Background: TRIC channels likely mediate counter-K+ movements during store Ca2+ release, but their roles are unknown in many cell types.
Results: Smooth muscle-specific Tric-a-transgenic mice developed hypotension due to facilitated hyperpolarization signaling in vascular muscle.
Conclusion: TRIC-A channels facilitate Ca2+ spark generation in vascular muscle.
Significance: TRIC-A channels may have unexplained roles in store Ca2+ distribution but provide a potential pharmaceutical target for vasomodulation.
Keywords: Calcium Imaging; Calcium Intracellular Release; Potassium Channels; Ryanodine Receptor; Vascular Smooth Muscle Cells; Blood Pressure; Ca2+ Spark; Ca2+-dependent K+ Channel; Inositol 1,4,5-Trisphosphate receptor; TRIC Channel
Abstract
The TRIC channel subtypes, namely TRIC-A and TRIC-B, are intracellular monovalent cation-specific channels and likely mediate counterion movements to support efficient Ca2+ release from the sarco/endoplasmic reticulum. Vascular smooth muscle cells (VSMCs) contain both TRIC subtypes and two Ca2+ release mechanisms; incidental opening of ryanodine receptors (RyRs) generates local Ca2+ sparks to induce hyperpolarization and relaxation, whereas agonist-induced activation of inositol trisphosphate receptors produces global Ca2+ transients causing contraction. Tric-a knock-out mice develop hypertension due to insufficient RyR-mediated Ca2+ sparks in VSMCs. Here we describe transgenic mice overexpressing TRIC-A channels under the control of a smooth muscle cell-specific promoter. The transgenic mice developed congenital hypotension. In Tric-a-overexpressing VSMCs from the transgenic mice, the resting membrane potential decreased because RyR-mediated Ca2+ sparks were facilitated and cell surface Ca2+-dependent K+ channels were hyperactivated. Under such hyperpolarized conditions, L-type Ca2+ channels were inactivated, and thus, the resting intracellular Ca2+ levels were reduced in Tric-a-overexpressing VSMCs. Moreover, Tric-a overexpression impaired inositol trisphosphate-sensitive stores to diminish agonist-induced Ca2+ signaling in VSMCs. These altered features likely reduced vascular tonus leading to the hypotensive phenotype. Our Tric-a-transgenic mice together with Tric-a knock-out mice indicate that TRIC-A channel density in VSMCs is responsible for controlling basal blood pressure at the whole-animal level.
Introduction
Two major classes of intracellular Ca2+ channels, inositol 1,4,5-trisphosphate receptors (IP3Rs)3 and ryanodine receptors (RyRs), provide the fundamental pathway for Ca2+ release from internal stores into the cytoplasm (1–4). IP3R-mediated Ca2+ release from IP3-sensitive stores in the sarco/endoplasmic reticulum (SR/ER) plays a central role in cellular signaling systems. RyR-mediated Ca2+ release from caffeine-sensitive stores also regulates important cellular functions such as excitation-contraction coupling, secretion/exocytosis, and gene expression, primarily in excitable cells. When the divalent cation Ca2+ is released from the SR/ER, a negative potential is likely generated on the luminal side and may inhibit subsequent Ca2+ release. Therefore, counterion movements may balance SR/ER membrane potential to establish efficient Ca2+ release mechanisms in various cell types (5, 6). Indeed, previous electrophysiological and fluorometric analyses detected several ionic currents, such as K+, Cl−, and H+ fluxes, across the SR/ER membrane (7–10). However, the channel/transporter components mediating such ionic flows have not been identified, and thus, their contributions to the proposed counterion movements are largely unknown.
Recently, we identified two TRIC (trimeric intracellular cation) channel subtypes, TRIC-A and TRIC-B, that assemble into bullet-shaped homotrimers to form monovalent cation-selective channels in the SR/ER and nuclear membranes (11–13). Double-knock-out mice lacking both the Tric-a and Tric-b genes show embryonic cardiac failure, and the mutant cardiomyocytes display weak RyR-mediated Ca2+ release from the SR (14). Tric-a knock-out mice develop hypertension associated with vascular hypertonicity, and the mutant vascular smooth muscle cells (VSMCs) show insufficient Ca2+ sparks for inducing hyperpolarization (15). Moreover, mutant skeletal muscle from Tric-a knock-out mice occasionally exhibits alternan contraction responses, likely due to destabilized RyR-mediated Ca2+ release (16). On the other hand, Tric-b knock-out mice develop respiratory failure at birth, and the mutant alveolar epithelial cells exhibit compromised IP3R-mediated Ca2+ release and insufficient handling of surfactant lipids (17). These defects observed in the knock-out mice support our hypothesis that TRIC channel subtypes partly mediate counterion movements to facilitate SR/ER Ca2+ release.
VSMCs possess both caffeine- and IP3-sensitive stores and also contain both TRIC-A and TRIC-B channels. Therefore, VSMCs are an ideal model system to examine the physiological functions of TRIC channel subtypes. We recently detected not only insufficient RyR-mediated Ca2+ sparks but also facilitated IP3R-mediated Ca2+ transients in Tric-a knock-out VSMCs (15). These observations led to the hypothesis that TRIC-A channels preferentially support RyR-mediated Ca2+ release and also regulate Ca2+ distribution between the caffeine- and IP3-sensitive stores. To further verify this hypothesis, we planned to generate mutant mice carrying the SMC-specific Tric-a transgene and to examine altered features in Tric-a-overexpressing VSMCs.
EXPERIMENTAL PROCEDURES
Transgenic Mice and Blood Pressure Monitoring
Transgenic mice overexpressing TRIC-A channels under the control of the human α-smooth muscle actin promoter were generated and genotyped as previously described (15). The transgenic and wild-type littermates (8–12 weeks old) were examined physiologically and biochemically in this study. All experiments were conducted with the approval of the Animal Research Committee at Kyoto University according to the regulations on animal experimentation at Kyoto University. To telemetrically monitor arterial blood pressure, PA-C10 transmitters (Data Sciences International, New Brighton, MN) were implanted into mice, and the pressure-sensing catheters were inserted into the aortic arch under anesthesia. Radio signals from the implanted transmitter were captured using the Physiotel RPC-1 receiver (Data Sciences International), and the data were stored online using the Dataquest ART data acquisition system (Data Sciences International). Blood pressure and heart rate in the conscious state were also monitored by tail-cuff plethysmography (Model BP-98A-L, Softron) during the daytime.
Anatomical Analysis
Histological and ultrastructural analyses were carried out as described previously (18). Briefly, mesenteric arteries from adult mice were fixed in 3% paraformaldehyde, 2.5% glutaraldehyde, and 0.1 m sodium cacodylate, pH 7.4. After the tissues were dehydrated and embedded in Epon, ultrathin sections (∼80 nm thick) were prepared and stained with toluidine blue for histological observation or uranyl acetate and lead citrate for electron microscopic analysis (JEM-200CX, JEOL).
Imaging Analysis
Single VSMCs were enzymatically isolated from mesenteric arteries and subjected to Ca2+ spark and membrane potential monitoring as described previously (15, 19). Briefly, single VSMCs were seeded onto glass-bottomed dishes and incubated with 5 μm Fluo-4AM (Dojindo) in HEPES-buffered saline: 137 mm NaCl, 5.9 mm KCl, 2.2 mm CaCl2, 1.2 mm MgCl2, 14 mm glucose, and 10 mm HEPES, pH 7.4, with NaOH). The Ca2+ spark images with excitation at 488 nm and emission at >520 nm were captured using a total internal reflection fluorescence microscopy system (TE-2000U, Nikon), and the data were analyzed using the custom software Aquacosmos (Hamamatsu Photonics). For membrane potential monitoring, single VSMCs on glass-bottomed dishes were perfused with HEPES-buffered saline containing 200 nm oxonol VI (Fluka). Fluorescence images with excitation at 559 nm and emission at >606 nm were captured at a sampling rate of ∼1.6 s using a confocal microscope system (FV1000, Olympus).
