466 | 76 (18.4–100) |
| Regular donors | 55,737 | 24 (0–81.6) |
| Total | 229,203 | |
| ID marker prevalence | ||
| HIV | 4,801 | 1.8* (0–7) |
| HBV | 17,211 | 6.8* (0.7–7.6) |
| HCV | 6,453 | 2.5* (0–7.5) |
| Syphilis | 4,426 | 1.7* (0–11) |
Percentage among all donors.
TABLE 4.
Donor medical history questionnaire content
| Number of centers asking each question | |||||
|---|---|---|---|---|---|
| Questionnaire content | Collection unit (n = 1) |
Hospital based (n = 5) |
Regional (n = 10) |
National (n = 6) |
Total (n = 22), n (%) |
| Questions pertaining to donor safety | |||||
| Prior donation | 1 | 4 | 5 | 4 | 14 (64) |
| Complications after donation | 1 | 3 | 1 | 2 | 7 (32) |
| Hypertension | 1 | 5 | 7 | 4 | 17 (77) |
| Asthma | 1 | 3 | 7 | 3 | 14 (64) |
| Epilepsy | 1 | 4 | 7 | 3 | 15 (68) |
| Sickle cell anemia | 0 | 4 | 4 | 0 | 8 (37) |
| Pregnancy or breast feeding | 1 | 5 | 6 | 6 | 18 (82) |
| Diabetes | 1 | 4 | 7 | 4 | 16 (73) |
| Heart, liver, or kidney diseases | 1 | 5 | 7 | 4 | 17 (77) |
| Cancer | 1 | 3 | 2 | 4 | 10 (45) |
| Questions pertaining to recipient safety | |||||
| Homosexuality | 1 | 3 | 6 | 2 | 12 (55) |
| Injection drug use | 0 | 5 | 10 | 3 | 18 (82) |
| Multiple or occasional sex partners | 1 | 4 | 10 | 4 | 19 (86) |
| Scarification | 1 | 3 | 4 | 1 | 9 (41) |
| Tattoos | 1 | 3 | 9 | 4 | 17 (77) |
| Piercing | 1 | 3 | 8 | 4 | 16 (73) |
| Recent fever | 1 | 4 | 10 | 4 | 19 (86) |
| Transfusion | 0 | 5 | 10 | 5 | 20 (91) |
| Jaundice | 1 | 4 | 3 | 6 | 14 (64) |
| Chronic coughing | 1 | 3 | 6 | 4 | 14 (64) |
| Recent hospitalization | 1 | 5 | 10 | 6 | 22 (100) |
| Diarrhea | 1 | 3 | 7 | 3 | 14 (64) |
| Recent immunization | 1 | 4 | 10 | 4 | 19 (86) |
| STD(s) | 1 | 5 | 7 | 6 | 19 (86) |
| Medications | 0 | 4 | 6 | 6 | 16 (73) |
| Sexual intercourse with partner with STD | 1 | 3 | 7 | 3 | 14 (64) |
| Sexual intercourse with prostitute | 0 | 3 | 10 | 1 | 14 (64) |
| History of hemorrhage | 0 | 3 | 2 | 1 | 6 (27) |
| Type of donor (volunteer, family, paid) | 0 | 3 | 4 | 2 | 9 (41) |
STD = sexually transmitted disease.
TABLE 5.
