Summary
Autophagy mediates the degradation of cytoplasmic contents in the lysosome and plays a significant role in innate and adaptive immune responses. Lipid second messengers are implicated in the regulation of autophagy but the nature of the lipids involved and their mechanisms of action have yet to be characterized. Here we demonstrate a novel signaling role for diacylglycerol (DAG) in antibacterial autophagy. DAG production was necessary for efficient autophagy of Salmonella and its localization to bacteria-containing phagosomes preceded autophagy. Previous studies have revealed a role for the ubiquitin binding adaptor molecules p62 and NDP52 in autophagy of S. Typhimurium. We observed bacteria-containing autophagosomes colocalizing individually with either DAG or ubiquitinated proteins, indicating that both signals can act independently to promote anti-bacterial autophagy. We determined that the actions of phospholipase D (PLD) and phosphatidic acid phosphatase (PAP) were required for DAG generation and autophagy. The DAG-responsive δ isoform of protein kinase C was required for anti-bacterial autophagy, as were its downstream targets JNK and NADPH oxidase. Pkc1, the single PKC isoform in yeast, was essential for starvation-induced autophagy in Saccharomyces cerevisiae. These findings reveal an important role for DAG-mediated PKC function in mammalian anti-bacterial autophagy, and suggest a conserved role for PKC in autophagy regulation in eukaryotes.
Introduction
Autophagy was identified as a cell survival mechanism in response to starvation but also participates in protein turnover, cell differentiation and defense against invading pathogens (Huang and Klionsky, 2007; Levine and Kroemer, 2008). While originally characterized as a non-specific process, it is now clear that autophagy can also be selective for the cargo it delivers to the lysosome. However, the signaling mechanism(s) by which autophagy targets are selected for degradation are unclear. Lipid second messengers are required for autophagy and are important candidates (Juhasz and Neufeld, 2006; Obara et al., 2008; Yamashita et al., 2006). Phosphatidylinositol 3-phosphate (PI3P) is required for yeast and mammalian autophagy (Axe et al., 2008; Obara et al., 2008). In yeast, PI3P may function by recruiting PI3P-binding autophagy-related (Atg) proteins such as Atg18 to the forming autophagosome (Xie and Klionsky, 2007). In mammalian cells, PI3P-enriched structures are observed in association with the endoplasmic reticulum and may recruit autophagy-related proteins to initiate autophagy in response to starvation (Axe et al., 2008). Generation of another signaling lipid, sphingosine 1-phosphate, which is formed by sphingosine kinase 1, also stimulates autophagy (Lavieu et al., 2006); however, the role of other lipid second messengers in autophagy regulation in mammalian cells has not been explored.
Autophagy is now recognized as a key component of innate immunity to bacterial infection (Deretic and Levine, 2009) and defects in anti-bacterial autophagy are linked to Crohn’s disease, a type of inflammatory bowel disease (Cadwell et al., 2008; Kuballa et al., 2008; McCarroll et al., 2008; Parkes et al., 2007; Rioux et al., 2007). A variant in the ATG16L1 gene associated with Crohn’s disease impairs autophagy of Salmonella enterica serovar Typhimurium (S. Typhimurium) (Kuballa et al., 2008), a Gram-negative intracellular pathogen with a broad host range (Haraga et al., 2008). During infection of the host, these bacteria typically replicate within a modified endosomal compartment in host cells, the Salmonella-containing vacuole (SCV) (Brumell and Grinstein, 2004). We have previously demonstrated that a population of intracellular S. Typhimurium is targeted by autophagy following invasion of host cells, and that autophagy restricts intracellular bacterial replication (Birmingham et al., 2006). We and others find that S. Typhimurium can be targeted by autophagy in a ubiquitin-dependent manner that requires the autophagy adaptors p62 (also called SQSTM1) and NDP52 (Thurston et al., 2009; Zheng et al., 2009). These adaptors bind to ubiquitin and microtubule-associated protein 1 light chain 3 (LC3) and serve to target ubiquitin-associated S. Typhimurium to the autophagy pathway (Thurston et al., 2009; Zheng et al., 2009). Knockdown of p62 and NDP52 expression leads to a partial, though not complete, decrease in autophagy of S. Typhimurium (Thurston et al., 2009; Zheng et al., 2009). In this regard it is noteworthy that only 50% of S. Typhimurium targeted by autophagy (LC3+) are associated with ubiquitinated proteins (Birmingham et al., 2006), indicating the potential for a ubiquitin-independent pathway for autophagic targeting of bacteria prior to escape into the cytosol. We hypothesized that a lipid second messenger generated on SCV membranes mediates anti-bacterial autophagy. Here we demonstrate that diacylglycerol (DAG) serves as a specific signal to initiate autophagy of S. Typhimurium during infection.
Results
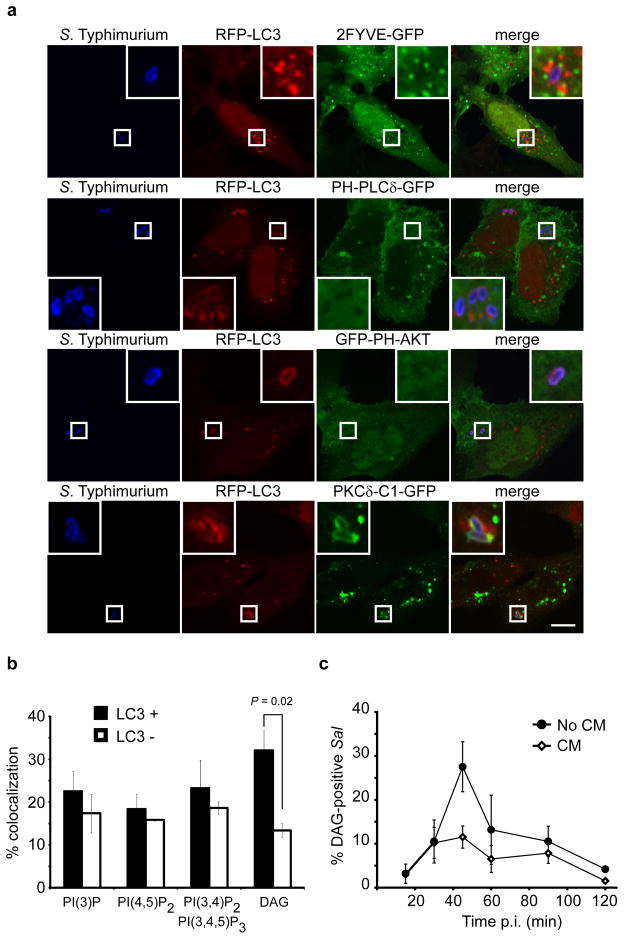
We performed a screen for lipid second messengers associated with bacteria targeted by autophagy. Lipid second messengers were visualized by transfecting HeLa cells with fluorescent lipid-binding probes prior to infection (Table S1). The colocalization of each lipid with intracellular bacteria was assessed at 1 h post-infection (p.i.), the time of maximal autophagy of S. Typhimurium (Birmingham et al., 2006). To visualize SCVs targeted by autophagy we cotransfected cells with RFP fusions to LC3, a marker of autophagosomes (Klionsky et al., 2008). As an internal control, lipid colocalization with bacteria not targeted by autophagy (LC3−) was also quantified.
Using 2FYVE-GFP we monitored PI3P localization in S. Typhimurium-infected cells. We observed PI3P-containing puncta associated with LC3+ bacteria (Figure 1a). However, these puncta were also observed in association with LC3− bacteria, and there was no significant difference in the colocalization of PI3P with the two populations (Figure 1b). Production of PI3P by the class III PI3-kinase Vps34 is essential for autophagy in mammals and yeast, and PI3-kinase inhibitors block autophagy of S. Typhimurium (Kihara et al., 2001; Klionsky et al., 2008; Obara et al., 2006). Therefore, our findings suggest that PI3P production, although essential for autophagy, does not provide a localized signal that selects SCVs as cargo for autophagy. PI(4,5)P2 (PLCδ-PH-GFP) and PI(3,4)P2/PI(3,4,5)P3 (GFP-PH-AKT) were similarly found to be equally associated with both LC3+ and LC3− bacteria (Figure 1a, b).
Figure 1. DAG colocalizes with bacteria-containing autophagosomes. (a).
HeLa cells were co-transfected with RFP-LC3 and either 2FYVE-GFP (PI(3)P probe), PLCδ-PH-GFP (PI(4,5)P2 probe), GFP-PH-AKT (PI(3,5)P2 and PI(3,4,5)P3 probe) or PKCδ-C1-GFP (DAG probe). Cells were infected with wild-type S. Typhimurium, fixed at 1 h p.i. and immunostained for S. Typhimurium. Representative confocal z-slices are shown. The inner panels represent a higher magnification of the boxed areas. Size bar, 10 μm. (b) The percentage of RFP-LC3+ or RFP-LC3− bacteria colocalizing with the lipid probes in a was determined by fluorescence microscopy. (c) HeLa cells were transfected with PKCδ-C1-GFP and infected as in a. Cells were treated with or without chloramphenicol (CM, 200 μg/mL) at 10 min p.i. for the remainder of the infection. Cells were fixed at the indicated time-points and immunostained for S. Typhimurium. The percentage of PKCδ-C1-GFP+ bacteria was enumerated by fluorescence microscopy. Data represent the mean ± standard error (s.e.m.) for three independent experiments.
An important lipid second messenger is diacylglycerol (DAG) which induces translocation of responsive proteins to DAG-rich membranes (Carrasco and Merida, 2007). Using the C1 domain from PKCδ as a probe we visualized DAG. The probe localized to perinuclear transferrin and Rab5-positive endosomes in control (not infected) cells (Figure S1a). In S. Typhimurium-infected cells, DAG was preferentially found on LC3+ SCVs, suggesting that this lipid may play a role in autophagy (Figure 1a, b). The association of DAG with autophagy-targeted bacteria was further confirmed using antibodies to endogenous LC3 (Figure S1b). In fact, quantification revealed that endogenous LC3 preferentially colocalizes with DAG+ bacteria (Figure S1c). Another component of the autophagy machinery is Atg16L1 which acts as a targeting factor for localized induction of autophagy (Mizushima et al., 2003). Using a transiently transfected construct we also observed preferential colocalization of Atg16L1 with DAG+ bacteria (Figure S1d, e). These findings suggest that LC3 may be conjugated to DAG+ SCVs.