For monitoring intracellular Ca2+ concentration ([Ca2+]i) in VSMCs, the inner surface of the artery was gently wiped with gauze to remove endothelial cells as described previously (20). The resulting arterial muscle segments were incubated with 5 μm Fura-PE3AM (Santa Cruz) and 0.02% cremophor EL (Sigma) and tightly fixed with fine steel pins onto a silicone rubber sheet, which was placed on a glass-bottomed dish. A CCD camera (ImagEM, Hamamatsu Photonics) mounted on the microscope (DMI 4000B, Leica) equipped with a polychromatic illumination system (MetaFluor ver7.7r3, Molecular Device) was used to capture fluorescence images with excitation at 340 and 380 nm and emission at >510 nm at room temperature (∼23 °C).
Patch Clamp and Membrane Potential Recording
Whole-cell voltage clamp recording was carried out using the Axopatch 200B amplifier (Molecular Device) essentially as described previously (21). For spontaneous transient outward current (STOC) monitoring in single VSMCs, the STOC pipette solution (140 mm KCl, 4 mm MgCl2, 5 mm Na2ATP, 0.05 mm EGTA, and 10 mm HEPES, pH 7.2, with KOH) and HEPES-buffered saline as a bathing solution were utilized. For the recording of total Ca2+-dependent K+ currents, the BK pipette solution (140 mm KCl, 1 mm MgCl2, 6.1 mm CaCl2, 1 mm Na2ATP, 10 mm EGTA, and 10 mm HEPES, pH 7.2, with KOH, pCa 6.5) and HEPES-buffered saline containing 100 μm CdCl2 as a bathing solution were utilized. All physiological measurements were carried out at room temperature.
The arterial muscle segments were subjected to transmembrane potential monitoring with high resistance glass microelectrodes (30–50 megaohms) filled with 3 m KCl solution as described previously (22). Membrane potential was determined as an average of the potentials obtained from stable impalements longer than 1 min from different areas in the muscle segment. The transmembrane potential was amplified by a high-input impedance amplifier with capacitance neutralization (MEZ-7200, Nihon Kohden), and the electrical signals were digitized and analyzed using a Power Lab system (AD Instruments). The bath solution used was modified Krebs solution babbled with 95% O2, 5% CO2 and maintained at 37 °C at pH ∼ 7.4 (112 mm NaCl, 4.7 mm KCl, 2.2 mm CaCl2, 1.2 mm MgSO4, 25 mm NaHCO3, 1.2 mm KH2PO4, and 14 mm glucose).
RT-PCR and Immunochemical Analysis
For quantitative PCR analysis, total RNA preparations from various tissues (at least three mice of each genotype) were used as templates for cDNA synthesis (ReverTra ACE qPCR-RT kit, Toyobo) and analyzed using a real-time PCR system according to the manufacturer's instructions (Thermal Cycler TP800, Takara). The cycle threshold was determined from the cDNA amplification curve as an index for relative mRNA content in each reaction. The PCR primer sets used in this study were listed previously (15). For immunochemical analysis, tissue lysates and fixed VSMCs were examined using primary antibodies to TRIC channel subtypes and calnexin (Santa Cruz) as described previously (14, 18).
RESULTS
Hypotension in SMC-specific Tric-a-Transgenic Mice
We previously generated transgenic mice with SMC-specific overexpression of TRIC-A channels using the α-smooth muscle actin promoter (23). Although the constructed Tric-a-transgene rescues the hypertensive phenotype developed in Tric-a knock-out mice (15), the effects of the transgene have not been examined in a wild-type genetic background. Therefore, we generated two Tric-a-transgenic mouse lines in wild-type backgrounds, which we designated as TgA3 and TgA20 mice. Both transgenic mouse lines grew and reproduced normally (supplemental Fig. S1A). RT-PCR analysis demonstrated that the transgene was highly expressed in blood vessels and other tissues containing SMCs, and its expression was faintly detected in the liver and skeletal muscle (supplemental Fig. S1B). In accordance with our previous observation that the transgene dosage in the TgA20 mouse line is higher than in the TgA3 line (15), TgA20 mice showed higher transgene expression in SMC-containing tissues than TgA3 mice in RT-PCR analysis (supplemental Fig. S1C).
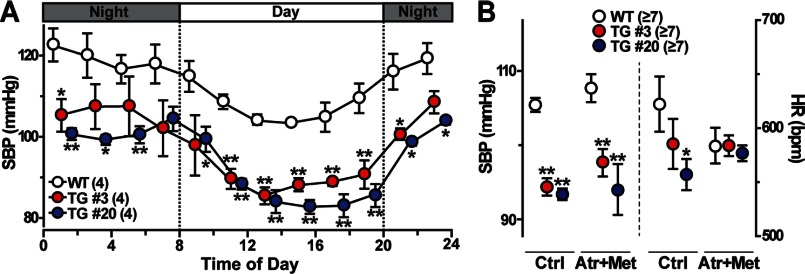
By telemetric blood pressure monitoring, both TgA20 and TgA3 mice, even at a young-adult age, showed low blood pressure levels throughout the entire day (Fig. 1A). This hypotensive phenotype was further confirmed by tail-cuff monitoring (Fig. 1B). Most likely reflecting the difference in transgene expression between the mouse lines, TgA20 mice developed slightly lower blood pressure than TgA3 mice. Elevated blood pressure stimulates the baroreceptor reflex and effectively decreases heart rate in normal animals (24). However, in both transgenic mice, a slow heart rate was clearly observed under hypotensive conditions. The blockage of cardiac autonomic controls by coapplication of the muscarinic blocker atropine and the β-adrenoreceptor blocker metoprolol ameliorated the bradycardic phenotype, whereas low blood pressure was clearly maintained in the transgenic mice (Fig. 1B). Therefore, ectopic Tric-a expression in baroreceptors and/or autonomic neurons likely produced vagal-dominant states in both transgenic mice. Moreover, the α1-adrenoreceptor blocker prazosin induced similar vasodilating effects in both wild-type and transgenic mice (supplemental Fig. S1E). These observations suggest that impaired sympathetic stimuli to the heart and vascular smooth muscle were not significant contributors to hypotension in the transgenic mice. It may be reasonable that altered vascular functions underlie the hypotensive phenotype in both transgenic mice because Tric-a knock-out mice develop hypertension due to increased vascular tonus (15).
FIGURE 1.
Hypotension in SMC-specific Tric-a-transgenic mice. A, telemetric blood pressure monitoring is shown. Circadian fluctuations in systolic blood pressure (SBP) were monitored, and the data were averaged over each 2-h interval during a 24-h period. B, systolic blood pressure and heart rate (HR) monitoring by tail-cuff plethysmography during the daytime. After the base-line monitoring (Ctrl), autonomic controls were blocked using an intraperitoneal injection of the muscarinic antagonist atropine (Atr, 4 mg/kg) and the β-antagonist metoprolol (Met, 4 mg/kg). Upon drug application, HR was quickly attenuated, but systolic blood pressure changed in a time-dependent manner (see supplemental Fig. S1E). The data represent the mean ± S.E., and the numbers of mice examined are shown in parentheses. Significant differences between the genotypes are marked with asterisks (*, p < 0.05; **, p < 0.01 by Student's t test).