Exclusion criteria for blood donors after the medical selection process
| Number of centers excluding donor candidates on these specific criteria | |||||
|---|---|---|---|---|---|
| Exclusion criteria | Collection unit (n = 4) |
Hospital based (n = 6) |
Regional (n = 12) |
National (n = 6) |
Total (n = 28), n (%) |
| Exclusion criteria relating to the safety of the donor | |||||
| Symptoms without medical consultation | 4 | 5 | 12 | 6 | 27 (96) |
| Hypertension | 4 | 6 | 12 | 6 | 28 (100) |
| Asthma | 4 | 6 | 12 | 6 | 28 (100) |
| Anemia | 4 | 6 | 12 | 6 | 28 (100) |
| Epilepsy | 4 | 6 | 11 | 5 | 26 (93) |
| Sickle cell anemia | 0 | 4 | 7 | 2 | 13 (46) |
| Pregnancy or breast feeding | 4 | 6 | 12 | 6 | 28 (100) |
| Diabetes | 4 | 6 | 12 | 6 | 28 (100) |
| Heart, liver, kidney conditions | 4 | 6 | 12 | 6 | 28 (100) |
| Cancer | 4 | 6 | 12 | 6 | 28 (100) |
| Exclusion criteria relating to the safety of the recipient | |||||
| Homosexuality | 4 | 4 | 12 | 4 | 24 (86) |
| Intravenous drug use | 4 | 6 | 12 | 4 | 26 (93) |
| Multiple or occasional partners | 4 | 5 | 12 | 4 | 25 (89) |
| Scarification | 4 | 3 | 10 | 3 | 20 (71) |
| Tattoo | 1 | 4 | 10 | 4 | 19 (68) |
| Piercing | 1 | 3 | 10 | 5 | 19 (68) |
| Recent fever | 4 | 6 | 12 | 6 | 28 (100) |
| Transfusion | 4 | 5 | 12 | 6 | 27 (96) |
| Jaundice | 4 | 6 | 12 | 6 | 28 (100) |
| Chronic cough | 4 | 6 | 12 | 5 | 27 (96) |
| Recent hospitalization | 4 | 6 | 12 | 6 | 28 (100) |
| Diarrhea | 4 | 6 | 12 | 5 | 27 (96) |
| Hemorrhage | 4 | 5 | 11 | 6 | 26 (93) |
| Sexual intercourse with a person having a STD | 4 | 6 | 11 | 6 | 27 (96) |
| Sexual relationship with a prostitute | 4 | 6 | 12 | 6 | 28 (100) |
| Recent vaccination | 4 | 5 | 11 | 4 | 24 (86) |
| STD | 4 | 6 | 11 | 5 | 26 (93) |
| Use of medication | 4 | 5 | 11 | 4 | 24 (86) |
| Family donation | 1 | 0 | 3 | 2 | 6 (2) |
| Paid donation | 4 | 5 | 9 | 4 | 22 (79) |
STD = sexually transmitted disease.
Donor education before the donation was done by all the centers but was systematically done in only 20 centers. The staff in charge of informing the donors was nonmedical personnel in nine centers, professionals (pharmacists, biologists, and physicians) in 23 centers, nurses in 17 centers, and technicians in 16 centers (Table 3). The information relayed to the donors included risk factors, possibility of self-exclusion, and criteria for donation blood. This information was conveyed verbally in 21 centers. Of 28 centers, 23 centers had a brochure or flyer as a way to educate donors. Among centers using posters to provide information, 60% were regional and 30% were collection units, but no posters were used in hospital-based settings. Among those using brochures or flyers, 52% were regional and 22% national. No collection units used verbal information. Among nurses, physicians, pharmacists, and biologists who provided predonation information and performed interviews, nearly half were in regional centers and less than 25% were in hospital-based centers. Among nonmedical personnel performing predonation interviews, 60% were in regional centers, 29% were in hospital-based settings, and none were in collection units.
TABLE 3.
Management of predonation donor-related information among 28 participating blood donation centers in francophone Africa in 2009*
| Characteristics | Collection unit (n = 4) | Hospital based (n = 6) | Regional (n = 12) | National (n = 6) |
|---|---|---|---|---|
| Frequency of donor information collection | ||||
| Always | 4 (20) | 4 (20) | 8 (40) | 4 (20) |
| Sometimes | 0 (0) | 2 (25) | 4 (50) | 2 (25) |
| Personnel† | ||||
| Nurse | 1 (6) | 3 (18) | 8 (47) | 5 (29) |
| Physician, pharmacist, or biologist | 2 (8) | 5 (22) | 11 (48) | 5 (22) |
| Technician | 3 (19) | 5 (31) | 6 (38) | 2 (12) |
| Nonmedical personnel | 0 (0) | 3 (29) | 5 (60) | 1 (11) |
| Information presentation | ||||
| Poster | 3 (30) | 0 (0) | 6 (60) | 1 (10) |
| Brochure or flyer | 4 (17) | 2 (9) | 12 (52) | 5 (22) |
| Verbal | 0 (0) | 6 (28) | 10 (48) | 5 (24) |
| Information provided | ||||
| Risk factors | 4 (15) | 6 (22) | 11 (41) | 6 (22) |
| Donation procedure | 4 (15) | 5 (20) | 11 (42) | 6 (23) |
| Self-exclusion | 4 (15) | 6 (21) | 12 (43) | 6 (21) |
Data are reported as number (%). Row percentages.
