Background: Pituitary growth hormone (GH) is a key hormone in the maintenance of glucose homeostasis.
Results: GH acts on the macrophage to blunt the deleterious effects of diet-induced obesity (DIO) on insulin sensitivity.
Conclusion: The effect of GH on glucose homeostasis is tissue-specific.
Significance: Administration of GH could have salutary effects on DIO-associated chronic inflammation and insulin resistance in humans.
Keywords: Adipose Tissue, Growth Hormone, Inflammation, Macrophages, Obesity
Abstract
We investigated GH action on macrophage (MΦ) by creating a MΦ-specific GH receptor-null mouse model (MacGHR KO). On a normal diet (10% fat), MacGHR KO and littermate controls exhibited similar growth profiles and glucose excursions on intraperitoneal glucose (ipGTT) and insulin tolerance (ITT) tests. However, when challenged with high fat diet (HFD, 45% fat) for 18 weeks, MacGHR KO mice exhibited impaired ipGTT and ITT compared with controls. In MacGHR KO, adipose-tissue (AT) MΦ abundance was increased with skewing toward M1 polarization. Expression of pro-inflammatory cytokines (IL1β, TNF-α, IL6, and osteopontin (OPN)) were increased in MacGHR KO AT stromal vascular fraction (SVF). In MacGHR KO AT, crown-like-structures were increased with decreased insulin-dependent Akt phosphorylation. The abundance of phosphorylated NF-κB and of OPN was increased in SVF and bone-marrow-derived MΦ in MacGHR KO. GH, acting via an NF-κB site in the distal OPN promoter, inhibited the OPN promoter. Thus in diet-induced obesity (DIO), lack of GH action on the MΦ exerts an unexpected deleterious effect on glucose homeostasis by accentuating AT inflammation and NF-κB-dependent activation of OPN expression. These novel results in mice support the possibility that administration of GH could have salutary effects on DIO-associated chronic inflammation and insulin resistance in humans.
Introduction
Pituitary growth hormone (GH)2 is a key hormone in the maintenance of glucose homeostasis. The canonical action of GH is to induce insulin resistance. The decrease in insulin sensitivity observed during puberty and in gestational diabetes is, in part, attributed to increased GH action (1, 2). Insulin resistance is a prominent clinical manifestation of GH treatment in adults and children, and glucose intolerance and diabetes mellitus are major clinical manifestations of GH excess in acromegaly (3, 4). Animal models of GH excess exhibit insulin resistance with hyperinsulinemia and hyperglycemia (5, 6) and endogenous GH contributes to impaired glucose metabolism in diabetic rats (7). Hence, the prevailing models of GH action predict that loss of GH action is likely to have salutary effects on insulin sensitivity.
Obesity is characterized by infiltration, accumulation, and activation of macrophage (MΦ) in adipose tissue (8, 9). MΦ in adipose tissue presents two distinct phenotypes, M1 and M2. In lean adipose tissue, MΦ expresses markers of M2 activation and secrete anti-inflammatory cytokines (10). Under this condition, glucose metabolic homeostasis and insulin sensitivity is maintained. Adipose tissues in obese individuals and in animal models of obesity are infiltrated by a large number of MΦ, which express M1 markers and secrete pro-inflammatory cytokines such as TNF-α, IL1b, and IL-6 (11, 12). These cytokines interact with adipocytes and cause chronic inflammation and insulin resistance (13). Certain chemoattractants, such as osteopontin (OPN), monocyte chemoattractant protein 1, and MΦ inflammatory proteins (MIPs) accelerate MΦ infiltration (14–17). OPN is a phosphorylated glycoprotein secreted by MΦ, monocytes, and activated T lymphocytes (18). Mice with global knock-out of OPN are resistant to diet-induced obesity (DIO) associated diabetes and this relative protection against DIO is attributed to decreased infiltration of MΦ in adipose tissue (19).
Whereas the role of MΦ in the pathogenesis of DIO associated diabetes is well established, the factor(s) regulating MΦ function in the development of DIO associated diabetes are not clear. One of the factors that could regulate the MΦ role in the pathogenesis of DIO is GH. Circulating levels of GH are low in obese individuals and both spontaneous GH secretion (20) and the GH secretory response to stimuli (e.g. arginine, GHRH-arginine, insulin-induced hypoglycemia) are impaired compared with normal subjects (21–25). The precise biological significance of low circulating levels of GH in obesity is not known. GH has been previously shown in vitro to regulate MΦ proliferation, migration, and cytokines production, and cellular uptake and degradation of low-density lipoprotein and rate of cholesterol esterification (26–30). Our previous studies indicate that GH can regulate MΦ cytokine production (27). To test these functions in vivo, we created a mouse model with targeted abrogation of GH receptor in the MΦ and using this model we now report novel, MΦ-dependent effects of GH on adipocyte function. Thus in DIO lack of GH action on the MΦ resulted in increased inflammation and decreased insulin sensitivity in adipose tissue. Furthermore, our studies reveal a direct effect of GH on OPN expression in the pathogenesis of these novel effects of GH on the MΦ.
EXPERIMENTAL PROCEDURES
Animals
Animals were housed in a specific pathogen-free animal facility at the University of Michigan, and all experiments were approved by the University of Michigan IACUC. Mice were maintained under a continuous light-dark cycle (light from 5:00 a.m. to 5:00 p.m.) and received either a standard diet or a high-fat diet (HFD) containing 45 kcal% fat (D12451, Research Diets, Inc). Mice were 7–8 weeks old at the beginning of the feeding of HFD. Body composition, measured at end of 18 weeks on HFD, was analyzed using NMR spectroscopy (minispec LF90; Bruker Optics).
Reagents
Phospho-Akt, total Akt antibodies, phospho-NF-κB, and total-NF-κB were purchased from Cell Signaling. β-Actin monoclonal antibody was obtained from Sigma-Aldrich. OPN and Anti-F4/80 antibodies were from Abcam. PE-conjugated anti-F4/80, PE/Cy7-conjugated anti-CD11c, Alexa 647-conjugated anti-CD206, and isotype antibodies were purchased from eBioscience. Anti-GH receptor AL-47 antibody was obtained from Dr. S. Frank (University of Alabama).
Generation of Mice with Targeted Deletion of GH Receptor in MΦ/Monocyte (MacGHR KO)
The generation of the homozygous GHR exon 4 floxed mice (Ghrfl/fl), MacGHR conditional knock-out mice (GhrΔ/fl;LysM-cre(+/−); designated as GHRMac KO), and GhrΔ/fl;LysM-cre(−/−) (designated as heterozygous control) have been previously described (27, 31).
Genotyping
DNA isolation and PCR were carried out using Terra PCR Direct Polymerase Mix (Clonetech). Primers for GH receptor genotyping: A: 5′-ctacacaaatacacacagatatac-3′; B: 5′-tcagcatgtttagaatcacaaga-3′; C: 5′-cttatagagaagccaagtattgga-3′.
Isolation of Peritoneal MΦ, Splenic Monocyte/Macrophage, and Stromal Vascular Fraction (SVF)
To isolate peritoneal MΦ, the mice were injected intraperitoneally with 3 ml of aged thioglycolate for 3 days prior to being euthanized. Peritoneal MΦ were obtained and purified by adherence as previously described (32). To isolate the adipose tissue stromal vascular fraction (SVF), the mice were perfused with PBS for 3 min to remove blood cells from circulation prior to harvesting of the epididymal and subcutaneous fat pads. The isolated fat pads were minced and incubated with 1 mg/ml collagenase (Sigma) for 20–30 min and filtered through a 100 μm nylon cell strainer. The strained cell mixtures were centrifuged at 1000 × g for 10 min and the pellet treated with ACK lysing buffer (Lonza) to remove erythrocytes to yield SVF. Splenic monocyte/macrophage cells were isolated by extruding the spleen through the cell strainer and rinsing the cell strainer with DMEM. Cells were collected by centrifugation and resuspended in 1 ml of ACK lysing buffer to remove erythrocytes Monocytes/macrophages were further purified by differential adherence to the culture plate; purity (>95%) of monocytes/macrophages was confirmed by staining for the macrophage marker, F4/80.