Tric-a-Overexpressing VSMCs from the Transgenic Mice
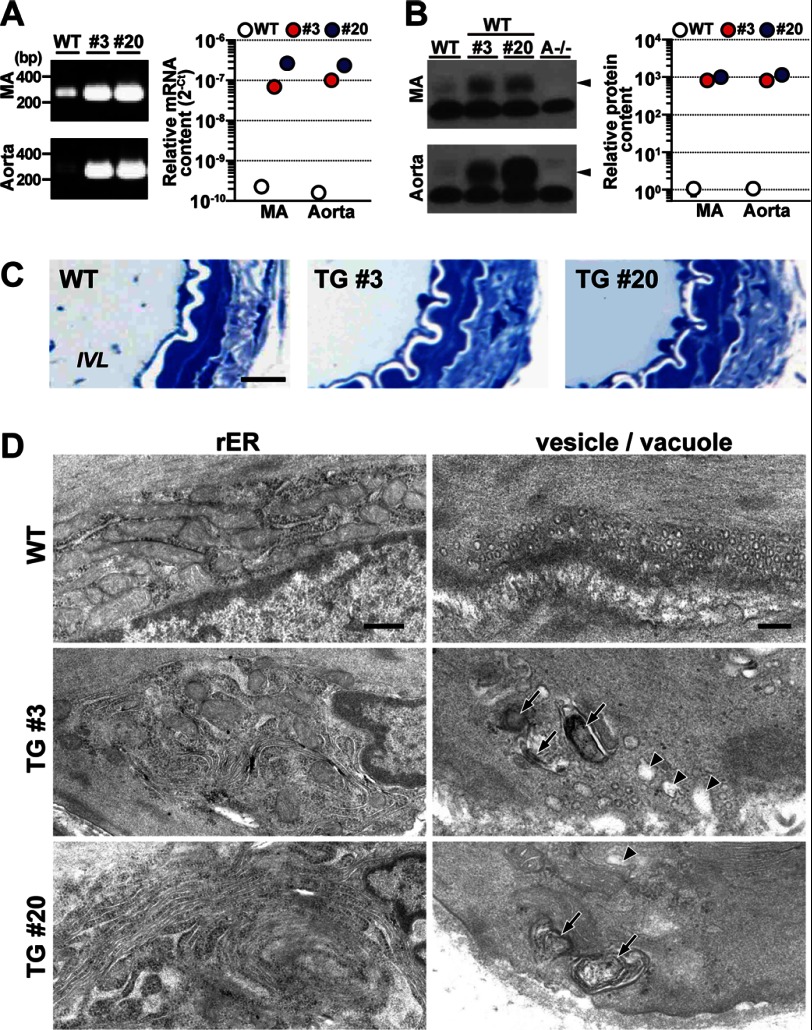
Resistance arteries distributed to peripheral tissues are primarily responsible for blood pressure maintenance. The mesenteric artery is a typical resistance vessel and was examined in our studies below. By RT-PCR analysis of mesenteric arteries, the Tric-a mRNA levels in TgA20 and TgA3 mice were at least 400-fold greater than those of wild-type controls (Fig. 2A). Western blot analysis confirmed Tric-a overexpression, and enhanced immunoreactivities provided estimates that the TRIC-A protein levels in the arteries from the transgenic mice were increased more than 500-fold compared with wild-type arteries (Fig. 2B). However, neither the heart nor skeletal muscle from the transgenic mice showed an obvious enhancement of TRIC-A immunoreactivity (supplemental Fig. S1D). Such SMC-specific overexpression of TRIC-A channels may not cause serious damage to the fundamental functions of resistance arteries because the transgenic mice retained circadian blood pressure variation (Fig. 1A) and regular blood pressure fluctuation in response to typical vasodilators targeting VSMCs (supplemental Fig. S1E).
FIGURE 2.
Irregular membranous features of Tric-a-overexpressing VSMCs. A, shown is quantitative detection of Tric-a mRNA in mesenteric artery (MA) and aorta. Total tissue RNA preparations from transgenic (#3 and #20) and wild-type mice (n = 3) were examined by quantitative RT-PCR using a primer set for amplifying Tric-a mRNA from both the endogenous gene and the transgene. The cDNA fragments amplified by 33 PCR cycles were analyzed by agarose gel electrophoresis. RT-PCR data obtained are summarized in the right graph. The cycle threshold (Ct) indicates the cycle number at which the amount of amplified cDNA reached a fixed threshold in each reaction. B, Western blot analysis of TRIC-A protein in MA and aorta is shown. The vessels dissected from the Tric-a-transgenic (#3 and #20), Tric-a knock-out (A−/−), and wild-type mice were homogenized to prepare total cell lysates (post-nuclear fractions). Wild-type lysates (10 μg of protein) with or without lysates from the transgenic mice (0.1 μg) were analyzed with an antibody against the TRIC-A protein. Lysates from the Tric-a knock-out mice (10 μg) served as negative controls. Arrowheads indicate TRIC-A protein bands. Immunoreactive signals were digitalized and statistically analyzed to estimate the relative contents of TRIC-A protein in the different genotypes as shown in the right graph. C, normal histology in MA from Tric-a-transgenic mice is shown. Mesenteric artery preparations were incubated in a Ca2+-free solution for ∼20 min and then fixed for anatomical analysis. Thin sections were stained with toluidine blue for photomicroscopic observation. IVL, intravascular lumen. Scale bar, 10 μm. D, shown is formation of stacked ER elements and vacuoles in Tric-a-overexpressing VSMCs. Rough ER (rER) elements at the cell-interior portion (left panels: scale bar, 500 nm) are shown. Stacks of rough ER elements were frequently observed in VSMCs from transgenic mice (∼27% of TgA3 cells and 80% of TgA20 cells), whereas such stacked ER was not detected in wild-type controls (n = 45–97 cells from 3–6 mice in each genotype). Surface vesicles and vacuoles were located at the cell periphery (right panels: scale bar, 200 nm). TRIC-A overexpression appeared to promote the formation of large-sized vacuoles containing myelin figures (arrows) or no electron-dense materials (arrowheads). Such vacuoles were frequently observed in >50% of VSMCs from TgA3 and TgA20 mice but only detected in 9.5% of wild-type VSMCs (n = 53–89 cells from 6 mice in each genotype).
Histological observations revealed no obvious abnormalities in mesenteric arteries from TgA20 and TgA3 mice (Fig. 2C). For example, under relaxation conditions after incubation in a Ca2+-free bathing solution, similar passive diameters and VSMC layer thicknesses of the arteries were observed in the transgenic and wild-type mice (supplemental Fig. S2A). These observations suggest that the hypotensive phenotype was mainly caused by insufficient myogenic tonus of resistance arteries under in vivo conditions in the transgenic mice.
Electron microscopic observation detected irregular membranous ultrastructures in mesenteric arteries from the transgenic mice. In Tric-a-overexpressing VSMCs, stacked rough ER elements accumulated around cell-interior regions (Fig. 2D, left panels). The generation of stacked rough ER has been repeatedly reported in various cell types overexpressing integral SR/ER membrane proteins (25, 26), and such irregular ER elements were not detected in wild-type VSMCs. Indeed, dense immunofluorescence signals of ectopic TRIC-A channels were predominantly observed in the cell-interior regions of VSMCs from transgenic mice (supplemental Fig. S2B), suggesting that the ER stacks were generated by nonspecific effects of excess TRIC-A protein. In SMCs, IP3Rs are distributed uniformly over the nuclear and ER/SR membranes (27, 28), whereas RyRs are predominantly localized in peripheral SR elements (29, 30). Therefore, it is possible that the formation of the stacked rough ER may have a functional impact on IP3-sensitive stores. However, such a morphological abnormality may minimally affect caffeine-sensitive stores.
In VSMCs, free-floating small vesicles, originally referred to as “surface vesicles” (31), were abundantly arranged underneath the cell membrane (Fig. 2D, right panels). Accompanied by the surface vesicles, larger-sized vacuoles with myelin figures (multilamellar lipid deposits) or without electron-dense material in the lumen were frequently observed in Tric-a-overexpressing VSMCs. Such vacuoles were rarely detected in wild-type VSMCs. To resolve the stacked ER elements, the large vacuoles may be aberrantly generated in Tric-a-overexpressing VSMCs. Alternatively, the excess amounts of TRIC-A protein may have promoted the swelling and fusion of normal small vesicles, generating the vacuoles. Swelling of SR elements is frequently accompanied by stored Ca2+ overloading in skeletal and cardiac muscle (16, 32, 33), and RyR-mediated Ca2+ sparks are predominantly generated underneath the cell membrane in VSMCs (3, 4). The facilitated formation of the vacuoles may imply altered Ca2+-handling characteristics in caffeine-sensitive stores of Tric-a-overexpressing VSMCs.