Personnel providing predonation information to donors and performing predonation interviews.
Interviewing donors on their medical history was performed systematically in 22 centers and “not always” in four centers. Six centers did not provide a standard questionnaire (verbal or written) to determine the donor’s medical history. The questionnaire was verbal in seven centers and written in 15 centers. The questions were mostly aimed at determining the safety of the donor (able to donate) and of the recipient (risk factors and behaviors). Among the four centers having a questionnaire addressing all the topics evaluated by this study, two were regional, one was national, and one was hospital based.
The questions on the type of donation, complications relating to the donation, religious scarification, and history of sickle cell anemia and hemorrhage were the least frequently asked (Table 4). Informed consent was required in 27 centers and was written in eight of them.
All the centers administered a physical examination to the donor before the donation. The most commonly examined variables were blood pressure (28 centers), weight (24 centers), and pulse (12 centers); a complete physical examination was performed in 25 centers. Three centers recorded temperature and two recorded the height. The minimum weight allowed was 50 kg in 20 centers and not specified in the others. At completion of the physical examination, the centers retained the blood donors who did not present any of the deferral criteria listed in Table 5.
The main behaviors considered at risk for infectious agents reported by the participating centers were, in order of frequency, recent fever (28 centers), jaundice (28 centers), recent hospitalization (28 centers), sexual relationship with a prostitute (28 centers), transfusion (27 centers), and chronic cough (27 centers; Table 5). Routine procedures (dental extractions, traditional bloodletting) and history of blood transfusions were identified as risk factors for infection by three centers.
Predonation testing was performed in 13 centers and was always accompanied by postdonation testing. The proportions of centers performing the screening tests before blood donation was 77, 70, 70, 46, and 31% for HIV, HBV, HCV, Hb concentration, and malaria, respectively. No biological screening for sickle cell anemia was completed. We did not gather data on the type of testing performed for each of these variables.
Regarding the centers’ hemovigilance after donation, 18 centers collected information from the donors in the hours or days after the donation. Ten centers placed the blood collected in quarantine for 1 to 5 days.
DISCUSSION
This study is the first international study performed in francophone African countries to analyze strategies for selecting blood donors that included a large number of transfusion centers and blood donors. The results confirm a high prevalence in major transfusion-transmissible infectious factors in blood donors in Africa, a high predominance of familial donation, low proportion of regular donors, and mobile collection of blood.1,2
The demand in blood products was met in 82% of the centers participating in this study, which appears to be an overestimate compared to WHO estimates.19 A limitation of our data is that each center estimated its own blood demand, typically estimated based on the requests of blood products registered by transfusion centers. To account for the unmentioned needs from remote areas, one needs to estimate the demand based on the number of people living in the area in question or based on the number of hospital beds that need to be covered.