We also monitored the kinetics of DAG colocalization with the total population of intracellular bacteria (without scoring for LC3 colocalization). DAG association with SCVs peaked at 45 min p.i. (Figure 1c), prior to maximal autophagy of the bacteria which occurs at 1 h p.i. (Birmingham et al., 2006). Live cell imaging confirmed that recruitment of the DAG probe to individual SCVs precedes LC3 (Figure S2 and Movie S1).
Previous studies have shown a requirement for bacterial protein synthesis after invasion and the bacterial SPI1-encoded Type III secretion system (T3SS) for autophagy of S. Typhimurium (Birmingham et al., 2006). We therefore looked at DAG colocalization in the presence of the bacterial protein synthesis inhibitor chloramphenicol (CM) and observed an impairment in DAG recruitment (Figure 1c). S. Typhimurium normally invades cells by translocating effector proteins into host cells via the SPI1-T3SS. We examined the invA/inv mutant of S. Typhimurium which lack a functional SPI1 T3SS and are instead internalized through expression of the Yersinia Invasin protein (Steele-Mortimer et al., 2002). We have previously shown that the invA/inv mutant is not targeted by autophagy (Birmingham et al., 2006). Here we observed that DAG did not colocalize with the invA/inv mutant (Figure S3a, b). These findings are consistent with a link between DAG production on SCVs and their targeting by autophagy.
To determine whether DAG is a signal for autophagy we next tested whether maintenance of the DAG signal would result in prolonged autophagy of S. Typhimurium at later time points p.i. Members of the DAG kinases (DGKs) act to attenuate DAG signaling levels by phosphorylating DAG to produce phosphatidic acid (PA) (Carrasco and Merida, 2007; Merida et al., 2008). Treatment with DGK inhibitor I (R59022) resulted in significantly more autophagy at 90 min p.i. when compared with vehicle-treated cells (Figure S4a, b). Therefore, decreased turnover of DAG maintains autophagic targeting of SCVs.
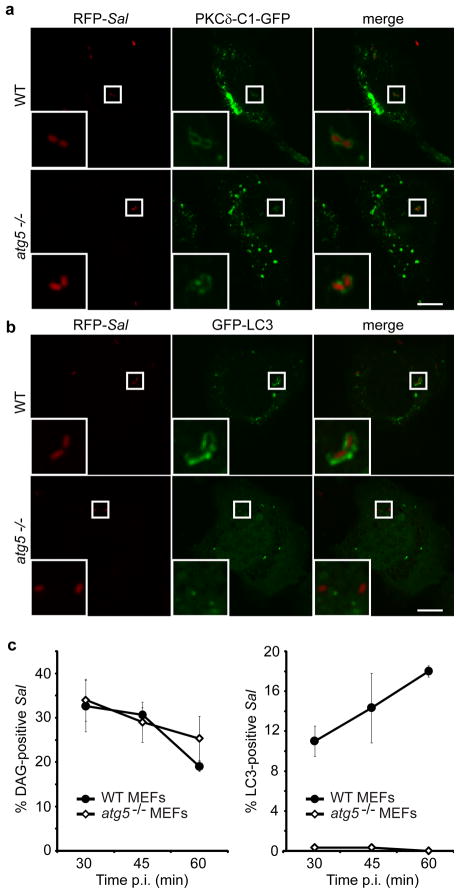
Since our data suggested a link between DAG and autophagy we wanted to determine whether DAG localization to bacteria was dependent on the cell’s autophagic machinery. It was reasonable to postulate that DAG accumulation on the SCV was the result of delivery of autophagic membrane. Indeed, the source(s) of membranes for autophagy and their lipid composition are not known (Mizushima, 2007). To address this question we examined wild-type and atg5−/− (autophagy-deficient) mouse embryonic fibroblasts (MEFs). These cells were infected with S. Typhimurium, and bacterial colocalization with the DAG probe or LC3 was characterized in separate experiments. We determined that DAG colocalization to the SCV was independent of autophagy and still occurred in the absence of a functional autophagy system (in Atg5−/− MEFs) (Figure 2a, b, c). We conclude that the DAG signal precedes autophagy and that its presence on SCVs is not due to delivery of autophagic membrane.
Figure 2. DAG production on SCVs is independent of autophagy. (a-b).
Wild-type (WT) and autophagy-deficient (atg5 −/−) MEFs were transfected with either PKCδ-C1-GFP (a) or GFP-LC3 (b). Cells were infected with S. Typhimurium expressing mRFP (RFP-Sal). Cells were fixed at 45 min a or 1 h b p.i. Representative confocal z-slices are shown. The inner panels represent a higher magnification of the boxed areas. Size bar, 10 μm. (c) WT and atg5−/− MEFs were transfected and infected as in a-b and fixed at the indicated time-points. PKCδ-C1-GFP+ or GFP-LC3+ RFP-Sal were determined by fluorescence microscopy. Data represent the mean ± standard error (s.e.m.) for at least three independent experiments.
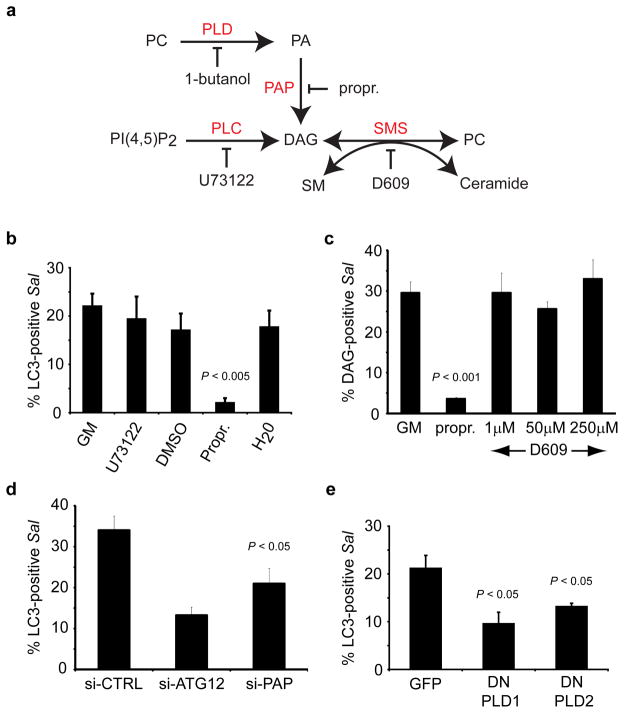
Next, we examined the pathway of DAG generation on SCVs. In addition to de novo DAG biosynthesis, DAG generation can also occur via phospholipase C (PLC), phosphatidic acid phosphatase (PAP) and sphingomyelin synthase (SMS) (Figure 3a) (Carrasco and Merida, 2007; Huitema et al., 2004). Treatment with the PLC inhibitor U73122 did not alter DAG or LC3 colocalization with S. Typhimurium, suggesting that PLC activity is not involved in autophagy (Figure S5a and Figure 3b). Additionally, treatment with the SMS inhibitor D609 did not have any effect on DAG colocalization with the bacteria (Figure 3c). In contrast, inhibition of PAP with propranolol hydrochloride resulted in significant decreases in both DAG colocalization with bacteria at 45 min p.i. and LC3 colocalization at 1 h p.i. (Figure 3b, c). siRNA to PAP also led to a significant decrease in LC3 colocalization with S. Typhimurium (Figure 3d and Figure S5b). These data demonstrate that DAG generation on SCVs occurs through the PAP pathway and that this pathway is required for anti-bacterial autophagy.
Figure 3. Autophagy of S. Typhimurium requires phosphatidic acid phosphatase and phospholipase D. (a).
Pathways for the production of DAG. PC (phosphatidylcholine), PLD (phopholipase D), PA (phosphatidic acid), PAP (phosphatidic acid phosphatase), propr. (propranolol hydrochloride), PLC (phospholipase C), SMS (sphingomyelin synthase), SM (sphingomyelin). (b) HeLa cells were transfected with GFP-LC3 and infected with RFP-Sal. Cells were treated with growth medium (GM), U73122(10 μM), DMSO, propranolol hydrochloride (propr., 250 μM) or H2O at 10 min p.i. for the remainder of the infection, and fixed at 1 h p.i. (c) HeLa cells were transfected with PKCδ-C1-GFP and infected with RFP-Sal. Cells were treated with GM, propranolol hydrochloride (propr., 250 μM) or D609 at the indicated concentrations at 10 min p.i. for the remainder of the infection, and fixed at 45 min p.i.. (d) HeLa cells were co-transfected with GFP-LC3 and either control siRNA (si-CTRL) or siRNA specifically targeting Atg12 (si-ATG12) or PAP2B (si-PAP). Cells were infected with RFP-Sal and fixed at 1 h p.i. (e) HeLa cells were transfected with RFP-LC3 and either GFP, HA-PLD1 DN (dominant negative) or HA-PLD2 DN. Cells were infected with S. Typhimurium, fixed at 1 h p.i. and immunostained for S. Typhimurium and HA tag. The percentage of DAG+ or LC3+ S. Typhimurium was determined by fluorescence microscopy. Data represent the mean ± standard error (s.e.m.) for at least three independent experiments.
PAP activity requires a pool of PA substrate, which can be generated through the action of PLD (Figure 3a) (Carrasco and Merida, 2007). Inhibition of PLD activity with 1-butanol led to a consistent decrease in both DAG and LC3 colocalization with S. Typhimurium (Figure S5 c, d). This effect was not observed with tert-butanol, an isomer of 1-butanol that does not inhibit PLD activity. Expression of catalytically inactive mutants of hPLD1 (K898R) and mPLD2 (K758R), which have been used previously as dominant negative constructs (DN) (Denmat-Ouisse et al., 2001), led to a significant decrease in autophagy of S. Typhimurium (Figure S5e and Figure 3e). Therefore, the generation of DAG on SCVs and subsequent autophagy of S. Typhimurium requires the action of both PAP and PLD.
Classical and novel PKC isoforms contain DAG-binding C1 domains, and DAG binding has an impact upon their function (Griner and Kazanietz, 2007; Kazanietz, 2000). To test a role for PKCs in autophagy we utilized pharmacological PKC inhibitors. Treatment with rottlerin, but not Go6983 led to a significant decrease in autophagy of S. Typhimurium (Figure S6a, b). Neither inhibitor affected DAG levels on the SCV (Figure. S6c, d). Rottlerin has affinity for PKCδ (Gschwendt et al., 1994). Therefore to further test a role for this PKC in anti-bacterial autophagy we used siRNA to target its expression (Figure S6e). Knockdown of PKCδ led to a significant decrease in autophagy of S. Typhimurium that was not observed upon siRNA targeting of PKCα expression (Figure 4a, b). The role for PKCδ in autophagy of S. Typhimurium was confirmed using PKCδ−/− MEFs (Figure 4c). Complementation of the PKCδ−/− MEFs with PKCδ expression from a plasmid restored autophagy to WT levels. To test whether PKCδ can restrict intracellular bacterial growth we also looked at bacterial replication in WT and PKCδ−/− MEFs and observed significantly more bacteria at 6 and 8 h p.i. in cells lacking PKCδ (Figure 4d) indicating that PKCδ plays a significant role in anti-bacterial autophagy.