Facilitated Hyperpolarization Signaling in Tric-a-Overexpressing VSMCs
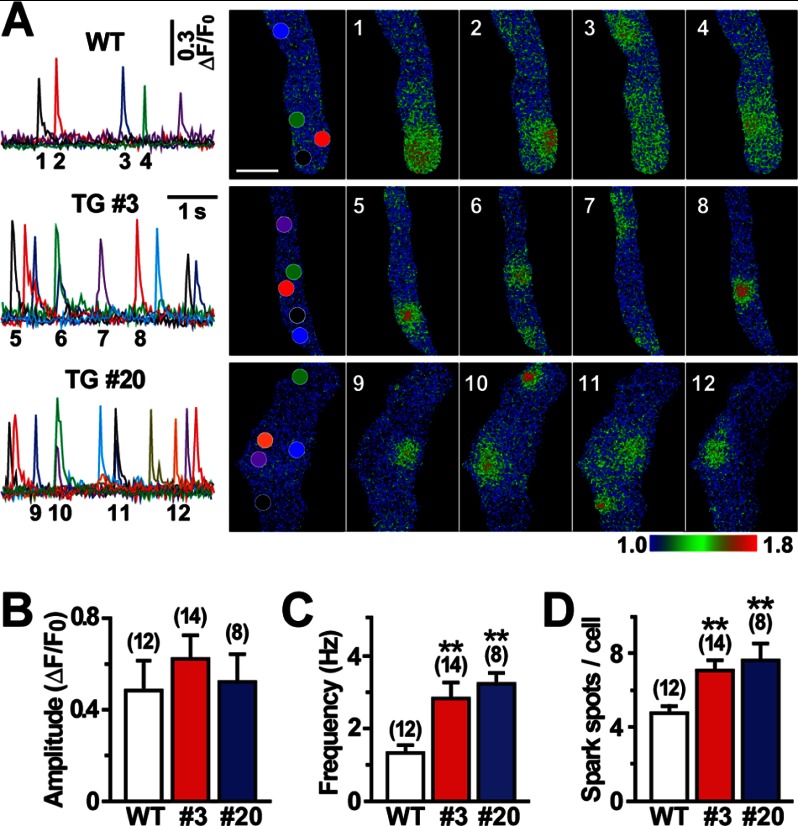
In VSMCs, incidental activation of RyRs generates Ca2+ sparks and then activates big-conductance Ca2+-dependent K+ (BK) channels to evoke STOCs, leading to hyperpolarization (3, 4). To identify the direct cause of the hypotensive phenotype, we investigated Ca2+ spark and STOC generation in VSMCs from the transgenic mice because hyperpolarization signaling is impaired in Tric-a knock-out VSMCs (15). Our Ca2+ spark imaging captured hyperactivated sparks at specified intracellular hotspots in single VSMCs prepared from the transgenic mice (Fig. 3A). Statistical analysis of the imaging data detected no difference in Ca2+ spark amplitude between the genotypes. However, Ca2+ spark frequency was substantially elevated, and the number of spark hotspots was significantly increased in Tric-a-overexpressing VSMCs from both TgA20 and TgA3 mice (Fig. 3, B–D). RT-PCR analysis detected no altered gene expression of the key components in intracellular Ca2+ signaling and G protein-coupled receptor signaling, such as RyR, IP3R, Ca2+-pump protein, α-adrenoreceptor, and phospholipase C, in mesenteric arteries and thoracic aorta from the transgenic mice (supplemental Fig. S3A). Therefore, Tric-a overexpression appears to hyperactivate Ca2+ spark generation without affecting the cellular levels of major Ca2+-handling proteins in VSMCs.
FIGURE 3.
Facilitated Ca2+ sparks in Tric-a-overexpressing VSMCs. Single VSMCs were prepared from mesenteric arteries and loaded with Fluo-4 for total internal reflection fluorescence imaging. A, shown are representative Ca2+-spark monitoring data in a normal bathing solution. The fluorescence intensity was normalized to the base-line intensity to yield the relative intensity (F/F0), and time courses of the intensity changes at the subcellular hotspots (see colored circles in the F0 cell images) are illustrated in the traces (scale bar, 5 μm). The F/F0 images were color-coded as indicated by the bar to prepare the Ca2+-spark images at the numbered time points. The data on spark amplitude (B), frequency (C), and spot number (D) are summarized for each genotype. Significant differences between the genotypes are indicated by asterisks (**, p < 0.01 by t test).
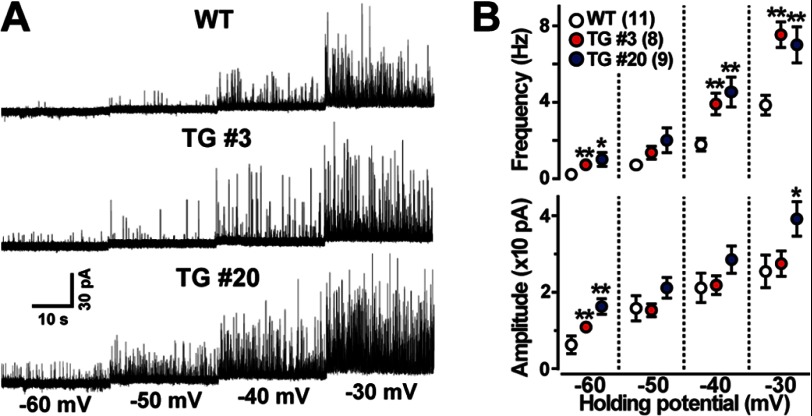
For monitoring BK channel-mediated STOCs, single VSMCs were examined by patch clamp recording using the nominally Ca2+-free STOC pipette solution. VSMCs from the transgenic mice showed highly activated STOCs (Fig. 4A). In particular, both STOC frequency and amplitude were markedly increased at a holding potential of −60 mV near the resting potential in the Tric-a-overexpressing cells (Fig. 4B). We also monitored total K+ currents using the Ca2+-containing BK pipette solution and examined the effects of the BK channel inhibitor iberiotoxin on VSMCs. VSMCs from the transgenic mice showed normal levels of total K+ currents both sensitive and insensitive to iberiotoxin (supplemental Fig. S3B). Therefore, despite normal cell-surface densities of BK channels, STOC generation was likely facilitated under physiological conditions in Tric-a-overexpressing VSMCs. The enhanced STOCs were fully consistent with the hyperactivated Ca2+ sparks observed (Fig. 3), suggesting that hyperpolarization signaling generated by functional cross-talk between the RyR and BK channels was stimulated in VSMCs from the transgenic mice.
FIGURE 4.
Facilitated STOCs in Tric-a-overexpressing VSMCs. The membrane potential of single VSMCs was controlled by the whole-cell patch clamp technique using the STOC pipette to monitor membrane currents. Representative STOC recording data are illustrated in A. The data for STOC frequency and amplitude are summarized in B. The data represent the mean ± S.E., and the numbers of cells examined from at least three mice are shown in parentheses. Significant differences between the genotypes are indicated by asterisks (*, p < 0.05; **, p < 0.01 by t test).