Medical selection before the donation was performed by all participating centers and allowed for identification and deferral of up to a third of the donor candidates. This proportion is very high compared to that of western countries,16,20 although the ratio of new to regular donors is also very different in industrialized countries compared to most African countries. Such a rate of exclusion may be due to the high prevalence of African donor candidates having one or more risk factors or an erroneously noted risk factor. While certain risk factors (multiple partners, occasional partners, homosexuality) have been described in depth,21,22 other risk factors attributed to routine practices in Africa have yet to be studied; such factors include religious scarification, traditional piercing in Sahelian populations, dental extractions, and bloodletting performed in North African countries. It appears that the epidemiology of infections transmissible by blood is not the same in all continents. Therefore, the criteria used for selecting donors are not universal and cannot be applied everywhere in the same manner. One needs to consider social differences: for instance, having multiple partners is defined as having had more than one partner in the past 4 months in French regulations, while polygamy is not considered as fitting this definition. The criteria used for selecting African donors must take into account local, social, and cultural differences: for instance, the mean age of the population in Africa is much lower than in industrialized countries. This demonstrates a need for epidemiologic studies of subjects known to have HIV when donating blood and knowing the epidemiologic characteristics (risk factors) of HIV infection in all the countries in Africa. In fact the lack of epidemiologic data hinders the establishment of accurate criteria for the selection of donor candidates.
The medical selection process is often sporadic and incomplete in many African countries with no specific reason to explain this inconsistency. However, one may notice the lack of specific personnel to greet and select blood donors in hospital transfusion centers and a shortage of staff trained in transfusion.23 Only few differences were noticed in terms of strategies in medical selection when comparing the type of blood donation centers. For example, all four collection units provided donor education and used medical personnel. No obvious explanation can be given for this apparently contradictory data, and the small number of centers precluded formal statistical analysis.
Informing the donor is the necessary first step in the selection process as it educates and assigns responsibility to the donor candidates regarding their own safety and that of the recipients. The importance of relaying this information was recognized by the participating centers, as all donor candidates were educated on the main topics, even though this information was relayed sporadically by some centers. The information was most often conveyed verbally and at times complemented by printed material. It was necessary to explain things verbally for a certain number of donors who did not understand the risk factors due to a low level of education.24–27 For such donor candidates, additional written information may be difficult to understand and does not lead to an effective medical selection process.28,29 The benefits of this discussion with the donor candidates also require further evaluation as they may hide certain information about themselves and lead to ineffective deferral for major transfusion risk factors.30 In certain centers, reviewing the donor’s medical history was not performed systematically, and important questions regarding the donor safety and that of the recipients were even omitted. Questions on complications that had occurred after past donations, on a history of sickle cell anemia and hemorrhage, were the least often addressed. In fact, donors having these risk factors (history of familial donation, sickle cell trait) were less often excluded due to lack of supply19 and due to a risk that was unknown or not well defined for the recipient.31 The participating centers asked a wide range of questions, despite a general consensus on the risk factors. Tables 4 and 5 show that questions regarding sexual behaviors (male homosexuality, having multiple sex partners, sexual relationships with an infected partner) are not always addressed, but are a definite contraindication for all the centers if a positive answer is found. Moreover, the blood donation centers excluding more than 20% of their blood donor candidates did not have a lower prevalence of ID markers. The efficiency of the medical selection of the blood donors seems to not absolutely be related to the number of excluded blood donors. This reflects an arbitrary choice of questions or an overcautious interpretation of the safety principles regarding risk factors reported in other countries. Certain deferrals remain empirical and were not the object of specific studies in these countries, as is also the case in industrialized countries.20,32
Only few differences were noticed in terms of the strategies in medical selection when comparing the type of blood donation centers. For instance, none of the four collection units utilizes verbal donor information and nonmedical personnel. Furthermore, three collection units did not use a questionnaire for medical selection. No obvious explanation can be given. Among the four centers having a questionnaire addressing all the topics evaluated by this study, two were regional, one was national, and one was hospital based. So, the type of blood donation center does not seem to influence good practice. But since the number of blood centers in the groups was small (only four for the collection unit and for the hospital-based blood bank), the results of this survey cannot suggest a reliable benefit for a model.