Isolation and Culture of Bone Marrow-derived MΦ (BMDM)
Femoral and tibial bones were isolated from control and MacGHR KO mice. Bone marrow cells were obtained by flushing the marrow cavity with DMEM using a 26 ½ gauge needle attached to a 1cc syringe. The bone-marrow cells were dispersed by pipetting and the erythrocytes were lysed with ACK lysing buffer. Cells were cultured in DMEM containing 10% heat-inactivated FBS, penicillin/streptomycin and 10 ng/ml M-CSF for 6 days. M-CSF was removed after 6 days of culture, and cells were treated with 10 ng/ml LPS or 40 ng/ml IL-4 for 24 h prior to collection for RNA extraction.
PCR Array
PCR array analysis was performed using RT2 profiler PCR array (Qiagen, SABiosciences mouse chemokines, and receptors, # PAMM-022) on the Applied Biosystems 7000 Prism using RT2 Real-Time SYBR Green PCR master mix. The total volume of the PCR was 25 μl. The thermocycler parameters were 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min.
Real-Time Quantitative PCR Assay (RT-qPCR)
Total RNA was isolated using Trizol reagent and then repurified using a column (RNeasy minikit; Qiagen, Valencia, CA) according to the manufacturer's protocol. In-column deoxyribonuclease digestion was performed for each sample to remove genomic DNA. Quantitative PCR was performed using QuantiTect SYBR Green RT-PCR Kit (Qiagen # 204243) as described previously (27). Primers for the cytokines were: IL1-b forward 5′-GGACCCATATGAGCTGAAAGC-3′, reverse 5′-TCGTGGCTTGGTTCTCCTTGT-3′; IL-6 forward 5′-TGGAGTCACAGAAGGAGTGGCTAAG-3′, reverse 5′-TCTGACCACAGTGAGGAATGTCCAC-3′; TNF-a, forward 5′-GACCCTCACACTCAGATCATCTTCT-3′, reverse 5′-CCACTTGGTGGTTTGCTACGA-3′; OPN forward 5′-CAGTATCCTGATGCCACAGATGA-3′, reverse 5′-ATGACATCGAGGGACTCCTTAGAC-3′.
Glucose and Insulin Tolerance Tests
Glucose tolerance tests were performed by intraperitoneal injection of glucose (2 g glucose per kg body weight) after a 16–18 h overnight fast. Insulin tolerance was performed by intraperitoneal injection of recombinant human regular insulin (1 unit insulin per kg body weight for mice on normal diet; 1.5 unit insulin per kg body weight for mice on HFD) in mice that had been fasted for 5 h. Blood glucose levels were measured using a glucometer from tail blood taken at indicated time points.
In Vivo and Ex Vivo Akt Activation
Akt activation in vivo was assessed in mice that had been fasted for 5 h and then injected intraperitoneally with 2 units/kg of insulin. 10 min after the injection, the respective tissue was harvested for further processing as described below. For assessing activation of Akt ex vivo, epididymal adipose tissue was harvested and preincubated for 10 min at 37 °C in Krebs-Ringer solution. For insulin stimulation, the adipose tissue was transferred directly to Krebs-Ringer solution containing 100 nm/ml insulin for 2 or 5 min at 37 °C. The tissue designated as the treatment control was similarly incubated but without insulin. After the treatment, the adipose tissues were washed with cold PBS and snap-frozen in dry ice, and stored at −80 °C until further processing. The tissues were homogenized in RIPA buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 2 mm EGTA, 0.1% Triton X-100) containing protease inhibitors and 0.1 mm NaOV4 and centrifuged (15,000 × g × 10 min). The protein extracts were subjected to Western blot analysis for quantification of total and phosphorylated Akt. Specific signals were detected using chemiluminescence techniques (ECL Plus; GE Healthcare) after incubation with appropriate horseradish peroxidase-conjugated secondary antibodies.
Flow Cytometry Analysis
SVF cells were incubated with Fc blocking solution for 5 min followed by fluorescence-conjugated antibodies (CD 206, CD11C, and F4/80) for 60 min at 4 °C. After staining, cells were washed twice with cold PBS, fixed in 0.5% PFA in PBS and analyzed using FACS Canto II.
Macrophage populations were identified by first gating singlets (by FSC-height versus FSC width) and identifying F4/80+ cells (PE). F4/80+ cells were gated and examined for CD11c (PE-Cy7) and CD206 (eFluor650) staining). The data analysis was performed using BD FACSDiva Software.
Histologic Analysis
Representative samples of adipose tissue isolated from the control and MacGHR KO mice were fixed in 1% of paraformaldehyde immediately after excision. These tissues were rinsed with PBS and blocked in 5% BSA in PBS for 1 h. Tissues were then stained with primary antibodies for 1 h prior to incubation with fluorescent-conjugated secondary antibodies for 1 h. After staining, sample was placed on a coverslip and examined using inverted fluorescence microscopy.
Luciferase Assay
OPN promoter regions were cloned into the pGL3 luciferase reporter vector (Promega) using the following primers. Forward primer: (F1 construct) 5′-GTCATATGGTTCAGCTCGAGGTGGCGCAGCTCCA-3′; Forward primer: (F2 construct) 5′-CACCCCTCGAGAGCAAGCATTCCAGTCTCACAAACTGCT-3′; Forward primer: (F3 construct) 5′-ATCAACTCGAGATTGTGTATCCATGTGGCCTTTATCTG-3′. Reverse primer for all the constructs: 5′-GGCCAAAGCTTCTGCAGGCTTACCTTGGCTGGTTTCCT-3′.
Renilla luciferase, pRL-TK (Promega) was used as an internal control. Luciferase activity was measured in cell extracts using the Dual Luciferase Assay system (Promega).
Mutagenesis
NF-κB binding site was mutated using mutagenesis kit from Promega. The sequence of the top strand was 5′-TGCTGCCTTAGGATTTGTGCCTGAACAACACTGAAAACA-3′; the sequence of the bottom strand was 5′-TGTTTTCAGTGTTGTTCAGGCACAAATCCTAAGGCAGCA.
Statistical Analysis
Mann-Whitney and Kruskal-Wallis nonparametric tests were used to analyze statistical differences between the distributions of two or multiple independent samples respectively. p values equal to or less than 0.05 were considered significant.