Deepened Resting Potential in Tric-a-Overexpressing VSMCs
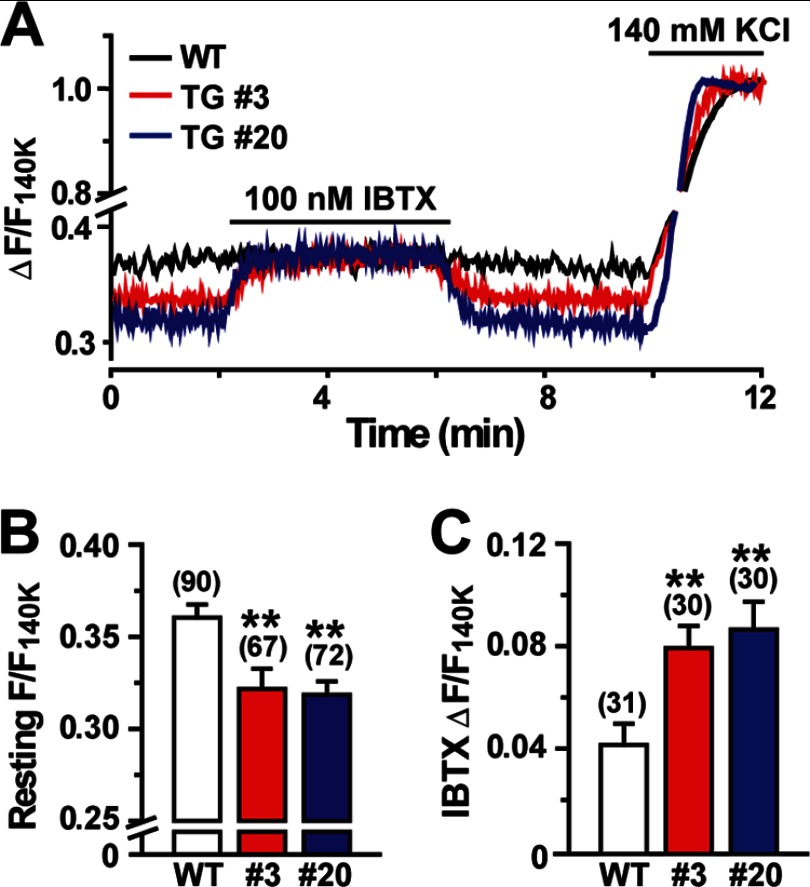
In SMCs, BK channel-mediated STOCs contribute to the maintenance of resting membrane potential and the fine-tuning of cellular excitability (3, 4). Based on the facilitated STOCs observed, we next focused on membrane potential in Tric-a-overexpressing VSMCs and leveraged confocal microscopic imaging using the voltage-dependent dye oxonol VI. Imaging analysis showed that fractional fluorescence intensity was clearly decreased in VSMCs from both TgA20 and TgA3 mice at basal conditions (Fig. 5A). The decreased intensities suggested that Tric-a-overexpressing VSMCs were deeply hyperpolarized during the resting state (Fig. 5B). We previously prepared the calibration plot representing the relationship between the fluorescence intensity and membrane potential using the reported resting potential of −59.9 mV in wild-type VSMCs (15, 34). The calibration plot estimated that the resting membrane potentials of TgA3, TgA20, and Tric-a knock-out VSMCs were −63.6, −63.9, and −54.6 mV, respectively. The estimated potentials agreed closely with actual measurement values from direct monitoring using high resistance microcapillary electrodes; the detected resting potentials of wild-type, TgA20, and Tric-a knock-out VSMCs were −59.4 ± 0.6, −64.8 ± 1.0, and −53.7 ± 0.7 mV, respectively (supplemental Fig. S3C). Moreover, from oxonol VI imaging, enlarged intensity shifts were evoked by iberiotoxin in VSMCs from the transgenic mice (Fig. 5C). The enhanced responses directly reflected the hyperactivation of BK channels at resting conditions in Tric-a-overexpressing VSMCs. However, while being exposed to iberiotoxin, similar fractional intensities were observed in wild-type and Tric-a-overexpressing VSMCs. Because many channels and transporters, in addition to BK channels, contribute to generating resting potential, the iberiotoxin-induced realignment of resting intensities suggested that the components other than BK channels functioned normally on the cell surface of Tric-a-overexpressing VSMCs. In summary, these results suggest that specific hyperactivation of BK channels lowered resting membrane potential in VSMCs from the transgenic mice.
FIGURE 5.
Decreased resting membrane potential in Tric-a-overexpressing VSMCs. Single VSMCs were examined by confocal microscopic imaging using the voltage-dependent dye oxonol VI. Cellular fluorescence intensities were normalized to the maximum value to yield the fractional intensity (F/F140K). A, shown are representative imaging data. B, shown are the summarized data of the resting intensity. C, shown are the summarized data of the intensity shift by iberiotoxin (IBTX). The data represent the mean ± S.E., and the numbers of cells examined from at least three mice are shown in parentheses. Significant differences between the genotypes are indicated by asterisks (**, p < 0.01 by t test).
Altered Ca2+ Handling in Tric-a-Overexpressing VSMCs
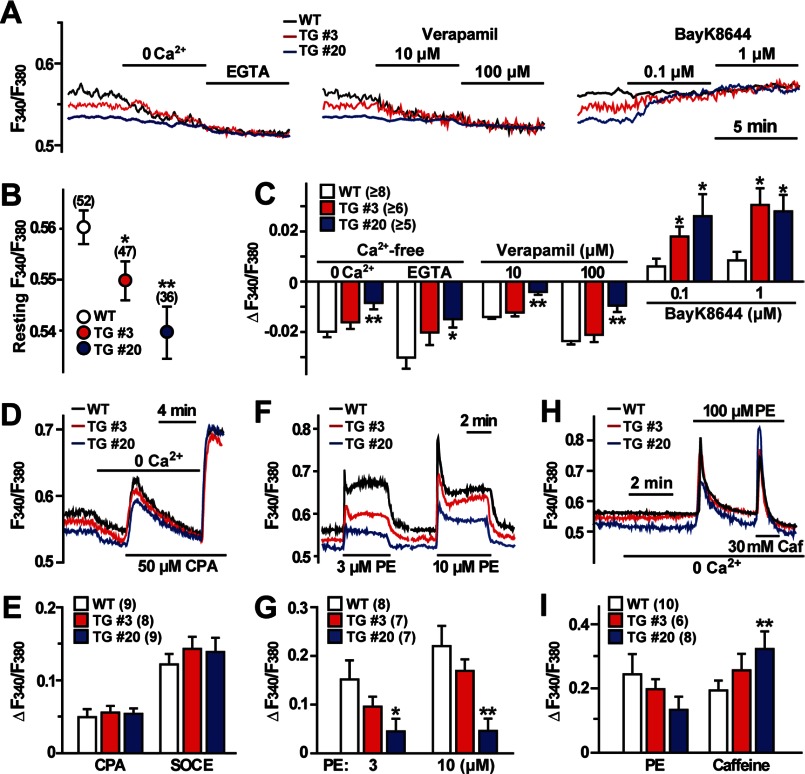
Of the excitable cell types, SMCs have relatively shallow resting membrane potentials. In VSMCs at basal conditions, L-type voltage-gated Ca2+ channels generate persistent Ca2+ influx and maintain both resting [Ca2+]i and intrinsic tonus (3, 4). We examined cellular Ca2+ handling in VSMCs from the transgenic mice and found several abnormal features. First, our ratiometric Fura-PE3 imaging detected that resting [Ca2+]i was markedly decreased in VSMCs from the transgenic mice (Fig. 6, A and B). In accordance with the detected membrane potentials, TgA20 cells exhibited slightly lower [Ca2+]i than TgA3 cells. In a Ca2+-free extracellular solution, similar resting [Ca2+]i levels were observed in wild-type and Tric-a-overexpressing VSMCs. Moreover, the downward-adjusted resting [Ca2+]i was clearly rescued by the L-type Ca2+ channel inhibitor verapamil and the activator BayK8644 in Tric-a-overexpressing VSMCs (Fig. 6C). Therefore, L-type Ca2+ channels were likely inactivated at basal conditions, and resting [Ca2+]i was thus decreased in Tric-a-overexpressing VSMCs.
FIGURE 6.
Abnormal Ca2+ handling in Tric-a-overexpressing VSMCs. VSMC segments were examined by Fura-PE3 Ca2+ imaging. The change in [Ca2+]i was expressed as ratio of the fluorescence intensity (F340/F380). A–C, shown is decreased resting [Ca2+]i in Tric-a-overexpressing VSMCs. A, representative traces under normal conditions and responses to Ca2+ removal and L-type Ca2+ channel modulators are illustrated. Resting [Ca2+]i data are summarized in B, and [Ca2+]i responses are analyzed in C. D and E, normal Ca2+ store contents in Tric-a-overexpressing VSMCs are shown. Representative CPA-induced responses are shown in D, and the data are summarized in E. F and G, impaired IP3-mediated Ca2+ release in Tric-a-overexpressing VSMCs . Representative PE-induced responses are shown in F, and the data are summarized in G. H and I, altered Ca2+ distribution to store compartments in Tric-a-overexpressing VSMCs are shown. Sequential responses to PE and caffeine (Caf) under Ca2+-free conditions are shown in (H), and the data are summarized in (I). The data represent the mean ± S.E., and the numbers of mice examined are shown in parentheses. Significant differences between the genotypes are indicated by asterisks (*, p < 0.05; **, p < 0.01 by t test).