Since all the transfusion centers in Africa do not have a full questionnaire that has been validated and adapted to the local epidemiology, this study relied on a questionnaire composed of questions pertaining to risk factors reported by several small studies in a few African countries and adapted from questionnaires currently used in transfusions centers in and outside of Africa. Hence, since medical questionnaires are important to ensure transfusion safety,16,33–35 it would be useful to establish an African questionnaire that is adapted to the epidemiologic and social characteristics of the region. The distribution of risk factors differs greatly based on the country and specific regions of the same country (capitals, villages, rural areas, etc.). In addition, certain questions considered embarrassing and even taboo (homosexuality) are likely to yield untruthful answers.36 While questions relating to sexuality are sensitive in all cultures, one should not avoid or tone them down. Instead, additional information and education is needed, like in other health-related disciplines. Such a questionnaire should be updated regularly as questionnaires evaluated periodically have shown to significantly reduce the prevalence of HIV and HCV.35
Physical examinations performed during the preliminary screenings were not always thorough. Since donor candidates may omit certain risk factors, voluntarily or not, it is therefore essential to examine them for evidence of multiple skin punctures related to intravenous drug use, religious scarifications, and tattoos. Body temperature was rarely recorded. Without a systematic screening for malaria, the presence of fever is the only symptom that could be detected and prevent widespread infection at a low cost. However, a recent fever is currently an exclusion criteria for donor candidates in all centers.
Familial blood donation still remains a very common practice in Africa due to a shortage in voluntary and non-remunerated donors resulting from insufficient donor recruitment and misperception of blood donation in African populations.1,19 Familial blood donation has been frequently reported to present a higher risk than a voluntary unremunerated donation. Few recent studies, yet to be widely confirmed, have not observed this difference between the two types of donations.37,38 Few African countries can afford to depend solely on unremunerated donations and exclude all familial donations. In certain transfusion centers, familial blood donations accounted for up to 98% of donations and excluding it would reduce the supply of donated blood by more than half. In addition, familial blood donations cost a lot less in the transfusion centers than voluntary unremunerated donation and seem more accepted by African populations from a social and cultural standpoint.39 While it will certainly be difficult, costly, and lengthy to inform and educate these populations on the benefits of voluntary unremunerated donations, it is the key to develop a safe blood donation system. However, such a transition must be gradual to be long-lasting.
Many participating centers selected their donors based on rapid tests before donation, mostly for HIV, HBV, and HCV. While it was beneficial to reduce the number of blood units discarded due to unusable blood, a disadvantage was the absence of dedicated personnel in hospital-based transfusion centers to support and counsel the rejected donor candidates. Counseling and follow-up of rejected donor candidates, once they have been made aware of their status, is very important for their psychological and physical well-being. It is also important to point out that all donations collected from nondeferred candidates are also subject to further enzyme-linked immunosorbent assay testing.
In summary, many risk factors investigated during the donor selection process are not well documented in the various regions of Africa; they are mostly based on empirical observations. In a continent where the supply of blood products is the lowest in the world and where the prevalence of major viral infections by transfusions is the highest in the world, it is urgent to determine the most critical risk factors for safe transfusions by thorough epidemiologic studies that would aim at preventing the epidemic instead of treating it. After using such research to create validated, socially, and culturally relevant questionnaires, it will be necessary to ensure that they are used rigorously and systematically under a system of quality control. One would also need to ensure that the questionnaire be adapted to the educational level of the donor, especially in countries where illiteracy is still widespread. It is essential to find the right balance between an adequate blood supply and reducing posttransfusion infections. In most cases, these strategies of medical screening for donor candidates will need to be accompanied by blood donation campaigns to not compromise the blood supply. Nonetheless, it will still be important to monitor the prevalence of viral markers and risk factors at the national and international level. Such ongoing surveillance and research will assure a safe and adequate blood supply in African countries.
ABBREVIATIONS
- ID
infectious disease
- TTI(s)
transfusion-transmissible infection(s)
Footnotes
CONFLICT OF INTEREST
The authors certify that they have no affiliation with or financial involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in this manuscript (e.g., employment, consultancies, board membership, stock ownership, honoraria), except as disclosed in an attachment.