Figure 4. Autophagy of S. Typhimurium requires protein kinase C. (a-b).
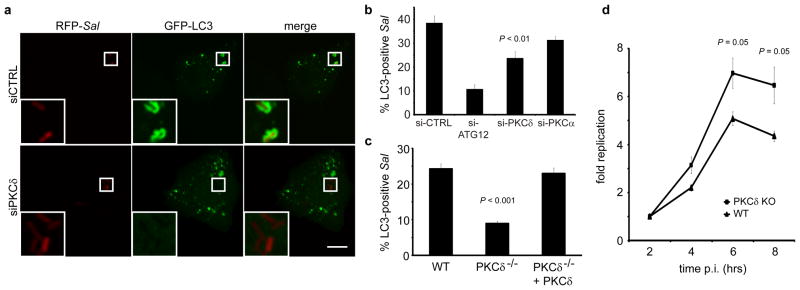
HeLa cells were co-transfected with GFP-LC3 and either control siRNA (si-CTRL) or siRNA specifically targeting Atg12 (si-ATG12), PKCδ (si-PKCδ) or PKCα (si-PKCα). Cells were infected with wild-type RFP-Sal and fixed at 1 h p.i. Representative confocal z-slices are shown a. The inner panels represent a higher magnification of the boxed areas. Size bar represents 10 μm. The percentage of GFP-LC3+ bacteria was determined by fluorescence microscopy b. (c) WT and PKCδ-deficient (PKCδ−/−) MEFs were transfected with GFP-LC3 and/or a PKCδ expression plasmid. Cells were infected with RFP-Sal and fixed at 1 h p.i. The percentage of GFP-LC3+ bacteria was determined by fluorescence microscopy. (d) WT and PKCδ-deficient (PKCδ −/−) MEFs were infected with wild-type S. Typhimurim and lysed at the indicated time points. The number of bacteria was determined by the number of colonies formed on agar plates. Data represent the mean ± standard error (s.e.m.) for at least three independent experiments.
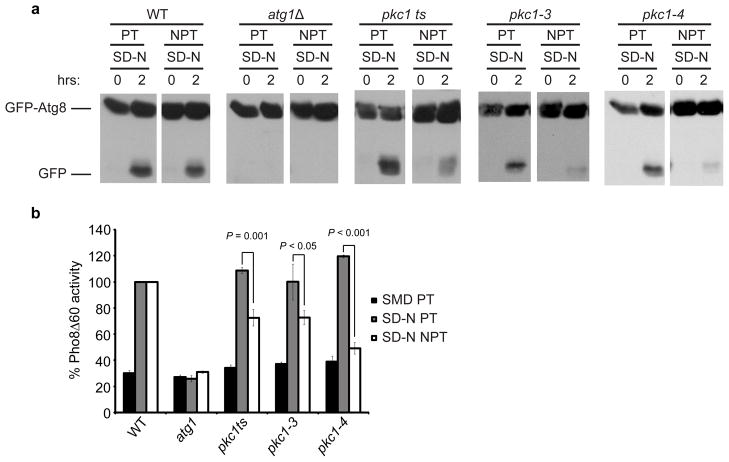
Saccharomyces cerevisiae (S. cerevisiae) contains a single PKC isozyme, Pkc1. Using temperature sensitive (ts) mutants of this kinase (Table S2) we examined its role in starvation-induced autophagy. In yeast, autophagosomes are delivered to the vacuole whereupon their contents are degraded. We used yeast expressing GFP-Atg8, which upon delivery to the vacuole via autophagosomes is degraded to produce free GFP. We found that pkc1 mutants (pkc1ts, pkc1-3 and pkc1-4) were defective in GFP-Atg8 processing in starvation-induced autophagy at the non-permissive temperature (NPT, 38°C) compared to the permissive temperature (PT, 24°C) (Figure 5a). As expected, cells lacking Atg1 (essential for autophagy) did not display GFP-Atg8 processing. We also measured alkaline phosphatase (Pho8Δ60) activity during starvation-induced autophagy. Pho8Δ60 is a truncation of the vacuolar alkaline phosphatase, Pho8, and is expressed in an inactive form that matures only upon delivery to the vacuole by autophagy (Noda et al., 1995). Pho8Δ60 activity was compromised in pkc1 mutants, indicating a required role for this kinase in starvation induced-autophagy in yeast (Figure 5b). Therefore, PKC plays a conserved role in autophagy regulation.
Figure 5. Pkc1 is required for starvation-induced autophagy in yeast. (a).
GFP-Atg8 processing was monitored in wild-type (TN124), atg1Δ (TYY127), pkc1ts, pkc1-3 and pkc1-4 cells expressing GFP-Atg8. Cells were grown in SD-N medium at the permissive (PT, 24°C) or non-permissive (NPT, 38 C) temperature. Full-length GFP-Atg8 and free GFP were detected by Western blotting using anti-YFP antibodies. (b) Autophagic activity was determined by obtaining extracts from wild-type (TN124), atg1Δ (TYY127), pkc1ts, pkc1-3 and pkc1-4 S. cerevisiae expressing Pho8Δ60 and analyzed for Pho8Δ60-dependent alkaline phosphatase activity. Synthetic defined media lacking nitrogen (SD-N). Data represent the mean ± standard error (s.e.m.) for at least three independent experiments.
Next, we addressed the mechanism by which PKCδ regulates autophagy in mammalian cells. Previously, it was shown that PKCδ regulates the interaction between Bcl-2 and Beclin 1 via JNK activation (Chen et al., 2008). Therefore, we treated cells with the JNK inhibitor SP600125 and observed impairment of anti-bacterial autophagy (Figure S7a). This role was further confirmed using DN constructs (Figure S7b). PKCδ also activates NADPH oxidases (Fontayne et al., 2002; Pendyala et al., 2008) which we have previously shown to be required for anti-bacterial autophagy (Huang et al., 2009). siRNA knockdown of p22phox, an essential component of NOX1-4 NADPH oxidases (Nauseef, 2008) impaired autophagy of Salmonella but had no effect on DAG localization to the bacteria (Figure S7c, d, e). Therefore, DAG localization to the SCV is not sufficient to promote autophagy of Salmonella in the absence of NADPH oxidase activity. These studies suggest that PKCδ promotes autophagy of bacteria through JNK and NADPH oxidase activation.
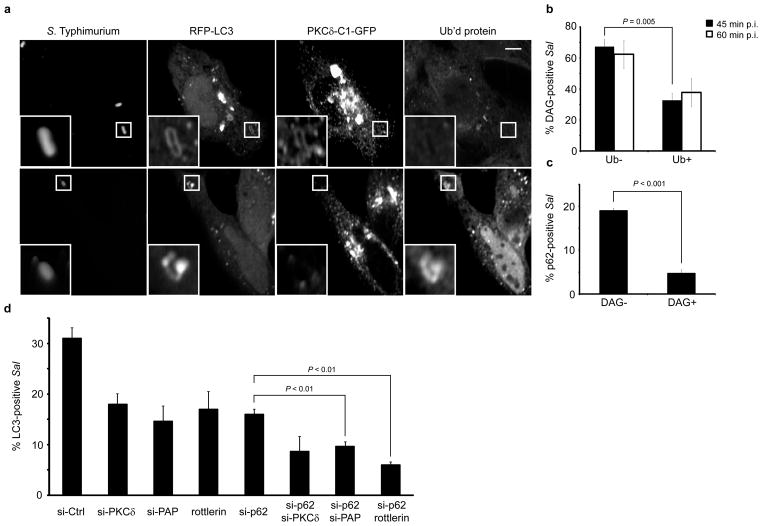
Having characterized DAG as a novel signal required for autophagy of S. Typhimurium, the possibility exists that it may act in conjunction with ubiquitin-dependent autophagy adaptors (p62/NDP52) in order to target bacteria to this pathway. To test this possibility we cotransfected HeLa cells with the DAG probe and RFP-LC3 and immunostained for endogenous ubiquitin (mono- and polyubiquitinated proteins). We examined LC3+ S. Typhimurium and were able to observe bacteria colocalizing individually with either DAG or ubiquitin (Figure 6a). We quantified colocalization of ubiquitin with DAG+ bacteria at 45 and 60 min p.i. and observed that the majority of DAG+ bacteria were negative for ubiquitin (~65% at both time points) (Figure 6b). We also quantified the colocalization of p62 with DAG+ and DAG− S. Typhimurium populations and observed that p62 colocalized significantly more with DAG− bacteria (Figure 6c). Additionally, we targeted components of each pathway (DAG and p62) separately and in combination in order to determine the contribution of each pathway to antibacterial autophagy. Using siRNA and pharmacological agents we observed additive inhibitory effects in the presence of siRNA to p62/PAP and p62/with rottlerin treatment (Figure 6d and Figure S7f). We also observed additive inhibitory effects in the presence of siRNA to p62/PKCδ although this effect was not statistically significant. These data are consistent with a model whereby both signals (DAG and ubiquitin) can act independently as inducers of anti-bacterial autophagy.
Figure 6. DAG and p62 act in independent signaling pathways for anti-bacterial autophagy. (a).
HeLa cells were co-transfected with PKCδ-C1-GFP and RFP-LC3. Cells were infected with wild-type S. Typhimurium, fixed at 45 min p.i. and immunostained for S. Typhimurium and mono- and polyubiquitinated proteins. Representative confocal z-slices are shown. The inner panels represent a higher magnification of the boxed areas. Size bar, 10 μm. (b) HeLa cells were transfected with PKCδ-C1-GFP, infected with wild-type S. Typhimurium and fixed at 45 and 60 min p.i.. Cells were immunostained for ubiquitinated protein and bacteria as in a. DAG+ bacteria were scored for colocalization with ubiquitin. (c) HeLa cells were transfected with PKCδ-C1-GFP, infected with wild-type S. Typhimurium, fixed at 45 min p.i. and immunostained for S. Typhimurium and p62. p62 colocalization was quantified for DAG+ and DAG− bacteria (d) HeLa cells were co-transfected with GFP-LC3 and either control siRNA (si-CTRL) or siRNA specifically targeting PKCδ (si-PKCδ) PAP (si-PAP) or p62 (si-p62). Cells were infected with wild-type S. Typhimurium and treated with 15 μM rottlerin where indicated. Cells were fixed at 1 h p.i. and quantified for colocalization with GFP-LC3. Data represent the mean ± standard error (s.e.m.) for three independent experiments.