RESULTS
Characterization of MacGHR KO Mice
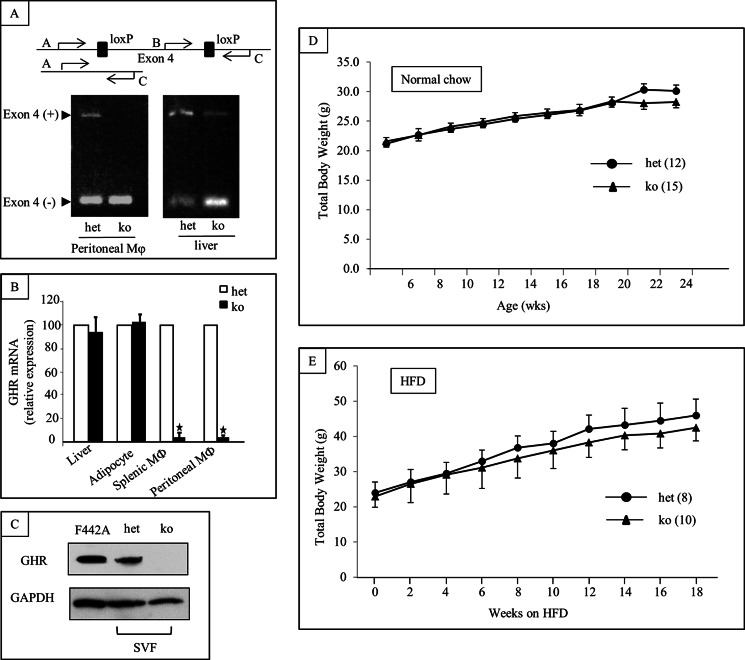
Targeted deletion of GH receptor in MΦ in MacGHR KO was confirmed by PCR of genomic DNA isolated from peritoneal MΦ (Fig. 1A), TaqMan RT-PCR (Fig. 1B), and Western blot analysis (Fig. 1C). Genomic DNA isolated from peritoneal MΦ from heterozygous controls and liver of both MacGHR KO and heterozygous controls yielded bands corresponding to both the intact Ghr allele (longer band), and the allele with the excised exon 4 (shorter band) because of their GhrΔ/fl genotype (Fig. 1A). In contrast, PCR of genomic DNA isolated from peritoneal MΦ from MacGHR KO yielded only the shorter band corresponding with excision of exon 4. These results indicate that excision of GH receptor exon 4 in MacGHR KO was restricted to MΦ. Whereas the expression level of GH receptor mRNA was not changed in liver and adipose tissue, expression was significantly decreased in monocytes, and MΦ isolated from spleen or peritoneal cavity of the MacGHR KO mice (Fig. 1B). Similarly, Western blot confirmed the absence of GH receptor protein expression in MacGHR KO MΦ (Fig. 1C).
FIGURE 1.
Targeted deletion of GH receptor in MΦ. A, genomic DNA PCR for Cre and the floxed GH receptor exon 4; absence of AC band containing exon 4 in peritoneal MΦ with retention of band in liver DNA. B, GHR mRNA expression is extinguished in MΦ (splenocytes and peritoneal) but retained in liver and adipocytes in MacGHR KO (ko, Ghr−/−, solid bar) versus het (Ghrfl/−, open bar); Internal control, GAPDH (mean ± S.E. (n = 4–5)), *, p < 0.05. C, Western blot with anti-GH receptor antibody (AL-47, 1:1000) demonstrating absence of GH receptor band in SVF MΦ from MacGHR KO. Positive control (F442A pre-adipocytes); loading control (GAPDH). D & E, weight profile of MacGHR KO (ko, Ghr−/−, ▴) and control mice (het (Ghrfl/−,●)) on normal chow (D) or high fat diet (E).
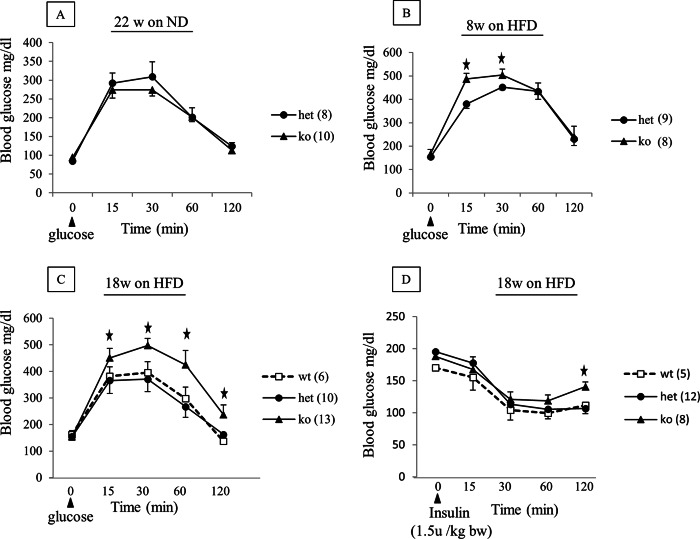
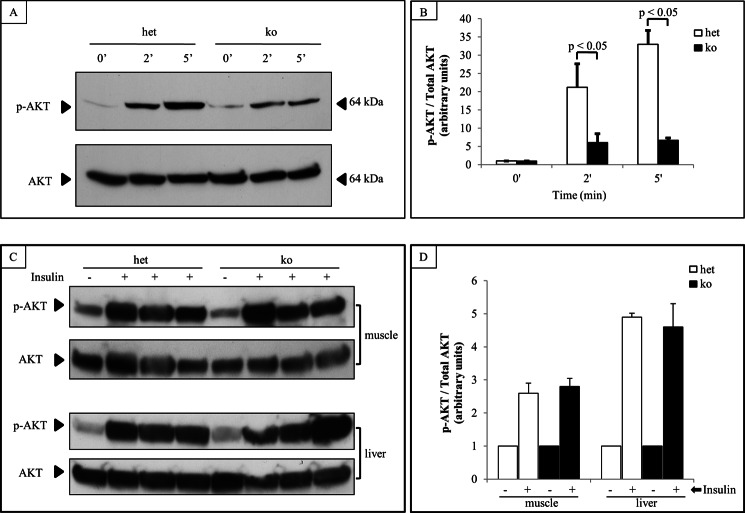
Diet-induced obesity associated impairment in glucose tolerance and increased insulin resistance are accentuated in MacGHR KO mice. On normal chow diet, there were no significant differences in the growth profile of MacGHR KO and their littermate controls (Fig. 1D). Similarly, feeding the animals with HFD for 18 weeks did not reveal significant differences between the weight profile of MacGHR KO and their littermate controls (Fig. 1E). To further investigate a potential role for GH in MΦ homeostasis in DIO, peritoneal glucose tolerance test (ipGTT) was performed on MacGHR KO and their littermate controls fed normal chow or HFD for 18 weeks. On normal chow diet there was no significant difference in the glucose profile of MacGHR KO compared with the control cohort (Fig. 2A). However, contrary to expectations based on the generally accepted notion that GH increases insulin resistance, on a HFD MacGHR KO progressively (at 8 and 18 weeks of HFD) exhibited higher glucose levels compared with the control cohort (Fig. 2, B & C). At 18 weeks glucose levels were significantly higher at 15, 30, 60, and 120 min after a glucose challenge in MacGHR KO versus control (Fig. 2C). To determine whether the impaired ipGTT observed in MacGHR KO mice was due to decreased insulin sensitivity, insulin tolerance test (ITT) was performed in both groups of mice. As seen in Fig. 2D, decreased insulin sensitivity was observed in MacGHR KO mice on HFD for 18 weeks. To further investigate the decrease in insulin sensitivity, insulin-stimulated phosphory-lation of Akt was profiled in adipose tissue, muscle, and liver. These results indicate that insulin stimulation of pAkt was significantly impaired in adipose tissue of MacGHR KO (Fig. 3, A & B). In contrast, there was no demonstrable difference in insulin stimulated p-Akt levels in muscle and liver between MacGHR KO versus control (Fig. 3, C & D).
FIGURE 2.
Progressive impairment in glucose tolerance and increased insulin resistance in MacGHR KO on HFD. MacGHR KO (ko, Ghr−/−, ▴) and control mice (wt [Ghrfl/fl,□], or het [Ghrfl/−,●]) were fasted overnight prior to ipGTT or 5 h prior to intraperitoneal ITT. Blood glucose levels were then measured using a glucometer from tail blood taken at indicated time points over a 2-h period. A, ipGTT in 22 week old male het and ko mice fed standard diet. B & C, ipGTT in male het and ko mice fed HFD (45% Kcal fat) for 8 weeks (panel B) or 18 weeks (panel C). D, ITT in male wt, het or ko mice fed a HFD (45% kcal fat) for 18 weeks. Data are mean ± S.E., *, p < 0.05 ko versus het or wt.