REFERENCES
- 1.Tagny CT, Mbanya D, Tapko JB, Lefrere JJ. Blood safety in sub-Saharan Africa: a multifactorial problem. Transfusion. 2008;48:1256–1261. doi: 10.1111/j.1537-2995.2008.01697.x. [DOI] [PubMed] [Google Scholar]
- 2.Tagny CT, Diarra A, Yahaya R, Hakizimana M, Nguessan A, Mbensa G, Nébié Y, Dahourou H, Mbanya D, Shiboski C, Murphy E, Lefrère JJ. Characteristics of blood donors and donated blood in sub-Saharan Francophone Africa. Transfusion. 2009;49:1592–1599. doi: 10.1111/j.1537-2995.2009.02137.x. [DOI] [PubMed] [Google Scholar]
- 3.Ouattara H, Siransy-Bogui L, Fretz C, Diane KM, Konate S, Koidio A, Minga KA, Hyda J, Koffi-Abe N, Offoumou AM, Abissey S. Residual risk of HIV, HVB and HCV transmission by blood transfusion between 2002 and 2004 at the Abidjan National Blood Transfusion Center. Transfus Clin Biol. 2006;13:242–245. doi: 10.1016/j.tracli.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Jayaraman S, Chalabi Z, Perel P, Guerriero C, Roberts I. The risk of transfusion-transmitted infections in sub-Saharan Africa. Transfusion. 2010;50:433–442. doi: 10.1111/j.1537-2995.2009.002402.x. [DOI] [PubMed] [Google Scholar]
- 5.Toure-Fall AO, Dieye TN, Sall A, Diop M, Seck M, Diop S, Thiam D, Diakhate L. Residual risk of transmission of HIV and HBV, in Senegalese national blood bank from 2003 to 2005. Transfus Clin Biol. 2009;16:439–443. doi: 10.1016/j.tracli.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Tapko JB. The road to safe blood supply in the African region of the World Health Organization: trends and current status: 1999–2006. Africa Sanguine. 2007;10:1–6. [Google Scholar]
- 7.Allain JP, Owusu-Ofori S, Bates I. Blood transfusion in sub-Saharan Africa. Transfusion alternatives. Transfus Med. 2004;6:16–23. [Google Scholar]
- 8.World Health Organization. Blood safety: a strategy for the African region. Brazzaville: Regional Office for Africa; 2001. AFR/RC51/R2 (Resolution). [Google Scholar]
- 9.Lefrère JJ, Dahourou H, Dokekias AE, Kouao MD, Diarra A, Diop S, Tapko JB, Murphy EL, Laperche S, Pillonel JJ. Estimate of the residual risk of transfusion-transmitted human immunodeficiency virus (HIV) infection in sub-Saharan Africa: a multi-national collaborative study. Transfusion. 2011;51:486–492. doi: 10.1111/j.1537-2995.2010.02886.x. [DOI] [PubMed] [Google Scholar]
- 10.Owusu-Ofori S, Temple J, Sarkodie F, Anokwa M, Candotti D, Allain JP. Predonation screening of blood donors with rapid tests: implementation and efficacy of a novel approach to blood safety in resource-poor settings. Transfusion. 2005;45:133–140. doi: 10.1111/j.1537-2995.2004.04279.x. [DOI] [PubMed] [Google Scholar]
- 11.Tagny CT, Mbanya D, Leballais L, Murphy E, Lefrere JJ, Laperche S. Reduction of the risk of transfusion-transmitted HIV infection by using a HIV Ag/Ab combination assay in blood donation screening in Cameroon. Transfusion. 2011;51:184–190. doi: 10.1111/j.1537-2995.2010.02782.x. [DOI] [PubMed] [Google Scholar]
- 12.Fang CT, Field SP, Busch MP, Heyns Adu P. Human immunodeficiency virus-1 and hepatitis C virus RNA among South African blood donors: estimation of residual transfusion risk and yield of nucleic acid testing. Vox Sang. 2003;85:9–19. doi: 10.1046/j.1423-0410.2003.00311.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Hu J, Tang S, Wood O, Francis K, Machuca A, Rios M, Daniel S, Vockley C, Awazi B, Zekeng L, Hewlett I. Evaluation of FDA licensed HIV assays using plasma from Cameroonian blood donors. J Med Virol. 2006;78(Suppl 1):S22–S23. doi: 10.1002/jmv.20602. [DOI] [PubMed] [Google Scholar]