Discussion
Selective autophagy is categorized based upon the cargo, which can include bacteria (xenophagy), mitochondria (mitophagy), peroxisomes (pexophagy) and other cytoplasmic contents. The mechanisms by which different cargo are specifically targeted for degradation by autophagy have remained unclear. Recent work in anti-bacterial autophagy of S. Typhimurium has characterized a role for protein ubiquitination in the targeted degradation of bacteria (Thurston et al., 2009; Zheng et al., 2009). This process is mediated by at least two different autophagy adaptors: p62 and NDP52, that bridge the ubiquitin and autophagy pathways and thereby restrict bacterial growth (Thurston et al., 2009; Zheng et al., 2009). A ubiquitin-dependent pathway involving p62 also contributes to autophagy of Shigella flexneri (Dupont et al., 2009) and Listeria monocytogenes (Yoshikawa et al., 2009). Despite the recognized importance of ubiquitin-dependent autophagy of bacteria, we have previously shown that only ~ 50 % of S. Typhimurium targeted by autophagy (LC3+) colocalize with ubiquitinated proteins, suggesting the presence of an alternative ubiquitin-independent pathway (Birmingham et al., 2006). Consistent with this notion, we recently observed that depletion of p62 only partially inhibits autophagy of S. Typhimurium (Zheng et al., 2009).
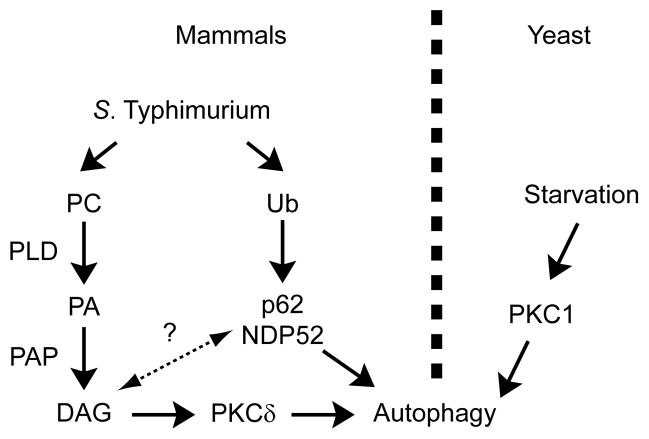
Here we provide several lines of evidence indicating that DAG can also serve as a signal to promote anti-bacterial autophagy: i) kinetic analysis and live cell imaging demonstrated that DAG is localized to bacteria prior to LC3 recruitment, ii) DAG colocalization with bacteria was independent of Atg5, indicating that DAG was not recruited to bacteria with autophagic membranes, iii) inhibition of DAG formation with genetic or pharmacological approaches impaired autophagy of bacteria, and iv) DAG was present on SCVs that did not colocalize with ubiquitin, and inhibition of both DAG and p62 pathways resulted in an additive inhibitory effect on anti-bacterial autophagy. Therefore, we propose that DAG-dependent and ubiquitin-dependent pathways contribute independently to autophagy of S. Typhimurium (Figure 7). We do not rule out the possibility that these pathways can interact and have overlapping impacts on autophagy of bacteria. It is also expected that the relative activity of these pathways will depend on the cell type examined and the virulence factors employed by the pathogen. Importantly, our data suggest that multiple pathways contribute to activation of anti-bacterial autophagy, providing a level of redundancy for innate immune defense.
Figure 7. Two signals target S. Typhimurium to the autophagy pathway.
Model depicting the dual pathways by which S. Typhimurium is targeted by autophagy in mammals and the conserved role for PKC in the regulation of this process in both mammals and yeast. Data is consistent with two independent pathways for induction of antibacterial autophagy in mammalian cells (DAG and ubiquitin pathways). Dashed line and question mark between pathways represents the possibility that the two pathways may interact with each other.
Much remains to be learned about these two signaling pathways. For example, with ubiquitin-dependent autophagy it is not clear which proteins, bacterial or eukaryotic, are ubiquitinated to recruit the cytosolic autophagy adaptors. Similarly, the E3 ligases that mediate these ubiquitination events to initiate autophagy have not been identified. It is noteworthy that both S. Typhimurium and S. flexneri encode ubiquitin E3 ligases that are translocated into the host cell by type III secretion systems during the early stages of infection (Quezada et al., 2009; Rohde et al., 2007; Rytkonen and Holden, 2007). Thus, it is tempting to speculate that bacterial E3 ligases promote anti-bacterial autophagy. Indeed, LaFont and colleagues have suggested the possibility that these bacteria initiate ubiquitin-dependent autophagy as a mechanism to suppress pro-inflammatory/cytotoxic signaling events localized to disrupted phagosomes (Dupont et al., 2009).
Whether DAG-dependent autophagy is a bacterial or host initiated process is unclear. Localization of DAG to S. Tyhimurium required the SPI1 T3SS. Consistent with this observation, we previously found that the SPI1 T3SS is also required for autophagy of S. Typhimurium (Birmingham et al., 2006). Membrane damage may provide a signal for DAG production and autophagy. The pore-forming translocon component of type III secretion systems causes membrane damage in eukaryotic cells, including disruption of SCVs during S. Typhimurium infection (Birmingham et al., 2006; Perrin et al., 2004; Roy et al., 2004; Veenendaal et al., 2007). Membrane damage has also been associated with autophagic targeting of latex bead phagosomes following their entry into non-phagocytic cells via coating with lipid-based transfection reagents (Kobayashi et al., 2010). Osmotic swelling is another method to induce endosomal rupture that may be analogous to bacterial type III secretion or other toxin damage. Interestingly, DAG is generated on endosomes recovering from osmotic shock (Shaughnessy et al., 2007), consistent with a role for autophagy in repairing damaged endosomes (Birmingham et al., 2006; Kobayashi et al., 2010). We have shown that the activity of PLD and PAP are required for DAG localization to bacteria and contribute to anti-bacterial autophagy (Figure 7). The nature of the upstream signals that lead to activation of these enzymes remains unclear, and will be the subject of future study. While we favor the model of membrane damage inducing autophagy, it is possible that S. Typhimurium translocates one or more type III secreted effector proteins into host cells to promote DAG production on SCVs and subsequent autophagy, analogous to the proposed initiation of autophagy by translocated E3 ligases.
How does DAG promote autophagy of S. Typhimurium? We propose that DAG acts at the target organelle (SCV) to recruit the DAG-binding PKC isoform PKCδ which can activate autophagy via the JNK and NADPH oxidase pathways. Interestingly, we also observed DAG on rapamycin-induced autophagosomes (Figure S8a, b). Other lipid-binding fluorescent probes were not significantly associated with these autophagosomes (Figure S8a, b). We also found that inhibition of PAP and PKCδ using an siRNA strategy resulted in significant inhibition of this type of autophagy (Figure S8c, d, e, f). These observations suggest a role for DAG/PKC in other types of autophagy. In fact, other DAG-responsive PKC isoforms (δ and θ) have also been implicated in hypoxia and ER stress-induced autophagy, respectively (Chen et al., 2008; Sakaki et al., 2008). However, it remains to be determined whether DAG plays a role in these forms of autophagy. We find that Pkc1 plays an important role in yeast autophagy, further suggesting a model wherein PKC isoforms are central and evolutionarily conserved regulators of autophagy. In mammalian cells it can be postulated that novel PKC isoforms are recruited and activated in a DAG-dependent manner to cargo selected for autophagy. The involvement of multiple PKC isoforms in autophagy regulation in mammals is reminiscent of Atg1, an essential autophagic factor whose regulatory role is conserved but has become more complex in higher eukaryotes (Chan and Tooze, 2009). To date, over 13 types of autophagy have been described in eukaryotes (Klionsky et al., 2007a). It remains to be seen whether DAG and PKC isoforms play a role in these processes and whether targeted modulation of their activities can provide therapeutic benefits for treatment of diseases where autophagy has been implicated in pathogenesis.
Experimental Procedures
Antibodies and Other Reagents
Primary antibodies used include rabbit polyclonal antibodies to S. Typhimurium O anti-serum Group B (Difco Laboratories), PKCα and δ (Santa Cruz Biotechnology), Atg12 (Cell Signaling), YFP (Clontech) and β-actin (Sigma). Rabbit polyclonal antibodies to LC3 (B-90) were a kind gift from Karla Kirkegaard (Stanford University). Rabbit polyclonal antibodies to p22 (R3179) were a kind gift from Mark T. Quinn (Montana State University). Mouse monoclonal antibodies used include those to mono- and polyubiquitinated conjugates, FK2 clone (Enzo Life Sciences) and p62 (BD Biosciences). All secondary antibodies used were Alexa Fluor conjugates (Molecular Probes). Pharmacological agents used include D609, Enzo Life Sciences. DGK inhibitor I (R59022), 3-methyladenine (3-MA), butanol, tert-butanol, phorbol 12-myristate 13-acetate (PMA), 4α-phorbol 12-myristate 13-acetate (4α-PMA) and SP600125 were purchased from Sigma. U73122, propranolol hydrochloride and rapamycin were purchased from Biomol International. Rottlerin and Go6983 were purchased from Calbiochem. siGENOME® SMARTpool® siRNA reagents (Dharmacon) were used for targeting human Atg12, Pap2B, p62, Pkcα, Pkcδ and p22 as well as scrambled control siRNA.
Bacterial Strains and Cell Culture
Wild-type S. Typhimurium SL1344 (Hoiseth and Stocker, 1981) or monomeric red fluorescent protein (mRFP)-expressing S. Typhimurium or invA/inv S. Typhimurium (Birmingham et al., 2006) were used for infection studies as indicated. atg5−/− and PKCδ −/− mouse embryonic fibroblasts (MEFs) have been previously described (Kuma et al., 2004; Miyamoto et al., 2002). HeLa, Henle and mouse embryonic fibroblasts (MEFs) were maintained in Dulbecco’s modified Eagle’s growth medium (Thermo Scientific) supplemented with 10% fetal bovine serum (Wisent) at 37°C at 5% CO2 without antibiotics. Cells were seeded in 24-well tissue culture plates (BD Biosciences) at 2.5 x 104 cells/well 48 h prior to use for experiments involving direct visual analysis. Cells were seeded at 5.0 x 104 cells/well 48 h prior to use for experiments involving siRNA treatment and Western blot analysis.