FIGURE 3.
Decreased insulin-stimulated-phospho-Akt in adipose tissue of MacGHR KO. A & B, ex vivo insulin stimulation in adipose tissue isolated from het [Ghrfl/−, open bar) or MacGHR KO (Ghr−/−, solid bar) mice on HFD for 18w. Adipose tissues were incubated with insulin for 0, 2, or 5 min. Akt phosphorylation and total Akt protein were assessed by Western blot. A, representative Western blots. B, quantification of Akt phosphorylation normalized to total Akt protein. Data are mean ± S.E. of three experiments, *, p < 0.05. C & D, in vivo insulin stimulation of muscle and liver Akt activation in het [Ghrfl/−, open bar) or MacGHR KO (Ghr−/−, solid bar) mice on HFD for 18w. 10 min after intraperitoneal injection of insulin, muscle, and liver tissue was harvested for measurement of Akt phosphorylation and total Akt protein by Western blot. A, representative Western blots. B, quantification of Akt phosphorylation normalized to total Akt protein. Data are mean ± S.E. of three experiments.
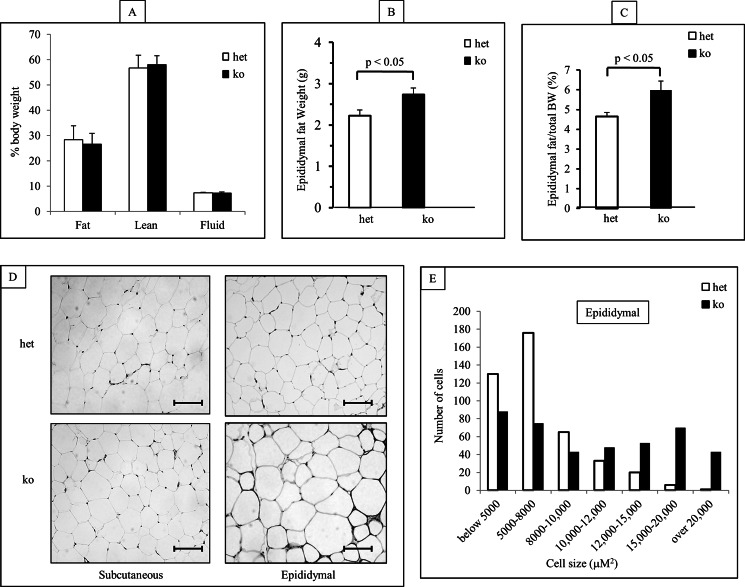
MacGHR KO Mice Exhibit Increased Epididymal Fat and Increased Adipocyte Cell Size
There were no differences in whole body composition measurements between MacGHR KO and the control cohort (Fig. 4A) mice fed HFD for 18w. However, examination of the epididymal (visceral) and subcutaneous fat depots revealed that the MacGHR KO mice had increased epididymal fat depots, expressed as either absolute weight or percent of total body weight (Fig. 4, B & C). On histological examination of epididymal fat depots, the size of adipocytes from MacGHR KO adipose tissue appeared larger than those from control animals (Fig. 4D, right panels). This was confirmed by morphometric analysis that demonstrated skewing toward the larger adipocyte cell sizes in the MacGHR KO mice (Fig. 4E). This size difference was not observed in the subcutaneous fat (Fig. 4D, left panels), suggesting that GH action on the MΦ disproportionately affected visceral fat depots in DIO.
FIGURE 4.
Increased epididymal fat and adipocyte cell size in MacGHR KO mice fed HFD for 18w. A, body composition of het (Ghrfl/−, open bar) and MacGHR KO (Ghr−/−, solid bar). B & C, epididymal fat pad weight in het (Ghrfl/−, open bar) and MacGHR KO (Ghr−/−, solid bar) mice depicted as either (B) absolute weight or (C) ratio of epididymal fat weight to total body weight (mean ± S.E.; n = 10); D, hematoxylin and eosin staining of subcutaneous and epididymal adipose tissues from het (Ghrfl/−) and MacGHR KO (Ghr−/−). E, analysis of adipocyte cell size distribution in het (Ghrfl/−, open bar) and MacGHR KO (Ghr−/−, solid bar) mice. Bar represent 100 μm. For the distribution of adipocyte size at least 120 cells were analyzed per animal.
MacGHR KO Mice Develop Increased Inflammation in Adipose Tissue
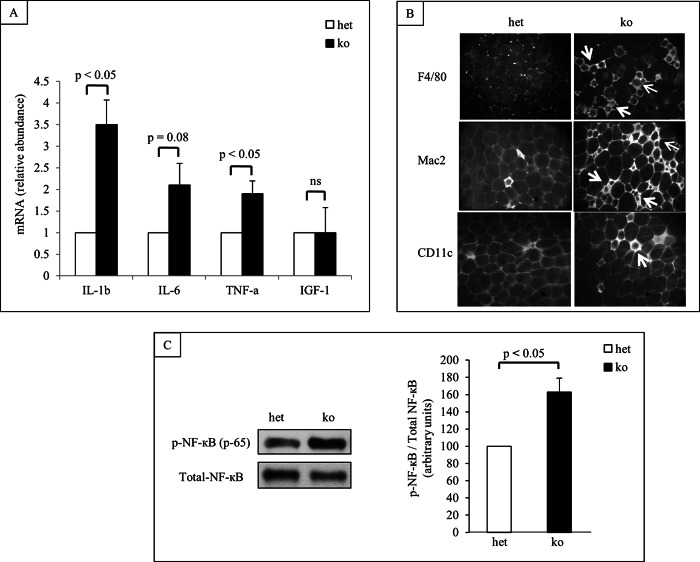
Glucose intolerance and insulin insensitivity in obesity are associated with inflammation in adipose tissue. To investigate whether lack of GH action on the MΦ modulates the inflammatory state of the adipose tissue, expression level of a cadre of relevant cytokines were measured by RT-qPCR. Our results indicated that when fed HFD for 18w pro-inflammatory cytokines, such as NF-κB, IL6, IL1β, and TNFα were expressed at higher levels in SVF isolated from MacGHR KO mice compared with SVF from litter mate controls (Fig. 5A). In contrast, there was no significant difference in the IGF-1 mRNA expression level between the MacGHR KO and control animals (Fig. 5A). Consistent with the increased expression of proinflammatory cytokines, crown-like-structures which typically associate with obesity associated chronic inflammation in adipose tissue were also increased in epididymal fat depots of MacGHR KO mice (Fig. 5B). Furthermore, Western blot analysis revealed increased levels of the phosphorylated 65 kDa subunit of NF-κB in MΦ isolated from MacGHR KO mice (Fig. 5C).
FIGURE 5.
Increased inflammation in adipose tissue of MacGHR KO mice fed HFD for 18w. A, increased expression of inflammatory cytokines. Total RNA was isolated from SVF of het (Ghrfl/−, open bar) or MacGHR KO (Ghr−/−, solid bar) mice and expression levels of the indicated cytokines anf IGF-1 were measured by SYBR Green RT-qPCR analysis. Expression levels were normalized to housekeeping gene GAPDH. The result (n = 3–5) is presented as mean ± S.E. B, increased crown-like structure in adipose tissue of MacGHR KO. Formadehyde-fixed adipose tissues were stained with pan MΦ antibodies F4/80 or Mac2, and M1 MΦ specific antibody CD11C. Crown-like structures are indicated with arrows. C, increased phopho-NF-κB protein in MΦ from MacGHR KO mice. Left panel: representative Western blot of three independent experiments. Right panel: quantification of NF-κB phosphorylation normalized to total NF-κB protein in het (open bar, set as 100) or MacGHR KO (solid bar) mice. Data are means ± S.E. of three times experiments, *, p < 0.05.