- 14.Busch MP, Lee LL, Satten GA, Henrard DR, Farzadegan H, Nelson KE, Read S, Dodd RY, Petersen LR. Time course of detection of viral and serologic markers preceding human immunodeficiency virus type 1 seroconversion: implications for screening of blood and tissue donors. Transfusion. 1995;35:91–97. doi: 10.1046/j.1537-2995.1995.35295125745.x. [DOI] [PubMed] [Google Scholar]
- 15.Seghatchia J, De Sousa G. Pathogen-reduction systems for blood components: the current position and future trends. Transfus Apher Sci. 2006;35:189–196. doi: 10.1016/j.transci.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Courtois F, Voultoury P, Ducot B, Boulard G, Poutier P, Tir R, Worms B, Bajos N, Spira A, Wild AM. Risk behavior among blood donors: efficacy of a new questionnaire. Transfus Clin Biol. 1999;6:227–235. doi: 10.1016/s1246-7820(99)80033-0. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Module 1, safe blood donation. Geneva: WHO; 2002. Safe blood and blood products. [Google Scholar]
- 18.Danic B. Clinical selection of blood donors. Transfus Clin Biol. 2003;10:227–233. doi: 10.1016/s1246-7820(03)00048-x. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Global Database on Blood Safety (GDBS) Geneva: WHO; 2004. [Google Scholar]
- 20.Erler A, Goldman M, Rossmann S, Waxman D, Bianco C. Selection criteria to protect the blood donor in North America and Europe: past (dogma), present (evidence), and future (hémovigilance) Transfus Med Rev. 2009;23:205–220. doi: 10.1016/j.tmrv.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Molé S. VIH chez les nouveaux donneurs de sang en milieu camerounais : profil comparé et facteurs de risque associés. Sidanet. 2008;5:1062–1064. [Google Scholar]
- 22.Minga A, Dohoun L, Abo Y, Coulibaly A, Konaté S, Ouattara HP, N’guessan BK, Dabis F, Salamon R, Lewden C ANRS 1220 PRIMO-CI Study Group. Risk behaviors in volunter blood donors who seroconverted for HIV, Abidjan, Côte d’Ivoire 1997 to 2005. Transfusion. 2010;50:888–893. doi: 10.1111/j.1537-2995.2009.02499.x. [DOI] [PubMed] [Google Scholar]
- 23.Tayou Tagny C, Kapamba G, Diarra A, Ngandu C, Deneys V, Sondag-Thull D. The training in transfusion medicine remains deficient in the centres of Francophone sub-Saharan Africa: results of a preliminary study. Transfus Clin Biol. 2011 Jun 14; doi: 10.1016/j.tracli.2011.02.026. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24.Agbovi KK, Kolou M, Fétéké L, Haudrechy D, North ML, Ségbéna AY. Knowledge, attitudes and practices about blood donation. A sociological study among the population of Lomé in Togo. Transfus Clin Biol. 2006;13:260–265. doi: 10.1016/j.tracli.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Cunha L, Plouzeau C, Ingrand P, Gudo JP, Ingrand I, Mondlane J, Beauchant M, Agius G. Use of replacement blood donors to study the epidemiology of major blood-borne viruses in the general population of Maputo, Mozambique. J Med Virol. 2007;79:1832–1840. doi: 10.1002/jmv.21010. [DOI] [PubMed] [Google Scholar]
- 26.Olaiya MA, Alakija W, Ajala A, Olatunji RO. Knowledge, attitudes, beliefs and motivations towards blood donations among blood donors in Lagos, Nigeria. Transfus Med. 2004;14:13–17. doi: 10.1111/j.0958-7578.2004.00474.x. [DOI] [PubMed] [Google Scholar]