Plasmids and Transfection
Transfection reagents GeneJuice (EMD Biosciences) and FuGene 6 (Roche Applied Sciences) were used according to the manufacturers’ instructions. Constructs used: 3xmyc-Atg16L1 (Kuballa et al., 2008), GFP-LC3 (Kabeya et al., 2000), RFP-LC3 (kindly provided by Walter Beron, Universidad Nacional de Cuyo), PKCδ-C1-GFP (Tse et al., 2005), PH-PLCδ-GFP (Stauffer et al., 1998), 2FYVE-GFP (Vieira et al., 2001), GFP-PH-AKT (Weernink et al., 2000). HA-PLD1 WT, HA-PLD2 WT, HA-PLD1 K898R and HA-PLD2 K758R were kindly provided by Michael Frohman (SUNY, New York). PKCδ expression plasmid pMT2-PKCδ−/− was a kind gift from Michael Leitges (University of Oslo). DN JNK constructs, DN JNK1 (plasmid 13846) and DN JNK2 (plasmid 13761) (Derijard et al., 1994; Gupta et al., 1996) were obtained from Addgene. siRNA transfections were performed using Oligofectamine (Invitrogen) according to the manufacturer’s instructions.
Bacterial Infection
Late-log S. Typhimurium cultures were used for infecting cells and prepared using a method optimized for bacterial invasion (Steele-Mortimer et al., 1999). Briefly, wild-type bacteria were grown in LB with antibiotics for approximately 16 h at 37°C with shaking and then diluted (1:33) in LB without antibiotics for 3 h. Bacterial inocula were prepared by pelleting at 10, 000x g for 2 min, diluted 1:100 in phosphate buffered saline (PBS), pH 7.2 and added to cells for 10 min at 37°C. After infection, extracellular bacteria were removed by extensive washing with PBS and addition of 100 μg/mL gentamicin to the growth medium at 30 min post-infection. For experiments requiring inhibition of bacterial protein synthesis, 200 μg/mL chloramphenicol was added directly to the growth medium after infection and kept present throughout the rest of the infection. invA/inv S. Typhimurium invasion has been previously described (Birmingham et al., 2006).
Immunofluorescence
Cells were fixed with 2.5% paraformaldehyde in PBS, pH 7.2, for 10 min at 37°C. Fixed cells were permeabilized and blocked in 0.2% saponin (Calbiochem) and 10% normal goat serum (Wisent) for 2–12 h and stained as previously described (Brumell et al., 2001). Samples were mounted on slides using fluorescence mounting medium (Dako).
Live Cell Imaging
Alexa Fluor 647 carboxylic acid, succinimidyl ester (Invitrogen) was used to label wild-type S. Typhimurium SL1344 as previously described (Birmingham et al., 2006). HeLa cells were maintained at 37°C in RPMI (Thermo Scientific) during the course of the infection. A Leica DMIRE2 inverted fluorescence microscope equipped with a Hamamatsu Back-Thinned EM-CCD camera and spinning disk confocal scan head with a 63x objective and LSM 510 software was used. Volocity software (Improvision) was used to acquire images and perform deconvolution and 3D rendering.
Confocal Microscopy and Image Preparation
All images shown are confocal z-slices taken using a Leica DMIRE2 inverted fluorescence microscope equipped with a Hamamatsu Back-Thinned EM-CCD camera and spinning disk confocal scan head with a 63x objective and LSM 510 software. Volocity software (Improvision) was used to acquire images. Confocal images were imported into AdobeR Photoshop and assembled in AdobeR Illustrator . Colocalization and enumeration studies were performed on a Leica DMIRE2 fluorescence microscope by direct visualization.
Yeast autophagy assays
GFP-Atg8 processing was determined using wild-type (TN124), atg1Δ (TYY127), pkc1ts, pkc1-3 and pkc1-4 cells expressing GFP-Atg8 as previously reported (Klionsky et al., 2007b). Briefly, cells were incubated in SD-N medium at the permissive (24°C) or non-permissive (38 C) temperature. Full-length GFP-Atg8 and free GFP were detected by Western blotting using anti-YFP antibodies. Bulk autophagic activity was determined using yeast strains expressing Pho8Δ60 as previously described (Klionsky et al., 2007b). Cells were grown in YPD at the permissive temperature. Half of the cell cultures were shifted to the non-permissive temperature for 30 min to inactivate Pkc1 while the other half remained at 24°C. Cells were then shifted to SD-N at either 24°C or 38°C for 2 h. Cell extracts were subsequently prepared for Pho8Δ60-dependent alkaline phosphatase activity measurements.
Statistics
The mean ± s.e.m. for at least three independent experiments is shown in figures unless otherwise indicated, and p values were calculated using a two-tailed two-sample unequal variance Student's t-test. A p value of less than 0.05 was determined to be statistically significant.
Supplementary Material
Figure S1 Characterization of the PKCδ-C1-GFP probe. (a) HeLa cells were transfected with PKCδ-C1-GFP and either Rab5-RFP or immunostained for transferrin receptor, LAMP-1, ERGIC 53, GM130, Bip or Giantin. Transferrin was visualized using Alexa Fluor 546 conjugated transferrin. Size bar, 10 μm. (b) HeLa cells were transfected with PKCδ-C1-GFP, infected with wild-type S. Typhimurium, fixed at 1 h p.i. and immunostained for LC3 using a rabbit polyclonal antibody. Bacteria were immunostained using a rabbit polyclonal antibody in conjuction with a Zenon Alexa Fluor 647 labeling kit. Representative confocal z-slices are shown. Size bar, 10 μm. (c) Quantification of the colocalization of LC3 with DAG+ or DAG− bacteria in b (d) HeLa cells were transfected with PKCδ-C1-GFP and 3xmyc-Atg16L1, infected with wild-type S. Typhimurium and fixed at 45 min p.i. Cells were immunostained for Atg16L1 using a mouse monoclonal antibody to myc and bacteria using a rabbit polyclonal antibody. Representative confocal z- slices are shown. Size bar, 10 μm. (e) Quantification of the colocalization of Atg16L1 with DAG+ or DAG− bacteria in d. Data represent the mean ± standard error (s.e.m.) for three independent experiments.
Figure S2 DAG colocalization to SCVs precedes LC3. HeLa cells were co-transfected with PKCδ-C1-GFP and RFP-LC3, infected with wild-type S. Typhimurium stained with Alexa Fluor 647 carboxylic acid, succinimidyl ester (blue) and imaged over the course of the infection. Images corresponding with the indicated time points p.i. are shown.
Figure S3 DAG does not colocalize with invA/inv S. Typhimurium. (a-b) HeLa cells were transfected with PKCδ-C1-GFP, infected with invA/inv S. Typhimurium, fixed at the indicated time points and immunostained for the bacteria using a rabbit polyclonal antibody. One representative experiment is also shown for wild-type S. Typhimurium in b. Representative fluorescence images are provided for the 45 min p.i. time point. Data represent the mean ± standard error (s.e.m.) for three independent experiments for invA/inv S. Typhimurium invasion.
Figure S4 Inhibition of DGK results in enhanced anti-bacterial autophagy. (a) HeLa cells were transfected with GFP-LC3 and treated with either DMSO or DGK inhibitor I (R59022, 10 μM). Cells were infected with RFP-Sal and fixed at 90 min p.i. Representative confocal z-slices are shown. The inner panels represent a higher magnification of the boxed areas. Size bar, 10 μm. (b) HeLa cells were transfected, treated and infected as in a, and fixed at the indicated time points. The percentage of GFP- LC3+ bacteria was determined by fluorescence microscopy for each condition. Data represent the mean ± standard error (s.e.m.) for three independent experiments.
Figure S5 DAG generation on SCVs requires phosphatidic acid phosphatase and phospholipase D. (a) HeLa cells were transfected with PKCδ-C1-GFP and infected with RFP-Sal. Cells were treated with U73122 (10 μM), DMSO, propranolol hydrochloride (propr., 250 μM) or H2O at 10 min p.i. for the remainder of the infection, and fixed at 45 min p.i. (b) HeLa cells were co-transfected with GFP-LC3 and either control siRNA (si-CTRL) or siRNA specifically targeting Atg12 (si-ATG12) or PAP2B (si-PAP). Cells were infected with wild-type RFP-Sal and fixed at 1 h p.i. Representative confocal z-slices are shown. The inner panels represent a higher magnification of the boxed areas. (c-d) HeLa cells were transfected with either PKCδ-C1-GFP c or GFP-LC3 d and treated with 0.3% v/v 1-butanol (1-but) or tert-butanol (tert-but). Cells were infected with S. Typhimurium, fixed at 45 min c, or 1 h d p.i. and stained for S. Typhimurium. The number of PKCδ-C1-GFP+ c, or GFP-LC3+ d bacteria was determined for at least 100 bacteria for each condition. Values represent the percentage of marker-positive bacteria compared to control cells. (e) HeLa cells were transfected with RFP-LC3 and either GFP, HA-PLD1 DN (dominant negative) or HA-PLD2 DN. Cells were infected with S. Typhimurium, fixed at 1 h p.i. and immunostained for S. Typhimurium and HA tag. Representative confocal z-slices are shown. The inner panels represent a higher magnification of the boxed areas. Size bar, 10 μm. Data represent the mean ± standard error (s.e.m.) for at least three independent experiments.
Figure S6 Protein kinase C is required for autophagy of S. Typhimurium. HeLa cells were transfected with GFP-LC3 (a) or PKCδ-C1-GFP (c) and treated with either DMSO, rottlerin (15 μM) or Go6983 (10 nM). Cells were infected with RFP-Sal and fixed at 1 h a, or 45 min c p.i. Representative confocal z-slices are shown. The inner panels represent a higher magnification of the boxed areas. Size bar, 10 μm. (b) HeLa cells were transfected and treated as in a. The percentage of GFP-LC3+ bacteria was determined by fluorescence microscopy. (d) HeLa cells were transfected and treated as in c. The percentage of PKCδ-C1-GFP+ was determined by fluorescence microscopy. Data represent the mean ± standard error (s.e.m.) for at least three independent experiments. (e) HeLa cells were transfected with either control siRNA (si-CTRL) or siRNA specifically targeting Atg12 (si-ATG12), PKCδ (si-PKCδ) or PKCα(si-PKCα). Western blotting was used to detect protein levels of Atg12, PKCα, PKCδ and β actin using anti-Atg12, anti-PKCα, anti-PKCδ and anti-β actin (as a loading control) antibodies, respectively.