Increased Inflammation in Adipose Tissue of MacGHR KO Is Associated with Increased MΦ Infiltration and M1/M2 Ratio
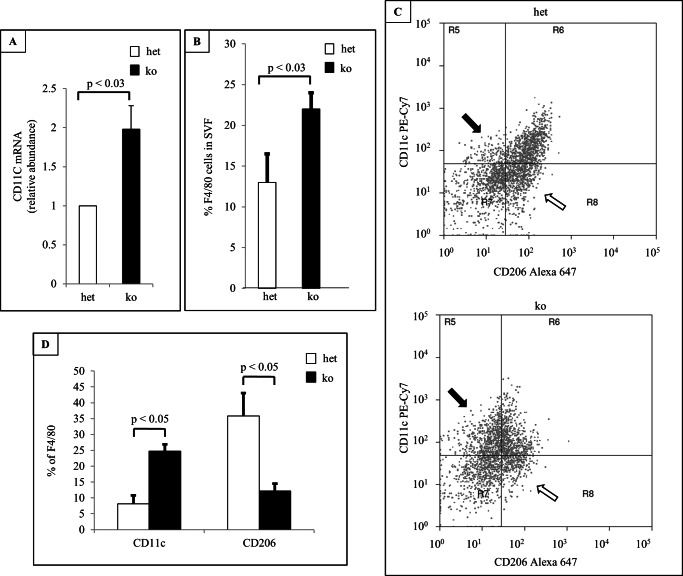
Inflammation is associated with increased macrophage infiltration and M1 macrophage population. To profile MΦ population in adipose tissue of MacGHR KO mice, SVF were isolated from the control or MacGHR KO mice fed HFD for 18w and phenotyped by flow cytometry analysis using M1(CD11c) and M2 (CD206) markers. As shown in Fig. 6B, the total number of adipose tissue MΦ was increased in epididymal fat from MacGHR KO mice. Additionally, in MacGHR KO, the proportion of M1 MΦ with pro-inflammatory phenotype (CD11c+) was significantly increased with corresponding decrease in the anti-inflammatory phenotype M2 MΦ tagged with CD206 (Fig. 6, C & D). Sybr-RT-PCR also showed increased M1 marker, CD11C in SVF from MacGHR KO mice (Fig. 6A).
FIGURE 6.
Increased MΦ and M1/M2 ratio in SVF of MacGHR KO mice fed HFD for 18w. A, expression of M1 marker, CD11c, in SVF of het (Ghrfl/−, open bar) or MacGHR KO (Ghr−/−, solid bar) measure by SYBR-RT-PCR. B, percentage of F4/80-positive cells in SVF of het (Ghrfl/−, open bar) or MacGHR KO (Ghr−/−, solid bar) analyzed using flow cytometry. C, representative flow cytometry results. CD11c-positive cells are indicated by black arrows and CD206-positive cells indicated by open arrows. D, percentage of CD11c and CD206 cells of F4/80-positive cells. The results are shown as means ± S.E. for six to seven animals per group. *, p < 0.05.
Lack of GH Action on MΦ Increases Expression of Osteopontin (OPN)
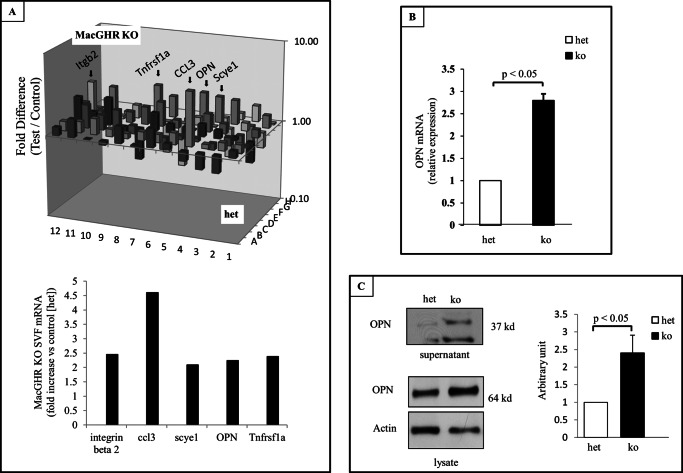
To elucidate mechanisms that link the absence of GH action in the MΦ with the observed increase in inflammation and MΦ accumulation in adipose tissue, we surveyed the expression of candidate genes using targeted microarray. These results revealed that the expression of OPN was significantly elevated in SVF from the MacGHR KO mice fed HFD for 18w (Fig. 7A). The increase in steady state levels of mRNA was confirmed by RT-qPCR of independent samples (Fig. 7B). Furthermore, Western blot analysis revealed that the expression of OPN protein, both the secreted form in the supernatant and the intracellular form in the cell lysate, was also increased in bone marrow-derived MΦ from MacGHR KO mice (Fig. 7B).
FIGURE 7.
Increased expression of OPN in MacGHR KO mice fed HFD for 18w. A, PCR array analysis of cytokine expression in SVF isolated from het (Ghrfl/−) or MacGHR KO (Ghr−/−) mice. Upper panel, three-dimensional representation of the change (positive and negative) in mRNA expression in MacGHR KO versus het. The bars representing proinflammatory genes increased in the MacGHR KO SVF are indicated by arrows. Lower panel, graphical representation of the fold increase in the proinflammatory genes, itgb2 (integrin β2), ccl3 (chemokine [C-C motif] ligand 3, also known as macrophage inflammatory protein-1α (MIP-1α),), scye 1 (small inducible cytokine subfamily E. member 1), spp1 (osteopontin), and Tnfrsaf1a (tumor necrosis factor receptor superfamily, member 1A); B, expression of OPN mRNA in SVF. Total RNA was isolated from SVF of het (Ghrfl/−, open bar) or MacGHR KO (Ghr−/−, solid bar) mice. SYBR Green RT-PCR was conducted as described under “Experimental Procedures” and results normalized to GAPDH level. The results are shown as means ± S.E. for three to five animals per group. *, p < 0.05. C, Western blot analysis of bone marrow-derived MΦ from either supernatant (upper panel) or total cell lysate (middle panel) from het (Ghrfl/−) or MacGHR KO (Ghr−/−). Bottom panel, densitometric analysis of Western blots of OPN in lysate normalized to actin.
GH Increases OPN Expression via Direct Effect on the OPN Promoter
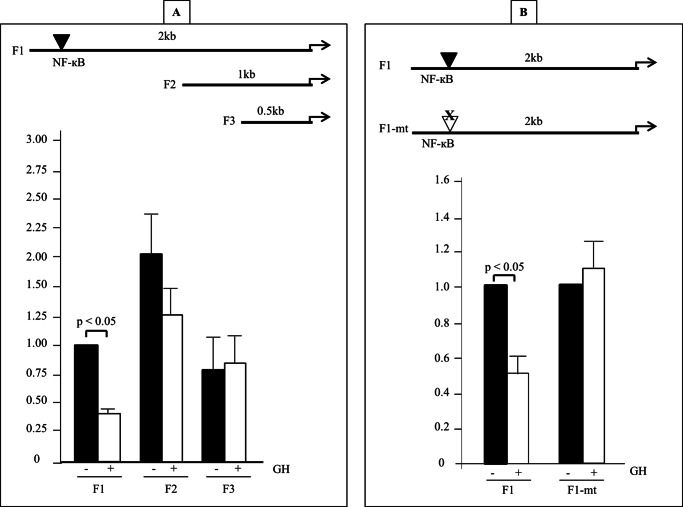
Increased levels of OPN expression in the MacGHR KO mice on HFD could be due to a direct effect of GH on OPN gene expression or indirect action mediated via effect of GH on regulators of OPN expression such as cytokines (e.g. TNFα that increase OPN expression or PPARγ and/or LXR ligands that antagonize OPN expression). To investigate the possibility of direct effects of GH on OPN gene expression we cloned varying lengths of the murine OPN promoter (F1 −2044 to −7, F2 −985 to −7 and F3 −482 to −7) into the luciferase reporter vector pGL3 and measured the effect of GH on the activity of the OPN promoter in transient transfection assays. These experiments revealed that GH significantly down-regulated F1 OPN promoter activity (Fig. 8A). In contrast, the promoter activities of F2 and F3 promoter fragments were not significantly altered by GH treatment, thus mapping the GH responsive region to between −2044 and −986 of the OPN promoter. In silico analysis of the −2044 to −986 region identified a putative NF-κB binding site (GGAATTTCCC). Previous reports had identified this NF-κB binding site to act as an inhibitor of OPN promoter activity (33, 34). To investigate the role of this NF-κB binding site in transducing GH action on the OPN promoter, we mutated the site in the F1 luciferase-promoter construct. Transient transfection experiments revealed that mutating the NF-κB site resulted in loss of GH inhibitory effect on the OPN promoter (Fig. 8B). These results indicate that GH inhibits OPN promoter via interaction with this NF-κB site in the distal OPN promoter.