VSMCs contain both IP3- and caffeine-sensitive stores, and they are functionally interconnected (3, 4). The SR/ER Ca2+-pump inhibitor cyclopiazonic acid (CPA) causes Ca2+ leakage from both stores, and the total stored Ca2+ contents can be estimated from CPA-induced responses. CPA-induced Ca2+ depletion from intracellular stores generally triggers store-operated Ca2+ entry (35). VSMCs from the transgenic mice exhibited normal CPA-induced and store-operated Ca2+ entry responses (Fig. 6, D and E). However, Tric-a-overexpressing VSMCs showed weak IP3R-mediated Ca2+ release in response to the α-adrenoreceptor agonist phenylephrine (PE) (Fig. 6, F and G). We also observed that PE-induced responses were diminished after normal caffeine-evoked transients in Tric-a-overexpressing VSMCs (supplemental Fig. S4A). Furthermore, in response to sequential applications of PE and caffeine under Ca2+-free conditions, Tric-a-overexpressing VSMCs exhibited poor IP3R-mediated transients immediately followed by enhanced RyR-mediated transients (Fig. 6, H and I). It is unlikely that poor PE-induced transients were due to compromised phosphoinositide turnover signaling because RT-PCR analysis suggested normal expression of major components of adrenoreceptor-mediated signaling in Tric-a-overexpressing VSMCs (supplemental Fig. S3A). Therefore, in addition to facilitating Ca2+ spark generation as described above, Tric-a overexpression appeared to affect IP3-sensitive stores in VSMCs.
DISCUSSION
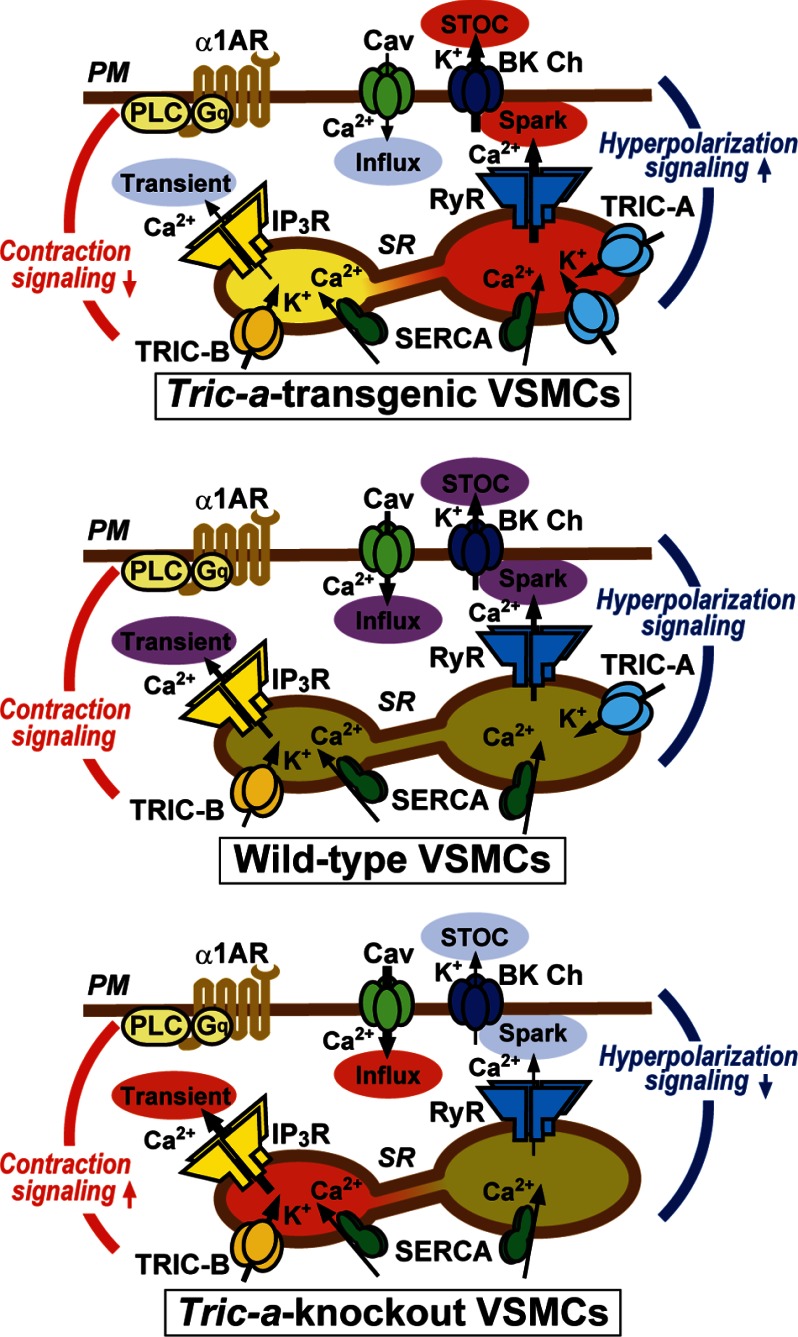
Single-channel recording in planar lipid bilayers demonstrated that purified TRIC-A and TRIC-B proteins form monovalent cation-specific channels (12–14). Therefore, TRIC channel subtypes likely function as SR/ER K+ channels under intracellular conditions. Although TRIC channels exhibit marked voltage dependence, they are likely to contribute to persistent K+ flux when membrane potential and osmotic pressure fluctuate in the SR/ER (11). In our previous study, Tric-a knock-out mice displayed high vascular tonus and developed hypertension because the hyperpolarization signaling triggered by Ca2+ sparks was compromised in the knock-out VSMCs (15). In the present study, as a polar opposite of the TRIC-A-null model, SMC-specific Tric-a-transgenic mice developed hypotension, and Ca2+ spark generation was facilitated in Tric-a-overexpressing VSMCs. Therefore, we reasonably conclude that TRIC-A channels support RyR-mediated Ca2+ spark generation and contribute to setting the basal tonus in VSMCs. From a biophysical point of view, the density-dependent contribution of TRIC-A channels to Ca2+ spark generation can be explained by an oversimplified scheme (supplemental Fig. S4B). Several lines of evidence indicate that RyR subtypes are nonselective cation channels equipped with large diameter pores and can bidirectionally conduct Ca2+ and K+ (36). Our model tentatively assumes that only K+ provided by TRIC-A or RyR channels acts as a counter ion for stabilizing SR membrane potential during Ca2+ release. In wild-type VSMCs, both TRIC-A and RyR channels may conduct counter-K+ to support Ca2+ release. However, the SR K+ currents are likely insufficient to stabilize membrane potential during vital Ca2+ release, and many events of incidental RyR opening may fail to grow to the Ca2+ sparks visualized in Ca2+-imaging analysis. Even lower SR K+ currents are proposed for Tric-a knock-out VSMCs, and thus, the growth to detectable Ca2+ sparks is further attenuated by the TRIC-A-null conditions. In contrast, in Tric-a-overexpressing VSMCs, enhanced SR K+ currents may no longer attenuate the growth of Ca2+ sparks. Additionally, the excess K+ currents mediated by overexpressed TRIC-A channels may persistently prevent negative-shifting of the membrane potential toward the Ca2+ reversal potential and could effectively maintain Ca2+ release from partially depleted stores. Such biophysical mechanisms likely underlie the facilitated Ca2+ sparks observed in Tric-a-overexpressing VSMCs. In the present study, several altered functions linked to the hypotensive phenotype were characterized in Tric-a-overexpressing VSMCs (Fig. 7). Excess TRIC-A channels facilitate Ca2+ spark (Fig. 3) and STOC generation (Fig. 4) and decrease resting membrane potential in VSMCs (Fig. 5). Under such hyperpolarized conditions, L-type Ca2+ channels are relatively inactivated, and resting [Ca2+]i is suppressed in Tric-a-overexpressing VSMCs (Fig. 6). Decreased [Ca2+]i in VSMCs likely reduces the intrinsic tonus of resistance arteries, leading to hypotension in the Tric-a-transgenic mice (Fig. 1).
FIGURE 7.