Figure S7 JNK and NADPH oxidases are required for autophagy of S. Typhimurium (a) HeLa cells were transfected with GFP-LC3 and infected with RFP-Sal. Cells were treated with DMSO or 5 μM SP600125 and fixed at 1 h p.i. (b) HeLa cells were transfected with GFP-LC3 and either DN JNK1, DN JNK2 or both, infected with RFP-Sal and fixed at 1 h p.i. The percentage of GFP-LC3+ bacteria in a and b was determined by fluorescence microscopy. (c-d) Henle cells were co-transfected with either control siRNA (si-CTRL) or siRNA specifically targeting Atg12 (si-ATG12) or p22 (si-p22) and GFP-LC3 c or PKCδ-C1-GFP d. Cells were infected with RFP-Sal and fixed at 1 h c, or 45 min d p.i. The percentage of GFP-LC3+ c and PKCδ-C1-GFP+ d bacteria was determined by fluorescence microscopy. Data represent the mean ± standard error (s.e.m.) for at least three independent experiments. (e) Western blotting was used to detect protein levels of p22 and β actin in the presence of si-CTRL or si-p22 using anti-p22 and anti-βactin (as a loading control) antibodies. (f) Western blotting was used to detect protein levels of p62 and β actin in the presence of si-CTRL or si-p62 using anti-p62 and anti-β actin (as a loading control) antibodies.
Figure S8 DAG, phosphatidic acid phosphatase and phospholipase D are required for rapamycin-induced autophagy (a) HeLa cells were co-transfected with RFP-LC3 and either 2FYVE-GFP (PI(3)P probe), PLCδ-PH-GFP (PI(4,5)P2 probe), GFP-PH-AKT (PI(3,5)P2 and PI(3,4,5)P3 probe) or PKCδ-C1-GFP (DAG probe). Cells were treated with rapamycin (25 μg/mL) and fixed 2 h post-treatment. Representative confocal z-slices are shown. The inner panels represent a higher magnification of the boxed areas. Size bar, 10 μm. (b) The percentage of autophagosomes colocalizing with the lipid probes in a was determined by fluorescence microscopy. (c-d) HeLa cells were co-transfected with GFP-LC3 and either control siRNA (si-CTRL) or siRNA specifically targeting Atg12 (si-ATG12) or PAP2B (si-PAP). Cells were treated with rapamycin (25 μg/mL) and fixed at 2 h post-treatment. 100 cells were counted and scored for autophagosomes/cell via fluorescence microscopy. Representative confocal z-slices are shown in d. Arrowheads represent autophagosomes. Size bar, 10 μm. (e-f) HeLa cells were transfected with either control siRNA (si-CTRL) or siRNA specifically targeting Atg12 (si-ATG12), PKCδ (si-PKCδ) or PKCα (si-PKCα). Cells were treated with rapamycin (25 μg/mL) and fixed at 2 h post-treatment. 100 cells were counted and scored for autophagosomes/cell via fluorescence microscopy. Representative confocal z-slices are shown f. Arrowheads indicate autophagosomes and size bar, 10 μm. Data represent the mean ± standard error (s.e.m.) for at least three independent experiments.
Table S1 - Lipid probes used in this study
Table S2 - Yeast strains used in this study
Highlights.
DAG is a novel signal for anti-bacterial autophagy
DAG is required for autophagy of Salmonella and generated by phosphatidic acid phosphatase and phospholipase D.
DAG is able to induce autophagy through the activation of PKCδ.
PKC has an evolutionarily conserved role in autophagy regulation.
Acknowledgments
We thank members of the Brumell lab for critical reading of the manuscript. We are grateful to T. Balla, W. Beron, C. Dall’Armi, G. Di Paolo, M. Frohman, S. Grinstein, N. Jones, K. Kirkegaard, P. Kim, M. Leitges, N. Mizushima, M. Quinn, P. Sherman, T. Yoshimori and R. Xavier for providing reagents and helpful suggestions. Special thanks to Michael Woodside and Paul Paroutis for assistance with microscopy. John H. Brumell, Ph.D., holds an Investigators in Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund. Lab infrastructure was provided by a New Opportunities Fund from the Canadian Foundation for Innovation and the Ontario Innovation Trust. S.S. was supported by a Canada Graduate Scholarship from the Canadian Institutes of Health Research. W.-L.Y. and D.J.K. were supported by Public Health Service Grant GM53396 from the National Institutes of Health.
Reference List
- Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol. 2008;182:685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham CL, Smith AC, Bakowski MA, Yoshimori T, Brumell JH. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J Biol Chem. 2006;281:11374–11383. doi: 10.1074/jbc.M509157200. [DOI] [PubMed] [Google Scholar]
- Brumell JH, Grinstein S. Salmonella redirects phagosomal maturation. Curr Opin Microbiol. 2004;7:78–84. doi: 10.1016/j.mib.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Brumell JH, Rosenberger CM, Gotto GT, Marcus SL, Finlay BB. SifA permits survival and replication of Salmonella typhimurium in murine macrophages. Cell Microbiol. 2001;3:75–84. doi: 10.1046/j.1462-5822.2001.00087.x. [DOI] [PubMed] [Google Scholar]
- Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, Kishi C, Kc W, Carrero JA, Hunt S, Stone CD, Brunt EM, Xavier RJ, Sleckman BP, Li E, Mizushima N, Stappenbeck TS, Virgin HW. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco S, Merida I. Diacylglycerol, when simplicity becomes complex. Trends Biochem Sci. 2007;32:27–36. doi: 10.1016/j.tibs.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Chan EY, Tooze SA. Evolution of Atg1 function and regulation. Autophagy. 2009;5 doi: 10.4161/auto.8709. [DOI] [PubMed] [Google Scholar]
- Chen JL, Lin HH, Kim KJ, Lin A, Forman HJ, Ann DK. Novel roles for Protein Kinase Cδ-dependent signaling pathways in acute hypoxic stress-induced autophagy. J Biol Chem. 2008;283:34432–34444. doi: 10.1074/jbc.M804239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denmat-Ouisse LA, Phebidias C, Honkavaara P, Robin P, Geny B, Min DS, Bourgoin S, Frohman MA, Raymond MN. Regulation of constitutive protein transit by phospholipase D in HT29-cl19A cells. J Biol Chem. 2001;276:48840–48846. doi: 10.1074/jbc.M104276200. [DOI] [PubMed] [Google Scholar]
- Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5:527–549. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Dupont N, Lacas-Gervais S, Bertout J, Paz I, Freche B, Van Nhieu GT, van der Goot FG, Sansonetti PJ, Lafont F. Shigella phagocytic vacuolar membrane remnants participate in the cellular response to pathogen invasion and are regulated by autophagy. Cell Host Microbe. 2009;6:137–149. doi: 10.1016/j.chom.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Fontayne A, Dang PM, Gougerot-Pocidalo MA, El Benna J. Phosphorylation of p47phox sites by PKC α, β II, δ, and ζ: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry. 2002;41:7743–7750. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- Gschwendt M, Muller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Derijard B, Davis RJ. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol. 2008;6:53–66. doi: 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Huang J, Canadien V, Lam GY, Steinberg BE, Dinauer MC, Magalhaes MA, Glogauer M, Grinstein S, Brumell JH. Activation of antibacterial autophagy by NADPH oxidases. Proc Natl Acad Sci U S A. 2009;106:6226–6231. doi: 10.1073/pnas.0811045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Klionsky DJ. Autophagy and human disease. Cell Cycle. 2007;6:1837–1849. doi: 10.4161/cc.6.15.4511. [DOI] [PubMed] [Google Scholar]
- Huitema K, van den DJ, Brouwers JF, Holthuis JC. Identification of a family of animal sphingomyelin synthases. EMBO J. 2004;23:33–44. doi: 10.1038/sj.emboj.7600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhasz G, Neufeld TP. Autophagy: a forty-year search for a missing membrane source. PLoS Biol. 2006;4:e36. doi: 10.1371/journal.pbio.0040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanietz MG. Eyes wide shut: protein kinase C isozymes are not the only receptors for the phorbol ester tumor promoters. Mol Carcinog. 2000;28:5–11. doi: 10.1002/(sici)1098-2744(200005)28:1<5::aid-mc2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kihara A, Noda T, Ishihara N, Ohsumi Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J Cell Biol. 2001;152:519–530. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, Bamber BA, Bassham DC, Bergamini E, Bi X, Biard-Piechaczyk M, Blum JS, Bredesen DE, Brodsky JL, Brumell JH, Brunk UT, Bursch W, Camougrand N, Cebollero E, Cecconi F, Chen Y, Chin LS, Choi A, Chu CT, Chung J, Clarke PG, Clark RS, Clarke SG, Clave C, Cleveland JL, Codogno P, Colombo MI, Coto-Montes A, Cregg JM, Cuervo AM, Debnath J, Demarchi F, Dennis PB, Dennis PA, Deretic V, Devenish RJ, Di Sano F, Dice JF, Difiglia M, Dinesh-Kumar S, Distelhorst CW, Djavaheri-Mergny M, Dorsey FC, Droge W, Dron M, Dunn WA, Jr, Duszenko M, Eissa NT, Elazar Z, Esclatine A, Eskelinen EL, Fesus L, Finley KD, Fuentes JM, Fueyo J, Fujisaki K, Galliot B, Gao FB, Gewirtz DA, Gibson SB, Gohla A, Goldberg AL, Gonzalez R, Gonzalez-Estevez C, Gorski S, Gottlieb RA, Haussinger D, He YW, Heidenreich K, Hill JA, Hoyer-Hansen M, Hu X, Huang WP, Iwasaki A, Jaattela M, Jackson WT, Jiang X, Jin S, Johansen T, Jung JU, Kadowaki M, Kang C, Kelekar A, Kessel DH, Kiel JA, Kim HP, Kimchi A, Kinsella TJ, Kiselyov K, Kitamoto K, Knecht E, Komatsu M, Kominami E, Kondo S, Kovacs AL, Kroemer G, Kuan CY, Kumar R, Kundu M, Landry J, Laporte M, Le W, Lei HY, Lenardo MJ, Levine B, Lieberman A, Lim KL, Lin FC, Liou W, Liu LF, Lopez-Berestein G, Lopez-Otin C, Lu B, Macleod KF, Malorni W, Martinet W, Matsuoka K, Mautner J, Meijer AJ, Melendez A, Michels P, Miotto G, Mistiaen WP, Mizushima N, Mograbi B, Monastyrska I, Moore MN, Moreira PI, Moriyasu Y, Motyl T, Munz C, Murphy LO, Naqvi NI, Neufeld TP, Nishino I, Nixon RA, Noda T, Nurnberg B, Ogawa M, Oleinick NL, Olsen LJ, Ozpolat B, Paglin S, Palmer GE, Papassideri I, Parkes M, Perlmutter DH, Perry G, Piacentini M, Pinkas-Kramarski R, Prescott M, Proikas-Cezanne T, Raben N, Rami A, Reggiori F, Rohrer B, Rubinsztein DC, Ryan KM, Sadoshima J, Sakagami H, Sakai Y, Sandri M, Sasakawa C, Sass M, Schneider C, Seglen PO, Seleverstov O, Settleman J, Shacka JJ, Shapiro IM, Sibirny A, Silva-Zacarin EC, Simon HU, Simone C, Simonsen A, Smith MA, Spanel-Borowski K, Srinivas V, Steeves M, Stenmark H, Stromhaug PE, Subauste CS, Sugimoto S, Sulzer D, Suzuki T, Swanson MS, Tabas I, Takeshita F, Talbot NJ, Talloczy Z, Tanaka K, Tanaka K, Tanida I, Taylor GS, Taylor JP, Terman A, Tettamanti G, Thompson CB, Thumm M, Tolkovsky AM, Tooze SA, Truant R, Tumanovska LV, Uchiyama Y, Ueno T, Uzcategui NL, van dKI, Vaquero EC, Vellai T, Vogel MW, Wang HG, Webster P, Wiley JW, Xi Z, Xiao G, Yahalom J, Yang JM, Yap G, Yin XM, Yoshimori T, Yu L, Yue Z, Yuzaki M, Zabirnyk O, Zheng X, Zhu X, Deter RL. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Cuervo AM, Dunn WA, Jr, Levine B, van dKI, Seglen PO. How shall I eat thee? Autophagy. 2007a;3:413–416. doi: 10.4161/auto.4377. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Cuervo AM, Seglen PO. Methods for monitoring autophagy from yeast to human. Autophagy. 2007b;3:181–206. doi: 10.4161/auto.3678. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Kojidani T, Osakada H, Yamamoto A, Yoshimori T, Hiraoka Y, Haraguchi T. Artificial induction of autophagy around polystyrene beads in nonphagocytic cells. Autophagy. 2010;6 doi: 10.4161/auto.6.1.10324. [DOI] [PubMed] [Google Scholar]