FIGURE 8.
GH inhibits OPN promoter activity. A, top panel, deletion analysis of OPN promoter. OPN promoters of varying length (F1, F2, and F3) were generated by PCR and inserted into the luciferase reporter vector pGL3. Bottom panel, J774A.1 cells were transfected with pGL3 basic, F1, F2, or F3 OPN-pGL3, and exposed to either vehicle (solid bar) or GH (500 ng/ml; open bar) × 24 h prior to measurement of luciferase activity. Luciferase activities (Renilla luciferase was used to normalize for transfection efficiency) are expressed as fold increase over activity in the absence of GH. Values are expressed as mean ± S.E. of five independent experiments. B, top panel, location of the NF-κB site in F1 segment that was mutated (mt). Bottom panel, J774A.1 cells were transfected with F1, or F1-mt and exposed to either vehicle (solid bar) or GH (500 ng/ml; open bar) × 48 h. Luciferase activities (Renilla luciferase was used to normalize for transfection efficiency) are expressed as fold increase over activity in the absence of GH. Values are expressed as mean ± S.E. of four independent experiments.
DISCUSSION
The seminal finding of this study is the discovery of a hitherto unknown role for GH action on the MΦ in the maintenance of adipose tissue homeostasis in DIO. Thus on a HFD, lack of GH action on the MΦ resulted in increased MΦ infiltration and chronic inflammation in adipose tissue, insulin resistance, and glucose intolerance. The increased inflammation in adipose tissue of mice with targeted knock-out of GH receptor (MacGHR KO) in MΦ is associated with increased adipose tissue expression of the chemokine osteopontin (OPN), a critical regulator of obesity-associated adipose tissue inflammation and insulin resistance. Our studies also identify a mechanism for this increased expression of OPN to be the loss of a suppressive effect of GH on a NF-κB cis-element in the distal OPN promoter. These phenotypic changes in MacGHR KO are not accompanied by changes in MΦ IGF1 expression, thus supporting a novel direct action of GH on the MΦ. The prevailing models of GH metabolic actions do not involve the MΦ; our findings argue for a paradigm shift in the current understanding of the metabolic sequelae of tissue-specific GH action.
The conventional model of GH action is based on the premise that the preponderance of GH action is mediated via generation of liver-derived IGF1 (35). This paradigm is being challenged by mouse genetic models that do not support an essential role for liver-derived IGF1 in transducing GH actions on statural growth (31, 36, 37). Consequently, these studies have highlighted the urgency to understand the physiological significance of direct action of GH at the cell/tissue level. The adipocyte is a major target for GH action. Traditionally, the actions of GH on the adipocyte are believed to be mediated through activation of GH receptors on the adipocyte which results in increased lipolytic activity via catecholamine-induced lipolysis secondary to up-regulation of β-adrenergic receptors, and decreased accumulation of triglyceride in the adipocyte via inhibition of lipoprotein lipase activity (3). However, our previous studies had identified a potential paracrine mechanism for GH effects on adipose tissue (4). Using in vitro cell culture models we had demonstrated that GH acts directly on the macrophage (MΦ) to alter the profile of cytokines secreted by the MΦ, and thus in a paracrine manner increases adipocyte differentiation. The present study identifies a physiological significance of this novel paradigm of GH acting on the MΦ to alter adipocyte function in a paracrine manner. Specifically, our studies establish that on a HFD, lack of GH action on the MΦ results in preferential accumulation of M1-polarized MΦ in adipose tissue. It is noteworthy that a recent study reported that targeted ablation of GHR expression in adipose tissue in mouse did not alter glucose metabolism on a normal diet (38). These results were obtained on a normal diet and it would be of interest to compare the effect of GH action on adipose tissue versus adipose tissue macrophage on a high fat diet.
The results of the ITT indicate that the lack of GH action on the MΦ results in worsening of whole body insulin resistance in DIO and the studies with ex vivo adipose tissue explants demonstrate adipose tissue insulin resistance in the MacGHR KO animals. In addition to the adipose tissue, MΦ are also resident in other tissues/organs, such as skeletal muscle and liver, that play direct roles in determining whole body insulin sensitivity. Our results indicate that insulin sensitivity is not impaired in muscle and liver tissue of the MacGHR KO animals, thus implicating the adipose tissue as the primary site for insulin resistance in this model. A recent study demonstrated that disruption of GH action in skeletal muscle in mice was associated with reduced adiposity and improved insulin sensitivity in DIO (39). In contrast, Mavalli et al. (40) reported that ablation of GHR in skeletal muscle resulted in increased body weight and adiposity, and deterioration in insulin sensitivity. The exact reason(s) for the variance in findings between these two studies is not clear, but could be related to the differences in the genetic background and the promoters used to drive the Cre-recombinase. Thus, these studies emphasize the importance of analyzing and understanding tissue-specific actions of GH on glucose homeostasis and insulin sensitivity. Furthermore, the demonstration of effects of GH action on adipose tissue MΦ in the present study highlights the importance and relevance of investigating the role of GH in macrophages resident in other organs and tissues such as liver (Kupffer cells) and brain (microglia).
Adipose tissue MΦ (ATMs) is a major source of inflammatory cytokines in DIO. Our results reveal that abrogating GH action on MΦ accentuates the inflammatory state of the adipose tissue in DIO. Proinflammatory cytokines, such as TNF-α, IL-1b, IL-6, and OPN, are expressed at higher level in SVF of MacGHR KO versus controls (Fig. 5A). The increase in crown-like structures observed in MacGHR KO mice compared with control mice provides further evidence of increased adipose tissue inflammation in MacGHR KO. Consistent with these findings, phosphorylation of NF-κB, a key component in inflammatory pathway, is also increased in MacGHR KO. The increase in NF-κB activity in the MacGHR KO ATMs is consistent with the inhibitory effect of GH on LPS stimulated NF-κB nuclear translocation in human monocytes (41) and our previous studies demonstrating GH-dependent inhibition of NF-κB activity in J774A.1 MΦ (27). In contrast in the lung, GH enhanced LPS stimulated NF- κB activity (42), suggesting that the effect of GH on NF-κB activation is tissue/cell type-specific.