Facilitated hyperpolarization signaling in Tric-a-overexpressing VSMCs. Tric-a overexpression appears to activate Ca2+ spark generation directly, thus facilitating the hyperpolarization signaling produced by functional coupling between RyRs and BK channels (BK Ch) and decreasing the resting membrane potential in VSMCs. In this situation, L-type Ca2+ channels (Cav) are inactivated in the plasma membrane (PM), and resting [Ca2+]i is decreased to develop insufficient spontaneous tonus in resistance arteries and hypotension in the transgenic mice. Upon sympathetic stimulation, the α1-adrenoreceptor (α1AR), trimeric GTP-binding protein Gq, and phospholipase C (PLC) are coordinately activated to trigger IP3R-mediated Ca2+ release. In Tric-a-overexpressing VSMCs, IP3R-mediated Ca2+ release is unexpectedly impaired. The poor agonist-induced Ca2+ release may be due to partial depletion of IP3-sensitive stores or TRIC-A-mediated inhibition of IP3Rs. In contrast, hyperpolarization signaling is impaired, and IP3-sensitive stores are likely overloaded in Tric-a knock-out VSMCs (15). SERCA, sarco(endo)plasmic reticulum calcium ATPase.
VSMCs possess both caffeine- and IP3-sensitive stores and contain both TRIC-A and TRIC-B channels with similar estimated expression levels (15). In accordance with previous studies (37–39), our Ca2+ imaging also detected that caffeine-induced responses were larger than agonist-induced responses in wild-type VSMCs from mesenteric arteries (Fig. 6H). In addition to the altered features directly linked to hypotension, Tric-a-overexpressing VSMCs exhibited an unexplainable abnormality in SR Ca2+ handling; PE-induced Ca2+ release was attenuated despite unchanged total Ca2+ content in the SR (Fig. 6D). In contrast, Tric-a knock-out VSMCs exhibit facilitated PE-induced Ca2+ release from the Ca2+-overloaded SR (15). The alterations in PE-induced response appear to display a mirror image between Tric-a-overexpressing and knock-out VSMCs (supplemental Fig. S4A). Low Ca2+ contents of IP3-sensitive stores may contribute to impaired PE-induced Ca2+ release in Tric-a-overexpressing cells because the sequential application of PE and caffeine implied poor loading in IP3-sensitive stores (Fig. 6H). In this case, overexpressed TRIC-A channels may selectively gather Ca2+ into caffeine-sensitive stores to relatively deplete IP3-sensitive stores in VSMCs. Because IP3R channels are activated by both high luminal and cytoplasmic Ca2+ (1, 38), lowered luminal and cytoplasmic Ca2+ levels may synergistically inhibit IP3R channels in Tric-a-overexpressing VSMCs, whereas elevated Ca2+ levels on both sides could facilitate IP3Rs in Tric-a knock-out VSMCs. Alternatively, altered PE-induced responses could be explained by differentially modulated IP3Rs in Tric-a-overexpressing and knock-out VSMCs. In addition to generating countercurrents favorable to RyR activation, the TRIC-A protein might inhibit IP3R channels in VSMCs. These postulated inhibitory effects might be enhanced to remarkably depress PE-induced Ca2+ release in Tric-a-overexpressing VSMCs but might be removed to facilitate PE-evoked responses in Tric-a knock-out VSMCs. Furthermore, other molecular mechanisms can also be hypothesized for the altered PE-induced Ca2+ release depending upon TRIC-A contents. To further investigate the mysterious role of TRIC-A channels in IP3-sensitive stores, physiological measurements of the mutant VSMCs under membrane-permeable “skinned” conditions together with comprehensive proteomic survey for its binding partners in Ca2+ stores would be required in future studies. From another point of view, it can be presumed that TRIC-A and TRIC-B channels may have distinct subtype-specific roles in SR Ca2+ handling. To verify this possibility, Tric-b-overexpressing VSMCs, producible with the use of similar transgenic technology, will provide an important model system.
In our clinical study the TRIC-A gene polymorphisms in the Japanese population were associated with both hypertension susceptibility and sensitivity to antihypertensive medications (15). As discussed above, TRIC-A channel density in VSMCs is an important determinant of basal blood pressure at the whole animal level. Therefore, the human hypertensive risk allele is likely associated with relatively low Tric-a expression in VSMCs. Hypertension represents a major health problem with an appalling annual toll, and various antihypertensive drugs have been developed. However, hypertension still remains resistant to multiple antihypertensive medications in a considerable number of patients. Although renal sympathetic denervation has recently come into use in patients with malignant hypertension, novel target proteins are still required for creating new medication strategies (40). Our transgenic mice together with the knock-out mice suggest that TRIC-A channel openers may potentially be drugs with benefits for malignant hypertension.
Supplementary Material
Acknowledgments
We thank Yuki Mizobe for technical assistance and Dr. Masamitsu Iino for valuable comments on the experimental data.
This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Grants-in-Aid for Scientific Research and Platform for Drug Design, Discovery, and Development), the Ministry of Health, Labour and Welfare, the Vehicle Racing Commemorative Foundation, the Astellas Foundation, the Suzuken Memorial Foundation, the Kanae Foundation, the Japan Foundation for Applied Enzymology, the Takeda Science Foundation, the Ono Medical Research Foundation, and the Kowa Life Science Foundation.

This article contains supplemental Figs. S1—S4.
- IP3R
- inositol trisphosphate (IP3) receptor
- BK channel
- big-conductance Ca2+-dependent K+ channel
- CPA
- cyclopiazonic acid
- ER
- endoplasmic reticulum
- PE
- phenylephrine
- RyR
- ryanodine receptor
- STOC
- spontaneous transient outward current
- SR
- sarcoplasmic reticulum
- SMC
- smooth muscle cell
- VSMC
- vascular smooth muscle cell.
REFERENCES
- 1. Bezprozvanny I., Ehrlich B. E. (1995) The inositol 1,4,5-trisphosphate (InsP3) receptor. J. Membr. Biol. 145, 205–216 [DOI] [PubMed] [Google Scholar]
- 2. Meissner G. (1994) Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annu. Rev. Physiol. 56, 485–508 [DOI] [PubMed] [Google Scholar]
- 3. Berridge M. J. (2008) Smooth muscle cell calcium activation mechanisms. J. Physiol. 586, 5047–5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wellman G. C., Nelson M. T. (2003) Signaling between SR and plasmalemma in smooth muscle. Sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium 34, 211–229 [DOI] [PubMed] [Google Scholar]
- 5. Somlyo A. V., Gonzalez-Serratos H. G., Shuman H., McClellan G., Somlyo A. P. (1981) Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle. An electron-probe study. J. Cell Biol. 90, 577–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fink R. H., Veigel C. (1996) Calcium uptake and release modulated by counter-ion conductances in the sarcoplasmic reticulum of skeletal muscle. Acta Physiol. Scand. 156, 387–396 [DOI] [PubMed] [Google Scholar]
- 7. Coronado R., Miller C. (1980) Decamethonium and hexamethonium block K+ channels of sarcoplasmic reticulum. Nature 288, 495–497 [DOI] [PubMed] [Google Scholar]
- 8. Meissner G. (1983) Monovalent ion and calcium ion fluxes in sarcoplasmic reticulum. Mol. Cell. Biochem. 55, 65–82 [DOI] [PubMed] [Google Scholar]
- 9. Ide T., Sakamoto H., Morita T., Taguchi T., Kasai M. (1991) Purification of a Cl−-channel protein of sarcoplasmic reticulum by assaying the channel activity in the planar lipid bilayer system. Biochem. Biophys. Res. Commun. 176, 38–44 [DOI] [PubMed] [Google Scholar]