- Kuballa P, Huett A, Rioux JD, Daly MJ, Xavier RJ. Impaired autophagy of an intracellular pathogen induced by a Crohn's disease associated ATG16L1 variant. PLoS ONE. 2008;3:e3391. doi: 10.1371/journal.pone.0003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- Lavieu G, Scarlatti F, Sala G, Carpentier S, Levade T, Ghidoni R, Botti J, Codogno P. Regulation of autophagy by sphingosine kinase 1 and its role in cell survival during nutrient starvation. J Biol Chem. 2006;281:8518–8527. doi: 10.1074/jbc.M506182200. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll SA, Huett A, Kuballa P, Chilewski SD, Landry A, Goyette P, Zody MC, Hall JL, Brant SR, Cho JH, Duerr RH, Silverberg MS, Taylor KD, Rioux JD, Altshuler D, Daly MJ, Xavier RJ. Deletion polymorphism upstream of IRGM associated with altered IRGM expression and Crohn's disease. Nat Genet. 2008;40:1107–1112. doi: 10.1038/ng.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merida I, Avila-Flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. Biochem J. 2008;409:1–18. doi: 10.1042/BJ20071040. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, Nakayama K, Imaki H, Hirose S, Jiang Y, Abe M, Tsukiyama T, Nagahama H, Ohno S, Hatakeyama S, Nakayama KI. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cdelta. Nature. 2002;416:865–869. doi: 10.1038/416865a. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Kuma A, Kobayashi Y, Yamamoto A, Matsubae M, Takao T, Natsume T, Ohsumi Y, Yoshimori T. Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12-Apg5 conjugate. J Cell Sci. 2003;116:1679–1688. doi: 10.1242/jcs.00381. [DOI] [PubMed] [Google Scholar]
- Nauseef WM. Biological roles for the NOX family NADPH oxidases. J Biol Chem. 2008;283:16961–16965. doi: 10.1074/jbc.R700045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T, Matsuura A, Wada Y, Ohsumi Y. Novel system for monitoring autophagy in the yeast Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1995;210:126–132. doi: 10.1006/bbrc.1995.1636. [DOI] [PubMed] [Google Scholar]
- Obara K, Noda T, Niimi K, Ohsumi Y. Transport of phosphatidylinositol 3-phosphate into the vacuole via autophagic membranes in Saccharomyces cerevisiae. Genes Cells. 2008;13:537–547. doi: 10.1111/j.1365-2443.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- Obara K, Sekito T, Ohsumi Y. Assortment of phosphatidylinositol 3-kinase complexes--Atg14p directs association of complex I to the pre-autophagosomal structure in Saccharomyces cerevisiae. Mol Biol Cell. 2006;17:1527–1539. doi: 10.1091/mbc.E05-09-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes M, Barrett JC, Prescott NJ, Tremelling M, Anderson CA, Fisher SA, Roberts RG, Nimmo ER, Cummings FR, Soars D, Drummond H, Lees CW, Khawaja SA, Bagnall R, Burke DA, Todhunter CE, Ahmad T, Onnie CM, McArdle W, Strachan D, Bethel G, Bryan C, Lewis CM, Deloukas P, Forbes A, Sanderson J, Jewell DP, Satsangi J, Mansfield JC, Cardon L, Mathew CG. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn's disease susceptibility. Nat Genet. 2007;39:830–832. doi: 10.1038/ng2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendyala S, Usatyuk P, Gorshkova IA, Garcia JG, Natarajan V. Regulation of NADPH Oxidase in Vascular Endothelium: The role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid Redox Signal. 2008;11:841–860. doi: 10.1089/ars.2008.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin AJ, Jiang X, Birmingham CL, So NS, Brumell JH. Recognition of bacteria in the cytosol of mammalian cells by the ubiquitin system. Curr Biol. 2004;14:806–811. doi: 10.1016/j.cub.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Quezada CM, Hicks SW, Galan JE, Stebbins CE. A family of Salmonella virulence factors functions as a distinct class of autoregulated E3 ubiquitin ligases. Proc Natl Acad Sci U S A. 2009;106:4864–4869. doi: 10.1073/pnas.0811058106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux JD, Xavier RJ, Taylor KD, Silverberg MS, Goyette P, Huett A, Green T, Kuballa P, Barmada MM, Datta LW, Shugart YY, Griffiths AM, Targan SR, Ippoliti AF, Bernard EJ, Mei L, Nicolae DL, Regueiro M, Schumm LP, Steinhart AH, Rotter JI, Duerr RH, Cho JH, Daly MJ, Brant SR. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde JR, Breitkreutz A, Chenal A, Sansonetti PJ, Parsot C. Type III secretion effectors of the IpaH family are E3 ubiquitin ligases. Cell Host Microbe. 2007;1:77–83. doi: 10.1016/j.chom.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Roy D, Liston DR, Idone VJ, Di A, Nelson DJ, Pujol C, Bliska JB, Chakrabarti S, Andrews NW. A process for controlling intracellular bacterial infections induced by membrane injury. Science. 2004;304:1515–1518. doi: 10.1126/science.1098371. [DOI] [PubMed] [Google Scholar]
- Rytkonen A, Holden DW. Bacterial interference of ubiquitination and deubiquitination. Cell Host Microbe. 2007;1:13–22. doi: 10.1016/j.chom.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki K, Wu J, Kaufman RJ. Protein kinase C-θ is required for autophagy in response to stress in the endoplasmic reticulum. J Biol Chem. 2008;283:15370–15380. doi: 10.1074/jbc.M710209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaughnessy LM, Lipp P, Lee KD, Swanson JA. Localization of protein kinase C ε to macrophage vacuoles perforated by Listeria monocytogenes cytolysin. Cell Microbiol. 2007;9:1695–1704. doi: 10.1111/j.1462-5822.2007.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer TP, Ahn S, Meyer T. Receptor-induced transient reduction in plasma membrane PtdIns(4, 5)P2 concentration monitored in living cells. Curr Biol. 1998;8:343–346. doi: 10.1016/s0960-9822(98)70135-6. [DOI] [PubMed] [Google Scholar]
- Steele-Mortimer O, Brumell JH, Knodler LA, Meresse S, Lopez A, Finlay BB. The invasion-associated type III secretion system of Salmonella enterica serovar Typhimurium is necessary for intracellular proliferation and vacuole biogenesis in epithelial cells. Cell Microbiol. 2002;4:43–54. doi: 10.1046/j.1462-5822.2002.00170.x. [DOI] [PubMed] [Google Scholar]
- Steele-Mortimer O, Meresse S, Gorvel JP, Toh BH, Finlay BB. Biogenesis of Salmonella Typhimurium-containing vacuoles in epithelial cells involves interactions with the early endocytic pathway. Cell Microbiol. 1999;1:33–49. doi: 10.1046/j.1462-5822.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- Thurston TL, Ryzhakov G, Bloor S, von Muhlinen N, Randow F. The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat Immunol. 2009;10:1215–1221. doi: 10.1038/ni.1800. [DOI] [PubMed] [Google Scholar]
- Tse SM, Mason D, Botelho RJ, Chiu B, Reyland M, Hanada K, Inman RD, Grinstein S. Accumulation of diacylglycerol in the Chlamydia inclusion vacuole: possible role in the inhibition of host cell apoptosis. J Biol Chem. 2005;280:25210–25215. doi: 10.1074/jbc.M501980200. [DOI] [PubMed] [Google Scholar]
- Veenendaal AK, Hodgkinson JL, Schwarzer L, Stabat D, Zenk SF, Blocker AJ. The type III secretion system needle tip complex mediates host cell sensing and translocon insertion. Mol Microbiol. 2007;63:1719–1730. doi: 10.1111/j.1365-2958.2007.05620.x. [DOI] [PubMed] [Google Scholar]
- Vieira OV, Botelho RJ, Rameh L, Brachmann SM, Matsuo T, Davidson HW, Schreiber A, Backer JM, Cantley LC, Grinstein S. Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J Cell Biol. 2001;155:19–25. doi: 10.1083/jcb.200107069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weernink PA, Guo Y, Zhang C, Schmidt M, Eichel-Streiber C, Jakobs KH. Control of cellular phosphatidylinositol 4, 5-bisphosphate levels by adhesion signals and rho GTPases in NIH 3T3 fibroblasts involvement of both phosphatidylinositol-4-phosphate 5-kinase and phospholipase C. Eur J Biochem. 2000;267:5237–5246. doi: 10.1046/j.1432-1327.2000.01599.x. [DOI] [PubMed] [Google Scholar]
- Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Oku M, Wasada Y, Ano Y, Sakai Y. PI4P-signaling pathway for the synthesis of a nascent membrane structure in selective autophagy. J Cell Biol. 2006;173:709–717. doi: 10.1083/jcb.200512142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa Y, Ogawa M, Hain T, Yoshida M, Fukumatsu M, Kim M, Mimuro H, Nakagawa I, Yanagawa T, Ishii T, Kakizuka A, Sztul E, Chakraborty T, Sasakawa C. Listeria monocytogenes ActA-mediated escape from autophagic recognition. Nat Cell Biol. 2009;11:1233–1240. doi: 10.1038/ncb1967. [DOI] [PubMed] [Google Scholar]
- Zheng YT, Shahnazari S, Brech A, Lamark T, Johansen T, Brumell JH. The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J Immunol. 2009;183:5909–5916. doi: 10.4049/jimmunol.0900441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Characterization of the PKCδ-C1-GFP probe. (a) HeLa cells were transfected with PKCδ-C1-GFP and either Rab5-RFP or immunostained for transferrin receptor, LAMP-1, ERGIC 53, GM130, Bip or Giantin. Transferrin was visualized using Alexa Fluor 546 conjugated transferrin. Size bar, 10 μm. (b) HeLa cells were transfected with PKCδ-C1-GFP, infected with wild-type S. Typhimurium, fixed at 1 h p.i. and immunostained for LC3 using a rabbit polyclonal antibody. Bacteria were immunostained using a rabbit polyclonal antibody in conjuction with a Zenon Alexa Fluor 647 labeling kit. Representative confocal z-slices are shown. Size bar, 10 μm. (c) Quantification of the colocalization of LC3 with DAG+ or DAG− bacteria in b (d) HeLa cells were transfected with PKCδ-C1-GFP and 3xmyc-Atg16L1, infected with wild-type S. Typhimurium and fixed at 45 min p.i. Cells were immunostained for Atg16L1 using a mouse monoclonal antibody to myc and bacteria using a rabbit polyclonal antibody. Representative confocal z- slices are shown. Size bar, 10 μm. (e) Quantification of the colocalization of Atg16L1 with DAG+ or DAG− bacteria in d. Data represent the mean ± standard error (s.e.m.) for three independent experiments.