Osteopontin (OPN; also known as ETA1 and encoded by SPP1) is a cytokine that is secreted by MΦ and other types of cells (43, 44). OPN modulates the function of MΦ including migration and MΦ accumulation (45). In DIO, expression of OPN is enhanced and is posited to increase the migration of M1 MΦ into adipose tissue and subsequently increase inflammation and decrease insulin sensitivity (46, 47). Total OPN knock-out mice fed a HFD display decreased MΦ infiltration and less insulin resistance than control mice (17). Our data show that OPN is up-regulated in adipose tissue SVF and bone marrow-derived MΦ of MacGHR KO (Fig. 7). The increase in OPN could be due to a direct effect of GH on OPN gene expression or an indirect action mediated via the increased inflammation in adipose tissue in MacGHR KO. It is noteworthy that the increase in OPN expression in bone marrow-derived MΦ of MacGHR KO was not accompanied by parallel changes in the expression of other cytokines (e.g. IL-1β and IL-6, data not shown), thus suggested a specific effect of GH on OPN expression. Our studies establish that GH action directly inhibits OPN promoter activity (Fig. 8). Furthermore, we demonstrate that this inhibitory effect is regulated through NF-κB pathway since mutating a NF-κB binding site on the OPN promoter, previously demonstrated to inhibit OPN promoter activity (33, 34) abolishes the inhibitory effect of GH on the OPN promoter (Fig. 8B).
Pituitary GH is essential for postnatal linear growth and also exerts pleotropic actions on metabolism of fat, carbohydrate, and protein. In the adult, wherein the linear growth promoting actions of GH are not apparent, GH effects on metabolism are preeminent. However, the precise biological significance and the essential nature of these actions in the adult remain unclear. Consequently, there is lack of consensus about the need to treat adult GH deficiency with GH (48). Our results support a model wherein GH plays an essential and biologically significant role in the adult via its actions on the MΦ. Our study indicates that GH actions on the MΦ blunt the deleterious effects of DIO on adipose tissue. These novel actions of GH highlight a hitherto not described physiological role for GH in the adult.
In summary, our results argue for a paradigm shift in our understanding of the biological roles of GH by placing the MΦ, a cell type hitherto not well studied as a GH target, central to the metabolic (non-growth) actions of GH in the intact animal. Our results support a model wherein in DIO, lack of GH action on the MΦ results in increased inflammation and decreased insulin sensitivity in adipose tissue. This effect is in part mediated by increased OPN expression resulting from loss of GH inhibitory effect on an NF-κB element in the OPN promoter. We conclude that one of the physiological roles of GH in the MΦ is to suppress NF-κB activation and OPN expression. Our results in mice provide novel insights into GH role in the maintenance of metabolic homeostasis in DIO and support the possibility that, despite the dogma of GH being a diabetogenic hormone that increases insulin resistance, administration of GH could have salutary effects on DIO-associated chronic inflammation and insulin resistance in humans.
Acknowledgments
We thank Dr. Stuart Frank (University of Alabama at Birmingham) for the generous provision of the anti-GH receptor antibody (AL-47).
This work was supported, in whole or in part, by grants from the National Institutes of Health (DK49845 (to R. K. M.) and P60DK-20572 Center) and the D.R.E.A.M. Foundation.
- GH
- growth hormone
- MΦ
- macrophage
- ipGTT
- intraperitoneal glucose
- ITT
- insulin tolerance
- HFD
- high fat diet
- OPN
- osteopontin
- SVF
- stromal vascular fraction
- DIO
- diet-induced obesity
- MIP
- MΦ inflammatory protein.
REFERENCES
- 1. Newbern D., Freemark M. (2011) Placental hormones and the control of maternal metabolism and fetal growth. Curr. Opin. Endocrinol. Diabetes Obes. 18, 409–416 [DOI] [PubMed] [Google Scholar]
- 2. Bloch C. A., Clemons P., Sperling M. A. (1987) Puberty decreases insulin sensitivity. J. Pediatr. 110, 481–487 [DOI] [PubMed] [Google Scholar]
- 3. Clayton P. E., Cowell C. T. (2000) Safety issues in children and adolescents during growth hormone therapy–a review. Growth Horm IGF Res. 10, 306–317 [DOI] [PubMed] [Google Scholar]
- 4. Møller N., Jørgensen J. O. (2009) Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 30, 152–177 [DOI] [PubMed] [Google Scholar]
- 5. Johansen T., Laurino C., Barreca A., Malmlöf K. (2005) Reduction of adiposity with prolonged growth hormone treatment in old obese rats: effects on glucose handling and early insulin signaling. Growth Horm IGF Res. 15, 55–63 [DOI] [PubMed] [Google Scholar]
- 6. Thirone A. C., Carvalho C. R., Saad M. J. (1999) Growth hormone stimulates the tyrosine kinase activity of JAK2 and induces tyrosine phosphorylation of insulin receptor substrates and Shc in rat tissues. Endocrinology 140, 55–62 [DOI] [PubMed] [Google Scholar]
- 7. Tatro J. B., Schwartz J. (1987) Metabolic effects of acute and prolonged growth hormone deficiency in streptozotocin-diabetic rats. Endocrinology 120, 373–380 [DOI] [PubMed] [Google Scholar]
- 8. Zeyda M., Stulnig T. M. (2007) Adipose tissue macrophages. Immunol. Lett. 112, 61–67 [DOI] [PubMed] [Google Scholar]
- 9. Wellen K. E., Hotamisligil G. S. (2005) Inflammation, stress, and diabetes. J. Clin. Invest. 115, 1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mantovani A. (2006) Macrophage diversity and polarization: in vivo veritas. Blood 108, 408–409 [Google Scholar]
- 11. Fujisaka S., Usui I., Bukhari A., Ikutani M., Oya T., Kanatani Y., Tsuneyama K., Nagai Y., Takatsu K., Urakaze M., Kobayashi M., Tobe K. (2009) Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 58, 2574–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W. (2003) Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Olefsky J. M., Glass C. K. (2010) Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72, 219–246 [DOI] [PubMed] [Google Scholar]
- 14. DiPietro L. A., Burdick M., Low Q. E., Kunkel S. L., Strieter R. M. (1998) MIP-1alpha as a critical macrophage chemoattractant in murine wound repair. J. Clin. Invest. 101, 1693–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanda H., Tateya S., Tamori Y., Kotani K., Hiasa K., Kitazawa R., Kitazawa S., Miyachi H., Maeda S., Egashira K., Kasuga M. (2006) MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 116, 1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maurer M., von Stebut E. (2004) Int. J. Biochem. Cell B 36, 1882–1886 [DOI] [PubMed] [Google Scholar]
- 17. Nomiyama T., Perez-Tilve D., Ogawa D., Gizard F., Zhao Y., Heywood E. B., Jones K. L., Kawamori R., Cassis L. A., Tschöp M. H., Bruemmer D. (2007) Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J. Clin. Invest. 117, 2877–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Denhardt D. T., Guo X. J. (1993) Osteopontin: a protein with diverse functions. Faseb J. 7, 1475–1482 [PubMed] [Google Scholar]
- 19. Kiefer F. W., Zeyda M., Gollinger K., Pfau B., Neuhofer A., Weichhart T., Säemann M. D., Geyeregger R., Schlederer M., Kenner L., Stulnig T. M. (2010) Neutralization of osteopontin inhibits obesity-induced inflammation and insulin resistance. Diabetes 59, 935–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Veldhuis J. D., Iranmanesh A., Ho K., Waters M. J., Johnson M. L., Lizarralde G. (1991) Dual defects in pulsatile growth hormone secretion and clearance subserve the hyposomatotropism of obesity in man. J. Clin. Endocr. Metab. 72, 51–59 [DOI] [PubMed] [Google Scholar]