- 10. Kamp F., Donoso P., Hidalgo C. (1998) Changes in luminal pH caused by calcium release in sarcoplasmic reticulum vesicles. Biophys. J. 74, 290–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Venturi E., Sitsapesan R., Yamazaki D., Takeshima H. (2013) TRIC channels supporting efficient Ca2+ release from intracellular stores. Pflugers Arch. 465, 187–195 [DOI] [PubMed] [Google Scholar]
- 12. Pitt S. J., Park K.-H., Nishi M., Urashima T., Aoki S., Yamazaki D., Ma J., Takeshima H., Sitsapesan R. (2010) Charade of the SR K+-channel. Two ion-channels, TRIC-A and TRIC-B, masquerade as a single K+-channel. Biophys. J. 99, 417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Venturi E., Matyjaszkiewicz A., Pitt S. J., Tsaneva-Atanasova K., Nishi M., Yamazaki D., Takeshima H., Sitsapesan R. (2013) TRIC-B channels display labile gating. Evidence from the TRIC-A knockout mouse model. Pflugers Arch., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yazawa M., Ferrante C., Feng J., Mio K., Ogura T., Zhang M., Lin P.-H., Pan Z., Komazaki S., Kato K., Nishi M., Zhao X., Weisleder N., Sato C., Ma J., Takeshima H. (2007) TRIC channels are essential for Ca2+ handling in intracellular stores. Nature 448, 78–82 [DOI] [PubMed] [Google Scholar]
- 15. Yamazaki D., Tabara Y., Kita S., Hanada H., Komazaki S., Naitou D., Mishima A., Nishi M., Yamamura H., Yamamoto S., Kakizawa S., Miyachi H., Yamamoto S., Miyata T., Kawano Y., Kamide K., Ogihara T., Hata A., Umemura S., Soma M., Takahashi N., Imaizumi Y., Miki T., Iwamoto T., Takeshima H. (2011) TRIC-A channels in vascular smooth muscle contribute to blood pressure maintenance. Cell Metab. 14, 231–241 [DOI] [PubMed] [Google Scholar]
- 16. Zhao X., Yamazaki D., Park K.-H., Komazaki S., Tjondrokoesoemo A., Nishi M., Lin P., Hirata Y., Brotto M., Takeshima H., Ma J. (2010) Ca2+ overload and sarcoplasmic reticulum instability in tric-a null skeletal muscle. J. Biol. Chem. 285, 37370–37376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamazaki D., Komazaki S., Nakanishi H., Mishima A., Nishi M., Yazawa M., Yamazaki T., Taguchi R., Takeshima H. (2009) Essential role of the TRIC-B channel in Ca2+ handling of alveolar epithelial cells and in perinatal lung maturation. Development 136, 2355–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takeshima H., Komazaki S., Nishi M., Iino M., Kangawa K. (2000) Junctophilins. A novel family of junctional membrane complex proteins. Mol. Cell 6, 11–22 [DOI] [PubMed] [Google Scholar]
- 19. Ohi Y., Yamamura H., Nagano N., Ohya S., Muraki K., Watanabe M., Imaizumi Y. (2001) Local Ca2+ transients and distribution of BK channels and ryanodine receptors in smooth muscle cells of guinea pig vas deferens and urinary bladder. J. Physiol. 534, 313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iwamoto T., Kita S., Zhang J., Blaustein M. P., Arai Y., Yoshida S., Wakimoto K., Komuro I., Katsuragi T. (2004) Salt-sensitive hypertension is triggered by Ca2+ entry via Na+/Ca2+ exchanger type-1 in vascular smooth muscle. Nat. Med. 10, 1193–1199 [DOI] [PubMed] [Google Scholar]
- 21. Hotta S., Morimura K., Ohya S., Muraki K., Takeshima H., Imaizumi Y. (2007) Ryanodine receptor type 2 deficiency changes excitation-contraction coupling and membrane potential in urinary bladder smooth muscle. J. Physiol. 582, 489–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kiyoshi H., Yamazaki D., Ohya S., Kitsukawa M., Muraki K., Saito S. Y., Ohizumi Y., Imaizumi Y. (2006) Molecular and electrophysiological characteristics of K+ conductance sensitive to acidic pH in aortic smooth muscle cells of WKY and SHR. Am. J. Physiol. Heart Circ. Physiol. 291, H2723–H2734 [DOI] [PubMed] [Google Scholar]
- 23. Nakano Y., Nishihara T., Sasayama S., Miwa T., Kamada S., Kakunaga T. (1991) Transcriptional regulatory elements in the 5′ upstream and first intron regions of the human smooth muscle (aortic type) α-actin-encoding gene. Gene 99, 285–289 [DOI] [PubMed] [Google Scholar]
- 24. Smith O. A. (1974) Reflex and central mechanisms involved in the control of the heart and circulation. Annu. Rev. Physiol. 36, 93–123 [DOI] [PubMed] [Google Scholar]
- 25. Pathak R. K., Luskey K. L., Anderson R. G. (1986) Biogenesis of the crystalloid endoplasmic reticulum in UT-1 cells. Evidence that newly formed endoplasmic reticulum emerges from the nuclear envelope. J. Cell Biol. 102, 2158–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Snapp E. L., Hegde R. S., Francolini M., Lombardo F., Colombo S., Pedrazzini E., Borgese N., Lippincott-Schwartz J. (2003) Formation of stacked ER cisternae by low affinity protein interactions. J. Cell Biol. 163, 257–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tasker P. N., Michelangeli F., Nixon G. F. (1999) Expression and distribution of the type 1 and type 3 inositol 1,4,5-trisphospate receptor in developing vascular smooth muscle. Circ. Res. 84, 536–542 [DOI] [PubMed] [Google Scholar]
- 28. Vermassen E., Van Acker K., Annaert W. G., Himpens B., Callewaert G., Missiaen L., De Smedt H., Parys J. B. (2003) Microtubule-dependent redistribution of the type-1 inositol 1,4,5-trisphosphate receptor in A7r5 smooth muscle cells. J. Cell Sci. 116, 1269–1277 [DOI] [PubMed] [Google Scholar]
- 29. Lesh R. E., Nixon G. F., Fleischer S., Airey J. A., Somlyo A. P., Somlyo A. V. (1998) Localization of ryanodine receptors in smooth muscle. Circ. Res. 82, 175–185 [DOI] [PubMed] [Google Scholar]
- 30. Moore E. D., Voigt T., Kobayashi Y. M., Isenberg G., Fay F. S., Gallitelli M. F., Franzini-Armstrong C. (2004) Organization of Ca2+ release units in excitable smooth muscle of the guinea pig urinary bladder. Biophys. J. 87, 1836–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Devine C. E., Somlyo A. V., Somlyo A. P. (1972) Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J. Cell Biol. 52, 690–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ikemoto T., Takeshima H., Iino M., Endo M. (1998) Effect of calmodulin on Ca2+-induced Ca2+ release of skeletal muscle from mutant mice expressing either ryanodine receptor type 1 or type 3. Pflugers Arch. 437, 43–48 [DOI] [PubMed] [Google Scholar]
- 33. Takeshima H., Komazaki S., Hirose K., Nishi M., Noda T., Iino M. (1998) Embryonic lethality and abnormal cardiac myocytes in mice lacking ryanodine receptor type 2. EMBO J. 17, 3309–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koshita M., Hidaka K., Ueno H., Yamamoto Y., Suzuki H. (2007) Properties of acetylcholine-induced hyperpolarization in smooth muscle cells of the mouse mesenteric artery. J. Smooth Muscle Res. 43, 219–227 [DOI] [PubMed] [Google Scholar]
- 35. Smyth J. T., Hwang S. Y., Tomita T., DeHaven W. I., Mercer J. C., Putney J. W. (2010) Activation and regulation of store-operated calcium entry. J. Cell Mol. Med. 14, 2337–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gillespie D., Chen H., Fill M. (2012) Is ryanodine receptor a calcium or magnesium channel? Roles of K+ and Mg2+ during Ca2+ release. Cell Calcium 51, 427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Iino M. (1990) Calcium release mechanisms in smooth muscle. Jpn. J. Pharmacol. 54, 345–354 [DOI] [PubMed] [Google Scholar]
- 38. Iino M. (2000) Molecular basis of spatio-temporal dynamics in inositol 1,4,5-trisphosphate-mediated Ca2+ signalling. Jpn. J. Pharmacol. 82, 15–20 [DOI] [PubMed] [Google Scholar]
- 39. Bolton T. B., Prestwich S. A., Zholos A. V., Gordienko D. V. (1999) Excitation-contraction coupling in gastrointestinal and other smooth muscles. Annu. Rev. Physiol. 61, 85–115 [DOI] [PubMed] [Google Scholar]
- 40. Laurent S., Schlaich M., Esler M. (2012) New drugs, procedures, and devices for hypertension. Lancet 380, 591–600 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.