Figure S2 DAG colocalization to SCVs precedes LC3. HeLa cells were co-transfected with PKCδ-C1-GFP and RFP-LC3, infected with wild-type S. Typhimurium stained with Alexa Fluor 647 carboxylic acid, succinimidyl ester (blue) and imaged over the course of the infection. Images corresponding with the indicated time points p.i. are shown.
Figure S3 DAG does not colocalize with invA/inv S. Typhimurium. (a-b) HeLa cells were transfected with PKCδ-C1-GFP, infected with invA/inv S. Typhimurium, fixed at the indicated time points and immunostained for the bacteria using a rabbit polyclonal antibody. One representative experiment is also shown for wild-type S. Typhimurium in b. Representative fluorescence images are provided for the 45 min p.i. time point. Data represent the mean ± standard error (s.e.m.) for three independent experiments for invA/inv S. Typhimurium invasion.
Figure S4 Inhibition of DGK results in enhanced anti-bacterial autophagy. (a) HeLa cells were transfected with GFP-LC3 and treated with either DMSO or DGK inhibitor I (R59022, 10 μM). Cells were infected with RFP-Sal and fixed at 90 min p.i. Representative confocal z-slices are shown. The inner panels represent a higher magnification of the boxed areas. Size bar, 10 μm. (b) HeLa cells were transfected, treated and infected as in a, and fixed at the indicated time points. The percentage of GFP- LC3+ bacteria was determined by fluorescence microscopy for each condition. Data represent the mean ± standard error (s.e.m.) for three independent experiments.
Figure S5 DAG generation on SCVs requires phosphatidic acid phosphatase and phospholipase D. (a) HeLa cells were transfected with PKCδ-C1-GFP and infected with RFP-Sal. Cells were treated with U73122 (10 μM), DMSO, propranolol hydrochloride (propr., 250 μM) or H2O at 10 min p.i. for the remainder of the infection, and fixed at 45 min p.i. (b) HeLa cells were co-transfected with GFP-LC3 and either control siRNA (si-CTRL) or siRNA specifically targeting Atg12 (si-ATG12) or PAP2B (si-PAP). Cells were infected with wild-type RFP-Sal and fixed at 1 h p.i. Representative confocal z-slices are shown. The inner panels represent a higher magnification of the boxed areas. (c-d) HeLa cells were transfected with either PKCδ-C1-GFP c or GFP-LC3 d and treated with 0.3% v/v 1-butanol (1-but) or tert-butanol (tert-but). Cells were infected with S. Typhimurium, fixed at 45 min c, or 1 h d p.i. and stained for S. Typhimurium. The number of PKCδ-C1-GFP+ c, or GFP-LC3+ d bacteria was determined for at least 100 bacteria for each condition. Values represent the percentage of marker-positive bacteria compared to control cells. (e) HeLa cells were transfected with RFP-LC3 and either GFP, HA-PLD1 DN (dominant negative) or HA-PLD2 DN. Cells were infected with S. Typhimurium, fixed at 1 h p.i. and immunostained for S. Typhimurium and HA tag. Representative confocal z-slices are shown. The inner panels represent a higher magnification of the boxed areas. Size bar, 10 μm. Data represent the mean ± standard error (s.e.m.) for at least three independent experiments.
Figure S6 Protein kinase C is required for autophagy of S. Typhimurium. HeLa cells were transfected with GFP-LC3 (a) or PKCδ-C1-GFP (c) and treated with either DMSO, rottlerin (15 μM) or Go6983 (10 nM). Cells were infected with RFP-Sal and fixed at 1 h a, or 45 min c p.i. Representative confocal z-slices are shown. The inner panels represent a higher magnification of the boxed areas. Size bar, 10 μm. (b) HeLa cells were transfected and treated as in a. The percentage of GFP-LC3+ bacteria was determined by fluorescence microscopy. (d) HeLa cells were transfected and treated as in c. The percentage of PKCδ-C1-GFP+ was determined by fluorescence microscopy. Data represent the mean ± standard error (s.e.m.) for at least three independent experiments. (e) HeLa cells were transfected with either control siRNA (si-CTRL) or siRNA specifically targeting Atg12 (si-ATG12), PKCδ (si-PKCδ) or PKCα(si-PKCα). Western blotting was used to detect protein levels of Atg12, PKCα, PKCδ and β actin using anti-Atg12, anti-PKCα, anti-PKCδ and anti-β actin (as a loading control) antibodies, respectively.
Figure S7 JNK and NADPH oxidases are required for autophagy of S. Typhimurium (a) HeLa cells were transfected with GFP-LC3 and infected with RFP-Sal. Cells were treated with DMSO or 5 μM SP600125 and fixed at 1 h p.i. (b) HeLa cells were transfected with GFP-LC3 and either DN JNK1, DN JNK2 or both, infected with RFP-Sal and fixed at 1 h p.i. The percentage of GFP-LC3+ bacteria in a and b was determined by fluorescence microscopy. (c-d) Henle cells were co-transfected with either control siRNA (si-CTRL) or siRNA specifically targeting Atg12 (si-ATG12) or p22 (si-p22) and GFP-LC3 c or PKCδ-C1-GFP d. Cells were infected with RFP-Sal and fixed at 1 h c, or 45 min d p.i. The percentage of GFP-LC3+ c and PKCδ-C1-GFP+ d bacteria was determined by fluorescence microscopy. Data represent the mean ± standard error (s.e.m.) for at least three independent experiments. (e) Western blotting was used to detect protein levels of p22 and β actin in the presence of si-CTRL or si-p22 using anti-p22 and anti-βactin (as a loading control) antibodies. (f) Western blotting was used to detect protein levels of p62 and β actin in the presence of si-CTRL or si-p62 using anti-p62 and anti-β actin (as a loading control) antibodies.
Figure S8 DAG, phosphatidic acid phosphatase and phospholipase D are required for rapamycin-induced autophagy (a) HeLa cells were co-transfected with RFP-LC3 and either 2FYVE-GFP (PI(3)P probe), PLCδ-PH-GFP (PI(4,5)P2 probe), GFP-PH-AKT (PI(3,5)P2 and PI(3,4,5)P3 probe) or PKCδ-C1-GFP (DAG probe). Cells were treated with rapamycin (25 μg/mL) and fixed 2 h post-treatment. Representative confocal z-slices are shown. The inner panels represent a higher magnification of the boxed areas. Size bar, 10 μm. (b) The percentage of autophagosomes colocalizing with the lipid probes in a was determined by fluorescence microscopy. (c-d) HeLa cells were co-transfected with GFP-LC3 and either control siRNA (si-CTRL) or siRNA specifically targeting Atg12 (si-ATG12) or PAP2B (si-PAP). Cells were treated with rapamycin (25 μg/mL) and fixed at 2 h post-treatment. 100 cells were counted and scored for autophagosomes/cell via fluorescence microscopy. Representative confocal z-slices are shown in d. Arrowheads represent autophagosomes. Size bar, 10 μm. (e-f) HeLa cells were transfected with either control siRNA (si-CTRL) or siRNA specifically targeting Atg12 (si-ATG12), PKCδ (si-PKCδ) or PKCα (si-PKCα). Cells were treated with rapamycin (25 μg/mL) and fixed at 2 h post-treatment. 100 cells were counted and scored for autophagosomes/cell via fluorescence microscopy. Representative confocal z-slices are shown f. Arrowheads indicate autophagosomes and size bar, 10 μm. Data represent the mean ± standard error (s.e.m.) for at least three independent experiments.
Table S1 - Lipid probes used in this study
Table S2 - Yeast strains used in this study