- 27.Wiwanitkit V. A study on attitude towards blood donation among people in a rural district, Thailand. Southeast Asian J Trop Med Public Health. 2000;31:609–611. [PubMed] [Google Scholar]
- 28.Lindstrom TC, Rosvik A. Ambiguity leads to uncertainty: ambiguous demands to blood donors. Scand J Caring Sci. 2003;17:74–77. doi: 10.1046/j.1471-6712.2003.00121.x. [DOI] [PubMed] [Google Scholar]
- 29.Rugege-Hakiza SE, Glynn SA, Hutching ST, Bethel J, Nass CC, McEntire RL, Hirschler NV, Campbell JG, Ladavac A, Schreiber GB. Retrovirus Epidemiology Donor Study. Do blood donors read and understand screening educational materials? Transfusion. 2003;43:1075–1083. doi: 10.1046/j.1537-2995.2003.00473.x. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien SF, Fan W, Ram SS, Goldman M, Nair RC, Chiavetta JA, Vamvakas EC. Face-to-face interviewing in predonation screening: lack of effect on detected human immunodeficiency virus and hepatitis C virus infections. Transfusion. 2006;46:1380–1387. doi: 10.1111/j.1537-2995.2006.00906.x. [DOI] [PubMed] [Google Scholar]
- 31.Oulf Amar AK. Red blood cells from donors with sickle cell trait: a safety issue for transfusion? Transfus Med. 2006;16:248–253. doi: 10.1111/j.1365-3148.2006.00661.x. [DOI] [PubMed] [Google Scholar]
- 32.Lawson-Ayayi S, Salmi R. Risque infectieux et efficacité des techniques de sélection clinique des volontaires au don du sang. Transfus Clin Biol. 1997;4:513–521. doi: 10.1016/s1246-7820(97)80076-6. [DOI] [PubMed] [Google Scholar]
- 33.Polizzotto MN, Wood EM, Ingham H, Keller AJ. Australian Red Cross Blood Service Donor and Product Safety Team. Reducing the risk of transfusion-transmissible viral infection through blood donor selection: the Australian experience 2000 through 2006. Transfusion. 2008;48:55–63. doi: 10.1111/j.1537-2995.2007.01482.x. [DOI] [PubMed] [Google Scholar]
- 34.Heyns Adu P, Benjamin RJ, Swanevelder JP, Laycock ME, Pappalardo BL, Crookes RL, Wright DJ, Busch MP. Prevalence of HIV-1 in blood donations following implementation of a structured blood safety policy in South Africa. JAMA. 2006;295:519–526. doi: 10.1001/jama.295.5.519. [DOI] [PubMed] [Google Scholar]
- 35.Zou S, Fuji K, Johnson S, Spencer B, Washington N, Iv EN, Musavi F, Newman B, Cable R, Rios J, Hillyer KL, Hillyer CD, Dodd RY ARCNET Study Group. Prevalence of selected viral infections among blood donors deferred for potential risk to blood safety. Transfusion. 2006;46:1997–2003. doi: 10.1111/j.1537-2995.2006.01008.x. [DOI] [PubMed] [Google Scholar]
- 36.Nebié KY, Olinger CM, Kafando E, Dahourou H, Diallo S, Kientega Y, Domo Y, Kienou K, Ouattara S, Sawadogo I, Ky L, Muller CP. Lack of knowledge among blood donors in Burkina Faso (West Africa). Potential obstacle to transfusion security. Transfus Clin Biol. 2007;14:446–452. doi: 10.1016/j.tracli.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Allain JP, Sarkodie F, Asenso-Mensah K, Owusu-Ofori S. Relative safety of first-time volunteer and replacement donors inWest Africa. Transfusion. 2010;50:340–343. doi: 10.1111/j.1537-2995.2009.02444.x. [DOI] [PubMed] [Google Scholar]
- 38.Mbanya DN, Feunou F, Tayou Tagny C. Volunteer or family/replacement donations: are the tides changing? Transfusion. 2010;50:1849–1850. doi: 10.1111/j.1537-2995.2010.02656.x. [DOI] [PubMed] [Google Scholar]
- 39.Bates I, Manyasi G, Medina Lara A. Reducing replacement donors in sub-Saharan Africa: challenges and affordability. Transfus Med. 2007;17:434–442. doi: 10.1111/j.1365-3148.2007.00798.x. [DOI] [PubMed] [Google Scholar]