- 21. Makimura H., Stanley T., Mun D., You S. M., Grinspoon S. (2008) The effects of central adiposity on growth hormone (GH) response to GH-releasing hormone-arginine stimulation testing in men. J. Clin. Endocr. Metab. 93, 4254–4260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rasmussen M. H., Juul A., Kjems L. L., Hilsted J.,. (2006) Effects of short-term caloric restriction on circulating free IGF-I, acid-labile subunit, IGF-binding proteins (IGFBPs)-1–4, and IGFBPs-1–3 protease activity in obese subjects. Eur. J. Endocrinol. 155, 575–581 [DOI] [PubMed] [Google Scholar]
- 23. Rasmussen M. H., Wildschiødtz G., Juul A., Hilsted J. (2008) Polysomnographic sleep, growth hormone insulin-like growth factor-I axis, leptin, and weight loss. Obesity 16, 1516–1521 [DOI] [PubMed] [Google Scholar]
- 24. Rasmussen M. H., Hvidberg A., Juul A., Main K. M., Gotfredsen A., Skakkebaek N. E., Hilsted J., Skakkebae N. E. (1995) Massive weight loss restores 24-hour growth hormone release profiles and serum insulin-like growth factor-I levels in obese subjects. J. Clin. Endocrinol. Metab. 80, 1407–1415 [DOI] [PubMed] [Google Scholar]
- 25. Rasmussen M. H., Juul A., Kjems L. L., Skakkebaek N. E., Hilsted J. (1995) Lack of stimulation of 24-hour growth hormone release by hypocaloric diet in obesity. J Clin. Endocrinol. Metab. 80, 796–801 [DOI] [PubMed] [Google Scholar]
- 26. Hull K. L., Thiagarajah A., Harvey S. (1996) Cellular localization of growth hormone receptors/binding proteins in immune tissues. Cell Tissue Res. 286, 69–80 [DOI] [PubMed] [Google Scholar]
- 27. Lu C., Kumar P. A., Fan Y., Sperling M. A., Menon R. K. (2010) A novel effect of growth hormone on macrophage modulates macrophage-dependent adipocyte differentiation. Endocrinology 151, 2189–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hochberg Z., Hertz P., Maor G., Oiknine J., Aviram M. (1992) Growth hormone and insulin-like growth factor-I increase macrophage uptake and degradation of low density lipoprotein. Endocrinology 131, 430–435 [DOI] [PubMed] [Google Scholar]
- 29. Smith J. R., Benghuzzi H., Tucci M., Puckett A., Hughes J. L. (2000) The effects of growth hormone and insulin-like growth factor on the proliferation rate and morphology of RAW 264.7 macrophages. Biomed. Sci. Instrum. 36, 111–116 [PubMed] [Google Scholar]
- 30. Tripathi A., Sodhi A. (2007) Production of nitric oxide by murine peritoneal macrophages in vitro on treatment with prolactin and growth hormone: involvement of protein tyrosine kinases, Ca(++), and MAP kinase signal transduction pathways. Mol. Immunol. 44, 3185–3194 [DOI] [PubMed] [Google Scholar]
- 31. Fan Y., Menon R. K., Cohen P., Hwang D., Clemens T., DiGirolamo D. J., Kopchick J. J., Le Roith D., Trucco M., Sperling M. A. (2009) Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J. Biol. Chem. 284, 19937–19944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schultz R. M., Woods W. A., Mohr S. J., Chirigos M. A. (1976) Immune response of BALB/c X DBA/2F1 mice to a tumor allograft during pyran copolymer-induced tumor enhancement. Cancer Res. 36, 1641–1646 [PubMed] [Google Scholar]
- 33. Williams E. S., Wilson E., Ramos K. S. (2012) NF-κB and matrix-dependent regulation of osteopontin promoter activity in allylamine-activated vascular smooth muscle cells. Oxidative Med. Cell. Longevity, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao Wei, L. W., Zhang Meng, Wang Peng, Zhang Lei, Yuan Chao, Qi Jianni, Qiao Yu, Kuo Paul C., Gao Chengjiang. (2011) NF-κB– and AP-1–mediated DNA looping regulates osteopontin transcription in endotoxin-stimulated murine macrophages. J. Immunol. 186, 3173–3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaplan S. A., Cohen P. (2007) The somatomedin hypothesis 2007: 50 years later. J. Clin. Endocrinol. Metab. 92, 4529–4535 [DOI] [PubMed] [Google Scholar]
- 36. Yakar S., Liu J. L., Stannard B., Butler A., Accili D., Sauer B., LeRoith D. (1999) Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl. Acad. Sci. U.S.A. 96, 7324–7329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yakar S., Sun H., Zhao H., Pennisi P., Toyoshima Y., Setser J., Stannard B., Scavo L., Leroith D. (2005) Metabolic effects of IGF-I deficiency: lessons from mouse models. Pediatr. Endocrinol. Rev. 3, 11–19 [PubMed] [Google Scholar]
- 38. List E. O., Berryman D. E., Funk K., Gosney E. S., Jara A., Kelder B., Wang X., Kutz L., Troike K., Lozier N., Mikula V., Lubbers E. R., Zhang H., Vesel C., Junnila R. K., Frank S. J., Masternak M. M., Bartke A., Kopchick J. J. (2013) The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol. Endocrinol. 27, 524–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vijayakumar A., Wu Y., Sun H., Li X., Jeddy Z., Liu C., Schwartz G. J., Yakar S., LeRoith D. (2012) Targeted loss of GHR signaling in mouse skeletal muscle protects against high-fat diet-induced metabolic deterioration. Diabetes 61, 94–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mavalli M. D., DiGirolamo D. J., Fan Y., Riddle R. C., Campbell K. S., van Groen T., Frank S. J., Sperling M. A., Esser K. A., Bamman M. M., Clemens T. L. (2010) Distinct growth hormone receptor signaling modes regulate skeletal muscle development and insulin sensitivity in mice. J. Clin. Invest. 120, 4007–4020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Haeffner A., Thieblemont N., Déas O., Marelli O., Charpentier B., Senik A., Wright S. D., Haeffner-Cavaillon N., Hirsch F. (1997) Inhibitory effect of growth hormone on TNF-α secretion and nuclear factor-κB translocation in lipopolysaccharide-stimulated human monocytes. J. Immunol. 158, 1310–1314 [PubMed] [Google Scholar]
- 42. Liu Z., Yu Y., Jiang Y., Li J. (2002) Growth hormone increases lung NF-κB activation and lung microvascular injury induced by lipopolysaccharide in rats. Ann. Clin. Lab. Sci. 32, 164–170 [PubMed] [Google Scholar]
- 43. Rittling S. R. (2011) Osteopontin in macrophage function. Expert. Rev. Mol. Med. 13, e15. [DOI] [PubMed] [Google Scholar]
- 44. Wang K. X., Denhardt D. T. (2008) Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 19, 333–345 [DOI] [PubMed] [Google Scholar]
- 45. Standal T., Borset M., Sundan A. (2004) Role of osteopontin in adhesion, migration, cell survival and bone remodeling. Exp. Oncol. 26, 179–184 [PubMed] [Google Scholar]
- 46. Chapman J., Miles P. D., Ofrecio J. M., Neels J. G., Yu J. G., Resnik J. L., Wilkes J., Talukdar S., Thapar D., Johnson K., Sears D. D. (2010) Osteopontin is required for the early onset of high fat diet-induced insulin resistance in mice. Plos One 5, e13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zeyda M., Gollinger K., Todoric J., Kiefer F. W., Keck M., Aszmann O., Prager G., Zlabinger G. J., Petzelbauer P., Stulnig T. M. (2011) Osteopontin is an activator of human adipose tissue macrophages and directly affects adipocyte function. Endocrinology 152, 2219–2227 [DOI] [PubMed] [Google Scholar]
- 48. Clemmons D. R. (2010) The diagnosis and treatment of growth hormone deficiency in adults. Curr. Opin. Endocrinol. Diabetes. Obes. 17, 377–383 [DOI] [PubMed] [Google Scholar]